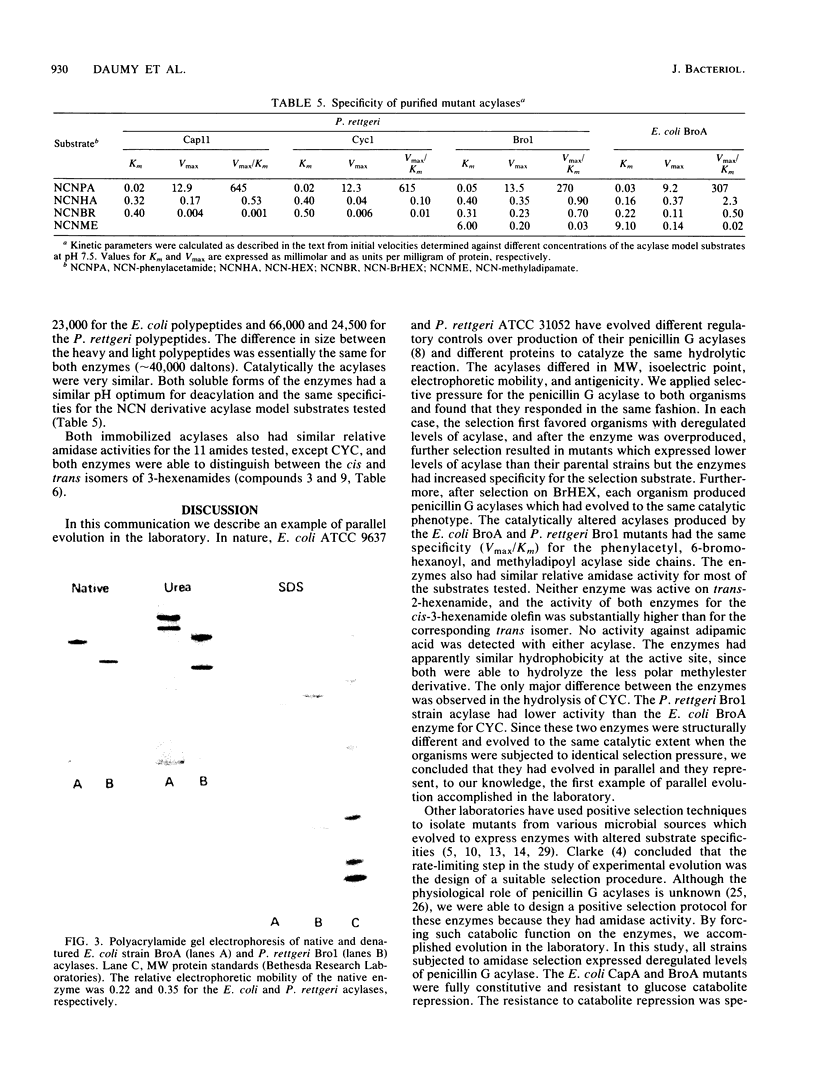
Abstract
Proteus rettgeri and Escherichia coli W were shown to express structurally different penicillin G acylases. The enzymes had similar substrate specificity but differed in molecular weight, isoelectric point, and electrophoretic mobility in polyacrylamide gels and did not antigenically cross-react. When the organisms were subjected to environmental conditions which made expression of this enzyme essential for growth, spontaneous mutants were isolated that used different amides as the only source of nitrogen. These mutants acquired the ability to use amides for growth by deregulating the penicillin G acylase and by their evolution to novel substrate specificities. The enzymes expressed by mutants isolated from each genus appeared to have evolved in parallel since each acylase attained similar new substrate specificities when the organisms were subjected to identical selection pressure.
Full text
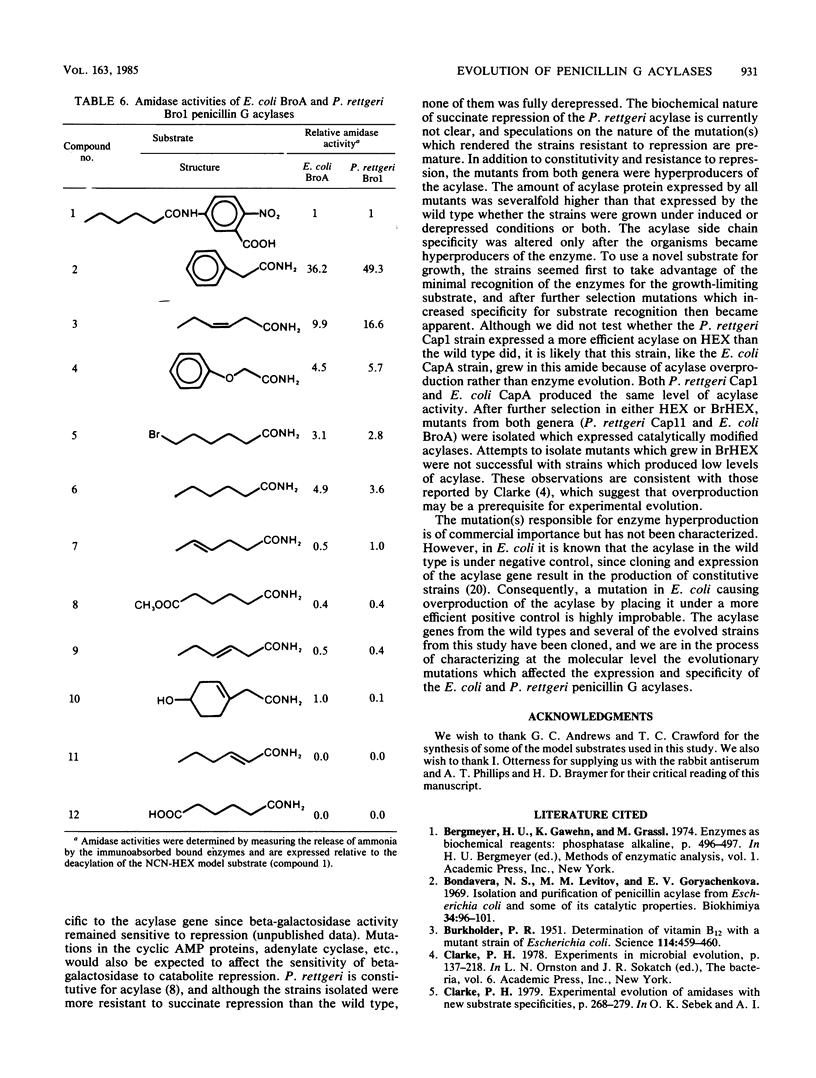
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURKHOLDER P. R. Determination of vitamin B12 with a mutant strain of Escherichia coli. Science. 1951 Nov 2;114(2966):459–460. doi: 10.1126/science.114.2966.459. [DOI] [PubMed] [Google Scholar]
- Bondareva N. S., Levitov M. M., Goriachenkova E. V. Vydelenie i ochistka penitsillinatsilazy iz kletok E. coli i izuchenie ee nekotorykh kataliticheskikh svoistv. Biokhimiia. 1969 Jan-Feb;34(1):96–101. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
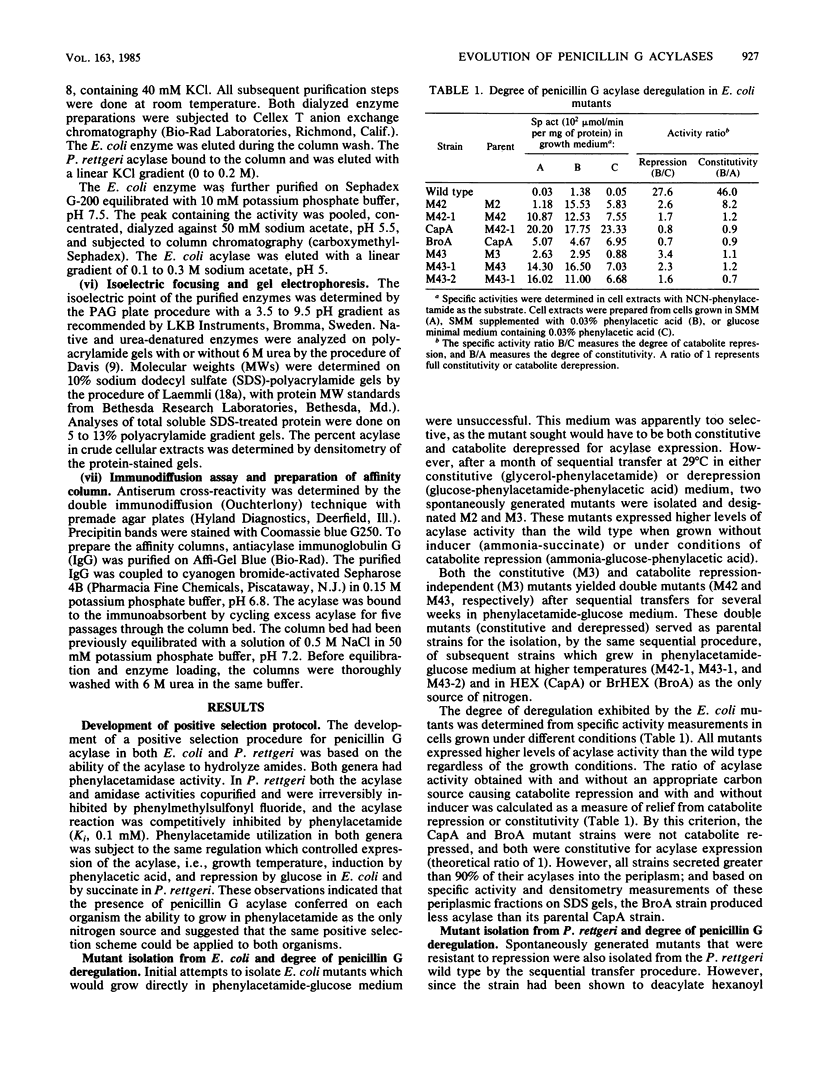
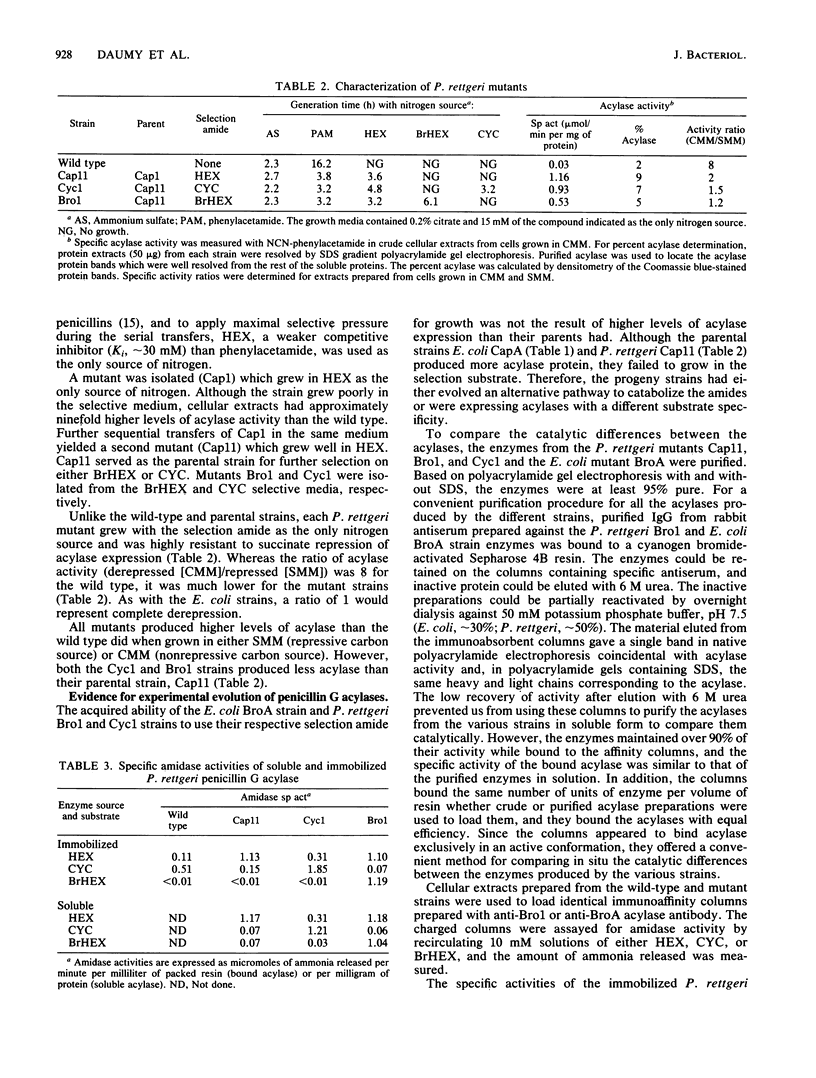
- Daumy G. O., McColl A. S., Apostolakos D. Repression of penicillin G acylase of Proteus rettgeri by tricarboxylic acid cycle intermediates. J Bacteriol. 1982 Oct;152(1):104–110. doi: 10.1128/jb.152.1.104-110.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis J. C., Hansche P. E. Directed evolution of metabolic pathways in microbial populations. I. Modification of the acid phosphatase pH optimum in S. cerevisiae. Genetics. 1972 Jan;70(1):59–73. doi: 10.1093/genetics/70.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gang D. M., Shaikh K. Regulation of penicillin acylase in Escherichia coli by cyclic AMP. Biochim Biophys Acta. 1976 Feb 18;425(1):110–114. doi: 10.1016/0005-2787(76)90220-3. [DOI] [PubMed] [Google Scholar]
- Groves W. E., Davis F. C., Jr, Sells B. H. Spectrophotometric determination of microgram quantities of protein without nucleic acid interference. Anal Biochem. 1968 Feb;22(2):195–210. doi: 10.1016/0003-2697(68)90307-2. [DOI] [PubMed] [Google Scholar]
- HUANG H. T., SETO T. A., SHULL G. M. Distribution and substrate specificity of benzylpenicillin acylase. Appl Microbiol. 1963 Jan;11:1–6. doi: 10.1128/am.11.1.1-6.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G. Changes in the substrate specificities of an enzyme during directed evolution of new functions. Biochemistry. 1981 Jul 7;20(14):4042–4049. doi: 10.1021/bi00517a015. [DOI] [PubMed] [Google Scholar]
- Kutzbach C., Rauenbusch E. Preparation and general properties of crystalline penicillin acylase from Escherichia coli ATCC 11 105. Hoppe Seylers Z Physiol Chem. 1974 Jan;355(1):45–53. doi: 10.1515/bchm2.1974.355.1.45. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levitov M. M., Klapovskaia K. I., Kleiner G. I. Indutsirovannyi biosintez atsilazy Escherichia coli. Mikrobiologiia. 1967 Sep-Oct;36(5):912–917. [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. Regulation of a membrane component required for protein secretion in Escherichia coli. Cell. 1982 Aug;30(1):311–319. doi: 10.1016/0092-8674(82)90037-x. [DOI] [PubMed] [Google Scholar]
- SZENTIRMAI A. PRODUCTION OF PENICILLIN ACYLASE. Appl Microbiol. 1964 May;12:185–187. doi: 10.1128/am.12.3.185-187.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Vandamme E. J., Voets J. P. Microbial penicillin acylases. Adv Appl Microbiol. 1974;17(0):311–369. doi: 10.1016/s0065-2164(08)70563-x. [DOI] [PubMed] [Google Scholar]
- Vojtísek V., Slezák J. Penicillinamidohydrolase in Escherichia coli. II. Synthesis of the enzyme, kinetics and specificity of its induction and the effect of O2. Folia Microbiol (Praha) 1975;20(4):289–297. doi: 10.1007/BF02878110. [DOI] [PubMed] [Google Scholar]
- Vojtísek V., Slezák J. Penicillinamidohydrolase in Escherichia coli. III. Catabolite repression, diauxie, effect of cAMP and nature of the enzyme induction. Folia Microbiol (Praha) 1975;20(4):298–306. doi: 10.1007/BF02878111. [DOI] [PubMed] [Google Scholar]
- Wills C., Phelps J. A technique for the isolation of yeast alcohol dehydrogenase mutants with altered substrate specificity. Arch Biochem Biophys. 1975 Apr;167(2):627–637. doi: 10.1016/0003-9861(75)90506-8. [DOI] [PubMed] [Google Scholar]