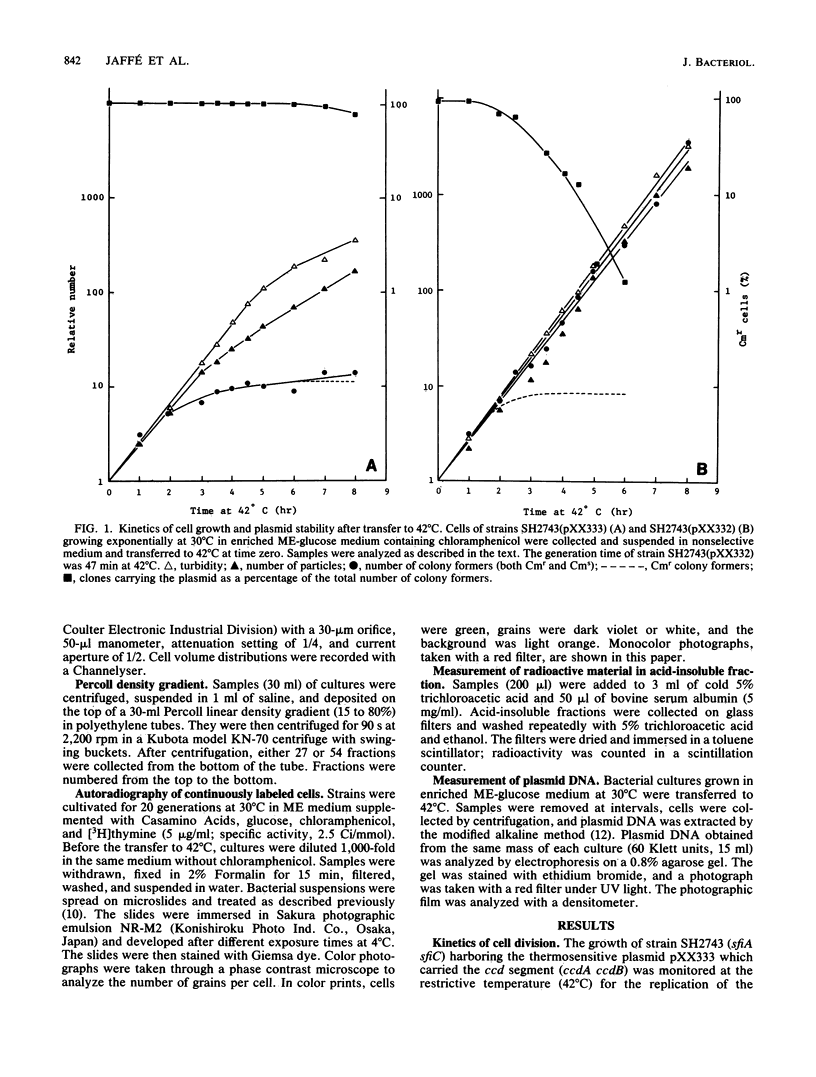
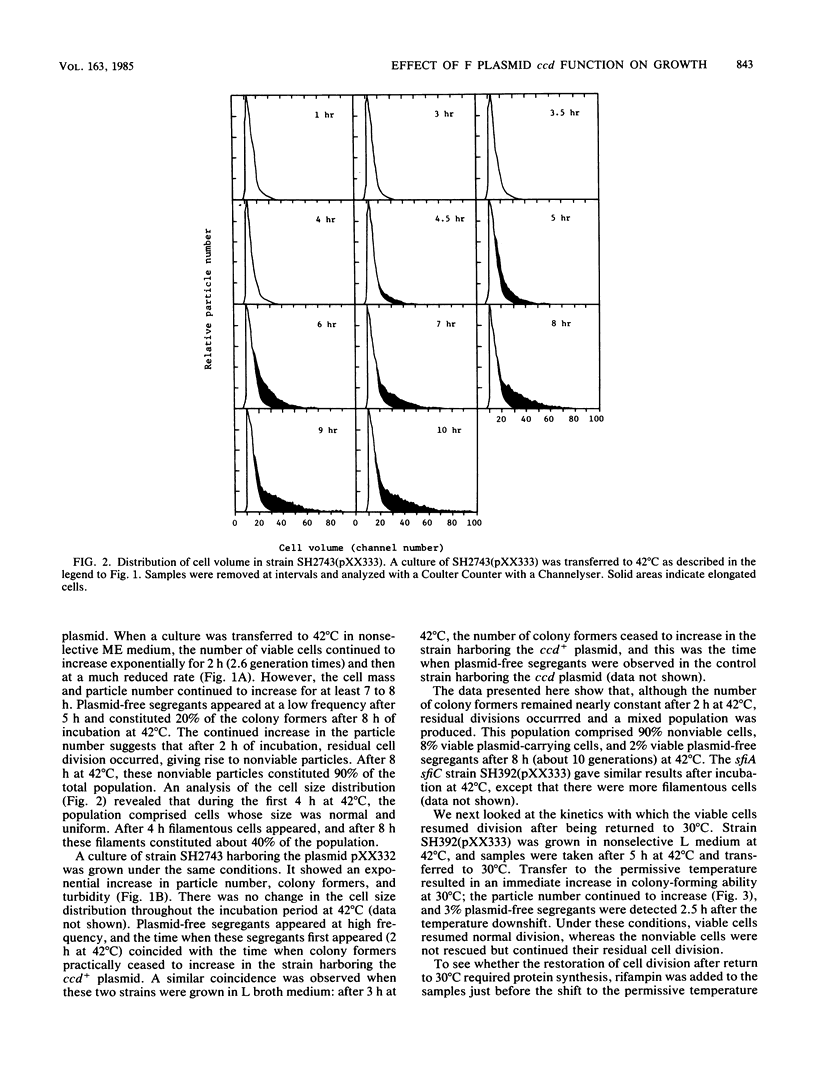
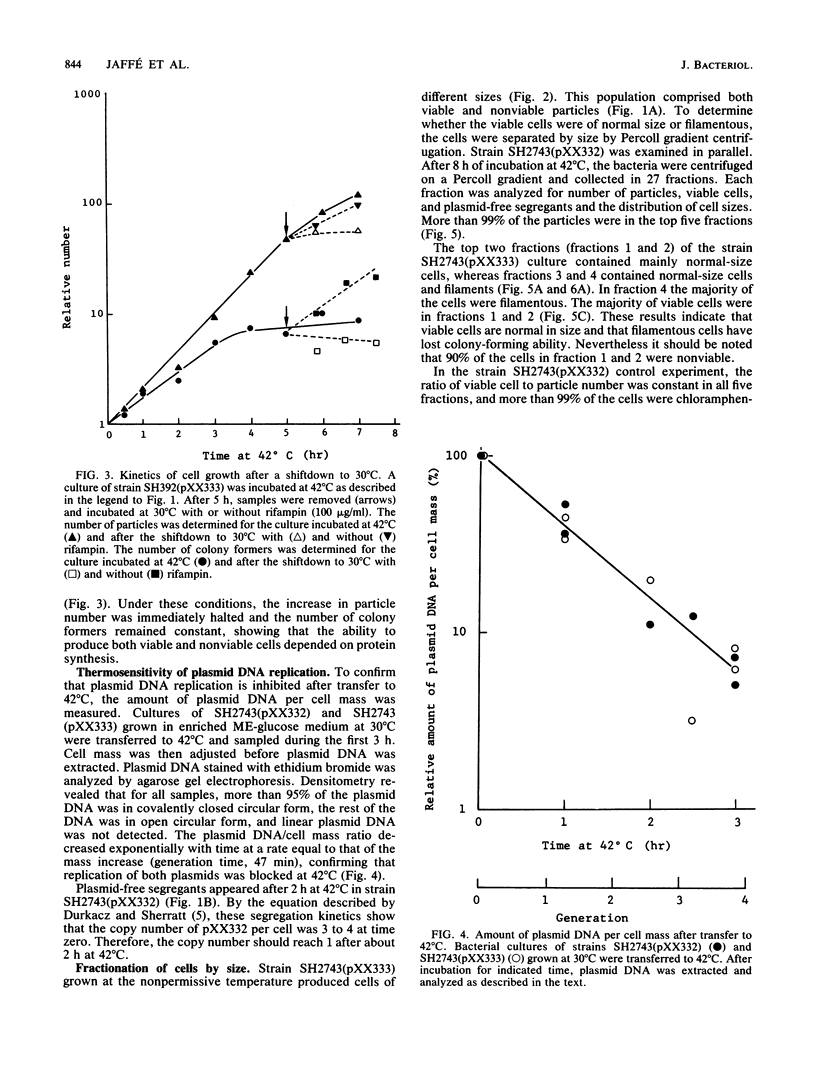
Abstract
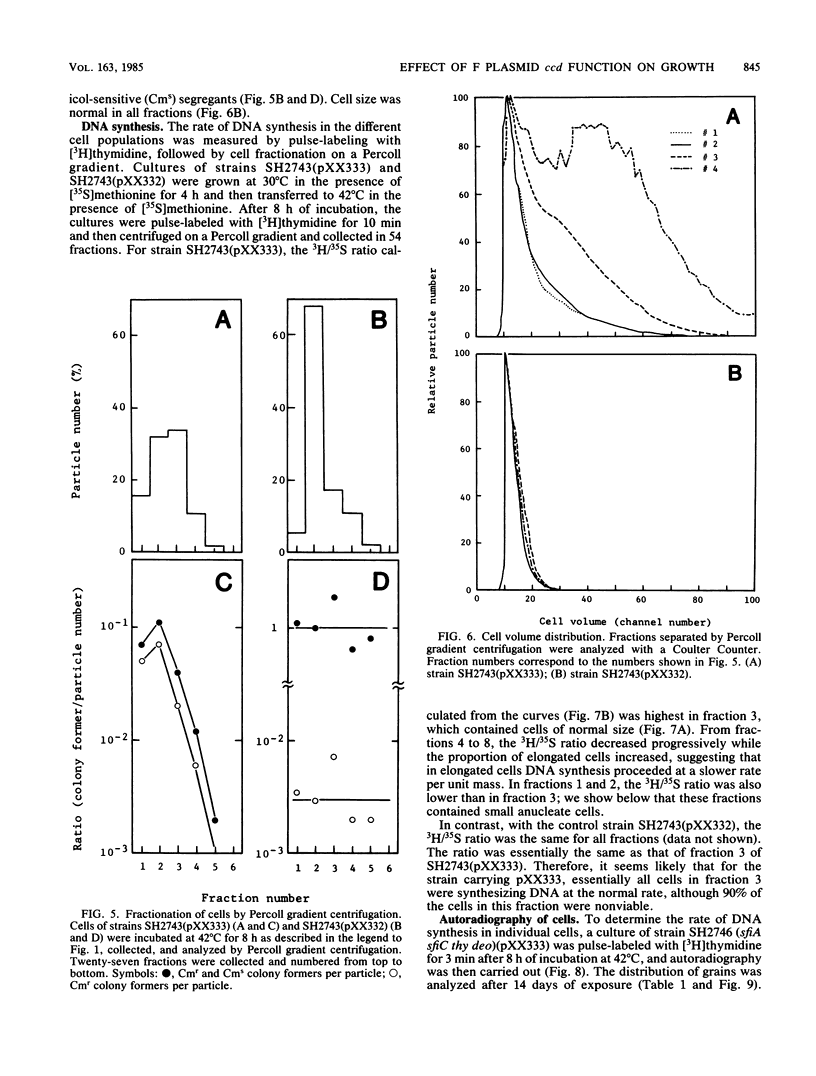
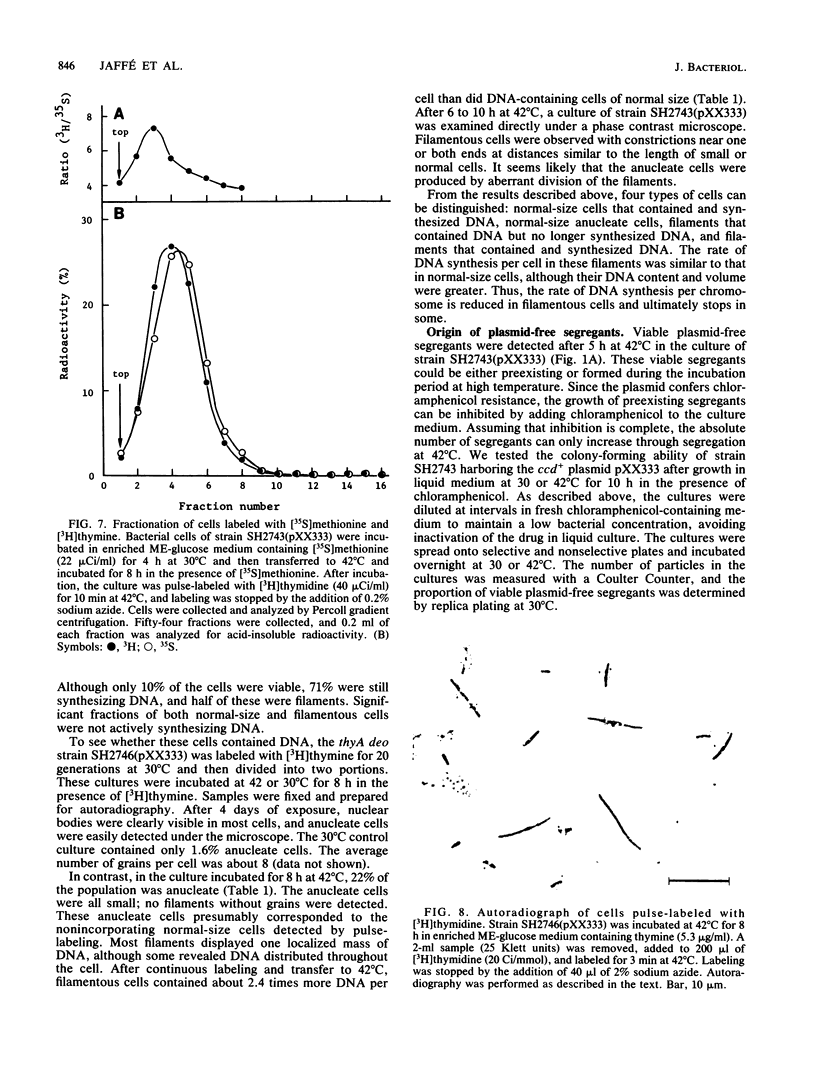
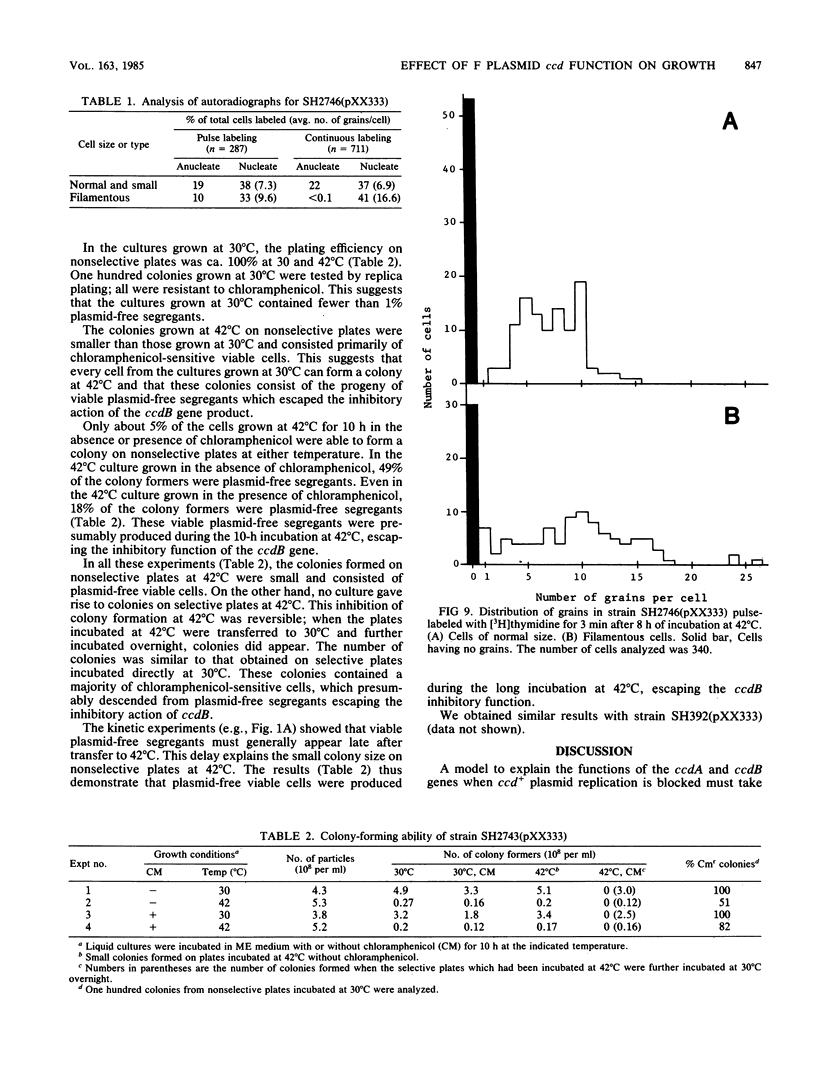
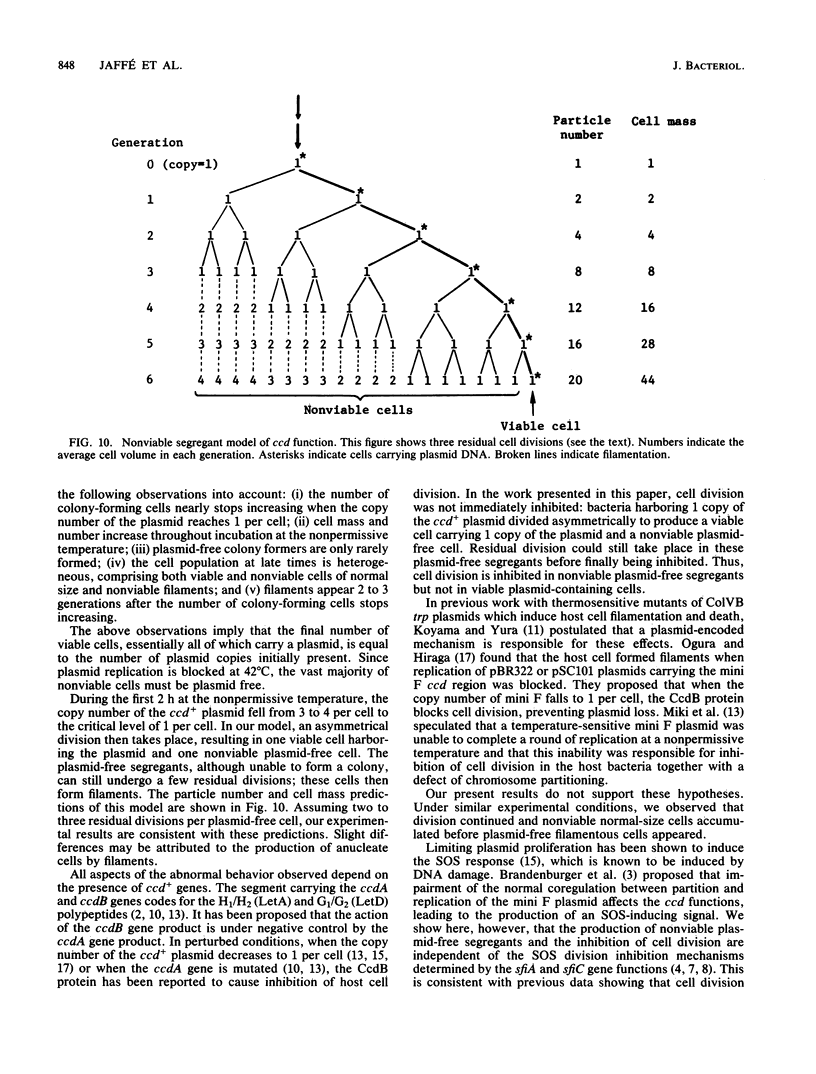
The ccd segment of the mini F plasmid containing the ccdA and ccdB genes controls the coordination between plasmid proliferation and cell physiology and fate. When the DNA replication of a thermosensitive-replication plasmid carrying the ccd segment of mini F is blocked, plasmid DNA molecules are progressively diluted through cell division until the copy number reaches 1 per cell. From this time on, there is little increase in the number of viable cells, although cells continue to divide, resulting in a mixed population of viable cells (mostly plasmid containing), nonviable but residually dividing cells, and nonviable nondividing cells. Results are presented suggesting that plasmid-containing cells are viable and continue to divide, whereas plasmid-free segregants are nonviable and form filaments after a few residual divisions, with DNA synthesis reduced or arrested in the filaments. Although the ccd functions are known to induce the SOS response when plasmid replication is blocked, the production of nonviable plasmid-free segregants is independent of the SOS cell division inhibition mechanism determined by the sfiA and sfiC genes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailone A., Brandenburger A., Lévine A., Pierre M., Dutreix M., Devoret R. Indirect SOS induction is promoted by ultraviolet light-damaged miniF and requires the miniF lynA locus. J Mol Biol. 1984 Nov 5;179(3):367–390. doi: 10.1016/0022-2836(84)90071-8. [DOI] [PubMed] [Google Scholar]
- Bex F., Karoui H., Rokeach L., Drèze P., Garcia L., Couturier M. Mini-F encoded proteins: identification of a new 10.5 kilodalton species. EMBO J. 1983;2(11):1853–1861. doi: 10.1002/j.1460-2075.1983.tb01671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenburger A., Bailone A., Lévine A., Devoret R. Gratuitous induction. J Mol Biol. 1984 Nov 5;179(3):571–576. doi: 10.1016/0022-2836(84)90082-2. [DOI] [PubMed] [Google Scholar]
- D'Ari R., Huisman O. Novel mechanism of cell division inhibition associated with the SOS response in Escherichia coli. J Bacteriol. 1983 Oct;156(1):243–250. doi: 10.1128/jb.156.1.243-250.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkacz B. W., Sherratt D. J. Segregation kinetics of colicinogenic factor col E1 from a bacterial population temperature sensitive for DNA polymerase I. Mol Gen Genet. 1973;121(1):71–75. doi: 10.1007/BF00353694. [DOI] [PubMed] [Google Scholar]
- Hashimoto-Gotoh T., Franklin F. C., Nordheim A., Timmis K. N. Specific-purpose plasmid cloning vectors. I. Low copy number, temperature-sensitive, mobilization-defective pSC101-derived containment vectors. Gene. 1981 Dec;16(1-3):227–235. doi: 10.1016/0378-1119(81)90079-2. [DOI] [PubMed] [Google Scholar]
- Huisman O., D'Ari R., George J. Inducible sfi dependent division inhibition in Escherichia coli. Mol Gen Genet. 1980;177(4):629–636. doi: 10.1007/BF00272673. [DOI] [PubMed] [Google Scholar]
- Huisman O., D'Ari R., Gottesman S. Cell-division control in Escherichia coli: specific induction of the SOS function SfiA protein is sufficient to block septation. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4490–4494. doi: 10.1073/pnas.81.14.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffé A., D'Ari R. Regulation of chromosome segregation in Escherichia coli. Ann Inst Pasteur Microbiol. 1985 Jan-Feb;136A(1):159–164. doi: 10.1016/s0769-2609(85)80036-3. [DOI] [PubMed] [Google Scholar]
- Karoui H., Bex F., Drèze P., Couturier M. Ham22, a mini-F mutation which is lethal to host cell and promotes recA-dependent induction of lambdoid prophage. EMBO J. 1983;2(11):1863–1868. doi: 10.1002/j.1460-2075.1983.tb01672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama A. H., Yura T. Plasmid mutations affecting self-maintenance and host growth in Escherichia coli. J Bacteriol. 1975 Apr;122(1):80–88. doi: 10.1128/jb.122.1.80-88.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T., Chang Z. T., Horiuchi T. Control of cell division by sex factor F in Escherichia coli. II. Identification of genes for inhibitor protein and trigger protein on the 42.84-43.6 F segment. J Mol Biol. 1984 Apr 25;174(4):627–646. doi: 10.1016/0022-2836(84)90087-1. [DOI] [PubMed] [Google Scholar]
- Miki T., Yoshioka K., Horiuchi T. Control of cell division by sex factor F in Escherichia coli. I. The 42.84-43.6 F segment couples cell division of the host bacteria with replication of plasmid DNA. J Mol Biol. 1984 Apr 25;174(4):605–625. doi: 10.1016/0022-2836(84)90086-x. [DOI] [PubMed] [Google Scholar]
- Mori H., Ogura T., Hiraga S. Prophage lambda induction caused by mini-F plasmid genes. Mol Gen Genet. 1984;196(2):185–193. doi: 10.1007/BF00328049. [DOI] [PubMed] [Google Scholar]
- Ogura T., Hiraga S. Mini-F plasmid genes that couple host cell division to plasmid proliferation. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4784–4788. doi: 10.1073/pnas.80.15.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura T., Hiraga S. Partition mechanism of F plasmid: two plasmid gene-encoded products and a cis-acting region are involved in partition. Cell. 1983 Feb;32(2):351–360. doi: 10.1016/0092-8674(83)90454-3. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]