Abstract
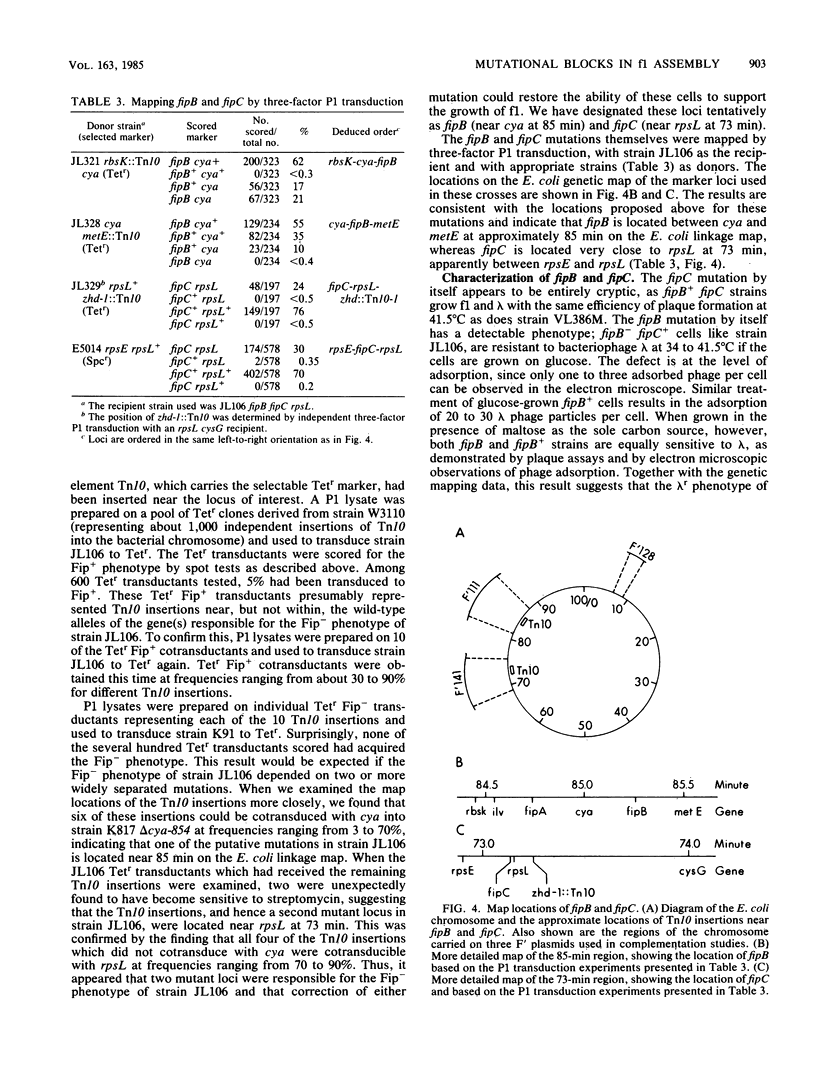
We describe the identification of two mutations in bacterial genes, designated as fipB and fipC, which resulted in temperature-sensitive morphogenesis of bacteriophage f1. These mutations mapped at separate loci but had to be present simultaneously to block f1 production at 41.5 degrees C. One mutation defined the locus fipB at 85.3 min on the Escherichia coli linkage map; the other defined the locus fipC, which mapped very close to rpsL at 73 min. Since these mutations did not appear to affect phage DNA replication, gene expression, or protein localization, they probably interfered with the its life cycle at the level of assembly. fipB mutants were partially deficient in adsorption of bacteriophage lambda, and fipB and fipC mutants leaked beta-lactamase into the medium, suggesting that the mutations affect outer-membrane structure or function.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J., Hewitt J. A., Perham R. N. Chemical modification of the coat protein in bacteriophage fd and orientation of the virion during assembly and disassembly. EMBO J. 1983;2(10):1641–1646. doi: 10.1002/j.1460-2075.1983.tb01638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelberg-Kulka H., Dekel L., Israeli-Reches M., Belfort M. The requirement of nonsense suppression for the development of several phages. Mol Gen Genet. 1979 Feb 26;170(2):155–159. doi: 10.1007/BF00337791. [DOI] [PubMed] [Google Scholar]
- Engelberg-Kulka H. UGA suppression by normal tRNA Trp in Escherichia coli: codon context effects. Nucleic Acids Res. 1981 Feb 25;9(4):983–991. doi: 10.1093/nar/9.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag C. S., Eisenstein B. I. Genetic mapping and transcriptional orientation of the fimD gene. J Bacteriol. 1983 Dec;156(3):1052–1058. doi: 10.1128/jb.156.3.1052-1058.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMANN BERLING H., MAZE R. RELEASE OF MALE-SPECIFIC BACTERIOPHAGES FROM SURVIVING HOST BACTERIA. Virology. 1964 Mar;22:305–313. doi: 10.1016/0042-6822(64)90021-2. [DOI] [PubMed] [Google Scholar]
- HOFFMANN-BERLING H., DUERWALD H., BEULKE I. EIN FAEDIGER DNS-PHAGE (FD) UND EIN SPHAERISCHER RNS-PHAGE (FR) WIRTSSPEZIFISCH FUER MAENNLICHE STAEMME VON E. COLI. III. BIOLOGISCHES VERHALTEN VON FD UND FR. Z Naturforsch B. 1963 Nov;18:893–898. [PubMed] [Google Scholar]
- Hofstetter H., Monstein H. J., Weissmann C. The readthrough protein A1 is essential for the formation of viable Q beta particles. Biochim Biophys Acta. 1974 Dec 6;374(2):238–251. doi: 10.1016/0005-2787(74)90366-9. [DOI] [PubMed] [Google Scholar]
- Lim C. J., Haller B., Fuchs J. A. Thioredoxin is the bacterial protein encoded by fip that is required for filamentous bacteriophage f1 assembly. J Bacteriol. 1985 Feb;161(2):799–802. doi: 10.1128/jb.161.2.799-802.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J., Webster R. E. Morphogenesis of filamentous bacteriophage f1: orientation of extrusion and production of polyphage. Virology. 1983 May;127(1):177–193. doi: 10.1016/0042-6822(83)90382-3. [DOI] [PubMed] [Google Scholar]
- Russel M., Model P. A bacterial gene, fip, required for filamentous bacteriophage fl assembly. J Bacteriol. 1983 Jun;154(3):1064–1076. doi: 10.1128/jb.154.3.1064-1076.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Model P. Characterization of the cloned fip gene and its product. J Bacteriol. 1984 Feb;157(2):526–532. doi: 10.1128/jb.157.2.526-532.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Model P. Filamentous phage pre-coat is an integral membrane protein: analysis by a new method of membrane preparation. Cell. 1982 Jan;28(1):177–184. doi: 10.1016/0092-8674(82)90387-7. [DOI] [PubMed] [Google Scholar]
- Russel M., Model P. Thioredoxin is required for filamentous phage assembly. Proc Natl Acad Sci U S A. 1985 Jan;82(1):29–33. doi: 10.1073/pnas.82.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilowitz H. Bacteriophage f1 infection: fate of the parental major coat protein. J Virol. 1974 Jan;13(1):94–99. doi: 10.1128/jvi.13.1.94-99.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegen U., Hofschneider P. H. Replication of the single-stranded DNA bacteriophage M13: absence of intracellular phages. J Mol Biol. 1970 Mar 14;48(2):361–364. doi: 10.1016/0022-2836(70)90168-3. [DOI] [PubMed] [Google Scholar]
- Timmis K., Marvin D. A. Filamentous bacterial viruses. XVI. Inherent temperature sensitivity of gene 5 protein and its involvement in abortive infection. Virology. 1974 May;59(1):293–300. doi: 10.1016/0042-6822(74)90225-6. [DOI] [PubMed] [Google Scholar]
- Trenkner E., Bonhoeffer F., Gierer A. The fate of the protein component of bacteriophage fd during infection. Biochem Biophys Res Commun. 1967 Sep 27;28(6):932–939. doi: 10.1016/0006-291x(67)90069-1. [DOI] [PubMed] [Google Scholar]
- Trenkner E. Pool sizes of fd bacteriophage components in infected bacterial cells. Virology. 1970 Jan;40(1):18–22. doi: 10.1016/0042-6822(70)90374-0. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Viñuela E., Algranati I. D., Ochoa S. Synthesis of virus-specific proteins in Escherichia coli infected with the RNA bacteriophage MS2. Eur J Biochem. 1967 Mar;1(1):3–11. doi: 10.1007/978-3-662-25813-2_2. [DOI] [PubMed] [Google Scholar]
- Zeevi M., Daniel V., Engelberg-Kulka H. A streptomycin-resistant Escherichia coli mutant with ribosomes temperature-sensitive in the suppression of a nonsense codon. Mol Gen Genet. 1979 Feb 26;170(2):149–153. doi: 10.1007/BF00337790. [DOI] [PubMed] [Google Scholar]




