Abstract
Histamine is considered one of the important mediators of immediate hypersensitivity and inflammation, and acts via G protein–coupled receptors. Here, we report that histamine may affect antigen receptor–mediated immune responses of T and B cells via a signal(s) from histamine H1 receptors (H1Rs). Histamine exhibited enhancing effects on the in vitro proliferative responses of anti-CD3ε– or anti-IgM–stimulated spleen T and B cells, respectively, at the culture condition that the fetal calf serum was dialyzed before culture and c-kit–positive cells were depleted from the spleen cells. In studies of histamine H1R knockout mice, H1R-deficient T cells had low proliferative responses to anti-CD3ε cross-linking or antigen stimulation in vitro. B cells from H1R-deficient mice were also affected, demonstrating low proliferative responses to B cell receptor cross-linking. Antibody production against trinitrophenyl-Ficoll was reduced in H1R-deficient mice. Other aspects of T and B cell function were normal in the H1R knockout mice. H1R-deficient T and B cells showed normal responses upon stimulation with interleukin (IL)-2, IL-4, CD40 ligand, CD40 ligand plus IL-4, and lipopolysaccharide. Collectively, these results imply that the signal generated by histamine through H1R augments antigen receptor–mediated immune responses, suggesting cross-talk between G protein–coupled receptors and antigen receptor–mediated signaling.
Keywords: G protein, antigen receptor, signaling, histamine H1 receptor, G protein– coupled receptor
Guanine-nucleotide binding (G)1 protein–coupled receptors (GPCRs) link to downstream signaling pathways through activation of heterotrimeric G proteins, which are composed of three subunits, termed α, β, and γ (1, 2) in an inactive state. Upon ligand/agonist binding, GPCRs stimulate the α subunit of heterotrimeric G protein to release GDP and to bind GTP in its place. In the GTP-bound form, a Gα dissociates from a Gβγ dimer, each of which independently binds and activates target effectors. The α subunits that bind and hydrolyze GTP are classified into four subfamilies based on sequence homology and shared effector molecules: Gαs, Gαi, Gαq, and Gα12 (1–3). Intracellular signaling pathways regulated by GPCRs include the cAMP/protein kinase A pathway, the phosphatidyl inositol/calcium/protein kinase pathway mediated by phospholipase Cβ (PLCβ) and the mitogen-activated protein kinase (MAPK) pathway (4). Recent studies have provided strong evidence that in some cell types activation of MAPK pathway by GPCR is tyrosine kinase dependent (5–8). The genetic and biochemical analysis clearly provides evidence that protein tyrosine kinase cascade bridges G protein and MAPK pathways in mammalian cells (9). The Gα subunits that regulate activity of PLCβ belong to the Gq class (Gαq, Gα11, Gα14, Gα15/16; references 10, 11). Tyrosine phosphorylation of the Gαq/11 subunit by protein tyrosine kinases (PTKs) contributes to GPCR- mediated activation of Gq/11 (12, 13) followed by hydrolysis of phosphatidylinositol phosphates and production of inositol-1,4,5-triphosphate (IP3) and diacylglycerol. Tyrosine-phosphorylated Gαq/11 has been shown to be more active in stimulating PLCβ in vitro (12).
Protein tyrosine phosphorylation is an important event in the initiation of cellular responses triggered by antigen receptors on both B and T cells (14–16). One of the initial intracellular signaling events after cross-linking of the B cell antigen-receptors (BCR) with antigens is the activation of non-receptor type PTKs, such as Src family kinases (Lyn, Blk, Fyn), Syk kinase, and Bruton's tyrosine kinase (Btk) (15). Activation of the PTKs of the Src family such as Lck and Fyn, followed by the activation of ZAP-70 kinase, has been implicated in an initial step of TCR signal transduction (14). These protein tyrosine kinases rapidly phosphorylate a number of intracellular substrates and activate signaling cascades that include activation of phospholipase Cγ-1 and 2 (PLCγ1, γ2), phosphatidylinositol 3 kinase (PI3 kinase), and the Ras–MAPK pathway, which transmits further biochemical events that eventually regulate cell cycle and gene expression.
It has been shown that GTP exchange within Gαq/11 and physical association of Gαq/11 with CD3ε are induced upon cross-linking of the TCR by anti-CD3ε antibody (17). Moreover, it was demonstrated that upon TCR engagement Gαq/11 is activated by a tyrosine kinase-dependent process that mediates both tyrosine phosphorylation of immunoreceptor tyrosine activation motif (ITAM) on CD3 molecules and IP3 generation through activation of PLCβ. Interestingly, tyrosine phosphorylation of TCR-ζ and CD3ε chains as well as ZAP-70 were diminished upon anti-CD3 antibody triggering in cells transfected with a function-loss mutant of Gα11 (17). These data suggest the involvement of the Gαq/11 family in TCR signaling and a reciprocal regulation between tyrosine kinases and G proteins during the initial stages of TCR-mediated signaling (3). Activation of tyrosine kinases Pyk2 and Src may link Gi- and Gq-coupled receptors to the MAPK pathway in certain cell types (18, 19). The cross-linking of antigen receptors also induced Pyk2 activation in T cells (20). Thus, Pyk2 may potentially serve as a convergence point for TCR and GPCR signaling. Moreover, it was shown that Gq-mediated signaling could trigger the translocation of the transcription factor, nuclear factor (NF)-AT, to the nucleus upon TCR-mediated T cell activation (21). Among chemokine receptors that are associated with GPCRs, RANTES (regulated on activation, normal T cell expressed and secreted) could activate ZAP-70, which plays a crucial role in TCR-mediated signaling (22).
In B cells, Gαq activates Btk (23), and Gβγ subunits have been also reported to bind and activate Btk (24, 25). Thus, engagement of either the BCR or a Gq-linked GPCR could activate Btk in B cells (3). Based on selective gene targeting in an avian B lymphoma cell line, DT40, MAPK activation through Gi-coupled receptors requires Btk and Syk, whereas Gq-coupled receptors require Csk, Lyn, and Syk (9).
Histamine is known to be a neurotransmitter (26), an inflammatory mediator, a factor in anaphylaxis, and a regulator of cardiac and gastrointestinal function. The pharmacological effects of histamine are mediated through three types of membrane receptors, H1, H2, and H3 (27). The H1R possesses all the structural features of GPCRs, including seven putative transmembrane domains, NH2-terminal glycosylation sites, and phosphorylation sites for protein kinase A and protein kinase C (28). It has been detected in mammalian brain (29), smooth muscle from airway (31, 32), gastrointestinal tract (33), genitourinary system (34), vascular smooth muscle (35), and lymphocytes (36, 37). Upon H1R stimulation, various intracellular responses, such as production of inositol phosphates, increase in Ca2+ influx, cyclic AMP and cyclic GMP accumulation, and arachidonic acid release (27, 38), are induced through coupling of the H1Rs to the heterotrimeric G proteins (27, 39, 40). The primary mechanism by which the H1R produces these functional responses in cells is the activation of PLC through a pertussis toxin–insensitive G protein that is related to the Gαq/11 family of G proteins (26, 27). It has also been reported that the histamine-induced production of inositol phosphates was inhibited by an antibody against Gαq-like proteins (41). These data suggest the involvement of a member of Gαq family of the G proteins in H1R- mediated signal transduction.
In this study, we have used H1R knockout mice (42) to demonstrate that histamine affects antigen receptor–mediated immune responses by T and B cells via signal(s) from histamine H1Rs, and we suggest the possibility of a reciprocal regulation between GPCRs and antigen receptor– mediated signaling.
Materials and Methods
Generation of H1R− /− Mice.
The histamine H1R knockout mice were established in our lab and have been described previously (42). The H1R−/− 129 × C57BL/6 mice were backcrossed to C57BL/6 in our animal facility under pathogen-free conditions with proper aeration, light, and temperature.
Genotype Determination by Southern Hybridization.
Genomic DNA (10 μg) was digested with EcoRI and subjected to agarose gel electrophoresis. DNA was transferred onto nucleic acid transfer membranes (Hybond TM− N+; Amersham) by alkali blotting. Filters were hybridized with radiolabeled DNA probes, and then washed in 0.1× SSC, 0.1% (wt/vol) SDS at 60°C for 30 min before autoradiography.
Cell Culture and Proliferation Assay.
Splenic B cells were prepared by the treatment of spleen cells with anti–mouse Thy 1.2 mAb for 30 min on ice and then with complement for 30 min at 37°C for depletion of T cells. To remove the adherent cells, cells were incubated at 37°C for 1 h and nonadherent cells were collected by Ficoll-Hypaque density centrifugation. Red blood cells were lysed with NH4Cl lysis buffer. After washing, cell purity was measured by FACS® analysis. Splenic T cells were prepared by passing spleen cells through a nylon wool column, and erythrocytes were lysed with NH4Cl lysis buffer. Cells were suspended in RPMI 1640 medium containing 10% heat-inactivated FCS (GIBCO BRL), 20 μg/ml gentamycin, 0.05 mM 2-ME, and 2 mM l-glutamine, and cultured at a final concentration of 5 × 105 cells/ml in 200-μl/well aliquots in flat-bottomed 96-well tissue culture plates (Becton Dickinson) at 37°C with 5% CO2 for 72 h. FCS was dialyzed twice against RPMI 1640 media at 4°C overnight. Various stimuli were added to the cells: LPS (Difco), goat F(ab)2 anti–mouse IgM (Southern Biotechnology Associates), rIL-4 (R & D Systems), soluble CD40 ligand (CD40L) (CD8 fusion protein contained in the culture supernatant [30% vol/vol] of a transfected myeloma cell line, provided by Dr. P. Lane, Basel Institute of Immunology, Basel, Switzerland), rIL-2 (R & D Systems), and goat anti–mouse CD3ε (PharMingen). The cultures were pulsed for the last 18 h with 0.5 μCi/ml [3H]thymidine (Amersham). After cell harvesting onto fiber glass filters (Printed Filter Mat A; Wallac OY, Turku, Finland), cell proliferation was determined by measuring [3H]thymidine incorporation with a beta plate counter (Wallac OY). FCS was dialyzed before use against RPMI 1640 medium overnight at 4°C by changing medium twice.
Depletion of c-kit–positive Cells.
Total spleen cells or purified B cells were suspended in serum-free RPMI 1640 media after red blood cell lysis. 107 cells/ml were incubated for 45 min on ice with 100 μl of 50 μg/ml rat IgG anti–c-kit mAb (clone ACK; a gift from Dr. S. Nishikawa, Kyoto University, Kyoto, Japan). After centrifugation at 4°C, cells were resuspended in 1 ml of serum-free media. Using a 21-gauge needle, 250 μl of 108 anti–rat IgG-coated beads (Dynabeads; Dynal AS) were added to the suspended cells and incubated for 5 min on ice. The incubated cells were centrifuged for 10 min at 4°C. After gentle pipetting, the Eppendorf tube was placed in front of a magnetic bar. The c-kit– positive cells were attached to the magnet. The nonattached cells were collected, washed, and finally resuspended in RPMI 1640 culture media containing 10% dialyzed FCS.
Immunization with OVA and Antigen-specific T cell Proliferation.
The wild-type (+/+) and H1R knockout (−/−) mice (8–10 wk of age) were immunized intraperitoneally with 100 μg of ovalbumin (Sigma Chemical Co.) emulsified in 1 mg aluminum hydroxide (alum) (Sigma Chemical Co.) in 200 μl of PBS. Mice were immunized twice with a 2-wk interval. Spleen cells were isolated 2 wk after the last injection and cultured for 3 d in the presence of different concentrations of OVA. Antigen-specific proliferative responses of spleen cells were measured by [3H]thymidine incorporation followed by a 12-h pulse at the end of 3 d of culture.
Immunization with TNP-Ficoll and Measurement of Anti-TNP Antibody.
Wild-type and H1R knockout mice (8–10 wk of age) were immunized intraperitoneally once with 25 μg of TNP- Ficoll (a gift from Dr. S. Ono, Osaka University, Osaka, Japan) in 200 μl PBS. After 10 d, the serum was collected and anti-TNP IgM antibody titer was measured by ELISA. For ELISA, flat-bottomed 96-well probind assay plates (Becton Dickinson) were coated with TNP-BSA at 4°C overnight. After blocking the nonspecific binding sites, serial dilutions of the sera were added (100 μl/well, in triplicate) and plates were incubated for 1 h at room temperature. After washing three times, horseradish peroxidase–conjugated anti–mouse IgM antibodies (Biosource International) were added and incubated at room temperature for 1 h. Finally, tetramethylbenzidine (TMB) peroxidase substrates (Kirkegaard and Perry Labs.) were added to each well and incubated for 5 min. The reaction was stopped by adding 1 M H3PO4. Absorbance at 450 nm was determined using a model 550 automatic microplate reader (Bio-Rad).
Titration of Isotype-specific Serum Igs and Anti-OVA Antibodies.
The 96-well flat-bottomed probind assay plates (Becton Dickinson) were coated with 10 μg/ml of isotype-specific goat anti–mouse antibodies overnight at 4°C. After washing uncoated antibodies, plates were blocked with 5% nonfat milk at room temperature for 1 h. The serially diluted sera from unimmunized mice were added to the wells and incubated at room temperature for 1 h. The HRP-labeled isotype-specific antibodies (Southern Biotechnology Associates) were applied to the plate and incubated for 2 h at room temperature. The TMB peroxidase substrate was added to each well for 5 min. Finally, the reaction was stopped by adding the stop solution (1 M H3PO4) and absorbance at 450 nm was measured using a model 550 automatic microplate reader (Bio-Rad Labs.).
For determination of isotype-specific anti-OVA antibodies in sera that were collected 2 wk after second immunization with OVA (100 μg/mouse) attached to alum, the assay plates were coated first with OVA (10 μg/ml) in 200 μl/well of PBS and incubated overnight. After washing plates with PBS, they were blocked with 5% nonfat milk at room temperature for 1 h. Serially diluted sera from OVA-immunized mice were added to the wells of plates and incubated at room temperature for 1 h. HRP-labeled isotype-specific antibodies were used for determination of the antibody titer of each Ig subclass.
Flow Cytometric Analysis.
Single cell suspensions from freshly isolated red blood cell lysed thymocytes, splenocytes, and bone marrow were prepared, and 2 × 105 cells/ml were stained with mAbs for 30 min on ice in PBS containing 2% FCS and 0.5% NaN3. Cells were washed with PBS and analyzed by FACScan® with Cellquest software (Becton Dickinson). mAbs used for the staining were labeled with FITC or PE. mAbs to B220, CD5, CD4, and CD8 were obtained from PharMingen, and anti-IgM and -IgD were from Southern Biotechnology Associates, Inc. Streptavidin red 670 (PharMingen) was used as a third color for FACS® analysis.
Measurement of Cytokine Production.
Spleen cells (4 × 106/ well) were cultured in a 24-well culture plate (Costar Co.) with OVA at various concentrations (0, 5, 10, 20, 30, 40, or 50 μg/ ml) or in anti-CD3ε antibody–coated (10 μg/ml) plates. The amounts of secreted cytokines were determined by ELISA. In brief, supernatants were collected after 72 h of culture. The amounts of IL-4 and IL-2 in the supernatants were determined by a quantitative sandwich ELISA method using the Biotrack and Amersham mouse IL-4 and IL-2 kit (Amersham International). Following the manufacturer's protocol, the cultured supernatants were added to plates that had been precoated with mouse IL-4 or IL-2 mAb. The HRP-conjugated anti–mouse IL-4 or IL-2 mAb was added to the plate and incubated at 37°C for 1 h. Bound cytokines were detected by the addition of TMB substrate. The stop solution (0.18 M sulphuric acid) was added to cease the reaction. The IFN-γ in the culture supernatants was determined by using the Titerzyme Mouse IFN-γ EIA Kit (Perseptive Diagnostics, Inc.). According to the described protocol, the culture supernatant was applied to the mouse IFN-γ antibody precoated well and incubated at room temperature for 2 h in horizontal orbital rotor. After a thorough washing of the plates, the wells were covered with 50 μl of mouse IFN-γ antibody and incubated at room temperature for 2 h. After washing, HRP-conjugated goat anti–rabbit IgG was applied to the wells and incubated at room temperature for 30 min. TMB substrate solution was added after proper washing and incubated at room temperature for 15 min. Finally, 1 N HCl was used to stop the reaction. The absorbance was determined at 450 nm using a Model 550 automatic microplate reader (Bio-Rad Labs.). Cytokine levels were calculated from a log–log plot of absorbence versus known concentration of recombinant cytokines. Results were expressed in picograms per milliliter.
Immunoprecipitation and Western Blot Analysis.
After depletion of c-kit–positive cells from H1R+/+ spleen cell suspension, 107 cells of wild-type or H1R−/− mice in 1 ml of RPMI 1640 media with 10% dialyzed FCS were stimulated with goat anti–mouse CD3ε (10 μg/ml) in the presence or absence of 10−5 M histamine at 37°C for 0, 1, 5, and 10 min. The anti-CD3ε-stimulated cells were centrifuged and the pellets were lysed in 1 ml cold lysis buffer (10 mM Tris, pH 7.4, 1.0% Triton X-100, 0.5% NP-40, 150 mM NaCl, 20 mM sodium fluoride, 0.2 mM sodium ortho-vanadate, 1.0 mM EDTA, and 1.0 mM EGTA) containing protease inhibitors (0.2 mM PMSF, 10 μg/ml aprotinine, 10 μg/ml leupeptin) for 30 min on ice with periodic vortexing. After lysis, the samples were centrifuged and the supernatants were frozen at −70°C before assay. Protein concentration of the lysates was assayed by using the BCA Reagents Kit (Pierce Chemical Co.) according to the manufacturer's instructions. ZAP-70 kinase was immunoprecipitated from lysates (0.4 mg total protein) with 2 μg of soluble anti–ZAP-70 antibody for 1 h at 4°C with end-over-end rotation. The immune complexes were captured with 10 μl of 50% suspended protein G–agarose (Sigma Chemical Co.) for 30 min at 4°C with constant rotation. The immunoprecipitates were washed three times with immunoprecipitation (IP) buffer (10 mM Tris, pH 7.4, 1.0% Triton X-100, 0.5% NP-40, 150 mM NaCl, 0.2 mM sodium ortho-vanadate, 1.0 mM EDTA, 1.0 mM EGTA, 0.2 mM PMSF), followed by a centrifugation at 15,000 rpm for 4 min at 4°C. The collected immunoprecipitates were then dissociated in boiled SDS-PAGE sample buffer containing 2-ME and fractionated on 10% SDS-polyacrylamide gels. The proteins on the gels were transferred onto nitrocellulose membrane filter (PROTRAN; Schleicher & Schnell, Inc.) and probed with HRP-labeled antiphosphotyrosine mAb, PY-20 (Transduction Labs.). The blots were developed by the chemiluminescence (ECL) reagents (Amersham Corp.) and Biomax TM MR film (Eastman Kodak Co.).
Results
Histamine-enhanced Antigen Receptor–mediated T and B Cell Proliferative Responses.
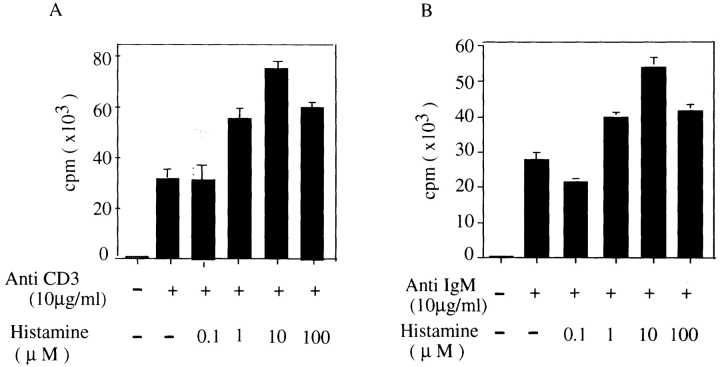
Mouse spleen T and B cells were initially cultured in medium containing 10% standard FCS and stimulated with anti-CD3ε (10 μg/ml) or anti-IgM (10 μg/ml) antibodies in the presence of varying concentrations of histamine. Histamine had no effect on anti-CD3ε or anti-IgM–stimulated T or B cell proliferation. To eliminate any effect of preexisting histamine or its derivatives in FCS, the serum was dialyzed against RPMI 1640 culture medium before use, and c-kit–positive spleen cells, which are presumably mast cells, were removed. Splenic T (Fig. 1 A) or B cells (Fig. 1 B) were stimulated with anti-CD3ε antibody or anti-IgM antibody, respectively, in the presence of various concentration of histamine. Histamine enhanced the proliferative responses of T and B cells at an optimum concentration of 10 μM for both cell types by about twofold. However, histamine did not show any effect on the proliferative responses unless the FCS was dialyzed before culture and c-kit–positive cells were depleted from the spleen cells (data not shown).
Figure 1.
Enhancing effect of histamine on proliferative responses of c-kit–positive cell-depleted spleen T and B cells cultured in media containing dialyzed FCS. c-kit–positive cells were removed from total spleen cells and splenic B cells of wild-type C57/Bl mice were purified as described in Materials and Methods. c-kit–positive cell-depleted spleen T and B cells (5 × 105 cells/ml) were cultured for 72 h in RPMI 1640 medium containing dialyzed FCS and stimulated with (A) anti-CD3ε (10 μg/ml) or (B) anti–mouse IgM (10 μg/ml) in the presence of different concentrations of histamine. The cells were pulsed for 12 h with [3H]thymidine before the end of culture. Shown is one representative experiment out of three.
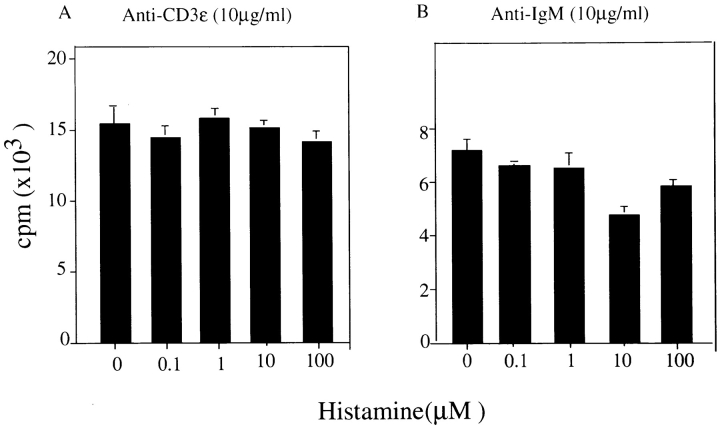
In contrast, the histamine-induced augmentation of antigen receptor–mediated proliferative responses of both T and B cells was not observed in spleen cells obtained from histamine H1R–deficient mice (Fig. 2) when cultured under the same conditions. These results indicate that histamine H1R–mediated signal(s) are responsible for the enhancing effect observed by the addition of histamine. It is noteworthy that the level of thymidine incorporation of H1R−/− splenic T and B cells upon antigen receptor cross-linking was very low when compared with that of spleen cells from wild-type mice in the absence of histamine, as shown in Fig. 1.
Figure 2.
Low proliferative responses of T and B cells of H1R−/− mice against anti-CD3ε and anti–mouse IgM stimulation. Total spleen cells and purified splenic B cells from H1R−/− mice were cultured in RPMI 1640 medium containing dialyzed FCS after depletion of c-kit–positive cells. Total spleen cells and purified B cells were stimulated with anti-CD3ε (A) and anti–mouse IgM (B), respectively, in the presence of different concentrations of histamine. The DNA synthesis was measured by pulsing with [3H]thymidine for 12 h before the end of culture. Shown is one representative experiment out of three.
Analysis of the Low Proliferative Responses Induced by Antigen Receptor Cross-linking in Histamine H1R− /− Mice.
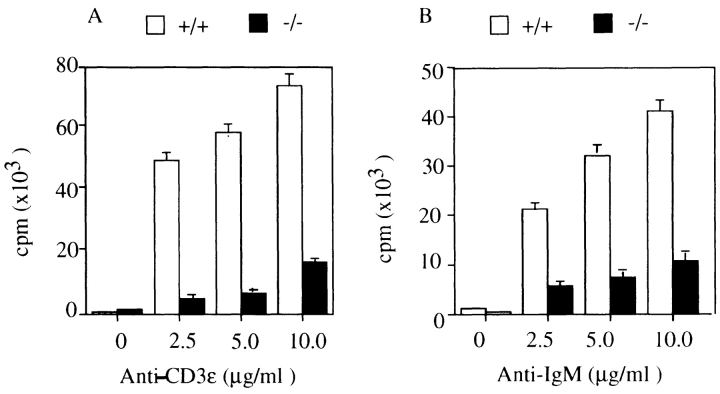
Splenic T and B cells prepared from histamine H1R−/− mice and normal littermates (+/+) were cultured in the presence of varying concentrations of anti-CD3ε (Fig. 3 A) and anti-IgM (Fig. 3 B), respectively. After 72 h of culture, proliferative responses were assayed by [3H]thymidine incorporation. The proliferative responses of T cells in H1R−/− mice to anti-CD3ε were five- to eightfold lower than those of normal littermate controls. Similarly, responses to anti-IgM stimulation of B cells from H1R−/− mice were decreased three- to fourfold compared to wild-type mice.
Figure 3.
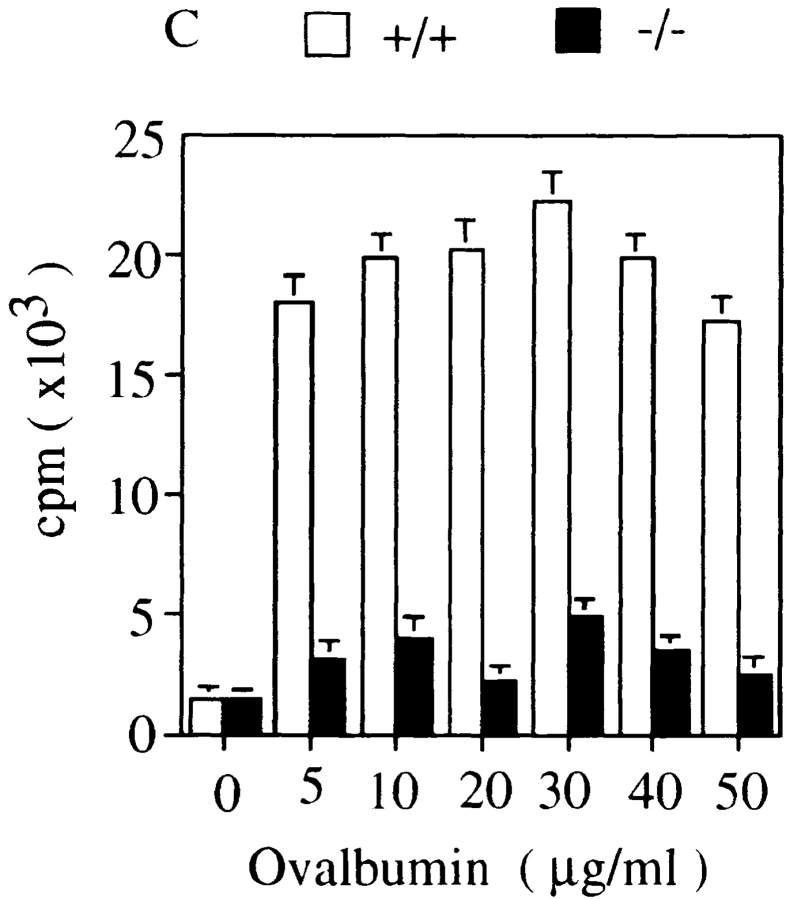
Low proliferative responses of splenic T and B cells of histamine H1R−/− mice upon cross-linking of antigen receptors. Total spleen cells and purified B cells from unimmunized wild-type mice (+/+, white bars) and H1R−/− mice (−/−, black bars) were cultured with various concentrations of (A) anti-CD3ε antibody or (B) anti-IgM antibody for 72 h. (C) The H1R−/− and wild-type mice were immunized intraperitoneally with OVA (100 μg/ mouse) emulsified in alum as described in Materials and Methods. Mice were immunized twice in a 2-wk interval. 2 wk after the last immunization, the mice were killed and spleen cells from both wild-type and H1R−/− mice were cultured in presence of OVA (5–50 μg/ml) for 3 d. Cell proliferation was measured with [3H]thymidine incorporation by pulsing for 12 h at the end of culture. Mean CPM and SD were calculated from triplicate cultures. Shown is one representative experiment out of four.
Both H1R−/− and wild-type mice were immunized twice with OVA to examine antigen-specific responses. Splenic T cells prepared from immunized mice were cultured in the presence of varying concentrations of OVA (Fig. 3 C). Proliferative responses to OVA antigen of H1R−/− T cells were four- to sixfold reduced as compared with the responses of the wild-type littermates. These data indicated that TCR- and BCR-mediated proliferative responses of T or B cells from mice lacking the histamine H1R were impaired, suggesting that a signal(s) from the histamine H1R upon ligand binding may play an indispensable role in signaling pathways originating from TCR and BCR complexes.
Decreased Antibody Production against a T Cell–independent Antigen in H1R− /− Mice.
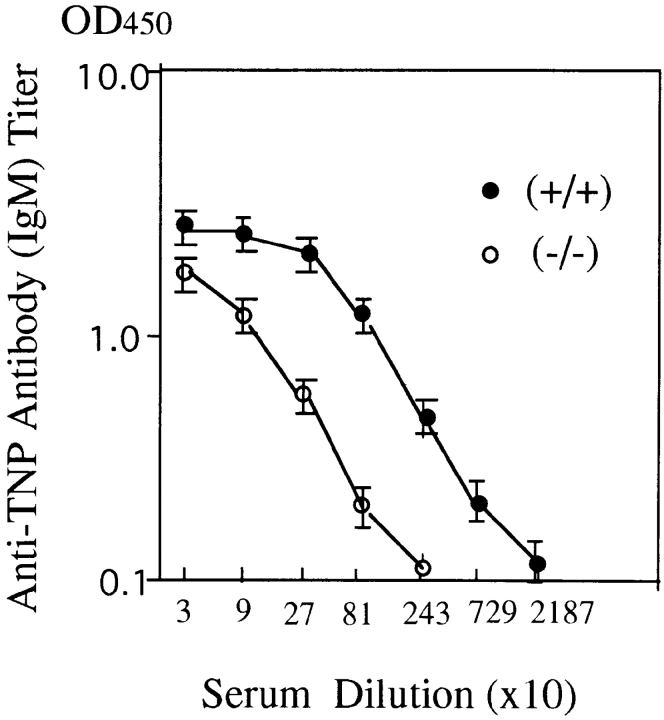
Impaired responses of splenic B cells to anti-IgM stimulation in H1R−/− mice suggested a significant role for the signal derived from the histamine H1R complex. H1R−/− and their wild-type littermates were challenged with the T cell–independent antigen, TNP-Ficoll (Fig. 4). After 10 d, sera were collected and antibodies to the TNP hapten were measured. Anti-TNP IgM antibody titer was low (about ninefold) in H1R−/− mice, indicating a crucial augmenting role for the interaction between ligands and H1Rs in responses triggered from BCRs.
Figure 4.
Reduced antibody response to T cell–independent antigen, TNP-Ficoll, in H1R−/− mice. Both the H1R−/− (−/−) and wild-type mice (+/+) received one intraperitoneal immunization of TNP-Ficoll (25 μg/mouse) in 200 μl of 1× PBS. Sera were collected from three mice of each group on day 10 after immunization. Anti-TNP IgM antibody titer in serum was measured by ELISA, as described in Materials and Methods. The antibody titer was determined by measuring the optical density in 450 nm. Data are plotted as mean ± SD.
Normal Proliferative Responses to Cytokines or Mitogens in H1R− /− Mice.
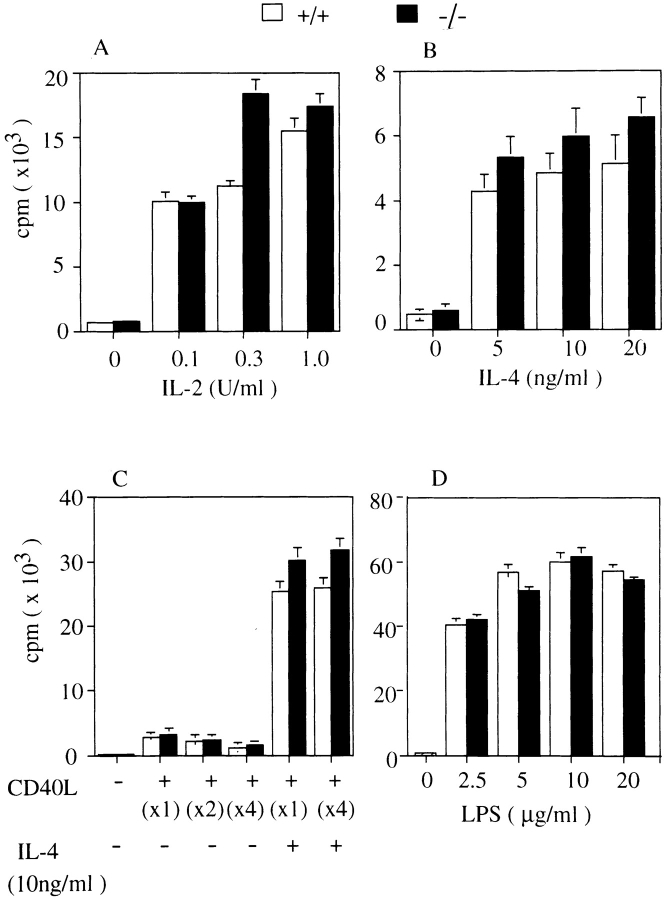
Splenic T cells of H1R−/− and wild-type mice were cultured in the presence of various concentration of IL-2. The thymidine incorporation was measured 72 h after culture and the level of the responses was not significantly changed (Fig. 5 A). Splenic B cells from H1R−/− and wild-type mice were cultured with IL-4 alone (Fig. 5 B) or in combination with CD40L (Fig. 5 C). Again, no significant difference was observed in the proliferative potential of splenic B cells from wild-type or H1R−/− mice, demonstrating that signals from histamine H1Rs do not modulate signaling in CD40-mediated proliferative response. Response of B cells to the mitogen, LPS (Fig. 5 D), and of T cells to the T cell mitogen, PHA, were also not significantly affected by H1R deficiency (data not shown). These data suggest that signals from H1Rs may not affect the intracellular signals induced by mitogen, cytokines, or costimulatory molecules, but exhibit augmenting effects on antigen receptor–mediated responses.
Figure 5.
Normal proliferative responses to IL-2, IL-4, CD40L, CD40L plus IL-4, and LPS in H1R−/− mice. Total spleen cells from H1R−/− (black bars) and their wild-type littermates (white bars) were cultured with medium alone or various concentrations of IL-2 (A) or IL-4 (B). Splenic B cells were cultured with CD40L–CD8 chimeric protein (CD40L) alone or in combination with IL-4 (C) or LPS (D). Cells were cultured for 3 d and pulse-labeled with [3H]thymidine for the final 18 h. All cultures were performed in triplicate.
Secondary Antibody Response against the T Cell–dependent Antigen, OVA, in H1R− /− Mice.
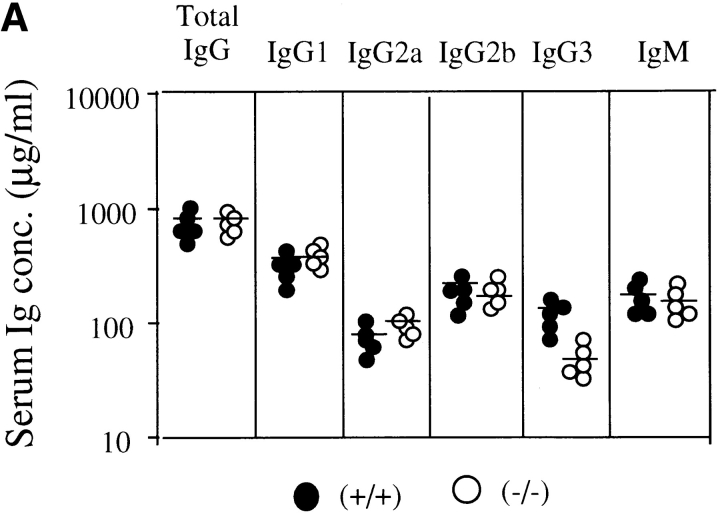
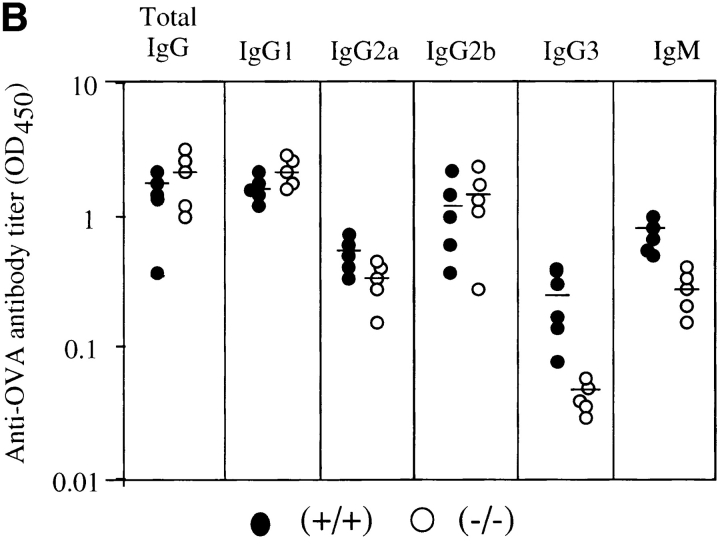
Mice were immunized twice with OVA (100 μg per mouse) emulsified with 1 mg Alum in 200 μl of PBS, and sera were collected 14 d after the second immunization. Antibody titer of each Ig class was measured by ELISA with isotype-specific antibodies. In unimmunized mice, serum level of each Ig subclass in H1R−/− mice was comparable to that of normal mice except for IgG3 class, which showed a slightly lower level in mutant mice as shown in Fig. 6 A. After immunization, total IgG of antibodies to OVA in H1R−/− mice was comparable to that of normal littermates. But, as shown in Fig. 6 B, IgG3 and IgM subclasses of anti-OVA antibodies in H1R−/− mice were significantly lower than those of control mice (P < 0.01). IgG2a subclass of antibodies was also somewhat low in mutant mice, but was not statistically significant. These data suggested that antibody production of the IgM and IgG3 subclasses may be impaired in the mice lacking H1Rs.
Figure 6.
Level of Ig subclass in H1R−/− mice before and after immunization with the T cell–dependent antigen, OVA. (A) Levels of serum Ig subclass in unimmunized mice. Serum Ig levels in 8–10-wk-old H1R−/− mice (−/−) and their wild-type littermates were determined by isotype-specific ELISA. •, control (+/+); ○, mutant (−/−) mice. Shown are the results from five mice of each group. (B) Levels of anti-OVA antibody titers of each Ig isotype after secondary immunization. Serum samples were collected 2 wk after the second immunization. Titers of OVA-specific antibodies were determined by isotype-specific ELISA. Serum antibody levels are expressed as OD450. Shown are the results from five mice of each group. Horizontal lines indicate mean.
Lymphocyte Profiles in Spleen, Bone Marrow, Thymus, and Peritoneal Cavity in H1R−/− Mice.
Single cell suspension of thymocytes, splenocytes, peritoneal exudated cells, and bone marrow cells were stained with various FITC-conjugated mAbs. No difference in total cell numbers was observed in each organ. FACS® analysis showed no significant difference in numbers of total or T and B cell subpopulation distribution in the profiles of thymus, bone marrow, and spleen in the histamine H1R−/− mice (data not shown). Level of expression of IgM on B cells or CD3 on T cells of H1R−/− mouse did not change and was comparable to that of wild-type littermates, although the numbers of CD5+, B220+ cells in peritoneal cavity in histamine H1R−/− mice were slightly decreased to about a half of those of normal littermates (21.0 ± 8.6% versus 39.5 ± 9.1% of total peritoneal exudated cells). These data indicated that the absence of the histamine H1Rs does not affect the development and differentiation of mature T and B lymphoid cells, but may affect the development of B1 cells in the peritoneal cavity.
Reduced Tyrosine Phosphorylation of ZAP-70 Kinase in H1R− /− Mouse T Cells.
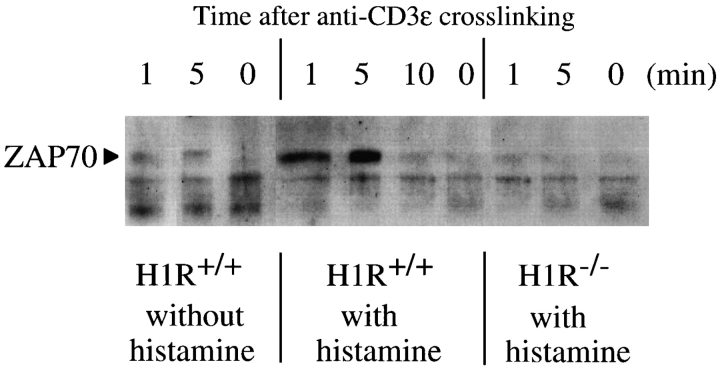
The low proliferative response of T cell of H1R−/− mice after triggering with anti-CD3ε or antigen (OVA) (Figs. 1 and 3) may suggest a low protein tyrosine phosphorylation in intracellular signaling events. ZAP-70 kinase is known to play a crucial role in transduction of TCR-mediated signaling. Experiments were performed to investigate whether the ZAP-70 kinase activation is normally induced in H1R−/− mouse T cells (Fig. 7). c-kit–positive cell-depleted spleen cells from wild-type or H1R−/− mice were stimulated with anti-CD3ε antibodies in RPMI 1640 medium with dialyzed FCS. Whole cell lysates from anti-CD3ε–stimulated spleen cells were immunoprecipitated with anti–ZAP-70 and blotted with antiphosphotyrosine antibodies. In wild-type mouse T cells, tyrosine phosphorylation of ZAP-70 kinase was induced after stimulation with anti-CD3ε (Fig. 7, left), and the tyrosine phosphorylation was strongly enhanced in the presence of histamine (10−5 M) (Fig. 7, middle). On the other hand, phosphorylation of ZAP-70 in spleen T cells was greatly reduced in the H1R−/− mouse (Fig. 7, right). These results suggest a crucial role of signaling from the histamine H1Rs in TCR-mediated protein tyrosine phosphorylation in the proximal signaling pathways.
Figure 7.
Tyrosine phosphorylation of ZAP-70 kinase after the cross-linking of TCR with anti-CD3ε in the absence or presence of histamine. c-kit–positive cell-depleted splenic T cells from wild-type (H1R+/+; left and center) or H1R−/− (right) mice were incubated with goat anti– mouse CD3ε antibody (10 μg/ml) with or without histamine for 0–10 min. Cell lysates were immunoprecipitated with affinity-purified goat anti–mouse ZAP-70 antibody. Immunoprecipitates were fractionated on 10% SDS-polyacrylamide gels. The proteins on the gels were transferred onto nitrocellulose membrane filter and blotted with an HRP-labeled antiphosphotyrosine mAb, PY-20. The blots were visualized with enhanced chemiluminescence reagents. The position of the band representing ZAP-70 phosphorylation is indicated by an arrow at 70 kD.
In Vitro Cytokine Production by Spleen Cells from H1R− /− Mice.
Cytokine production in response to OVA or anti-CD3ε cross-linking was assessed by using spleen cells from the wild-type and H1R−/− mice that had been immunized with OVA. Cells were cultured in presence of various concentration of OVA (5–50 μg/ml) or in anti-CD3ε–coated plates for 72 h. The culture supernatants were collected to determine the amount of IL-2, IL-4, and IFN-γ. The results are shown in Table I. Compared with the level of cytokines produced by wild-type mouse spleen cells upon stimulation with OVA antigen or anti-CD3ε cross-linking, the amounts of IL-2 and IFN-γ produced by spleen cells from H1R−/− mice was significantly reduced, whereas the IL-4 level was not decreased, but rather was enhanced. These results suggest that the production of Th1-derived cytokines, which is triggered by TCR signals, might be more dependent on the signal(s) from histamine H1Rs than Th2-type cytokine production, and that Th2 activation might be negatively regulated by the signal(s) from histamine H1Rs.
Table I.
Cytokine Production from Spleen Cells of Wild-type and H1R−/− Mice upon Stimulation with Antigen (OVA) or Anti-CD3ε Antibody
| Stimulator (cytokine produced) | OVA (40 μg/ml) | Anti-CD3ε (10 μg/ml) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-2 | IFN-γ | IL-4 | IL-2 | IFN-γ | IL-4 | |||||||
| pg/ml | pg/ml | |||||||||||
| H1R+/+ | 280 ± 5 | 9 ± 2 | 10 ± 2 | 620 ± 20 | 280 ± 5 | 25 ± 5 | ||||||
| H1R−/− | 22 ± 5 | 5 ± 1 | 20 ± 2 | 35 ± 5 | 10 ± 2 | 100 ± 11 | ||||||
Spleen cells were cultured in vitro with OVA (40 μg/ml) or goat anti– mouse CD3ε antibody (40 μg/ml) for 3 d. The culture supernatants were used for detection of IL-2, IL-4, and IFN-γ by quantitative ELISA.
Discussion
In this study, we describe the effect of histamine H1R signals on antigen receptor–mediated T and B cell signaling. Histamine caused a dose-dependent increase in the proliferation of splenic T and B cells induced by cross-linking of antigen receptors with anti-CD3 and anti-IgM, respectively. The enhanced response was only observed if the population of c-kit–positive cells, which includes mast cells, was depleted before culture, and if the FCS was dialyzed before use as a media supplement. In vitro T cell proliferative responses triggered by anti-CD3ε antibody or antigen stimulation with OVA were markedly decreased in mice lacking H1R. Anti-IgM–stimulated proliferation of B cells was also impaired in H1R−/− mice. In contrast, the responses to IL-2, LPS, IL-4, CD40L, and CD40L plus IL-4 were unaffected by the absence of H1R. These results strongly suggest a significant contribution of signal(s) from the histamine H1R to the antigen receptor–mediated signaling pathways that induce proliferative responses in T and B cells.
TCR signaling has been reported to act on Gαq, and results in its physical association with CD3ε (17). Activation of protein tyrosine kinases could result in Gαq and Gαq/11 phosphorylation, thus augmenting GPCR-mediated Gαq/11 signaling (12, 13). Therefore, Gαq/11 could mediate both tyrosine phosphorylation of CD3 and IP3 generation upon TCR engagement. The cross-linking also induced Pyk2 activation in T cells, which may potentially serve as a convergence point for TCR and GPCR signaling (18–20). It is noteworthy that the Gαq protein appears to associate with the histamine H1R as a signal transducer (26, 27, 41). Gq-mediated signaling also triggers the translocation of the transcription factor nuclear factor (NF)-AT to the nucleus, which is involved in IL-2 gene activation, providing another mechanism by which TCR and GPCR-linked signaling pathways may communicate with each other (21). It has been reported that the cells derived from the human Jurkat T cell line, which carried a function-deficient mutant of Gαq/11, displayed diminished tyrosine phosphorylation of TCR/CD3ζ and ε chains, as well as ZAP-70, upon anti-CD3 antibody triggering (17). Reduced tyrosine phosphorylation of ZAP-70 was also observed in H1R−/− spleen T cells after cross-linking of TCR by anti-CD3 antibody (Fig. 7). Moreover, addition of histamine enhanced tyrosine phosphorylation of ZAP-70 in anti-CD3–stimulated T cells of wild-type mice. Present data prove the speculation that signals from GPCR may regulate the TCR-mediated ZAP-70 activated tyrosine phosphorylation. Thus, the low T cell proliferative responses observed in the H1R−/− T cells may suggest a possible interaction between TCR and GPCR signaling triggered by H1R. Interestingly, not only proliferative responses, but also production of cytokines, such as IL-2 and IFN-γ, were impaired in H1R−/− spleen T cells as compared with T cells of the normal littermates upon cross-linking of TCR with anti-CD3 or antigen (OVA) in vitro (Table I). On the other hand, in the case of IL-4 production was not reduced, but rather was enhanced. These results suggested that H1R-mediated signal(s) may play a crucial role in antigen-receptor signaling mainly in Th1-type T cells, and it might be possible that signal(s) from H1R may function as a feedback regulator for activation of Th2-type T cells.
It has been reported that a Gαq activates Btk (23), and the Gβγ subunit binds and activates Btk (24, 25), which implicates Gαq in B and mast cell function. Thus, engagement of either the BCR or a Gq-linked GPCR could activate Btk in B cells. The xid (CBA/N) mice carry a point mutation in the pleckstrin homology domain of Btk. These mice exhibit reduced numbers of mature B cells, reduced serum levels of IgM and IgG3, and low proliferative responses to anti-IgM and LPS stimulation in vitro (43), and also lack CD5+ B-1 cells in the peritoneal cavity. The H1R−/− mice showed normal differentiation pattern of B and T cells, and the numbers of mature B and T cells were within normal range. However, the response to anti-IgM cross-linking was reduced (Fig. 2) and serum levels of IgG3 and IgM class antibodies were low (Fig. 6). These data suggest that signal(s) from the G protein coupled to the histamine H1Rs may contribute through Btk to the activation of B cells induced by antigen receptor signaling, but not during B cell maturation or in the case of a mitogenic response. Interestingly, the proportion of CD5+ B-1 cells was found to be decreased in the peritoneal cavity of the H1R−/− mice to half of that of normal littermates, suggesting the impaired activation of Btk in B1 cells in the H1R gene knockout mice. All these findings related to GPCR-mediated signaling lead us to postulate that ligand–GPCR binding triggers the tyrosine phosphorylation of members of the antigen receptor complex, thereby modifying antigen receptor signaling. Furthermore, activation of the histamine H1R may produce functional signals in cells via the Gq/11 family of G proteins and communicate with antigen receptor-mediated signaling, which results in the augmentation of proliferative responses induced by cross-linking of the TCR and BCR.
Proliferative responses of H1R−/− T and B cells in response to stimulation with anti-CD3 or anti-IgM antibodies were lower than those of control littermates in the absence of histamine (Fig. 1 versus Fig. 2). This may suggest the existence of an endogenous or exogenous H1R ligand(s) other than histamine, which may also affect antigen receptor–mediated activation of the lymphocytes, and thus the lack of H1R results in more pronounced effects. One of the possibilities is that a signal(s) from histamine H2Rs may play a role in H1R−/− mice since there was a report that the signal(s) from H2R appeared to suppress the production of certain types of cytokines, such as IL-10 and IL-12, from Th cells (44). Therefore, it is possible to speculate that the H1R-mediated signal(s) may be augmented in H1R−/− T cells. To discover the role of histamine H2R in immune response in H1R−/− mice, the spleen T and B cells of H1R−/− mice were stimulated with anti-CD3ε and anti IgM, respectively, in the presence of varying concentrations of an H2 antagonist, famotidine, (10−4 M to 10−7 M). The addition of Famotidine revealed a 20–40% reduction of proliferative responses of both T and B cells (data not shown). These data implicate the part of dependency of the remaining proliferative responses of H1R−/− T and B cells on the H2R-mediated signaling, but low proliferative responses of H1R−/− T and B cells was not due to the suppression through the H2R-mediated signaling. Alternatively, H1R knockout mice may carry an unknown genetic polymorphism that causes a low response to antigen receptor–mediated signaling, since the background of H1R−/− mice and their control littermates is not homogenous, although they have been backcrossed to C57BL/6 for more than five generations.
Mast cells were once considered mere effector cells for IgE-mediated hypersensitivity that released pharmacologically active mediators, including histamine. However, in recent years, with the realization that mast cells also secrete cytokines, the involvement of this cell type in regulating the immune response has become appreciated (45, 46). Our studies indicate that the mast cell product histamine may play an even more direct role in acquired immunity than was previously suspected.
Although the precise molecular mechanism remains obscure, further detailed investigation of the interaction of the H1R with the G proteins should reveal the importance of the cross-talk between GPCR-mediated and antigen receptor–mediated signaling pathways.
Footnotes
We express our sincere thanks to Dr. Peter Burrows for reading the manuscript and for his helpful comments. We also thank Dr. M. Nakashima for his invaluable assistance with the experiments.
Abbreviations used in this paper: BCR, B cell receptor; Btk, Bruton's tyrosine kinase; GPCR, G protein–coupled receptor; G protein, guanine-nucleotide binding protein; HRP, horseradish peroxidase; MAPK, mitogen-activated protein kinase; PLC, phospholipase C; PTK, protein tyrosine kinases; TMB, tetramethylbenzidine.
References
- 1.Neer EJ. Heterotrimeric G proteins: organizers of transmembrane signaling. Cell. 1995;80:249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- 2.Hamm HE, Gilchrist A. Heterotrimeric G proteins. Curr Opin Cell Biol. 1996;8:189–196. doi: 10.1016/s0955-0674(96)80065-2. [DOI] [PubMed] [Google Scholar]
- 3.Kehrl HJ. Heterotrimeric G protein signaling: roles in immune function and fine-tuning by RGS protein. Immunity. 1998;8:1–10. doi: 10.1016/s1074-7613(00)80453-7. [DOI] [PubMed] [Google Scholar]
- 4.Post GR, Brown JH. G-protein-coupled receptors and signaling pathways regulating growth responses. FASEB (Fed Am Soc Exp Biol) J. 1996;10:741–749. doi: 10.1096/fasebj.10.7.8635691. [DOI] [PubMed] [Google Scholar]
- 5.Wan Y, Kurosaki T, Huang XY. Tyrosine kinase in activation of the MAP kinase cascade by G-protein coupled receptors. Nature. 1996;380:541–544. doi: 10.1038/380541a0. [DOI] [PubMed] [Google Scholar]
- 6.Sadoshima JI, Izumo S. The heterotrimeric Gq protein coupled angiotensine II receptor activates P21ras via the tyrosine kinase Shc-Grb2-Sos pathway in cardiac myocytes. EMBO (Eur Mol Biol Organ) J. 1996;15:775–787. [PMC free article] [PubMed] [Google Scholar]
- 7.Luttrell LM, Hawes BE, Van Biesen T, Luttrell DK, Lansing TJ, Lefkowitz RJ. Role of c-Src tyrosine kinase in G-protein coupled receptor and Gβγ subunit-mediated activation of mitogen-activated protein kinases. J Biol Chem. 1996;271:19443–19450. doi: 10.1074/jbc.271.32.19443. [DOI] [PubMed] [Google Scholar]
- 8.Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role of Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 9.Wan Y, Bence K, Hata A, Kurosaki T, Velillete A, Huang XY. Genetic evidence for a tyrosine kinase cascade preceding the mitogen-activated protein kinase cascade in vertebrate G protein signaling. J Biol Chem. 1997;4:17209–17215. doi: 10.1074/jbc.272.27.17209. [DOI] [PubMed] [Google Scholar]
- 10.Simon MI, Strathmann MP, Gautam N. Diversity of G-proteins in signal transduction. Science. 1991;252:802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- 11.Kaizuka Y, Itoh H, Kozasa T, Nakafuku M, Satoh T. Structure and function of signal transducing GTP-binding protein. Annu Rev Biochem. 1991;60:349–400. doi: 10.1146/annurev.bi.60.070191.002025. [DOI] [PubMed] [Google Scholar]
- 12.Liu WW, Mattingly RR, Garrison JC. Transformation of Rat-1 fibroblast with the V-src oncogene increases the tyrosine phosphorylation state and activity of the α subunit of Gq/G11. Proc Natl Acad Sci USA. 1996;93:8258–8263. doi: 10.1073/pnas.93.16.8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umemori H, Inoue T, Kume S, Sekiyama N, Nagao M, Itoh H, Nakanishi S, Mikoshiba K, Yamamoto T. Activation of the G protein Gq/11 through tyrosine phosphorylation of the α subunit. Science. 1997;276:1878–1881. doi: 10.1126/science.276.5320.1878. [DOI] [PubMed] [Google Scholar]
- 14.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptor. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 15.Defranco AL. The complexity of signaling pathway activated by the BCR. Curr Opin Immunol. 1997;9:296–308. doi: 10.1016/s0952-7915(97)80074-x. [DOI] [PubMed] [Google Scholar]
- 16.Kurosaki T. Molecular dissection of B cell antigen receptor signaling. Int J Mol Med. 1998;1:515–527. doi: 10.3892/ijmm.1.3.515. [DOI] [PubMed] [Google Scholar]
- 17.Stanners J, Kabouridis PS, McGuire KL, Tsoukas CD. Interaction between G proteins and tyrosine kinases upon T cell receptor CD3-mediated signaling. J Biol Chem. 1995;270:30635–30642. doi: 10.1074/jbc.270.51.30635. [DOI] [PubMed] [Google Scholar]
- 18.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein tyrosine kinase Pyk2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 19.Della Rocca, G.J., T. Van Biesen, Y. Daaka, D.K. Luttrell, L.M. Luttrell, and R.J. Lefkowitz. Ras-dependent mitogen-activated protein kinase activation by G-protein-coupled receptors. Convergence of Gi and Gq-mediated pathways on calcium/calmodulin, Pyk2 and Src kinase. J Biol Chem. 1997;272:19125–19132. doi: 10.1074/jbc.272.31.19125. [DOI] [PubMed] [Google Scholar]
- 20.Qian D, Lev S, Vanoers NSC, Dikic I, Schlessinger J, Weiss A. Tyrosine phosphorylation of Pyk2 is selectively regulated by Fyn during TCR signaling. J Exp Med. 1997;185:1253–1259. doi: 10.1084/jem.185.7.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boss V, Talpade DJ, Murphy TJ. Induction of NFAT-mediated transcription by Gq coupled receptors in lymphoid and non-lymphoid cells. J Biol Chem. 1996;271:10429–10432. doi: 10.1074/jbc.271.18.10429. [DOI] [PubMed] [Google Scholar]
- 22.Bacon KB, Szabo MC, Yssel H, Bolen JB, Schall TJ. RANTES induces tyrosine kinase activity of stable complexed P125fakand ZAP-70 in human T cells. J Exp Med. 1996;184:873–882. doi: 10.1084/jem.184.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bence K, Ma W, Kozasa T, Huang XY. Direct stimulation of Bruton's tyrosine kinase by Gq protein α-subunit. Nature. 1997;389:296–299. doi: 10.1038/38520. [DOI] [PubMed] [Google Scholar]
- 24.Tsukada S, Simon MI, Witte ON, Katz A. Binding of βγ subunits of heterotrimeric G proteins to the PH domain of Bruton tyrosine kinase. Proc Natl Acad Sci USA. 1994;91:11256–11260. doi: 10.1073/pnas.91.23.11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langhans-Rajasekaran SA, Wan Y, Huang XY. Activation of Tsk and Btk tyrosine kinases by G protein β subunits. Proc Natl Acad Sci USA. 1995;92:8601–8632. doi: 10.1073/pnas.92.19.8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leurs R, Smit MJ, Timmerman H. Molecular pharmacological aspects of histamine receptors. Pharmacol Ther. 1995;66:413–463. doi: 10.1016/0163-7258(95)00006-3. [DOI] [PubMed] [Google Scholar]
- 27.Hill SJ. Distribution, properties and functional characteristics of three classes of histamine receptor. Pharmacol Rev. 1990;42:45–83. [PubMed] [Google Scholar]
- 28.Yamashita M, Fukui H, Sugama K, Horio Y, Ito S, Mizuguchi H, Wada H. Expression cloning of cDNA encoding the bovine histamine H1 receptor. Proc Natl Acad Sci USA. 1991;88:11515–11519. doi: 10.1073/pnas.88.24.11515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill SJ, Emson PC, Young JM. The binding of [3H]mepyramine to histamine H1-receptors in guinea-pig brain. J Neurochem. 1978;31:997–1004. doi: 10.1111/j.1471-4159.1978.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 30.Carswell H, Nahorski SR. Distribution and characteristics of histamine H1 receptors in guinea-pig airways identified by [3H]mepyramine. Eur J Pharmacol. 1982;81:301–307. doi: 10.1016/0014-2999(82)90448-4. [DOI] [PubMed] [Google Scholar]
- 31.Casale TB, Rodbard D, Kalinger M. Characterization of histamine H1 receptors on human peripheral lung. Biochem Pharmacol. 1985;34:3285–3292. doi: 10.1016/0006-2952(85)90347-8. [DOI] [PubMed] [Google Scholar]
- 32.Driver AG, Mustafe SJ. Correlation of histamine H1 receptor function and [3H]mepyramine binding in porcine tracheal tissue. Eur J Pharmacol. 1987;139:287–295. doi: 10.1016/0014-2999(87)90586-3. [DOI] [PubMed] [Google Scholar]
- 33.Hill SJ, Young JM. Characterization of [3H] mepyramine binding to the longitudinal muscle of guinea-pig small intestine. Mol Pharmacol. 1981;19:379–387. [PubMed] [Google Scholar]
- 34.Kondo M, Taniyama K, Tanaka C. Histamine H1 receptors in guinea-pig urinary bladder. Eur J Pharmacol. 1985;114:89–92. doi: 10.1016/0014-2999(85)90526-6. [DOI] [PubMed] [Google Scholar]
- 35.Hide M, Fukui H, Watanabe T, Wada H, Yamamoto S. Histamine H1 receptor in endothelial and smooth muscle cells of guinea-pig aorta. Eur J Pharmacol. 1988;148:161–169. doi: 10.1016/0014-2999(88)90560-2. [DOI] [PubMed] [Google Scholar]
- 36.Cameron W, Doyle K, Rockline RE. Histamine type 1 (H1) receptor radioligand binding studies on normal T cell subsets, B cells and monocytes. J Immunol. 1986;136:2116–2120. [PubMed] [Google Scholar]
- 37.Villemain FM, Bach JF, Chatenoud LM. Characteristics of histamine H1 binding sites on human T lymphocytes by means of 125I-iodobolpyramin. Preferential expression of H1 receptors on CD8 T lymphocytes. J Immunol. 1990;144:1449–1459. [PubMed] [Google Scholar]
- 38.Schwartz JC, Arrang JM, Garbarg M, Pollard H, Ruat M. Histaminergic transmission in mammalian brain. Physiol Rev. 1991;71:1–51. doi: 10.1152/physrev.1991.71.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Donaldson J, Hill SJ. Histamine induced inositol phospholipid breakdown in the longitudinal smooth muscle of guinea-pig ileum. Br J Pharmacol. 1985;85:499–512. doi: 10.1111/j.1476-5381.1985.tb08887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Litosch I. Guanine nucleotides mediate stimulatory and inhibitory effects on cerebral-cortical membrane phospholipase C activity. Biochem J. 1989;261:245–251. doi: 10.1042/bj2610245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gutowski S, Smrcka A, Nowak L, Wu D, Simon M, Sternweis PC. Antibodies to the αq subfamily of guanine nucleotide-binding regulatory protein α subunit of attenuate activation of phosphatidylinositol 4,5-bisphosphate hydrolysis by hormones. J Biol Chem. 1991;266:20519–20524. [PubMed] [Google Scholar]
- 42.Inoue I, Yanai K, Kitamura D, Taniuchi I, Kobayashi T, Niimura K, Watanabe T, Watanabe T. Impaired locomotor activity and exploratory behavior in mice lacking histamine H1 receptors. Proc Natl Acad Sci USA. 1996;93:13316–13320. doi: 10.1073/pnas.93.23.13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rawling DJ, Saffran DC, Tsukada S, Largaespada DA, Grimaldi JC, Cohen L, Mohr RN, Bazan JF, Howard M, Copeland NG, et al. Mutation of unique region of Bruton tyrosine kinase in immunodeficient XID mice. Science. 1993;261:358–360. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- 44.Elenkov IJ, Webster E, Papanicolaou DA, Fleisher TA, Chrousos GP, Wilder RL. Histamine potently suppresses human IL-12 and stimulates IL-10 production via H2 receptors. J Immunol. 1998;161:2586–2593. [PubMed] [Google Scholar]
- 45.Mecheri S, David B. Unravelling the mast cell dilemma: culprit or victim of its genocity? . Immunol Today. 1997;18:212–215. doi: 10.1016/s0167-5699(97)01034-7. [DOI] [PubMed] [Google Scholar]
- 46.Galli SJ. New concept about the mast cell. N Engl J Med. 1993;328:257–265. doi: 10.1056/NEJM199301283280408. [DOI] [PubMed] [Google Scholar]