Abstract
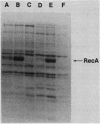
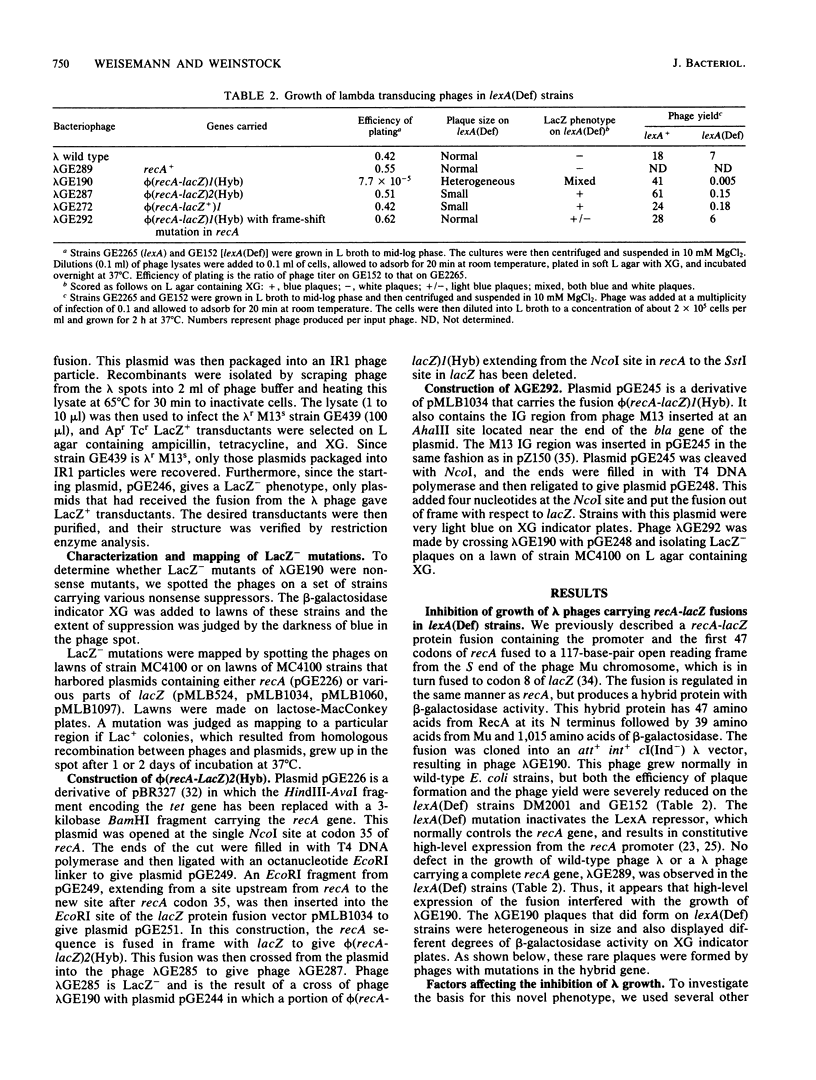
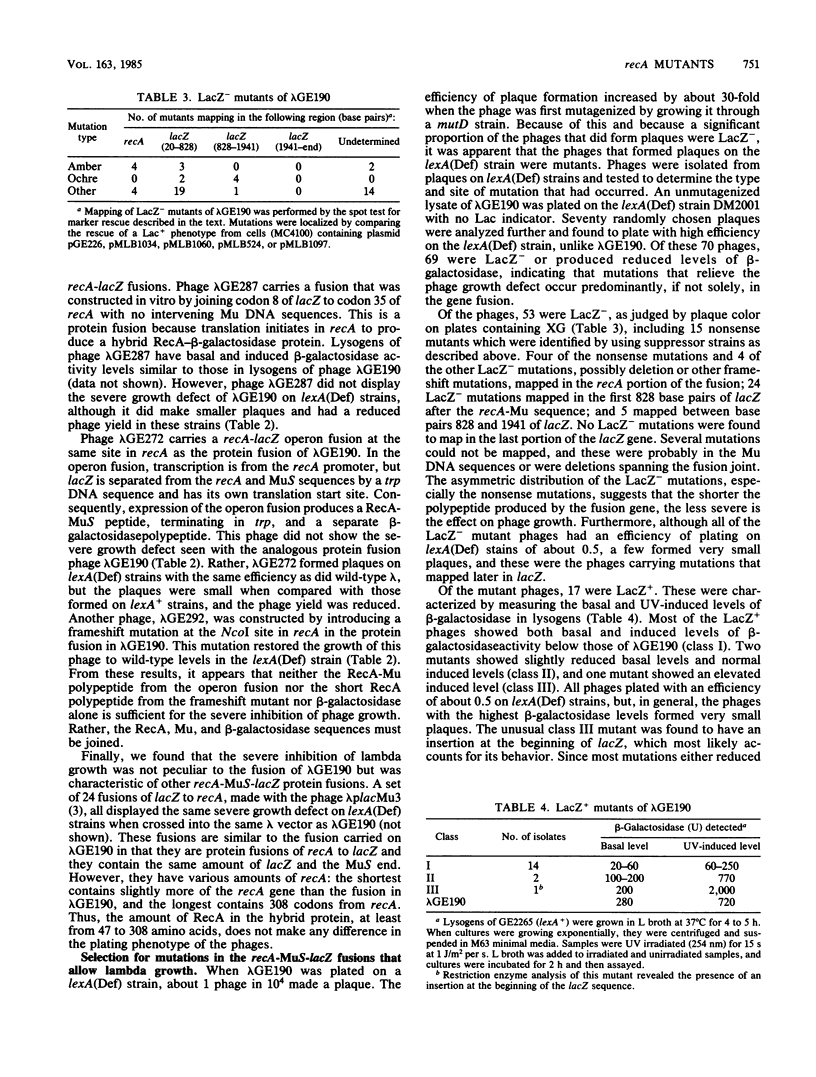
When a recA-lacZ protein fusion was cloned into phage lambda, the resulting transducing phage grew normally on wild-type Escherichia coli, but its growth was severely inhibited in lexA(Def) mutant strains that express recA constitutively at high levels. Mutants of the transducing phage that grew on the lexA(Def) strains were isolated and were found to affect production of the RecA-beta-galactosidase hybrid protein. Most mutants, including a number of nonsense mutants, were phenotypically LacZ-. LacZ+ mutants were also isolated; most of these expressed lower basal and induced levels of beta-galactosidase activity. DNA sequence analysis revealed that some of the LacZ+ mutations were in the recA promoter. One of these was found to prevent induction. Unexpectedly, three of the mutations that reduced expression were located in the recA structural gene, at codons 10, 11, and 12. Further analysis of the codon 10 mutant showed that it most likely affected translation since it had little effect on transcription as measured by beta-galactosidase synthesis from a recA-lacZ operon fusion. This expression defect was not limited to the protein fusion, since the codon 10 mutation also reduced synthesis of RecA protein when present in a complete recA gene. Analysis of the recA DNA sequence in the fusion revealed that each of the mutations at codons 10, 11, and 12 increases the homology between this region of the mRNA and a sequence found at codons 1 to 4. Thus, the secondary structure of the mutant recA mRNAs may be affecting translation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandsma J. A., Bosch D., Backendorf C., van de Putte P. A common regulatory region shared by divergently transcribed genes of the Escherichia coli SOS system. Nature. 1983 Sep 15;305(5931):243–245. doi: 10.1038/305243a0. [DOI] [PubMed] [Google Scholar]
- Bremer E., Silhavy T. J., Weisemann J. M., Weinstock G. M. Lambda placMu: a transposable derivative of bacteriophage lambda for creating lacZ protein fusions in a single step. J Bacteriol. 1984 Jun;158(3):1084–1093. doi: 10.1128/jb.158.3.1084-1093.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent R., Ptashne M. Mechanism of action of the lexA gene product. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4204–4208. doi: 10.1073/pnas.78.7.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Casaregola S., D'Ari R., Huisman O. Quantitative evaluation of recA gene expression in Escherichia coli. Mol Gen Genet. 1982;185(3):430–439. doi: 10.1007/BF00334135. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. [Prophage induction and cell division in E. coli. II. Linked (recA, zab) and unlinked (lex) suppressors of tif-1-mediated induction and filamentation]. Mol Gen Genet. 1972;119(2):153–174. doi: 10.1007/BF00269134. [DOI] [PubMed] [Google Scholar]
- Chattoraj D. K., Cordes K., Berman M. L., Das A. Mutagenesis and mutation transfer induced by ultraviolet light in plasmid-cloned DNA. Gene. 1984 Feb;27(2):213–222. doi: 10.1016/0378-1119(84)90142-2. [DOI] [PubMed] [Google Scholar]
- Craig N. L., Roberts J. W. E. coli recA protein-directed cleavage of phage lambda repressor requires polynucleotide. Nature. 1980 Jan 3;283(5742):26–30. doi: 10.1038/283026a0. [DOI] [PubMed] [Google Scholar]
- Enea V., Zinder N. D. Interference resistant mutants of phage f1. Virology. 1982 Oct 15;122(1):222–226. doi: 10.1016/0042-6822(82)90395-6. [DOI] [PubMed] [Google Scholar]
- Horii T., Ogawa T., Nakatani T., Hase T., Matsubara H., Ogawa H. Regulation of SOS functions: purification of E. coli LexA protein and determination of its specific site cleaved by the RecA protein. Cell. 1981 Dec;27(3 Pt 2):515–522. doi: 10.1016/0092-8674(81)90393-7. [DOI] [PubMed] [Google Scholar]
- Horii T., Ogawa T., Ogawa H. Organization of the recA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):313–317. doi: 10.1073/pnas.77.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman O., D'Ari R., George J. Dissociation of tsl-tif-induced filamentation and recA protein synthesis in Escherichia coli K-12. J Bacteriol. 1980 Jun;142(3):819–828. doi: 10.1128/jb.142.3.819-828.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karu A. E., Belk E. D. Induction of E. coli recA protein via recBC and alternate pathways: quantitation by enzyme-linked immunosorbent assay (ELISA). Mol Gen Genet. 1982;185(2):275–282. doi: 10.1007/BF00330798. [DOI] [PubMed] [Google Scholar]
- Konigsberg W., Godson G. N. Evidence for use of rare codons in the dnaG gene and other regulatory genes of Escherichia coli. Proc Natl Acad Sci U S A. 1983 Feb;80(3):687–691. doi: 10.1073/pnas.80.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Edmiston S. H., Pacelli L. Z., Mount D. W. Cleavage of the Escherichia coli lexA protein by the recA protease. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Little J. W., Mount D. W., Yanisch-Perron C. R. Purified lexA protein is a repressor of the recA and lexA genes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4199–4203. doi: 10.1073/pnas.78.7.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W. A mutant of Escherichia coli showing constitutive expression of the lysogenic induction and error-prone DNA repair pathways. Proc Natl Acad Sci U S A. 1977 Jan;74(1):300–304. doi: 10.1073/pnas.74.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. E., Hawley D. K., Entriken R., McClure W. R. Escherichia coli promoter sequences predict in vitro RNA polymerase selectivity. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):789–800. doi: 10.1093/nar/12.1part2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacelli L. Z., Edmiston S. H., Mount D. W. Isolation and characterization of amber mutations in the lexA gene of Escherichia coli K-12. J Bacteriol. 1979 Jan;137(1):568–573. doi: 10.1128/jb.137.1.568-573.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles B., Paoletti C. Control of UV induction of recA protein. Proc Natl Acad Sci U S A. 1983 Jan;80(1):65–69. doi: 10.1073/pnas.80.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar G. B., Sancar A., Little J. W., Rupp W. D. The uvrB gene of Escherichia coli has both lexA-repressed and lexA-independent promoters. Cell. 1982 Mar;28(3):523–530. doi: 10.1016/0092-8674(82)90207-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Benson S. A., Emr S. D. Mechanisms of protein localization. Microbiol Rev. 1983 Sep;47(3):313–344. doi: 10.1128/mr.47.3.313-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Weinstock G. M., Berman M. L., Silhavy T. J. Chimeric genetics with beta-galactosidase. Gene Amplif Anal. 1983;3:27–64. [PubMed] [Google Scholar]
- Weisemann J. M., Funk C., Weinstock G. M. Measurement of in vivo expression of the recA gene of Escherichia coli by using lacZ gene fusions. J Bacteriol. 1984 Oct;160(1):112–121. doi: 10.1128/jb.160.1.112-121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagursky R. J., Berman M. L. Cloning vectors that yield high levels of single-stranded DNA for rapid DNA sequencing. Gene. 1984 Feb;27(2):183–191. doi: 10.1016/0378-1119(84)90139-2. [DOI] [PubMed] [Google Scholar]