Abstract
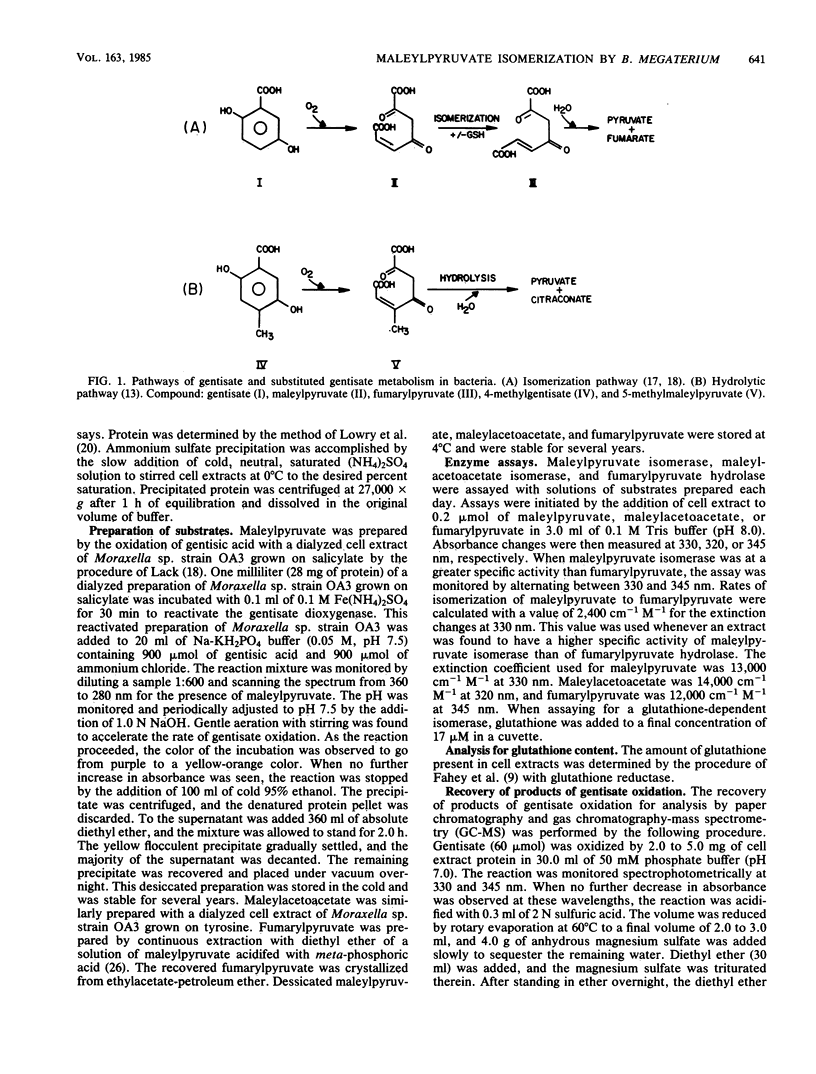
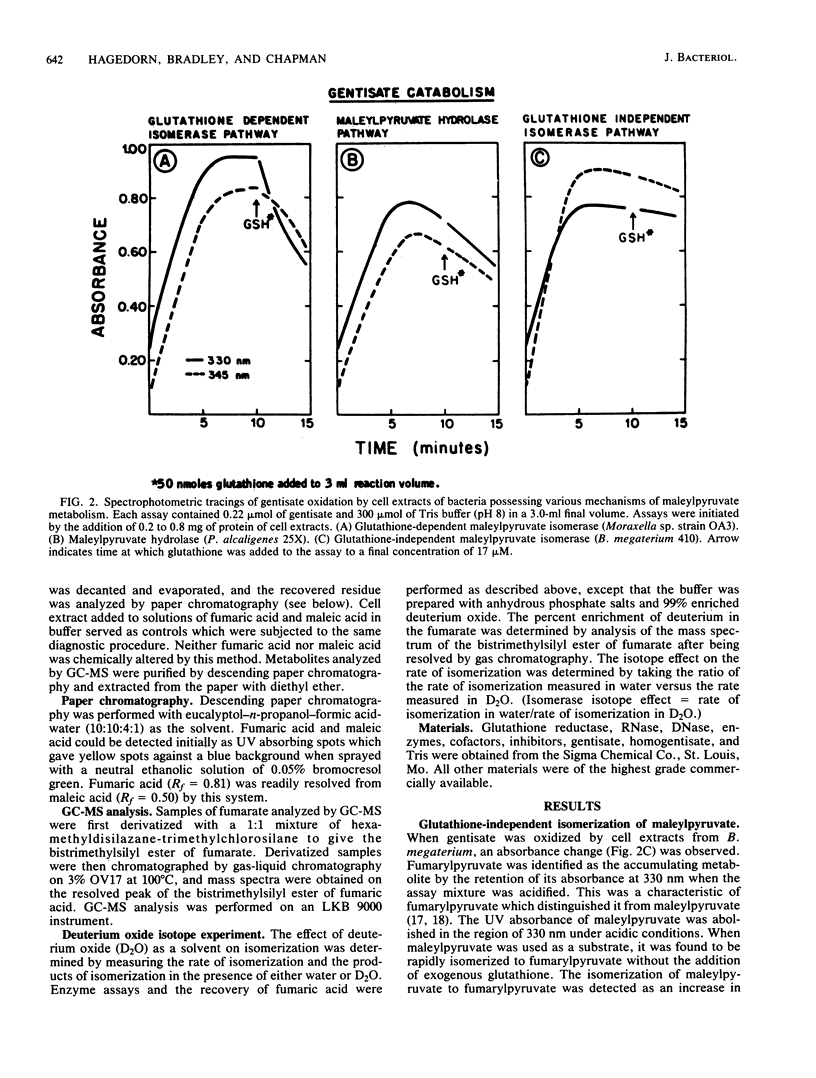
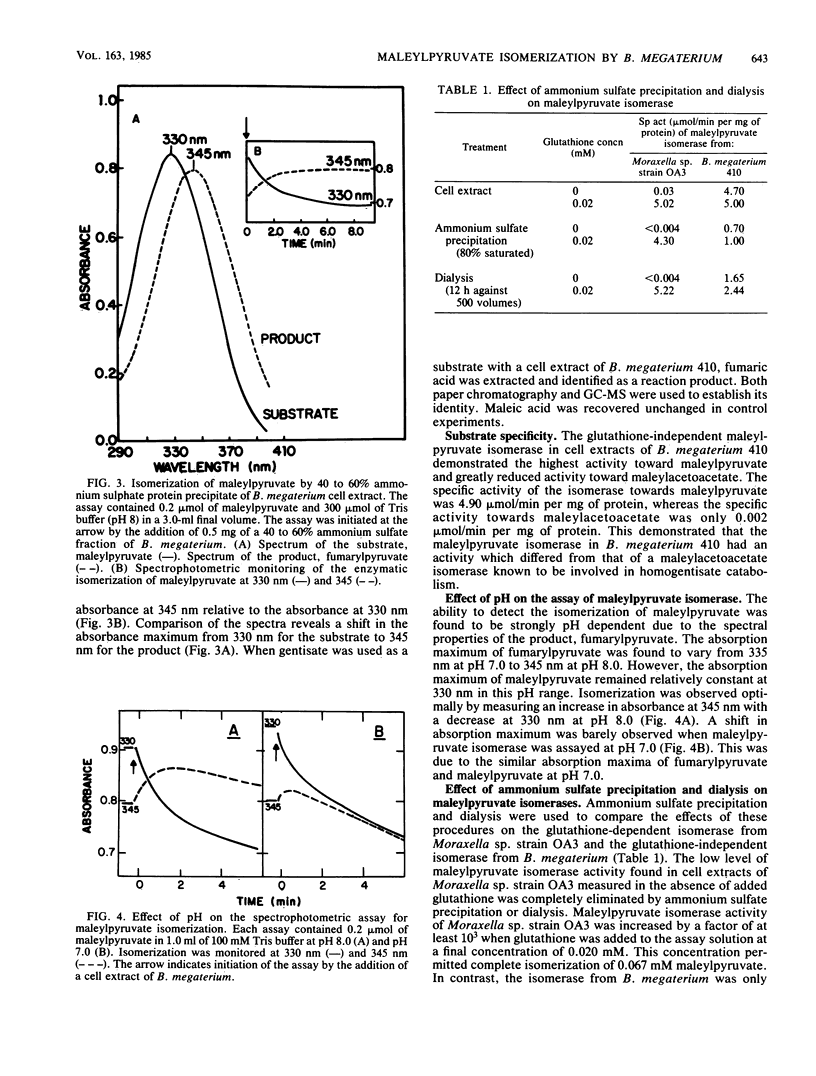
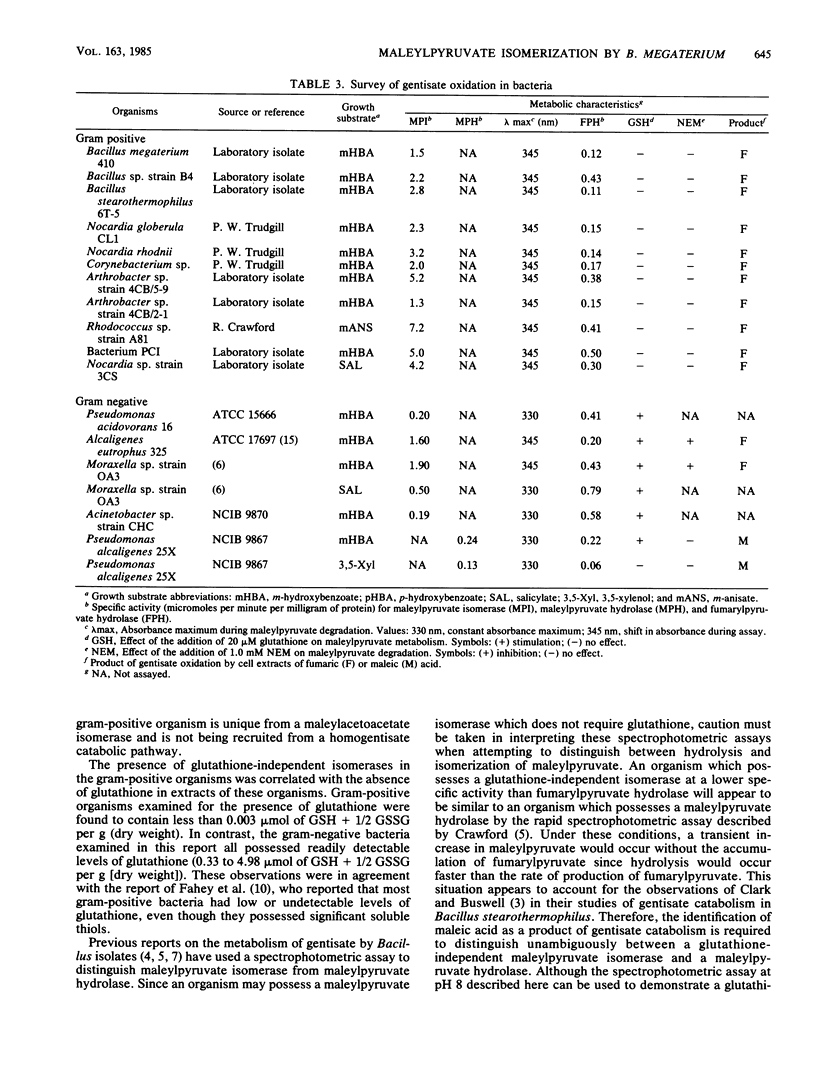
Maleylpyruvate, the ring fission product of gentisic acid, was found to be isomerized to fumarylpyruvate without a requirement for glutathione by an enzyme activity found in cell extracts of m-hydroxybenzoate-grown Bacillus megaterium 410. The isomerization reaction was detected as a shift in the absorbance maximum from 330 nm, the maximum for maleylpyruvate, to 345 nm, the maximum for fumarylpyruvate, when assayed at pH 8.0. Ammonium sulfate precipitation and dialysis of B. megaterium cell extracts resolved the isomerase activity from low-molecular-weight compounds such as glutathione but did not eliminate the isomerase activity. Iodoacetate and p-chloromercuribenzoate were potent inhibitors of the isomerase from B. megaterium. However, N-ethylmaleimide and iodoacetamide did not significantly inhibit this activity. In addition, fumaric acid was demonstrated as a product of gentisate oxidation by dialyzed cell extracts of B. megaterium. Glutathione-independent maleylpyruvate isomerases with properties similar to the isomerase found in B. megaterium were also found in other genera of gram-positive organisms. Eleven different organisms representing the genera Bacillus, Arthrobacter, Corynebacterium, Nocardia, and Rhodococcus were all found to possess this novel type of glutathione-independent maleylpyruvate isomerase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayly R. C., Chapman P. J., Dagley S., Di Berardino D. Purification and some properties of maleylpyruvate hydrolase and fumarylpyruvate hydrolase from Pseudomonas alcaligenes. J Bacteriol. 1980 Jul;143(1):70–77. doi: 10.1128/jb.143.1.70-77.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buswell J. A., Clark J. S. Oxidation of aromatic acids by a facultative thermophilic Bacillus sp. J Gen Microbiol. 1976 Sep;96(1):209–213. doi: 10.1099/00221287-96-1-209. [DOI] [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Crawford R. L. Degradation of 3-hydroxybenzoate by bacteria of the genus Bacillus. Appl Microbiol. 1975 Sep;30(3):439–444. doi: 10.1128/am.30.3.439-444.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. L., Frick T. D. Rapid spectrophotometric differentiation between glutathione-dependent and glutathione-independent gentisate and homogentisate pathways. Appl Environ Microbiol. 1977 Aug;34(2):170–174. doi: 10.1128/aem.34.2.170-174.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. L., Hutton S. W., Chapman P. J. Purification and properties of gentisate 1,2-dioxygenase from Moraxella osloensis. J Bacteriol. 1975 Mar;121(3):794–799. doi: 10.1128/jb.121.3.794-799.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey R. C., Brody S., Mikolajczyk S. D. Changes in the glutathione thiol-disulfide status of Neurospora crassa conidia during germination and aging. J Bacteriol. 1975 Jan;121(1):144–151. doi: 10.1128/jb.121.1.144-151.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey R. C., Brown W. C., Adams W. B., Worsham M. B. Occurrence of glutathione in bacteria. J Bacteriol. 1978 Mar;133(3):1126–1129. doi: 10.1128/jb.133.3.1126-1129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs J. A., Warner H. R. Isolation of an Escherichia coli mutant deficient in glutathione synthesis. J Bacteriol. 1975 Oct;124(1):140–148. doi: 10.1128/jb.124.1.140-148.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES D. E. A press for disrupting bacteria and other micro-organisms. Br J Exp Pathol. 1951 Apr;32(2):97–109. [PMC free article] [PubMed] [Google Scholar]
- Hopper D. J., Chapman P. J., Dagley S. Enzymic formation of D-malate. Biochem J. 1968 Dec;110(4):798–800. doi: 10.1042/bj1100798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper D. J., Chapman P. J., Dagley S. The enzymic degradation of alkyl-substituted gentisates, maleates and malates. Biochem J. 1971 Mar;122(1):29–40. doi: 10.1042/bj1220029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. F., Stanier R. Y. Dissimilation of aromatic compounds by Alcaligenes eutrophus. J Bacteriol. 1971 Aug;107(2):468–475. doi: 10.1128/jb.107.2.468-475.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOX W. E., EDWARDS S. W. The properties of maleylacetoacetate, the initial product of homogentisate oxidation in liver. J Biol Chem. 1955 Oct;216(2):489–498. [PubMed] [Google Scholar]
- LACK L. Enzymic cis-trans isomerization of maleylpyruvic acid. J Biol Chem. 1961 Nov;236:2835–2840. [PubMed] [Google Scholar]
- LACK L. The enzymic oxidation of gentisic acid. Biochim Biophys Acta. 1959 Jul;34:117–123. doi: 10.1016/0006-3002(59)90239-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee Y. L., Sparnins V. L., Dagley S. Catabolism of 2,4,5-trimethyoxybenzoic acid and 3-methoxycrotonic acid. Appl Environ Microbiol. 1978 Apr;35(4):817–819. doi: 10.1128/aem.35.4.817-819.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poh C. L., Bayly R. C. Evidence for isofunctional enzymes used in m-cresol and 2,5-xylenol degradation via the gentisate pathway in Pseudomonas alcaligenes. J Bacteriol. 1980 Jul;143(1):59–69. doi: 10.1128/jb.143.1.59-69.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. The mechanism of action of glyoxalase. J Biol Chem. 1951 Jun;190(2):685–696. [PubMed] [Google Scholar]
- STRITTMATTER P., BALL E. G. Formaldehyde dehydrogenase, a glutathionedependent enzyme system. J Biol Chem. 1955 Mar;213(1):445–461. [PubMed] [Google Scholar]
- Wheelis M. L., Palleroni N. J., Stanier R. Y. The metabolism of aromatic acids by Pseudomonas testosteroni and P. acidovorans. Arch Mikrobiol. 1967;59(1):302–314. doi: 10.1007/BF00406344. [DOI] [PubMed] [Google Scholar]


