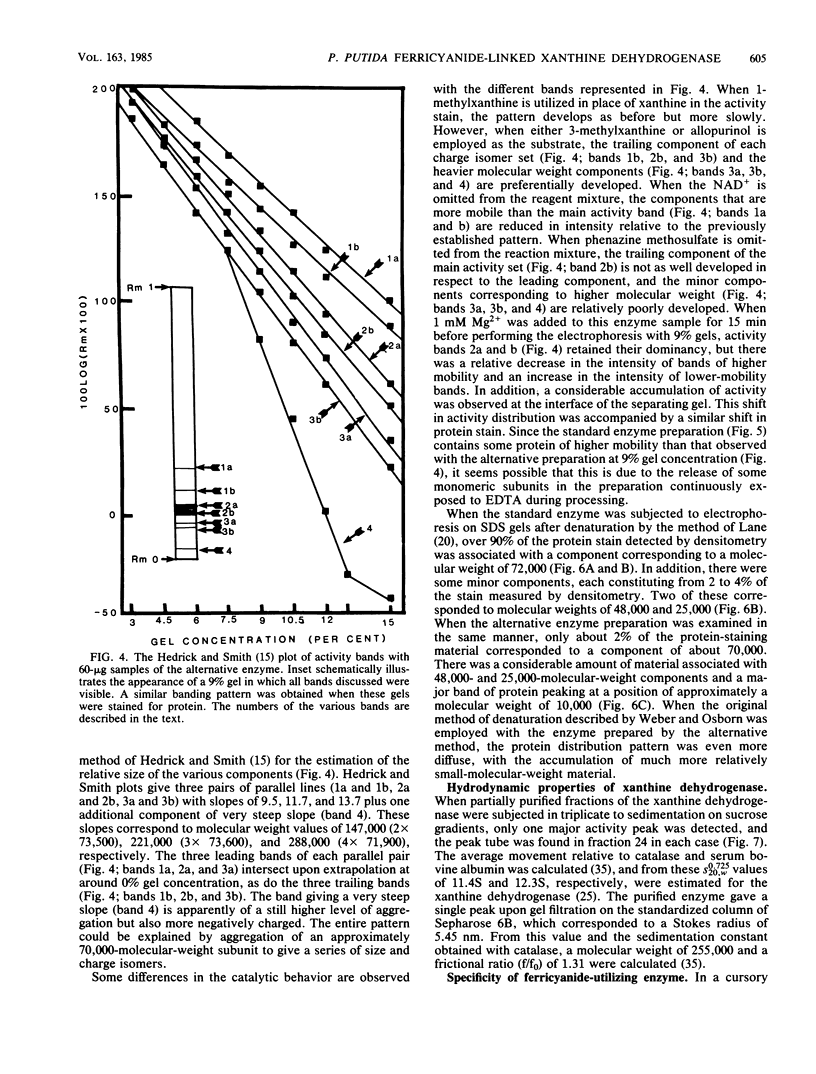
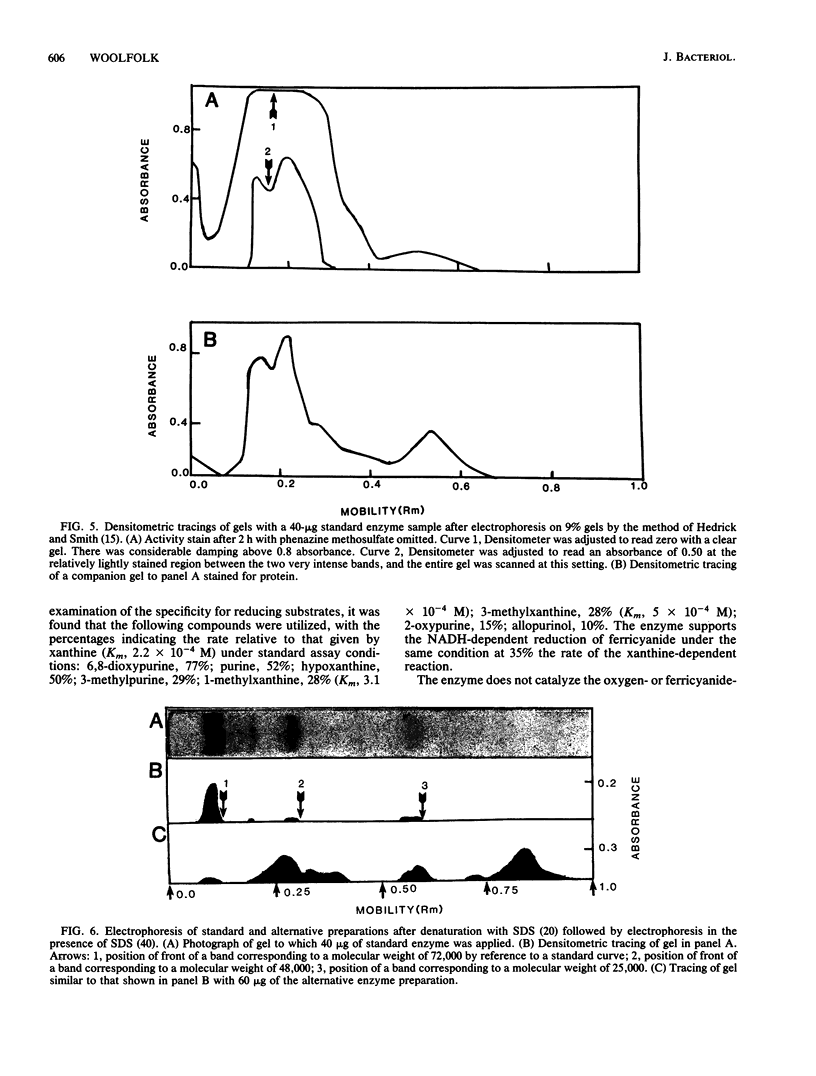
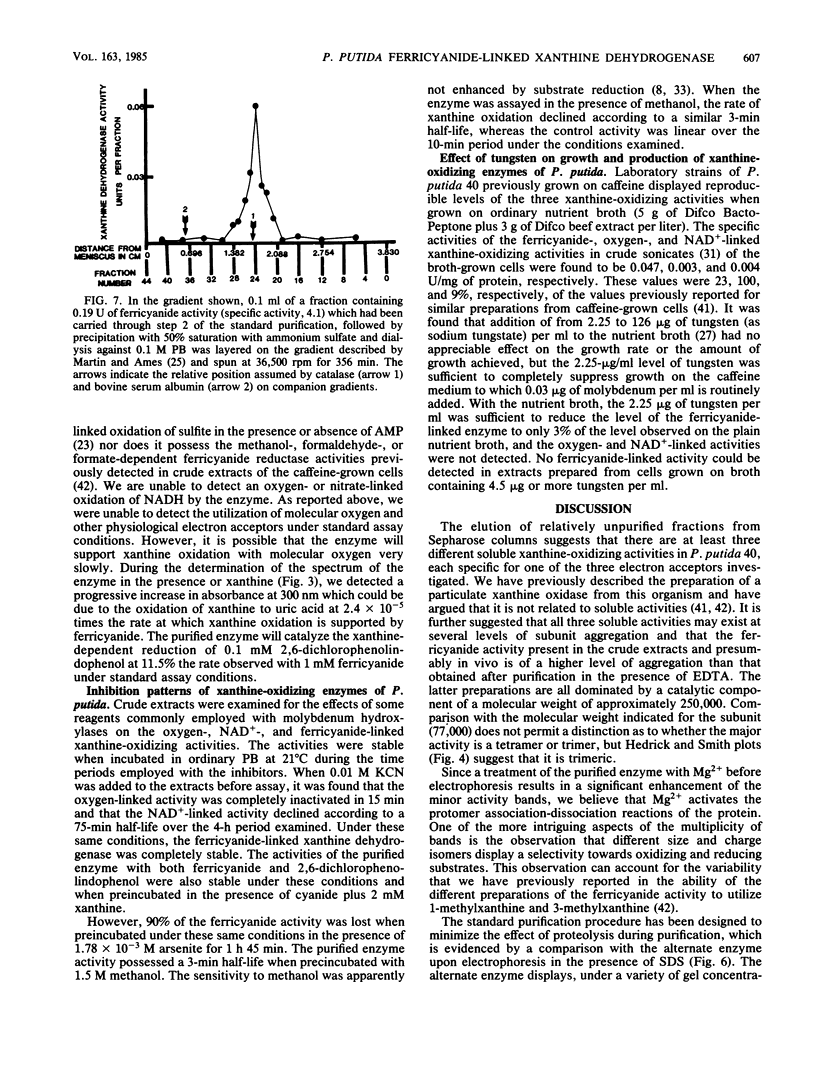
Abstract
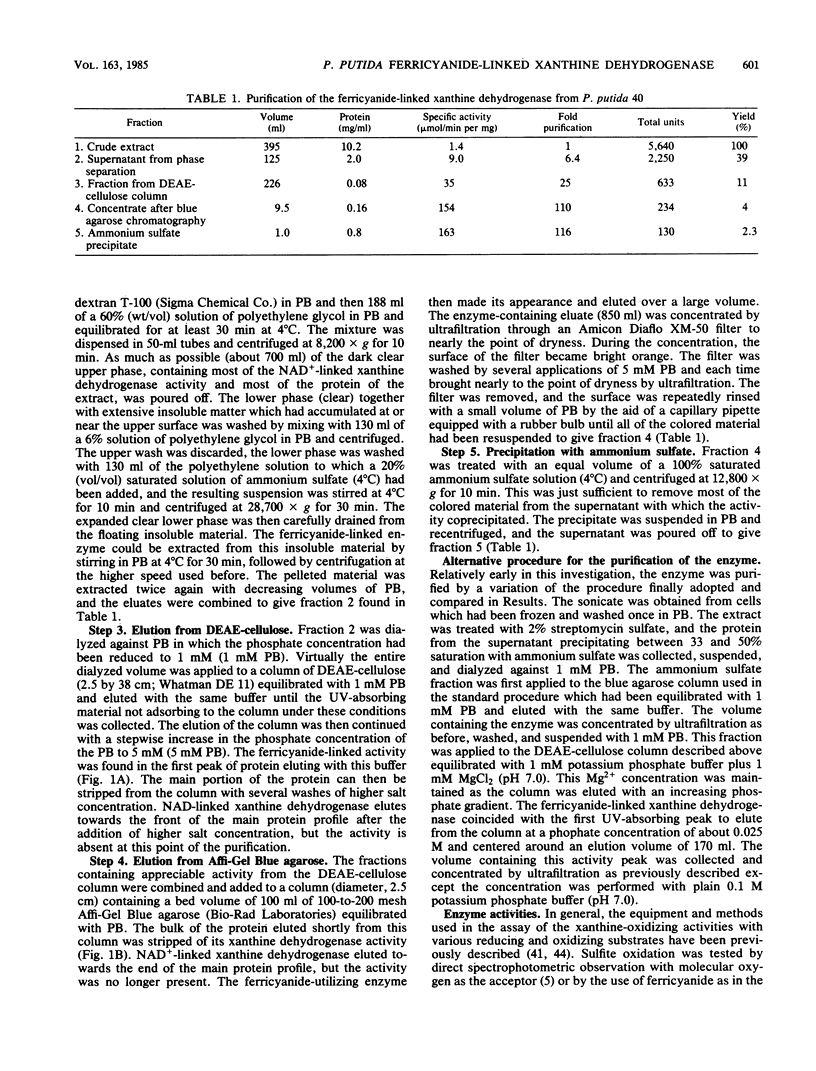
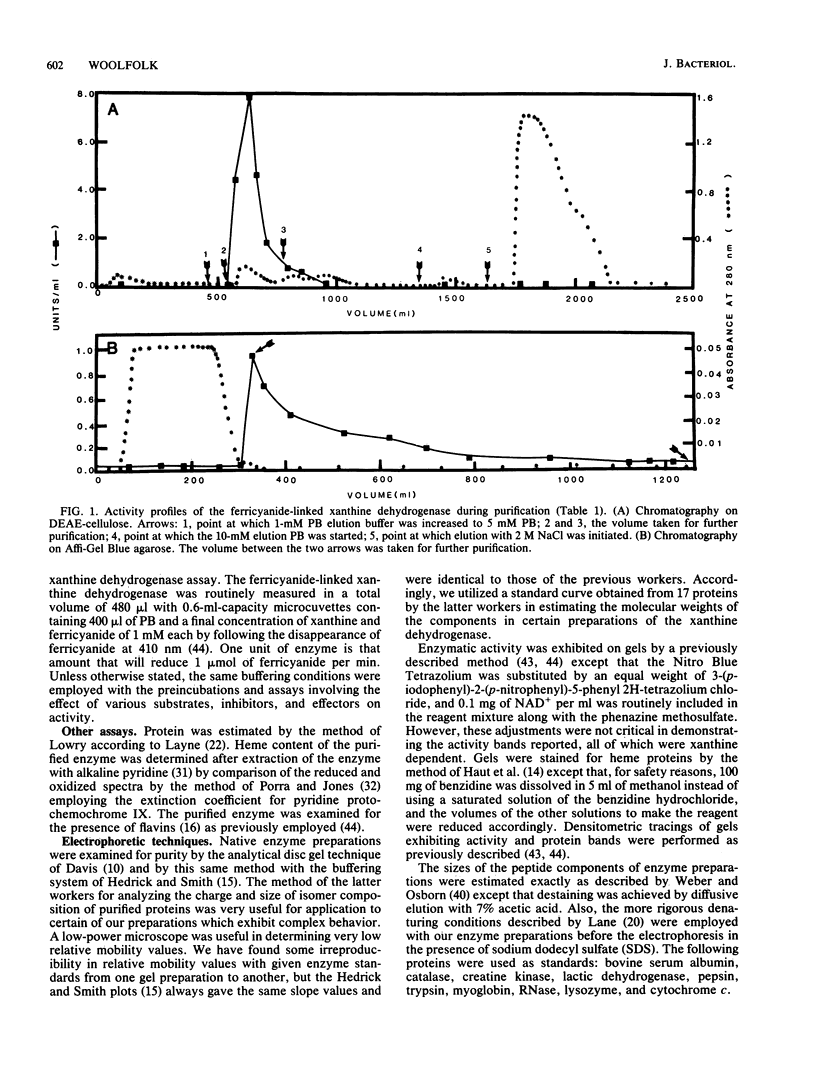
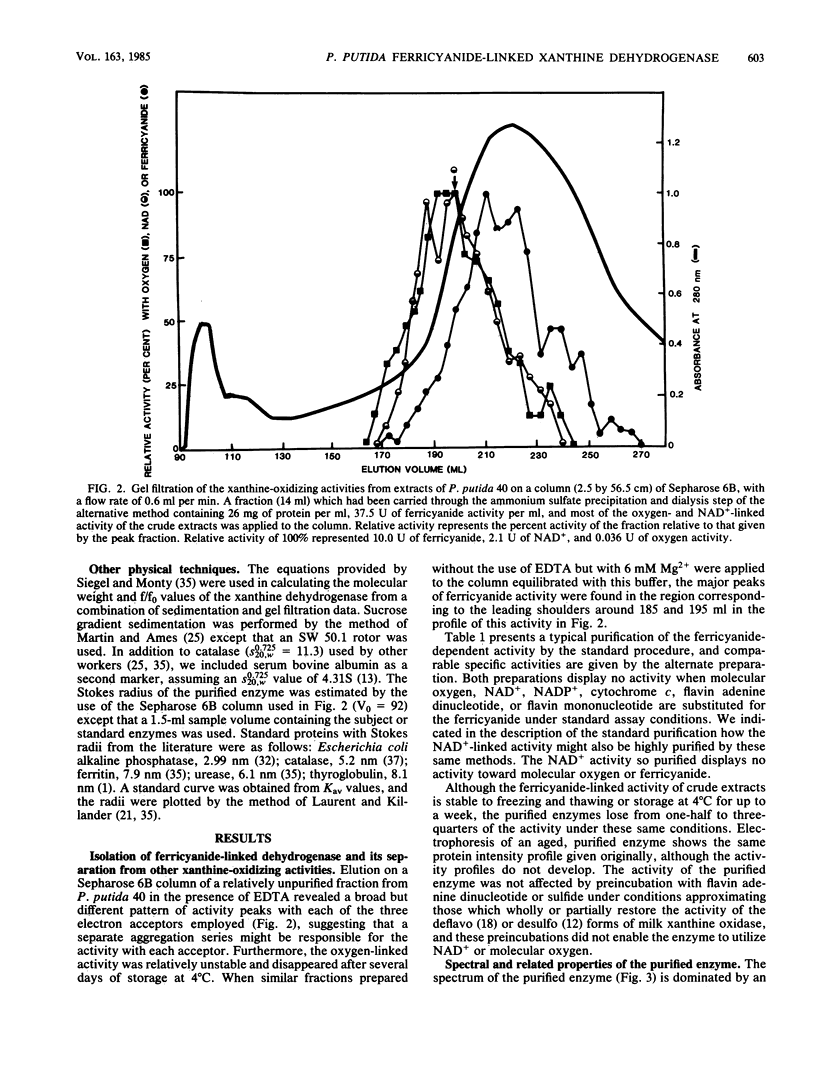
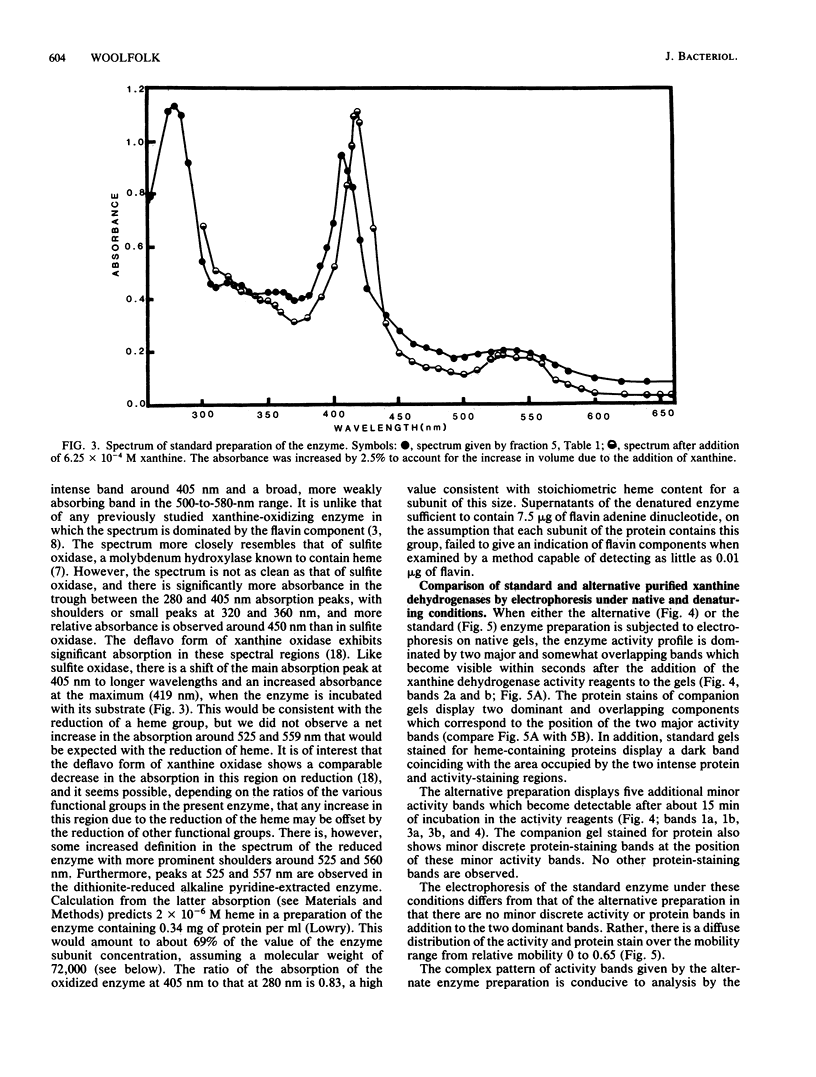
The isolation of a xanthine dehydrogenase from Pseudomonas putida 40 which utilizes ferricyanide as an electron acceptor at high efficiency is presented. The new activity is separate from the NAD+ and oxygen-utilizing activities of the same organism but displays a broad pattern for reducing substrates typical of those of previously studied xanthine-oxidizing enzymes. Unlike the previously studied enzymes, the new enzyme appears to lack flavin but possess heme and is resistant to cyanide treatment. However, sensitivity of the purified enzyme to methanol and the selective elimination of the activity when tungstate is added to certain growth media suggest a role for molybdenum. The enzyme is subject to a selective proteolytic action during processing which is not accompanied by denaturation or loss of activity and which is minimized by the continuous exposure of the activity to EDTA and phenylmethylsulfonyl fluoride. Electrophoresis of the denatured enzyme in the presence of sodium dodecyl sulfate suggests that the enzyme is constructed of subunits with a molecular weight of approximately 72,000. Electrophoresis under native conditions of a purified enzyme previously exposed to magnesium ion reveals a series of major and minor activity bands which display some selectivity toward both electron donors and acceptors. An analysis of the effect of gel concentration on this pattern suggests that the enzyme forms a series of charge and size isomers with a pair of trimeric forms predominating. Comparison of the rate of sedimentation of the enzyme in sucrose gradients with its elution profile from standardized Sepharose 6B columns suggests a molecular weight of 255,000 for the major form of the native enzyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERS G. K. MOLECULAR EXCLUSION AND RESTRICTED DIFFUSION PROCESSES IN MOLECULAR-SIEVE CHROMATOGRAPHY. Biochemistry. 1964 May;3:723–730. doi: 10.1021/bi00893a021. [DOI] [PubMed] [Google Scholar]
- Cleere W. F., Coughlan M. P. Avian xanthine dehydrogenases. I. Isolation and characterization of the turkey liver enzyme. Comp Biochem Physiol B. 1975 Feb 15;50(2B):311–322. doi: 10.1016/0305-0491(75)90280-1. [DOI] [PubMed] [Google Scholar]
- Cohen H. J., Fridovich I. Hepatic sulfite oxidase. Purification and properties. J Biol Chem. 1971 Jan 25;246(2):359–366. [PubMed] [Google Scholar]
- Cohen H. J., Fridovich I. Hepatic sulfite oxidase. The nature and function of the heme prosthetic groups. J Biol Chem. 1971 Jan 25;246(2):367–373. [PubMed] [Google Scholar]
- Cohen H. J., Fridovich I., Rajagopalan K. V. Hepatic sulfite oxidase. A functional role for molybdenum. J Biol Chem. 1971 Jan 25;246(2):374–382. [PubMed] [Google Scholar]
- Corte E. D., Stirpe F. The regulation of rat liver xanthine oxidase. Involvement of thiol groups in the conversion of the enzyme activity from dehydrogenase (type D) into oxidase (type O) and purification of the enzyme. Biochem J. 1972 Feb;126(3):739–745. doi: 10.1042/bj1260739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Edmondson D., Massey V., Palmer G., Beacham L. M., 3rd, Elion G. B. The resolution of active and inactive xanthine oxidase by affinity chromatography. J Biol Chem. 1972 Mar 10;247(5):1597–1604. [PubMed] [Google Scholar]
- Fhaoláin I. N., Hynes M. J., Coughlan C. P. Turkey liver xanthine dehydrogenase: effects of methanol on the enzyme-catalysed oxidation of reduced nicotinamide-adenine dinucleotide. Biochem Soc Trans. 1976;4(5):901–903. doi: 10.1042/bst0040901. [DOI] [PubMed] [Google Scholar]
- HAUT A., TUDHOPE G. R., CARTWRIGHT G. E., WINTROBEMM The nonhemoglobin erythrocytic proteins, studied by electrophoresis on starch gel. J Clin Invest. 1962 Mar;41:579–587. doi: 10.1172/JCI104512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Komai H., Massey V., Palmer G. The preparation and properties of deflavo xanthine oxidase. J Biol Chem. 1969 Apr 10;244(7):1692–1700. [PubMed] [Google Scholar]
- Krenitsky T. A., Neil S. M., Elion G. B., Hitchings G. H. A comparison of the specificities of xanthine oxidase and aldehyde oxidase. Arch Biochem Biophys. 1972 Jun;150(2):585–599. doi: 10.1016/0003-9861(72)90078-1. [DOI] [PubMed] [Google Scholar]
- Lane L. C. A simple method for stabilizing protein-sulfhydryl groups during SDS-gel electrophoresis. Anal Biochem. 1978 Jun 1;86(2):655–664. doi: 10.1016/0003-2697(78)90792-3. [DOI] [PubMed] [Google Scholar]
- Lyric R. M., Suzuki I. Enzymes involved in the metabolism of thiosulfate by Thiobacillus thioparus. I. Survey of enzymes and properties of sulfite: cytochrome c oxidoreductase. Can J Biochem. 1970 Mar;48(3):334–343. doi: 10.1139/o70-056. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Massey V., Komai H., Palmer G., Elion G. B. On the mechanism of inactivation of xanthine oxidase by allopurinol and other pyrazolo[3,4-d]pyrimidines. J Biol Chem. 1970 Jun 10;245(11):2837–2844. [PubMed] [Google Scholar]
- Mitidieri E., Affonso O. R. Molybdenum requirement for bacterial xanthine dehydrogenase activity. Biochim Biophys Acta. 1965 Aug 24;105(2):371–373. doi: 10.1016/s0926-6593(65)80161-8. [DOI] [PubMed] [Google Scholar]
- Nagler L. G., Vartanyan L. S. Subunit structure of bovine milk xanthine oxidase. Effect of limited cleavage by proteolytic enzymes on activity and structure. Biochim Biophys Acta. 1976 Mar 18;427(1):78–90. doi: 10.1016/0005-2795(76)90287-7. [DOI] [PubMed] [Google Scholar]
- Ohe T., Watanabe Y. Purification and properties of xanthine dehydrogenase from Streptomyces cyanogenus. J Biochem. 1979 Jul;86(1):45–53. [PubMed] [Google Scholar]
- PORRA R. J., JONES O. T. Studies on ferrochelatase. 1. Assay and properties of ferrochelatase from a pig-liver mitochondrial extract. Biochem J. 1963 Apr;87:181–185. doi: 10.1042/bj0870181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan K. V., Handler P. Purification and properties of chicken liver xanthine dehydrogenase. J Biol Chem. 1967 Sep 25;242(18):4097–4107. [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Sin I. L. Purification and properties of xanthine dehydrogenase from Pseudomonas acidovorans. Biochim Biophys Acta. 1975 Nov 20;410(1):12–20. doi: 10.1016/0005-2744(75)90203-x. [DOI] [PubMed] [Google Scholar]
- Smith S. T., Rajagopalan K. V., Handler P. Purification and properties of xanthine dehydroganase from Micrococcus lactilyticus. J Biol Chem. 1967 Sep 25;242(18):4108–4117. [PubMed] [Google Scholar]
- Waud W. R., Rajagopalan K. V. The mechanism of conversion of rat liver xanthine dehydrogenase from an NAD+-dependent form (type D) to an O2-dependent form (type O). Arch Biochem Biophys. 1976 Feb;172(2):365–379. doi: 10.1016/0003-9861(76)90088-6. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Woolfolk C. A., Downard J. S. Bacterial xanthine oxidase from Arthrobacter S-2. J Bacteriol. 1978 Aug;135(2):422–428. doi: 10.1128/jb.135.2.422-428.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfolk C. A., Downard J. S. Distribution of xanthine oxidase and xanthine dehydrogenase specificity types among bacteria. J Bacteriol. 1977 Jun;130(3):1175–1191. doi: 10.1128/jb.130.3.1175-1191.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfolk C. A. Metabolism of N-methylpurines by a Pseudomonas putida strain isolated by enrichment on caffeine as the sole source of carbon and nitrogen. J Bacteriol. 1975 Sep;123(3):1088–1106. doi: 10.1128/jb.123.3.1088-1106.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfolk C. A., Woolfolk B. S., Whiteley H. R. 2-oxypurine dehydrogenase from Micrococcus aerogenes. I. Isolation, specificity, and some chemical and physical properties. J Biol Chem. 1970 Jun;245(12):3167–3178. [PubMed] [Google Scholar]



