Abstract
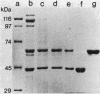
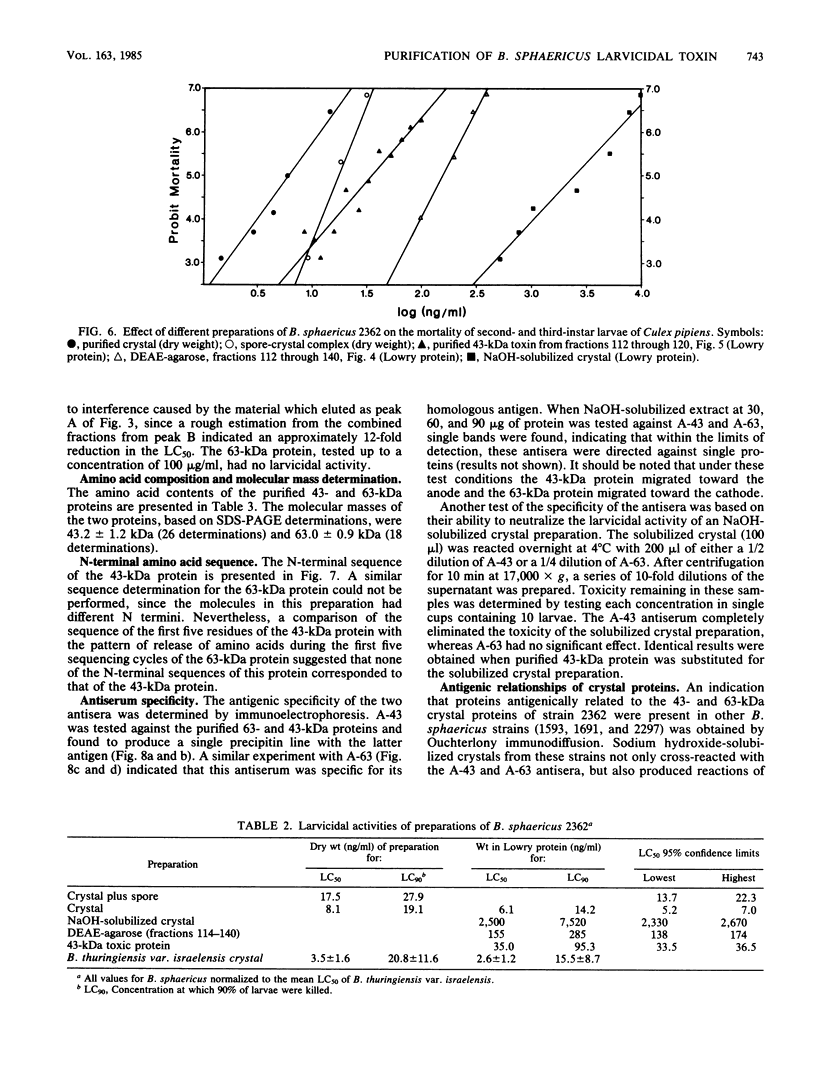
Crystals were purified from spore-crystal complexes of Bacillus sphaericus 2362 by disruption in a French pressure cell followed by centrifugation through 48% (wt/vol) NaBr. Crystals from such preparations had a 50% lethal concentration of 6 ng of protein per ml for the larvae of the mosquito Culex pipiens. When subjected to polyacrylamide gel electrophoresis under denaturing conditions, the proteins in B. sphaericus crystals migrated in positions corresponding to 43, 63, 98, 110, and 125 kilodaltons (kDa); solubilization of the crystal at pH 12 with NaOH eliminated all but the bands at 43 and 63 kDa. Since NaOH-solubilized preparations were toxic to mosquito larvae, these proteins were purified to electrophoretic homogeneity and antiserum was obtained to each. Analysis of the two purified proteins indicated that the 43-kDa protein was toxic to mosquito larvae (50% lethal concentration, 35 ng of protein per ml), whereas the 63-kDa protein was not. Further differences between them were their amino acid compositions, their lack of immunological cross-reactivity, their opposite net charges at pH 7.5, and their susceptibility to digestion by larval midgut proteases (the 63-kDa protein was highly susceptible, whereas the 43-kDa protein was not). The sequence of the 40 N-terminal residues of the 43-kDa protein was determined and found to contain a high percentage of hydrophobic amino acids. The sequence of the 63-kDa protein could not be determined, since it had multiple N termini. By electrophoretically separating the crystal proteins and then electroblotting onto nitrocellulose paper and visualizing the bands with antisera to the 43- and 63-kDa proteins in conjunction with an immunoblot assay, it was found that the high-molecular-mass crystal proteins (98 to 125 kDa) contained antigenic determinants of both proteins. These results suggested that the lower-molecular-weight crystal proteins detected in polyacrylamide gels after electrophoresis under denaturing conditions were derivatives of one or more of the higher-molecular-weight crystal proteins. In vivo studies of the products of crystal degradation by larvae of Culex pipiens indicated that the high-molecular-weight proteins and the 63-kDa antigenic determinants were rapidly degraded and that a 40-kDa protein related to the 43-kDa toxin persisted for the duration of the experiment (4 h). Some of the studies performed with B.sphaericus 2362 were extended to strains 1593, 1691, and 2297 of this species with results which indicated a high degree of similarity between the crystal proteins of all these larvicidal strains.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann P., Baumann L., Woolkalis M. J., Bang S. S. Evolutionary relationships in vibrio and Photobacterium: a basis for a natural classification. Annu Rev Microbiol. 1983;37:369–398. doi: 10.1146/annurev.mi.37.100183.002101. [DOI] [PubMed] [Google Scholar]
- Bulla L. A., Jr, Kramer K. J., Cox D. J., Jones B. L., Davidson L. I., Lookhart G. L. Purification and characterization of the entomocidal protoxin of Bacillus thuringiensis. J Biol Chem. 1981 Mar 25;256(6):3000–3004. [PubMed] [Google Scholar]
- Davidson E. W. Alkaline extraction of toxin from spores of the mosquito pathogen, Bacillus sphaericus strain 1593. Can J Microbiol. 1983 Feb;29(2):271–275. doi: 10.1139/m83-044. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hagedorn H. H., Turner S., Hagedorn E. A., Pontecorvo D., Greenbaum P., Pfeiffer D., Wheelock G., Flanagan T. R. Postemergence growth of the ovarian follicles of Aedes aegypti. J Insect Physiol. 1977;23(2):203–206. doi: 10.1016/0022-1910(77)90030-0. [DOI] [PubMed] [Google Scholar]
- Kalfon A., Charles J. F., Bourgouin C., de Barjac H. Sporulation of Bacillus sphaericus 2297: an electron microscope study of crystal-like inclusion biogenesis and toxicity to mosquito larvae. J Gen Microbiol. 1984 Apr;130(4):893–900. doi: 10.1099/00221287-130-4-893. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Louis J., Jayaraman K., Szulmajster J. Biocide gene(s) and biocidal activity in different strains of Bacillus sphaericus. Expression of the gene(s) in E. coli maxicells. Mol Gen Genet. 1984;195(1-2):23–28. doi: 10.1007/BF00332718. [DOI] [PubMed] [Google Scholar]
- Myers P., Yousten A. A., Davidson E. W. Comparative studies of the mosquito-larval toxin of Bacillus sphaericus SSII-1 and 1593. Can J Microbiol. 1979 Nov;25(11):1227–1231. doi: 10.1139/m79-193. [DOI] [PubMed] [Google Scholar]
- Payne J. M., Davidson E. W. Insecticidal activity of the crystalline parasporal inclusions and other components of the Bacillus sphaericus 1593 spore complex. J Invertebr Pathol. 1984 May;43(3):383–388. doi: 10.1016/0022-2011(84)90084-3. [DOI] [PubMed] [Google Scholar]
- Sharpe E. S., Nickerson K. W., Bulla L. A., Jr, Aronson J. N. Separation of spores and parasporal crystals of Bacillus thuringiensis in gradients of certain x-ray contrasting agents. Appl Microbiol. 1975 Dec;30(6):1052–1053. doi: 10.1128/am.30.6.1052-1053.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas W. E., Ellar D. J. Bacillus thuringiensis var israelensis crystal delta-endotoxin: effects on insect and mammalian cells in vitro and in vivo. J Cell Sci. 1983 Mar;60:181–197. doi: 10.1242/jcs.60.1.181. [DOI] [PubMed] [Google Scholar]
- Tinelli R., Bourgouin C. Larvicidal toxin from Bacillus sphaericus spores: isolation of toxic components. FEBS Lett. 1982 Jun 1;142(1):155–158. doi: 10.1016/0014-5793(82)80241-x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E. S., Ellar D. J., Todd J. A. Cloning and expression in Escherichia coli of the insecticidal delta-endotoxin gene of Bacillus thuringiensis var. israelensis. FEBS Lett. 1984 Oct 1;175(2):377–382. doi: 10.1016/0014-5793(84)80772-3. [DOI] [PubMed] [Google Scholar]
- Yousten A. A. Bacillus sphaericus: microbiological factors related to its potential as a mosquito larvicide. Adv Biotechnol Processes. 1984;3:315–343. [PubMed] [Google Scholar]
- Yousten A. A., Davidson E. W. Ultrastructural Analysis of Spores and Parasporal Crystals Formed by Bacillus sphaericus 2297. Appl Environ Microbiol. 1982 Dec;44(6):1449–1455. doi: 10.1128/aem.44.6.1449-1455.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]