Abstract
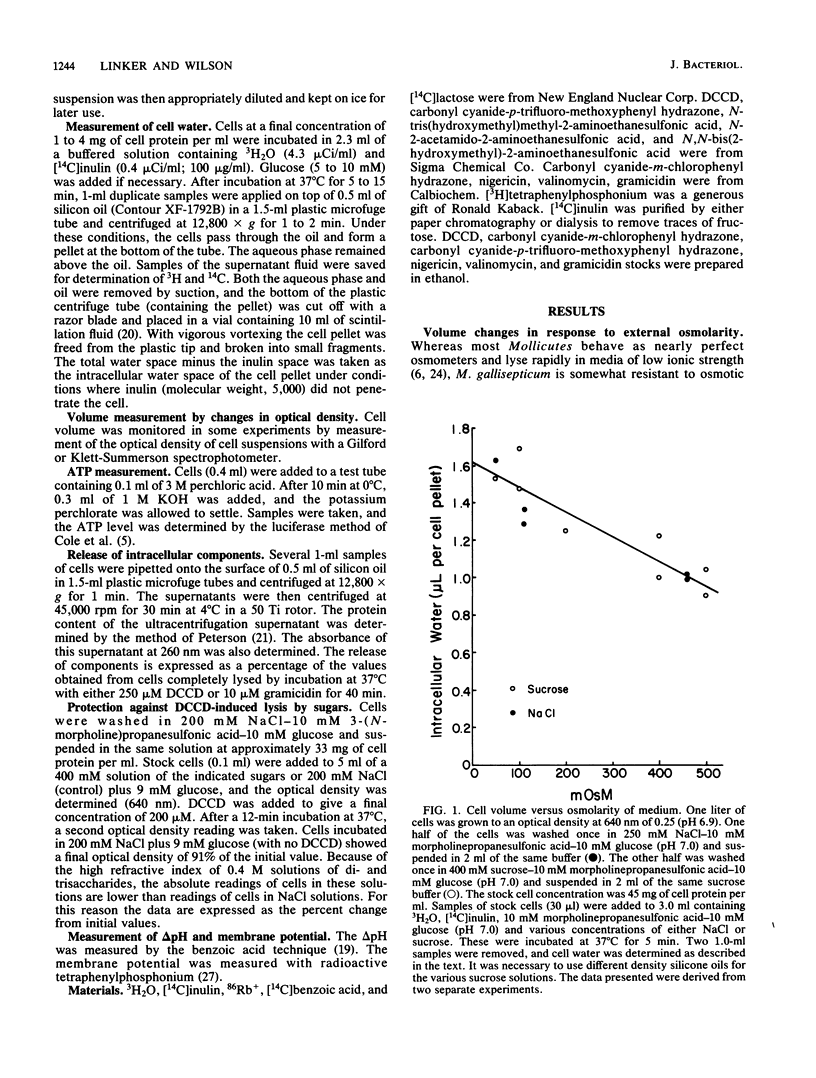
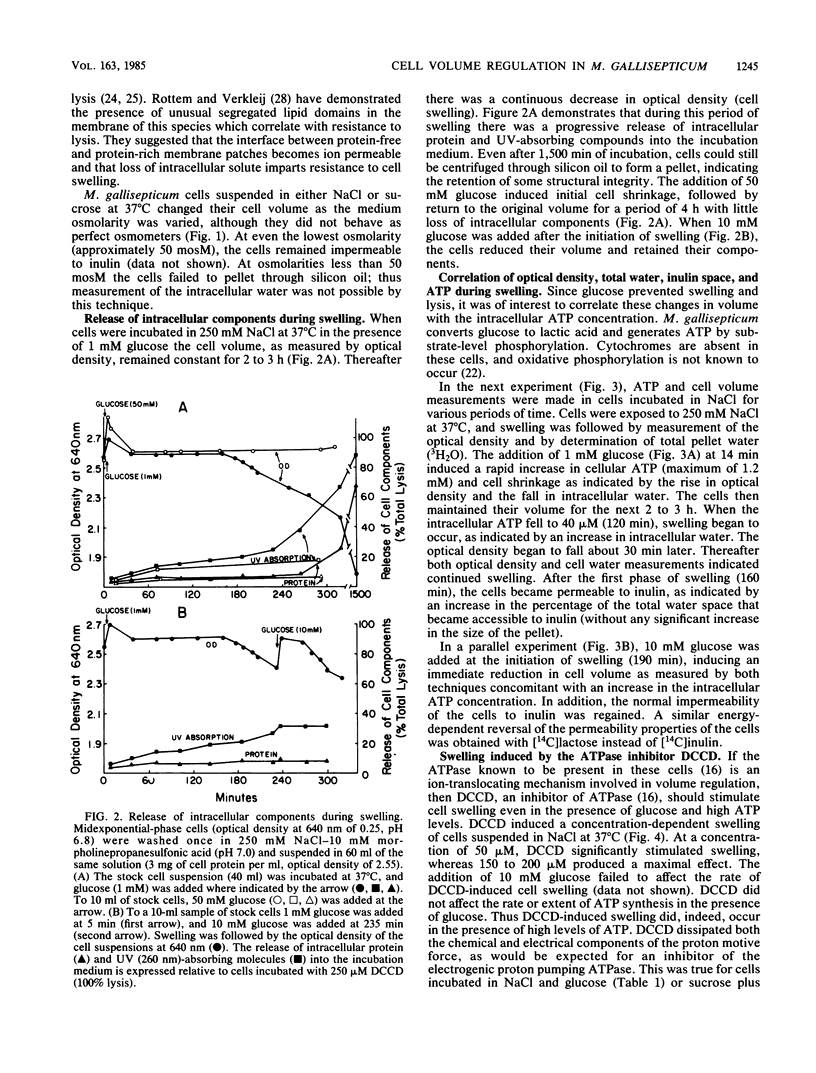
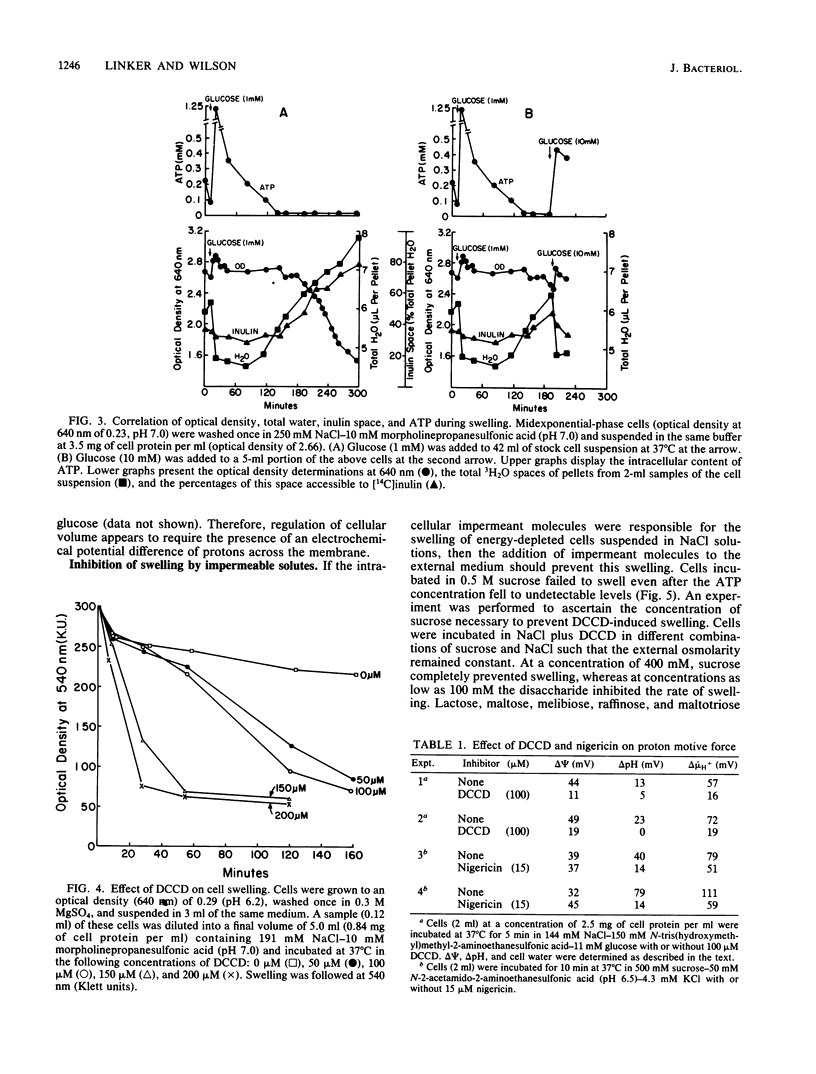
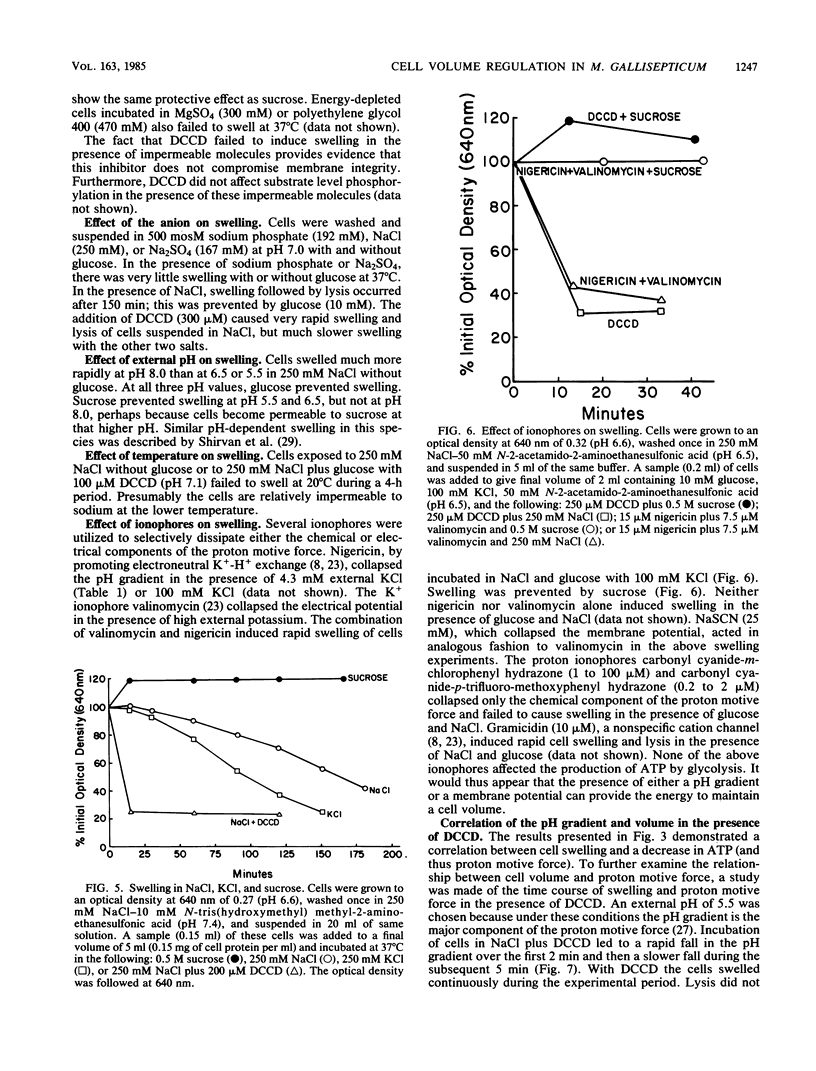
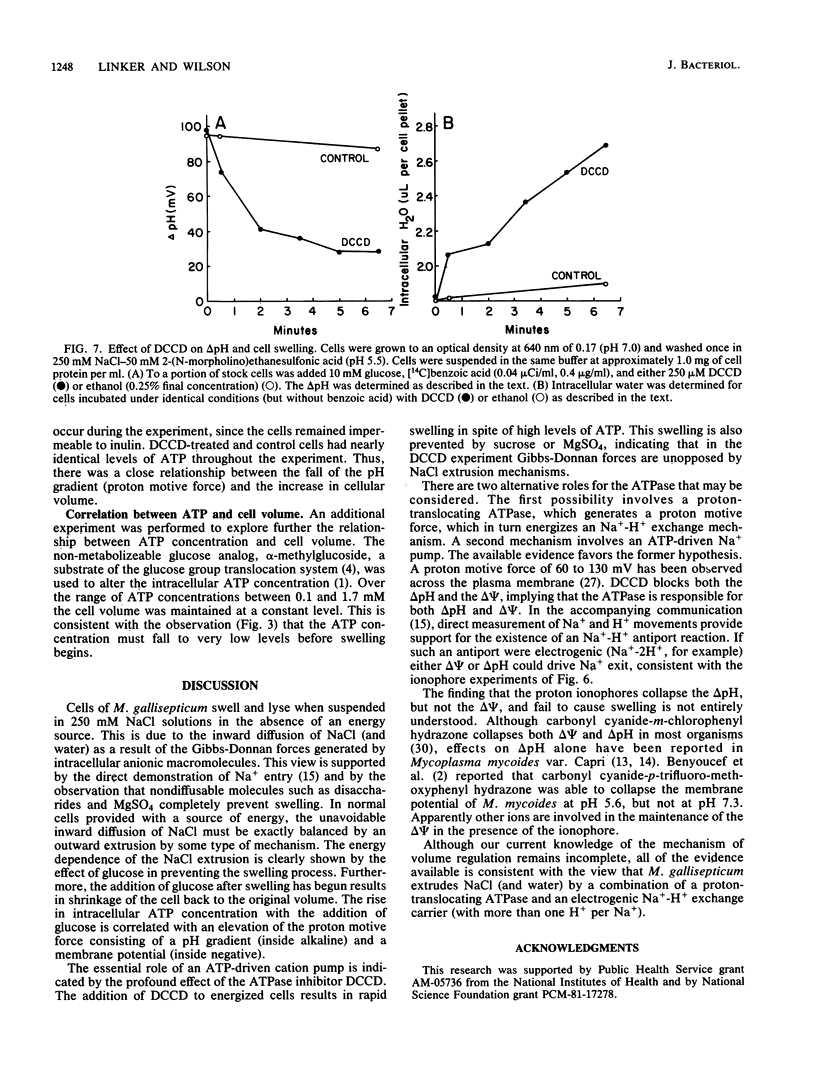
Mycoplasma gallisepticum cells incubated in 250 mM NaCl solutions in the absence of glucose showed a progressive fall in intracellular ATP concentration over a period of 2 to 3 h. When the ATP level fell below 40 microM the cell began to swell and become progressively permeable to [14C]inulin and leak intracellular protein and nucleotides. The addition of nondiffusable substances such as MgSO4 or disaccharides prevented swelling, suggesting that NaCl (and water) entry was due to Gibbs-Donnan forces. The addition of glucose after the initiation of cell swelling increased intracellular ATP, induced cell shrinkage, and prevented the release of intracellular components. The ATPase inhibitor dicyclohexylcarbodiimide, which collapsed the chemical and electrical components of the proton motive force, caused rapid cell swelling in the presence of glucose (and high intracellular ATP levels). Extracellular impermeable solutes such as MgSO4 and disaccharides prevented swelling of dicyclohexylcarbodiimide-treated cells incubated in NaCl. It was postulated that Na+ that diffused into the cell was extruded by an electrogenic Na+-H+ exchange (antiport) energized by the proton motive force established by the dicyclohexylcarbodiimide-sensitive H+-ATPase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benyoucef M., Rigaud J. L., Leblanc G. Cation transport mechanisms in Mycoplasma mycoides var. Capri cells. Na+-dependent K+ accumulation. Biochem J. 1982 Dec 15;208(3):529–538. doi: 10.1042/bj2080529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyoucef M., Rigaud J. L., Leblanc G. Cation transport mechanisms in Mycoplasma mycoides var. Capri cells. The nature of the link between K+ and Na+ transport. Biochem J. 1982 Dec 15;208(3):539–547. doi: 10.1042/bj2080539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyoucef M., Rigaud J. L., Leblanc G. Gradation of the magnitude of the electrochemical proton gradient in Mycoplasma cells. Eur J Biochem. 1981 Jan;113(3):499–506. doi: 10.1111/j.1432-1033.1981.tb05091.x. [DOI] [PubMed] [Google Scholar]
- Cole H. A., Wimpenny J. W., Hughes D. E. The ATP pool in Escherichia coli. I. Measurement of the pool using modified luciferase assay. Biochim Biophys Acta. 1967;143(3):445–453. doi: 10.1016/0005-2728(67)90050-3. [DOI] [PubMed] [Google Scholar]
- Jinks D. C., Silvius J. R., McElhaney R. N. Physiological role and membrane lipid modulation of the membrane-bound (Mg2+, na+)-adenosine triphosphatase activity in Acholeplasma laidlawii. J Bacteriol. 1978 Dec;136(3):1027–1036. doi: 10.1128/jb.136.3.1027-1036.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEAF A. Maintenance of concentration gradients and regulation of cell volume. Ann N Y Acad Sci. 1959 Feb 6;72(12):396–404. doi: 10.1111/j.1749-6632.1959.tb44168.x. [DOI] [PubMed] [Google Scholar]
- LEAF A. On the mechanism of fluid exchange of tissues in vitro. Biochem J. 1956 Feb;62(2):241–248. doi: 10.1042/bj0620241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grimellec C., Lajeunesse D., Rigaud J. L. Effects of energization on membrane organization in mycoplasma. Biochim Biophys Acta. 1982 May 7;687(2):281–290. doi: 10.1016/0005-2736(82)90556-9. [DOI] [PubMed] [Google Scholar]
- Leblanc G., Le Grimellec C. Active K+ transport in Mycoplasms mycoides var. Capri. Relationships between K+ distribution, electrical potential and ATPase activity. Biochim Biophys Acta. 1979 Jun 13;554(1):168–179. doi: 10.1016/0005-2736(79)90016-6. [DOI] [PubMed] [Google Scholar]
- Linker C., Wilson T. H. Characterization and solubilization of the membrane-bound ATPase of Mycoplasma gallisepticum. J Bacteriol. 1985 Sep;163(3):1258–1262. doi: 10.1128/jb.163.3.1258-1262.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker C., Wilson T. H. Sodium and proton transport in Mycoplasma gallisepticum. J Bacteriol. 1985 Sep;163(3):1250–1257. doi: 10.1128/jb.163.3.1250-1257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macknight A. D., Leaf A. Regulation of cellular volume. Physiol Rev. 1977 Jul;57(3):510–573. doi: 10.1152/physrev.1977.57.3.510. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pollack J. D., Tryon V. V., Beaman K. D. The metabolic pathways of Acholeplasma and Mycoplasma: an overview. Yale J Biol Med. 1983 Sep-Dec;56(5-6):709–716. [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- RAZIN S. FACTORS INFLUENCING OSMOTIC FRAGILITY OF MYCOPLASMA. J Gen Microbiol. 1964 Sep;36:451–459. doi: 10.1099/00221287-36-3-451. [DOI] [PubMed] [Google Scholar]
- Rottem S., Linker C., Wilson T. H. Proton motive force across the membrane of Mycoplasma gallisepticum and its possible role in cell volume regulation. J Bacteriol. 1981 Mar;145(3):1299–1304. doi: 10.1128/jb.145.3.1299-1304.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Verkleij A. J. Possible association of segregated lipid domains of Mycoplasma gallisepticum membranes with cell resistance to osmotic lysis. J Bacteriol. 1982 Jan;149(1):338–345. doi: 10.1128/jb.149.1.338-345.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH P. F., SASAKI S. Stability of pleuropneumonialike organisms to some physical factors. Appl Microbiol. 1958 May;6(3):184–189. doi: 10.1128/am.6.3.184-189.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni R. D., Postma P. W. The energetics of bacterial active transport. Annu Rev Biochem. 1975;44:523–554. doi: 10.1146/annurev.bi.44.070175.002515. [DOI] [PubMed] [Google Scholar]
- TOSTESON D. C., HOFFMAN J. F. Regulation of cell volume by active cation transport in high and low potassium sheep red cells. J Gen Physiol. 1960 Sep;44:169–194. doi: 10.1085/jgp.44.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON T. H. Ionic permeability and osmotic swelling of cells. Science. 1954 Jul 16;120(3107):104–105. doi: 10.1126/science.120.3107.104. [DOI] [PubMed] [Google Scholar]
- de Kruyff B., Demel R. A., van Deenen L. L. The effect of cholesterol and epicholesterol incorporation on the permeability and on the phase transition of intact Acholeplasma laidlawii cell membranes and derived liposomes. Biochim Biophys Acta. 1972 Jan 17;255(1):331–347. doi: 10.1016/0005-2736(72)90032-6. [DOI] [PubMed] [Google Scholar]


