Abstract
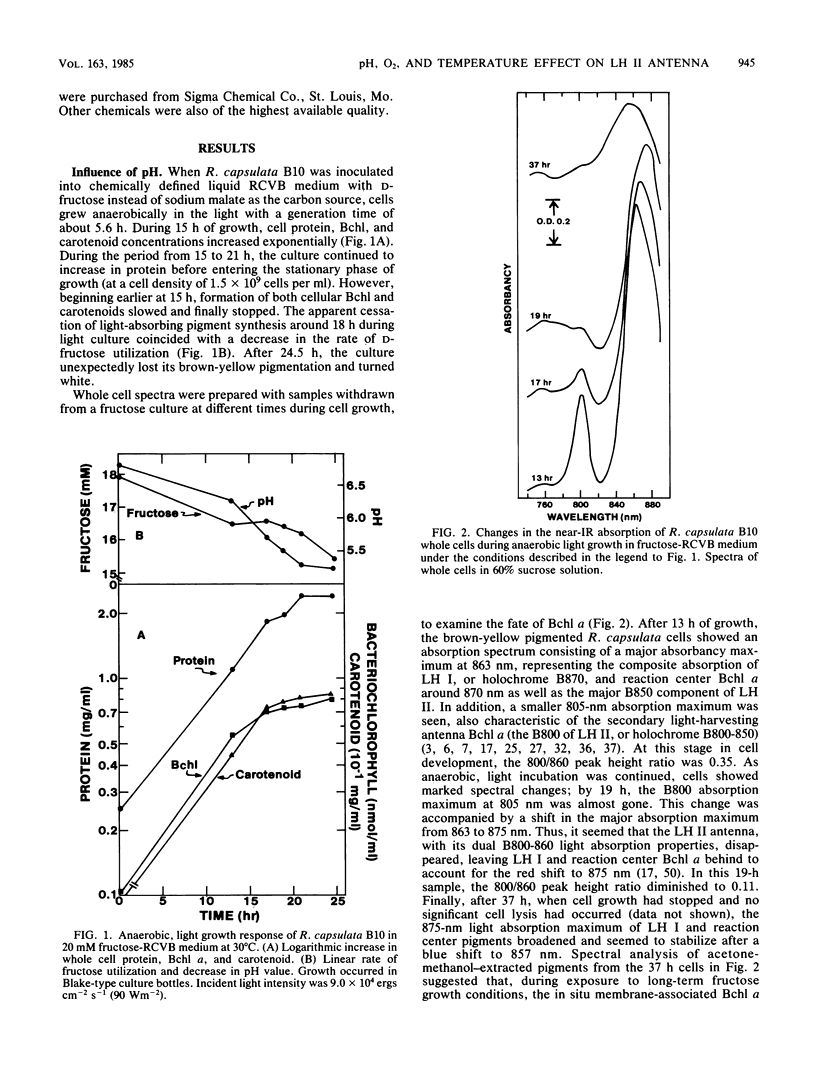
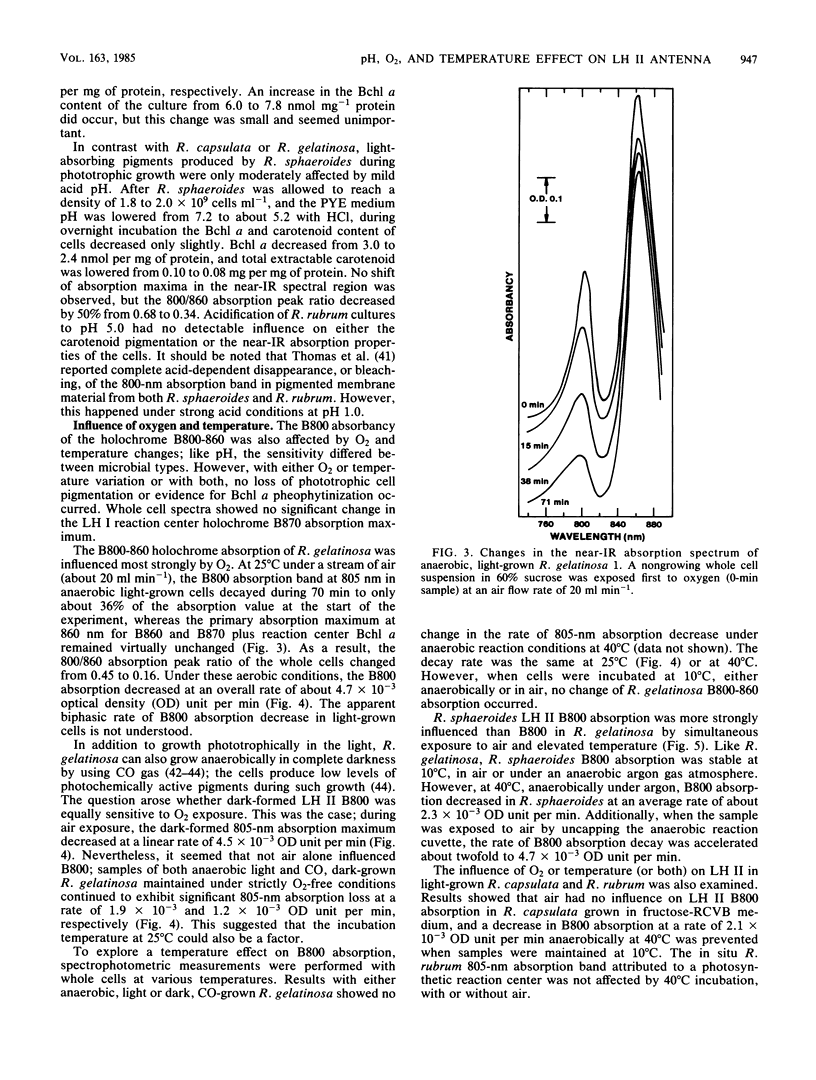
In some Rhodospirillaceae, the primary light-harvesting (LH I) antenna absorbs near-infrared light around 870 nm, whereas LH II (holochrome B800-860) has a major absorption band between 850 and 860 nm (B860) and a minor absorbancy around 800 nm (B800). Results show that, unlike LH I, holochrome B800-860 (LH II) exhibits unstable light absorption properties in whole cells. This was observed in Rhodopseudomonas capsulata grown anaerobically in light in weakly buffered carbohydrate medium; cultures lost both carotenoid-dependent brown-yellow pigmentation and LH II absorbancy. The whole cell spectrophotometric changes were attributed to mild acid conditions generated during sugar metabolism. LH II absorbancy was also destroyed in both R. capsulata and Rhodopseudomonas gelatinosa when cultures growing at neutral pH were acidified to a pH value around 5.0 with HCl. In contrast, during the same time period of exposure to pH 5.0, only a 50% decrease in Rhodopseudomonas sphaeroides LH II B800 absorbancy was measured. At neutral pH, LH II absorbancy in suspensions of nongrowing Rhodopseudomonas spp. was also sensitive to O2 exposure and to incubation at 30 to 40 degrees C. During treatment with O2, the rate of LH II B800 absorption decrease in R. gelatinosa and R. sphaeroides was 60 and 40% per h, respectively, compared with their absorbancy maximum around 860 nm. Both 860-nm absorbancy and the total bacteriochlorophyll content of the cells remained unchanged. On the other hand, no significant decrease in B800 if LH II in R. capsulata occurred during O2 exposure, but a 20% absorption decay rate per h of B800 was observed in cells incubated anaerobically at 40 degrees C. These B800 LH II spectral changes Rhodopseudomonas spp. were prevented by maintaining cells at neutral pH and at 10 degrees C. The near-infrared absorption spectrum of Rhodospirillum rubrum, which does not form LH II, was not significantly influenced by these different pH, aerobic, or temperature conditions.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiking H., Sojka G. Response of Rhodopseudomonas capsulata to illumination and growth rate in a light-limited continuous culture. J Bacteriol. 1979 Aug;139(2):530–536. doi: 10.1128/jb.139.2.530-536.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranki A., Freter R. Use of anaerobic glove boxes for the cultivation of strictly anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1329–1334. doi: 10.1093/ajcn/25.12.1329. [DOI] [PubMed] [Google Scholar]
- CLAYTON R. K. TOWARD THE ISOLATION OF A PHOTOCHEMICAL REACTION CENTER IN RHODOPSEUDOMONAS SPHEROIDES. Biochim Biophys Acta. 1963 Nov 29;75:312–323. doi: 10.1016/0006-3002(63)90618-8. [DOI] [PubMed] [Google Scholar]
- Clayton B. J., Clayton R. K. Properties of photochemical reaction centers purified from Rhodopseudomonas gelatinosa. Biochim Biophys Acta. 1978 Mar 13;501(3):470–477. doi: 10.1016/0005-2728(78)90114-7. [DOI] [PubMed] [Google Scholar]
- Conrad R., Schlegel H. G. Different degradation pathways for glucose and fructose in Rhodopseudomonas capsulata. Arch Microbiol. 1977 Feb 4;112(1):39–48. doi: 10.1007/BF00446652. [DOI] [PubMed] [Google Scholar]
- Conrad R., Schlegel H. G. Different pathways for fructose and glucose utilization in Rhodopseudomonas capsulata and demonstration of 1-phosphofructokinase in phototrophic bacteria. Biochim Biophys Acta. 1974 Jul 17;358(1):221–225. doi: 10.1016/0005-2744(74)90273-3. [DOI] [PubMed] [Google Scholar]
- Cox J. C., Madigan M. T., Favinger J. L., Gest H. Redox mechanisms in "oxidant-dependent" hexose fermentation by Rhodopseudomonas capsulata. Arch Biochem Biophys. 1980 Oct 1;204(1):10–17. doi: 10.1016/0003-9861(80)90002-8. [DOI] [PubMed] [Google Scholar]
- Feick R., Drews G. Isolation and characterization of light harvesting bacteriochlorophyll.protein complexes from Rhodopseudomonas capsulata. Biochim Biophys Acta. 1978 Mar 13;501(3):499–513. doi: 10.1016/0005-2728(78)90117-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Odelson D. A., Breznak J. A. Volatile Fatty Acid production by the hindgut microbiota of xylophagous termites. Appl Environ Microbiol. 1983 May;45(5):1602–1613. doi: 10.1128/aem.45.5.1602-1613.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelze J. Composition and development of the bacterial photosynthetic apparatus. Subcell Biochem. 1981;8:1–73. doi: 10.1007/978-1-4615-7951-9_1. [DOI] [PubMed] [Google Scholar]
- Oelze J. Control of the formation of bacteriochlorophyll, and B875- and B850-bacteriochlorophyll complexes in Rhodopseudomonas sphaeroides mutant strain H5. Arch Microbiol. 1983 Dec;136(4):312–316. doi: 10.1007/BF00425223. [DOI] [PubMed] [Google Scholar]
- Picorel R., Lefebvre S., Gingras G. Oxido-reduction of B800-850 and B880 holochromes isolated from three species of photosynthetic bacteria as studied by electron-paramagnetic resonance and optical spectroscopy. Eur J Biochem. 1984 Jul 16;142(2):304–311. doi: 10.1111/j.1432-1033.1984.tb08286.x. [DOI] [PubMed] [Google Scholar]
- Schultz J. E., Weaver P. F. Fermentation and anaerobic respiration by Rhodospirillum rubrum and Rhodopseudomonas capsulata. J Bacteriol. 1982 Jan;149(1):181–190. doi: 10.1128/jb.149.1.181-190.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleat R., Mah R. A. Quantitative method for colorimetric determination of formate in fermentation media. Appl Environ Microbiol. 1984 Apr;47(4):884–885. doi: 10.1128/aem.47.4.884-885.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaiman D., Uffen R. L. Pyruvate-dependent diauxic growth of Rhodospirillum rubrum in light. J Bacteriol. 1982 Dec;152(3):1175–1187. doi: 10.1128/jb.152.3.1175-1187.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uffen R. L. Anaerobic growth of a Rhodopseudomonas species in the dark with carbon monoxide as sole carbon and energy substrate. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3298–3302. doi: 10.1073/pnas.73.9.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uffen R. L. Metabolism of carbon monoxide by Rhodopseudomonas gelatinosa: cell growth and properties of the oxidation system. J Bacteriol. 1983 Sep;155(3):956–965. doi: 10.1128/jb.155.3.956-965.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uffen R. L., Wolfe R. S. Anaerobic growth of purple nonsulfur bacteria under dark conditions. J Bacteriol. 1970 Oct;104(1):462–472. doi: 10.1128/jb.104.1.462-472.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall J. D., Johansson B. C., Gest H. A pleiotropic mutant of Rhodopseudomonas capsulata defective in nitrogen metabolism. Arch Microbiol. 1977 Dec 15;115(3):259–263. doi: 10.1007/BF00446450. [DOI] [PubMed] [Google Scholar]
- Weaver P. F., Wall J. D., Gest H. Characterization of Rhodopseudomonas capsulata. Arch Microbiol. 1975 Nov 7;105(3):207–216. doi: 10.1007/BF00447139. [DOI] [PubMed] [Google Scholar]
- Yen H. C., Marrs B. Growth of Rhodopseudomonas capsulata under anaerobic dark conditions with dimethyl sulfoxide. Arch Biochem Biophys. 1977 Jun;181(2):411–418. doi: 10.1016/0003-9861(77)90246-6. [DOI] [PubMed] [Google Scholar]
- Youvan D. C., Hearst J. E., Marrs B. L. Isolation and characterization of enhanced fluorescence mutants of Rhodopseudomonas capsulata. J Bacteriol. 1983 May;154(2):748–755. doi: 10.1128/jb.154.2.748-755.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youvan D. C., Ismail S. Light-harvesting II (B800-B850 complex) structural genes from Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1985 Jan;82(1):58–62. doi: 10.1073/pnas.82.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]



