Abstract
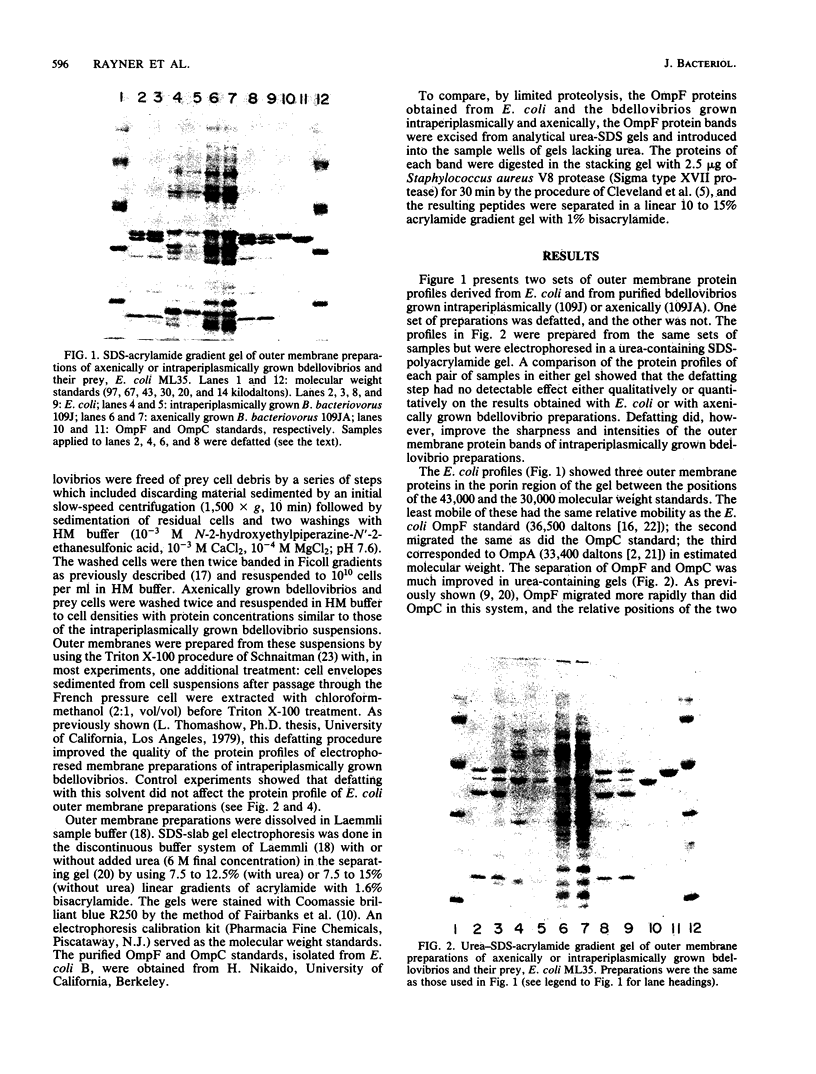
Outer membrane preparations of Bdellovibrio bacteriovorus grown intraperiplasmically on Escherichia coli containing OmpF were prepared by the Triton X-100 procedure of Schnaitman (J. Bacteriol. 108:545-552, 1971). They contained a protein that migrated to almost the same position as E. coli OmpF in sodium dodecyl sulfate-acrylamide gradient gel electrophoresis and to the same position as E. coli OmpF when urea was incorporated into the gel. The mobility of this protein increased relative to that of OmpC in urea-containing gels as does E. coli OmpF. However, the same protein was also produced during axenic growth and during intraperiplasmic growth on prey lacking OmpF. The peptide profile generated by partial proteolysis of this protein showed no homology to that produced from E. coli OmpF. We conclude that B. bacteriovorus synthesizes an OmpF-like protein. Previous claims that the bdellovibrio incorporates an intact E. coli OmpF are not consistent with these observations.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Organization of proteins in the native and reformed outer membrane of Escherichia coli. Biochim Biophys Acta. 1972 Aug 9;274(2):478–488. doi: 10.1016/0005-2736(72)90193-9. [DOI] [PubMed] [Google Scholar]
- Chai T., Wu V., Foulds J. Colicin A receptor: role of two Escherichia coli outer membrane proteins (OmpF protein and btuB gene product) and lipopolysaccharide. J Bacteriol. 1982 Aug;151(2):983–988. doi: 10.1128/jb.151.2.983-988.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cover W. H., Martinez R. J., Rittenberg S. C. Permeability of the boundary layers of Bdellovibrio bacteriovorus 109J and its bdelloplasts to small hydrophilic molecules. J Bacteriol. 1984 Feb;157(2):385–390. doi: 10.1128/jb.157.2.385-390.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decad G. M., Nikaido H. Outer membrane of gram-negative bacteria. XII. Molecular-sieving function of cell wall. J Bacteriol. 1976 Oct;128(1):325–336. doi: 10.1128/jb.128.1.325-336.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich D. L., Duran C. P., Conti S. F. Acquisition of Escherichia coli outer membrane proteins by Bdellovibrio sp. strain 109D. J Bacteriol. 1984 Jul;159(1):329–334. doi: 10.1128/jb.159.1.329-334.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Guerrini F., Romano V., Valenzi M., Di Giulio M., Mupo M. R., Sacco M. Molecular parasitism in the Escherichia coli-Bdellovibrio bacteriovorus system: translocation of the matrix protein from the host to the parasite outer membrane. EMBO J. 1982;1(11):1439–1444. doi: 10.1002/j.1460-2075.1982.tb01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning U., Rehn K., Hoehn B. Cell envelope and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2033–2036. doi: 10.1073/pnas.70.7.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning U., Schmidmayr W., Hindennach I. Major proteins of the outer cell envelope membrane of Escherichia coli K-12: multiple species of protein I. Mol Gen Genet. 1977 Sep 9;154(3):293–298. doi: 10.1007/BF00571285. [DOI] [PubMed] [Google Scholar]
- Hofstra H., Dankert J. Porin from the outer membrane of Escherichia coli: immunological characterization of native and heat-dissociated forms. J Gen Microbiol. 1981 Aug;125(2):285–292. doi: 10.1099/00221287-125-2-285. [DOI] [PubMed] [Google Scholar]
- Ichihara S., Mizushima S. Characterization of major outer membrane proteins O-8 and O-9 of Escherichia coli K-12. Evidence that structural genes for the two proteins are different. J Biochem. 1978 Apr;83(4):1095–1100. doi: 10.1093/oxfordjournals.jbchem.a131998. [DOI] [PubMed] [Google Scholar]
- Kuenen J. G., Rittenberg S. C. Incorporation of long-chain fatty acids of the substrate organism by Bdellovibrio bacteriovorus during intraperiplasmic growth. J Bacteriol. 1975 Mar;121(3):1145–1157. doi: 10.1128/jb.121.3.1145-1157.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Rittenberg S. C. Partial characterization of lipid A of intraperiplasmically grown Bdellovibrio bacteriovorus. J Bacteriol. 1981 Sep;147(3):869–874. doi: 10.1128/jb.147.3.869-874.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Factors affecting the electrophoretic mobility of the major outer membrane proteins of Escherichia coli in polyacrylamide gels. Biochim Biophys Acta. 1979 Nov 23;581(1):163–178. doi: 10.1016/0005-2795(79)90233-2. [DOI] [PubMed] [Google Scholar]
- Reithmeier R. A., Bragg P. D. Molecular characterization of a heat-modifiable protein from the outer membrane of Escherichia coli. Arch Biochem Biophys. 1977 Jan 30;178(2):527–534. doi: 10.1016/0003-9861(77)90223-5. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. 3. Evidence that the major protein of Escherichia coli O111 outer membrane consists of four distinct polypeptide species. J Bacteriol. 1974 May;118(2):442–453. doi: 10.1128/jb.118.2.442-453.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]