Abstract
Cellular immunity against Mycobacterium tuberculosis controls infection in the majority of infected humans. Studies in mice have delineated an important role for CD4+ T cells and cytokines including interferon γ and tumor necrosis factor α in the response to infection with mycobacteria. Recently, the identification of CD8+ CD1-restricted T cells that kill M. tuberculosis organisms via granulysin and the rapid death after infection of β2 microglobulin deficient mice in humans has drawn attention to a critical role for CD8+ T cells. The nature of mycobacterial-specific CD8+ T cells has been an enigma because few have been identified in any species. Here, we delineate the contribution of class I MHC–restricted T cells in the defense against tuberculosis as transporter associated with antigen processing (TAP)1-deficient mice died rapidly, bore a greater bacterial burden, and had more severe tissue pathology than control mice. In contrast, CD1D−/− mice were not significantly different in their susceptibility to infection than control mice. This data demonstrates a critical role for TAP-dependent peptide antigen presentation and provides further evidence that class I MHC–restricted CD8+ T cells, the major T cell subset activated by this antigen processing pathway, play an essential role in immunity to tuberculosis.
Keywords: CD1, CD8, transporter associated with antigen processing, infection, Mycobacterium tuberculosis
Tuberculosis is the most common cause of death from an infectious pathogen in the world (1). The causative agent, Mycobacterium tuberculosis, infects and grows intracellularly in macrophages, induces an intense immune response, and leads to the development of caseating granulomas, the pathological hallmark of the disease. It is well established that CD4+ T cells are critical in the immune response to M. tuberculosis in rodents and humans. This may be a consequence of the intracellular compartmentalization of M. tuberculosis in the phagolysosome, which could favor entry of its proteins into the class II MHC antigen-processing pathway (2).
The participation of CD8+ T cells in immunity to M. tuberculosis is less clearly defined. Prior studies using antibody mediated T cell subset depletion (3) and adoptive transfer of purified T cell subsets (4–6) were able to show that CD8+ T cells could reduce CFU of M. tuberculosis in the spleen of infected mice, although the effect was consistently weaker than that observed for CD4+ T cells (3, 4). Infection of mice with the less virulent Mycobacterium bovis (Bacillus Calmette-Guerin, BCG) consistently found that CD8+ T cells made no contribution to immunity (7–9). This difference between the immune response to M. tuberculosis and M. bovis BCG may be a reflection of their differing virulences. However, none of these studies examined the immune response in the lung, the site where rodents fail to control the infection after intravenous infection, nor were any other variables examined, such as pathology or survival. Furthermore, it has been difficult even to demonstrate the presence of mycobacterial-specific CD8+ T cells in human tuberculosis patients until recently (10, 11). Given the relative lack of data implicating CD8+ T cells in immunity to M. tuberculosis, it was a remarkable finding that mice genetically deficient in β2 microglobulin (β2m),1 and as a consequence lacking in CD8+ T cells, were unable to control infection, particularly in the lung, and succumbed prematurely to tuberculosis (12). Although the susceptible phenotype observed in β2m-deficient mice was thought to be secondary to the loss of class I MHC–restricted CD8+ T cells, β2m is also a component of a number of other antigen-presenting molecules, including H2-M3, TL, Qa-1, Qa-2, and CD1d. Because the MHC-encoded class I, H2-M3, Qa-1, and Qa-2 molecules have all been shown to bind peptide antigens loaded in a transporter-associated with antigen processing (TAP)-dependent fashion, whereas CD1 molecules, including CD1d, are characterized to bind lipid-based antigens, we sought to discriminate these pathways.
The CD1 family of β2m-associated, non-MHC locus– encoded proteins are able to present hydrophobic lipids and glycolipids to T cells. Specifically, the human group 1 CD1 proteins (CD1a, -b, and -c) have been shown to present mycobacterial antigens such as mycolic acid, glucose monomycolate, and lipoarabinomannan to human α/β-TCR+ T cells (13–15). The mouse has a pair of CD1 genes that are likely to represent a recent duplication, and are homologous to the human group 2 CD1 protein CD1d. Phosphatidylinositol-containing compounds have been eluted from murine CD1d (16), and both murine and human CD1d can present the glycolipid α-galactosylphytosphingosine to T cells in a TAP-independent manner (17, 18). The ability of murine CD1d to bind glycolipids that are structurally similar to CD1-restricted mycobacterial antigens, together with the delineation of CD1d-restricted T cells that use a diverse TCR repertoire (19–21), led us to hypothesize that CD1d may also be capable of presenting mycobacterial antigens to murine T cells.
In contrast to the presentation of lipid antigens by CD1, class I MHC presents peptides to CD8+ T cells. The class I MHC antigen-processing pathway is dependent upon cleavage and processing of protein antigens (by the proteosome), followed by transport of the peptides from the cytosol into the endoplasmic reticulum (ER) by the TAP complex. Here, the processed peptides associate with the class I MHC and β2m proteins to form a trimeric complex (22). The TAP protein is a heterodimer of the tap1 and tap2 gene products, and mediates the translocation of peptides from the cytoplasm into the ER. Cells that are deficient in either TAP1 or TAP2 are unable to efficiently process peptides derived from cytosolic proteins by the class I MHC pathway. In tap1-deficient mice, this defect in the class I MHC antigen-processing pathway results in greatly reduced numbers of CD8+ T cells in all lymphoid organs, as CD8+ T cells are not positively selected during T cell maturation in the thymus (23). In contrast, TAP deficiency is not believed to lead to a deficiency of CD1-restricted T cells, as all examples of CD1-restricted antigen recognition by T cells have been determined to be TAP independent (17, 24, 25). We therefore felt that the TAP1-deficient (TAP1−/−) and CD1D-deficient (CD1D−/−) mice would be important independent models to determine the significance of CD8+ T cells in immunity to M. tuberculosis.
Materials and Methods
Mice.
6–8-wk-old male (129/Sv,C57BL/6) TAP1−/− (26) and control (C57BL/6 × 129/Sv) F1 or F2 mice were obtained from The Jackson Laboratory. C57BL/6 β2m-deficient mice (β2m−/−) and control C57BL/6 mice were also obtained from The Jackson Laboratory. CD1D−/− mice and their littermate controls were used at the F2 and F6 backcross to C57BL/6 (27) or the F8 backcross to BALB/c mice (28). CD1D−/−TAP1−/− mice were generated by backcrossing the CD1D−/− onto the TAP1−/− background. All mice were housed in a biosafety level 3 facility under specific pathogen–free conditions at the Animal Biohazard Containment Suite (Dana-Farber Cancer Institute, Boston, MA) and used in a protocol approved by the institution.
Bacteria and Infections.
Virulent M. tuberculosis (Erdman strain; originally obtained from Barry Bloom, Albert Einstein College of Medicine, Bronx, NY) was passaged through mice and then grown in Middlebrook 7H9 supplemented with oleic acid- albumin-dextrose complex (OADC; Difco), before freezing aliquots at −80°C. Before inoculation of mice, an aliquot was thawed, diluted in normal saline (0.9% NaCl) containing 0.02% Tween 80, and sonicated twice for 10 s using a cup horn sonicator (Branson Ultrasonics Corp.). Mice were infected intravenously via the lateral tail vein with 106 live bacilli. The inoculum dose was confirmed by plating an aliquot onto 7H10 agar plates (Hardy or Remel).
Flow Cytometry.
Venous blood from mice infected with M. tuberculosis was obtained by retro-orbital puncture. 50 μl of blood anti-coagulated with heparin was stained with PE-conjugated anti-CD8 antibody (clone 53-6.72) (PharMingen) or a control antibody. The RBCs were lysed with NH4Cl and after extensive washing with buffer the samples were resuspended in 1% paraformaldehyde-PBS and analyzed after 24 h using a FACSort™ (Becton Dickinson). The percentage of CD8+ T cells within the lymphoid gate was determined.
CFU Determination.
To quantify viable mycobacteria in the infected mouse organs, the lungs, liver, and spleen were aseptically removed from each killed animal. The left lung, left lobe of the liver, and half of the spleen were homogenized in 0.02% Tween 80 in normal saline using Teflon homogenizers (Fischer). 10-fold serial dilutions were plated onto 7H10 agar plates and colonies were counted after incubation for 3 wk at 37°C.
Histology.
Tissues for histological studies were fixed in 10% buffered formalin and then embedded in paraffin blocks. 5-μm sections were stained with hematoxylin and eosin or by the Fite-Faraco method for acid-fast bacilli (AFB) (29).
Results
The Effect of CD1d on Survival of M. tuberculosis–infected Mice.
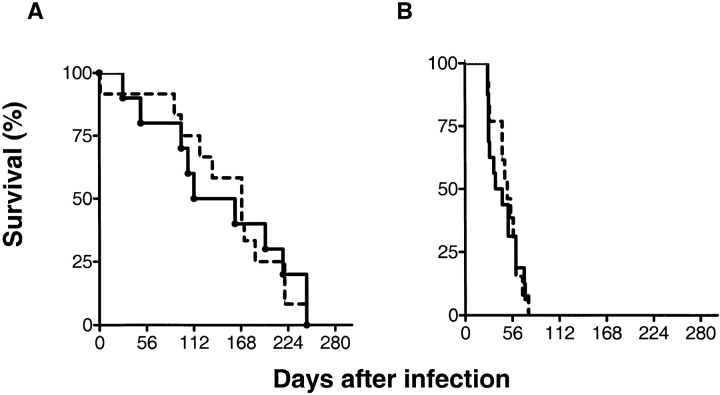
To test the hypothesis that the increased susceptibility of β2m−/− mice to M. tuberculosis was due to the absence of CD1d-restricted T cells, mice that had both the CD1D1 and CD1D2 genes disrupted by homologous recombination (CD1D−/− mice) were infected with virulent M. tuberculosis (27). No significant difference between the mortality of CD1D−/− mice and that of their heterozygous littermate controls was observed after intravenous infection with 106 CFU (Fig. 1 A). The median survival time (MST) was 169 d for the CD1D−/− mice and 136 d for the CD1D+/− mice. These CD1D−/− mice were used after the second backcross to C57BL/6 mice, and further studies using CD1D−/− mice after the sixth backcross gave similar results (data not shown). We also considered whether deletion of CD1D increased the resistance of mice to infection with M. tuberculosis. Since both C57BL/6 and 129/Sv mice are relatively resistant to tuberculosis, an increase in resistance of CD1D−/− mice on these genetic backgrounds would be difficult to detect. Therefore, CD1D−/− mice on the susceptible BALB/c genetic background were infected. CD1D−/− mice backcrossed eight generations to BALB/c mice showed no significant differences in survival compared with CD1D+/+ BALB/c mice after infection with M. tuberculosis (Fig. 1 B). Additional experiments using a higher (3 × 106) or lower (2 × 105) inoculum did not reveal any differences in survival (data not shown). Experiments done in parallel demonstrated a significant reduction in survival for β2m−/− mice compared with β2m+/+ mice (data not shown), as had been reported previously (12). These results indicate that the increased mortality of β2m−/− mice was not due to an absence of CD1d-restricted T cells.
Figure 1.
Survival of CD1D−/− mice after infection with M. tuberculosis. CD1D−/− mice on a resistant genetic background (C57BL/6) (A) or a susceptible genetic background (BALB/c) (B) were inoculated with 106 M. tuberculosis intravenously. Each group contained 12–13 mice and there were no statistically significant differences in survival of the CD1D−/− mice compared with CD1D+/+ mice using a Mann-Whitney test. A, P = 0.91; B, P = 0.95.
The Effect of TAP1 on Survival of M. tuberculosis–infected Mice.
As the absence of the CD1D1 and CD1D2 genes did not significantly alter the survival of mice, TAP1−/− mice were infected with M. tuberculosis to independently verify that the susceptibility of β2m−/− mice to tuberculosis was secondary to the absence of T cells restricted to MHC molecules loaded in the ER in a transporter-dependent manner. The vast majority of such T cells are class I MHC– restricted CD8+ T cells, and mice with disruption of the TAP1 gene are known to have a profound deficiency in CD8+ T cells (26). We confirmed the loss of CD8+ T cells from peripheral blood after intravenous infection with M. tuberculosis. We found that in infected TAP1+/+ mice, 9.0 ± 0.7% (mean ± SEM) of PBLs were CD8+, whereas only 1.2 ± 0.1% of PBLs were CD8+ in TAP1−/− mice (Fig. 2). These results were similar in uninfected TAP1−/− and control mice (30).
Figure 2.
Peripheral blood CD8+ T cells in TAP1−/− mice and controls after infection with M. tuberculosis. 5 wk after infection, blood was obtained and stained with a CD8-specific antibody. The percentage of CD8+ cells in peripheral blood lymphocytes was determined by flow cytometry for four mice/ group. The bar represents the mean for each group. This difference was statistically significant using a Mann-Whitney test (P = 0.0022).
In three separate experiments, a total of 42 TAP1−/− mice and 42 control mice were infected intravenously with 106 CFU of M. tuberculosis. It is striking that the TAP1−/− mice were more vulnerable to death from infection than were control mice (P < 0.0001 by the log-rank test) (Fig. 3). The TAP1−/− mice had a MST of 63 d, and with the exception of one mouse all were dead by day 91 (Fig. 2). In contrast, the MST for the control mice was >150 d (Fig. 3). The difference between the survival of the TAP1−/− and the TAP1+/+ mice was highly statistically significant, and the P values for the individual experiments were P < 0.0001, P = 0.0047, and P < 0.0001. These results demonstrate the importance of an intact TAP-dependent peptide loading antigen presentation pathway for immunity to tuberculosis and strongly supports a critical role for class I MHC–restricted CD8+ T cells in the immune response to M. tuberculosis.
Figure 3.
Survival of TAP1−/− mice infected with M. tuberculosis. TAP1−/− mice or controls were infected with 106 M. tuberculosis intravenously. The data presented are from three separate experiments and represent a total of 42 mice in each group. The survival curves were generated using the Kaplan-Meier method and the difference is statistically significant with a P < 0.0001 by the log-rank test.
The Survival of TAP1−/−CD1D−/− Mice after Infection with M. tuberculosis.
To exclude the possibility of a subtle CD1d-dependent effect that was obscured in the presence of CD8+ T cells, the survival of TAP1−/−CD1D−/− mice was compared with TAP1−/−CD1D+/+ mice, on a mixed 129/Sv and C57BL/6 genetic background. No significant difference was observed in the survival of these strains of mice (Fig. 4). The MST was 79 d for the TAP1−/−CD1D−/− mice and 65 d for the TAP1−/−CD1D+/+ mice, which was similar to other experiments in which TAP1−/−CD1D+/+ mice were infected (Fig. 3). This experiment is consistent with the conclusion that CD1d does not contribute to a protective immune response after intravenous inoculation with M. tuberculosis.
Figure 4.
Survival of TAP1−/− CD1−/− mice infected with M. tuberculosis. CD1D−/−TAP1−/− or CD1D+TAP1−/− littermate controls were infected with 106 M. tuberculosis. There were no statistically significant differences in survival (P = 0.95).
The Effect of TAP1 Deficiency on Bacterial Burden.
The TAP1−/− mice were unable to control the progression of the infection. The number of bacteria deposited in the spleen, liver, and lungs was determined 1 d after infection and was comparable to the numbers reported by other investigators (Fig. 5, reference 12). The ability of the mice to limit the mycobacterial growth was studied at several time points after infection. In all three organs examined, the TAP1−/− mice were not able to control the infection as efficiently as the control mice (Fig. 5). For example, the TAP1−/− mice had a 10–100-fold increase in the number of mycobacteria isolated from the lung 10 wk after infection. Similar differences were seen in the spleen and liver. In contrast, the early phase of the infection (days 1–21) was similar in TAP1−/− and TAP1+/+ mice (Fig. 5 and data not shown). This result is consistent with the finding that protective CD4+ T cells are present by day 10 after infection, whereas protective CD8+ T cells do not become apparent until 3–4 wk after infection (4). These data indicate that the absence of TAP1 affected the adaptive immune response. We observed some variability between experiments, particularly in the colony count data. We believe that this variability arose from the use of two different batches of M. tuberculosis. One of the batches was more virulent than the other. Formalin fixed sections of lung, spleen, and liver, were stained for mycobacteria (AFB) to confirm the increased bacterial burden in the TAP1−/− mice. In all tissues, but most dramatically in the lung, AFB were more abundant in tissue obtained from TAP1−/− mice compared with TAP1+/+ mice (Fig. 6, A and E). Although enumeration of AFB was not done, the tissues from the TAP1−/− mice had more numerous foci containing AFB, and those foci contained greater numbers of bacilli compared with tissue taken from TAP1+/+ mice. This is consistent with the colony count data, and suggests that TAP1−/− mice are defective in their capacity to control infectious foci.
Figure 5.
Mycobacterial burden in TAP1−/− and control mice after infection. The number of CFU recovered from the lung, spleen, and liver was determined at the time points indicated after intravenous infection with 106 M. tuberculosis. The baseline mycobacterial inoculum was determined 24 h after infection. Each data point is the mean bacterial counts from three to five mice ± SEM.
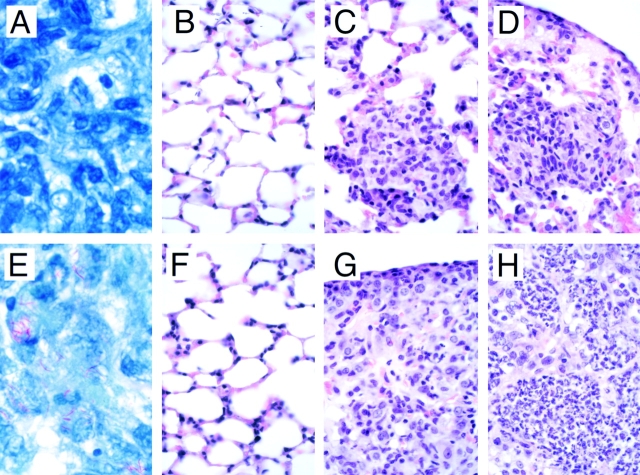
Figure 6.
Histopathology of the lung from TAP1+/+ (A–D) and TAP1−/− (E–H) mice after infection with M. tuberculosis. Mice were killed at the indicated times after infection, tissues were harvested, and formalin-fixed, paraffin-embedded sections were stained with Fite-Faraco stain for AFB or with the hematoxylin and eosin 1 d (B and F), 3 wk (A, C, E, and G), or 7 wk (D and H) after infection. Original magnification: A and E, ×2,000; B–D and F–H, ×300.
Pathological Changes in Target Organs.
The TAP1−/− mice had a much greater degree of hepatosplenomegaly and enlargement of the lungs compared with the control mice (data not shown). Lung tissue examined 1 d after infection appears normal by light microscopy and similar in both the TAP1+/+ and TAP1−/− mice (Fig. 6, B and F). 1 mo after infection, the lungs of the TAP1+/+ mice contain abundant foci of infection containing mixed inflammatory cells and most major blood vessels were surrounded by inflammatory cells; however, the airspaces of the lungs were well preserved (Fig. 6 C). In contrast, the TAP1−/− mice had severe pneumonia characterized by massive inflammatory cell infiltrates, and severe reductions in airspace (Fig. 6 G). The inflammatory cells were chiefly mononuclear cells, with some areas of granulomatous inflammation where the predominant cell types were epithelioid cells and foamy macrophages. By wk 7, well defined granulomas were observed grossly. Microscopically, the lungs of the TAP1+/+ mice also had severe granulomatous pneumonia with reduction of lung aeration (Fig. 6 D). In the TAP1−/− lungs, there was nearly complete obliteration of the airspace by pneumonia with spread via the large airways; neutrophilic infiltrates and early signs of tissue necrosis were apparent (Fig. 6 H). Although there were similar qualitative changes in the nature of the inflammatory infiltrate in the TAP1−/− mice compared with the control mice, the amount of cellular infiltrate was greater in the TAP1−/− mice at every time point. In contrast, the infiltrate observed in the TAP1+/+ mice was more focal and was distributed primarily in a perivascular location with better preservation of the alveolar air space (Fig. 6 D).
A similar pattern was seen with the spleens and livers. The spleens of the TAP1−/− animals were more disrupted, and the spleens of both the TAP1−/− and control mice had numerous giant cells. The livers of both types of mice had well-defined granulomata, with a tendency for the granulomas in the TAP1−/− mice to be slightly more cellular.
Discussion
Although it is clear that CD8+ T cells play a critical role in host defense against viral infections and some intracellular infections such as Toxoplasma gondii and Listeria monocytogenes, the role of CD8+ T cells in immunity to tuberculosis remains controversial despite numerous studies that have examined this question. The 1992 study by Flynn et al. redressed the role of CD8+ T cells in M. tuberculosis infection using mice deficient in β2m (12). Since β2m forms a heterodimer with the class I MHC heavy chain, mice deficient in β2m lack surface expression of class I MHC molecules, and therefore are unable to positively select CD8+ T cells during thymic T cell development. As a result such mice are largely deficient in CD8+ T cells. When β2m−/− mice were infected with the Erdman strain of M. tuberculosis, they quickly succumbed to infection. Associated with the decreased survival time was an increased mycobacterial burden in the lungs and more severe tissue pathology compared with infected β2m+/+ mice (12).
One interpretation of the increased vulnerability of the β2m−/− mice to M. tuberculosis is that class I MHC–restricted CD8+ T cells are critical in immunity to tuberculosis. However, alternative explanations exist. The β2m molecule forms heterodimers with molecules other than the class I MHC heavy chain, such as class Ib MHC heavy chains (i.e., H2-M3) and the non-MHC–encoded CD1 heavy chain, both of which are antigen-presenting molecules that present unique bacterial antigens to T cells. H2-M3 is known to specifically present N-formylated peptides derived from bacterial proteins to murine CD8+ T cells (31). CD1 is known to present antigens from M. tuberculosis and Hemophilus influenzae to human CD8+ and CD4−8− T cells. For example, human CD1b and CD1c present lipid and glycolipid antigens that are unique to mycobacterial species, including mycolic acid and lipoarabinomannan to human T cells, and antigen-specific CD1-restricted human T cells are able to lyse infected cells and kill the intracellular mycobacteria (13, 15, 32, 33). Thus, although the β2m−/− experiments strongly implicated a crucial role for class I MHC–restricted CD8+ T cells in immunity to M. tuberculosis, it is now clear that the effect of deleting β2m also might be mediated by T cell subsets other than class I MHC–restricted CD8+ T cells. One strategy to clarify the role of β2m-dependent T cells in immunity to tuberculosis was to examine the susceptibility of TAP1−/− or CD1D−/− mice to infection with M. tuberculosis. TAP1−/− mice are largely deficient in class I MHC–restricted T cells; however, because the recognition of CD1-restricted antigens is TAP independent, the CD1-restricted T cell populations should be largely unaffected (34, 35). Conversely, CD1D−/− mice have intact CD8+ T cell populations.
The finding that the absence of CD1d did not affect the outcome of M. tuberculosis infection in mice does not exclude the possibility that the human CD1 proteins play an important role in immunity to tuberculosis. Presentation of microbial antigens to T cells has been elucidated for the human group I CD1 proteins (i.e., CD1a, CD1b, and CD1c), but not for CD1d, which may have a greater role in immunoregulation (36). Furthermore, although humans are inherently more susceptible to tuberculosis than are mice, 95% of infected individuals develop long-lived immunity. If the group I CD1 proteins were to participate in the human immune response to M. tuberculosis, evolutionary selection may explain why group I CD1 genes are preserved in the human but not the murine genome. In this regard, it is of great interest that guinea pigs, another species that is highly susceptible to tuberculosis, has also retained the group I CD1 genes (Dascher, C.C., manuscript in preparation). The guinea pig may be a more suitable experimental animal for investigating the role of the group I CD1 proteins in the immune response to M. tuberculosis.
We found that TAP1−/− mice had an increased susceptibility to tuberculosis that was manifested by a decreased survival after intravenous infection, increased mycobacterial burden in the lungs, liver, and spleen, and overall more severe pathological changes in the target organs. These data establish that antigen-processing pathways that require TAP-dependent peptide loading are critical in the development and maintenance of protective immunity to virulent M. tuberculosis. In considering the β2m-associated antigen-presenting molecules, CD1 and TL are TAP independent (24, 37, 38). Presentation of antigens by H2-M3 and Qa-1 can be either TAP dependent or independent (35, 35, 37, 39). There exist examples of antigen presentation during intracellular infection with Listeria monocytogenes that are TAP independent for H2-M3 (39) and TAP dependent for Qa-1 (40). Qa-2 is TAP dependent (41), and although Qa-2 can bind peptides, T cell recognition of Qa-2 has not been demonstrated. Therefore, the TAP-dependent antigen-processing pathway primarily activates class I MHC–restricted CD8+ T cells.
CD8+ class I MHC–restricted T cells are not the only cellular subset that is abnormal in the TAP1−/− mice. Although their numbers and capacity to kill the YAC-1 cell line are normal, the repertoire of NK cells may be altered secondary to a change in the peptides bound by the class I MHC molecules and the overall decreased surface expression of class I MHC (30). Likewise, there is a relative expansion of NK1+ T cells in TAP1−/− mice (42), although these cells, which require CD1d1 for their positive selection, do not appear to be critical for the long-term survival of mice infected under the conditions used in this study.
Further work will be needed to clarify the role of CD8+ T cells during M. tuberculosis infection. It appears that progression of M. tuberculosis infection in both perforin- and fas-deficient mice is unaltered, and suggests that the cytolytic function of CD8+ T cells is not critical in immunity to tuberculosis (43, 44). Other work suggests that the crucial function of CD8+ T cells is mediated by IFN-γ (45). We have found that 40–60% of the CD4+ T cells in the lungs of infected mice are primed to produce IFN-γ (Chackerian, A., and S.M. Behar, manuscript in preparation), and it remains to be determined whether CD8+ T cells serve a role other than the production of IFN-γ. For example, CD8+ CTLs and NK cells produce granulysin, a protein found in cytotoxic granules that has direct microbicidal action against a variety of microorganisms (33). Granulysin does not have activity against intracellular bacteria unless it can gain access via a pore-forming molecule such as perforin (33). Although perforin-deficient mice are initially able to control mycobacterial infections (43), perforin and granulysin may have a role late in infection, for example in preventing recrudescence of disease.
Immunity to intracellular bacterial infections has been shown to be a cooperative effort between the innate and adaptive immune responses. Optimum protection against M. tuberculosis, L. monocytogenes, and Listeria major requires synergism between CD4+ and CD8+ T cells. Although the unique roles of each T cell subset during the course of infection remain to be elucidated, an understanding of these roles is critical to the rational development of vaccines and immunotherapeutic strategies.
Acknowledgments
The authors thank Chris Roy for his excellent technical assistance; Steve Jean, Linda Callahan, and the staff of the Animal Biohazard Containment Suite at the Dana-Farber Cancer Institute for their help in facilitating these experiments; and Frank Borriello for helpful discussions.
This work was supported by National Institutes of Health grants AR39582 (to M.B. Brenner), AR01978 (to S.M. Behar), AI43407 (to C.-R. Wang), and AI40171, as well as a gift from the Mathers Foundation to M.J. Grusby. M.J. Grusby is a Scholar of the Leukemia Society of America.
Abbreviations used in this paper
- AFB
acid fast bacilli
- β2m
β2 microglobulin
- ER
endoplasmic reticulum
- MST
mean survival time
- TAP
transporter associated with antigen processing
References
- 1.World Health Organization. 1998. Global Tuberculosis Programme. Tuberculosis Fact Sheet. WHO, Geneva. http://www.who.int/gtb/publications/factsheet/index.htm
- 2.Russell DG, Dant J, Sturgill-Koszycki S. Mycobacterium avium- and Mycobacterium tuberculosis-containing vacuoles are dynamic, fusion-competent vesicles that are accessible to glycosphingolipids from the host cell plasmalemma. J Immunol. 1996;156:4764–4773. [PubMed] [Google Scholar]
- 3.Muller I, Cobbold SP, Waldmann H, Kaufmann SH. Impaired resistance to Mycobacterium tuberculosisinfection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells. Infect Immun. 1987;55:2037–2041. doi: 10.1128/iai.55.9.2037-2041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orme IM. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. . J Immunol. 1987;138:293–298. [PubMed] [Google Scholar]
- 5.Orme IM, Collins FM. Protection against Mycobacterium tuberculosisinfection by adoptive immunotherapy. Requirement for T cell–deficient recipients. J Exp Med. 1983;158:74–83. doi: 10.1084/jem.158.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orme IM, Collins FM. Adoptive protection of the Mycobacterium tuberculosis-infected lung. Dissociation between cells that passively transfer protective immunity and those that transfer delayed-type hypersensitivity to tuberculin. Cell Immunol. 1984;84:113–120. doi: 10.1016/0008-8749(84)90082-0. [DOI] [PubMed] [Google Scholar]
- 7.Pedrazzini T, Hug K, Louis JA. Importance of L3T4+ and Lyt-2+ cells in the immunologic control of infection with Mycobacterium bovisstrain bacillus Calmette-Guerin in mice. Assessment by elimination of T cell subsets in vivo. J Immunol. 1987;139:2032–2037. [PubMed] [Google Scholar]
- 8.Cox JH, Knight BC, Ivanyi J. Mechanisms of recrudescence of Mycobacterium bovisBCG infection in mice. Infect Immun. 1989;57:1719–1724. doi: 10.1128/iai.57.6.1719-1724.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izzo AA, North RJ. Evidence for an alpha/beta T cell–independent mechanism of resistance to mycobacteria. Bacillus Calmette-Guerin causes progressive infection in severe combined immunodeficient mice, but not in nude mice or in mice depleted of CD4+ and CD8+T cells. J Exp Med. 1992;176:581–586. doi: 10.1084/jem.176.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lalvani A, Brookes R, Wilkinson RJ, Malin AS, Pathan AA, Andersen P, Dockrell H, Pasvol G, Hill AV. Human cytolytic and interferon gamma-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. . Proc Natl Acad Sci USA. 1998;95:270–275. doi: 10.1073/pnas.95.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewinsohn DM, Alderson MR, Briden AL, Riddell SR, Reed SG, Grabstein KH. Characterization of human CD8+ T cells reactive with Mycobacterium tuberculosis– infected antigen-presenting cells. J Exp Med. 1998;187:1633–1640. doi: 10.1084/jem.187.10.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I–restricted T cells are required for resistance to Mycobacterium tuberculosisinfection. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 14.Moody DB, Reinhold BB, Guy MR, Beckman EM, Frederique DE, Furlong ST, Ye S, Reinhold VN, Sieling PA, Modlin RL, et al. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science. 1997;278:283–286. doi: 10.1126/science.278.5336.283. [DOI] [PubMed] [Google Scholar]
- 15.Sieling PA, Chatterjee D, Porcelli SA, Prigozy TI, Mazzaccaro RJ, Soriano T, Bloom BR, Brenner MB, Kronenberg M, Brennan PJ. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science. 1995;269:227–230. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- 16.Joyce S, Woods AS, Yewdell JW, Bennink JR, De Silva AD, Boesteanu A, Balk SP, Cotter RJ, Brutkiewicz RR. Natural ligand of mouse CD1d1: cellular glycosylphosphatidylinositol. Science. 1998;279:1541–1544. doi: 10.1126/science.279.5356.1541. [DOI] [PubMed] [Google Scholar]
- 17.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, et al. CD1d-restricted and TCR-mediated activation of vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 18.Spada FM, Koezuka Y, Porcelli SA. CD1d- restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J Exp Med. 1998;188:1529–1534. doi: 10.1084/jem.188.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behar SM, Podrebarac TA, Roy CJ, Wang CR, Brenner MB. Diverse TCRs recognize murine CD1. J Immunol. 1999;162:161–167. [PubMed] [Google Scholar]
- 20.Cardell S, Tangri S, Chan S, Kronenberg M, Benoist C, Mathis D. CD1-restricted CD4+T cells in major histocompatibility complex class II–deficient mice. J Exp Med. 1995;182:993–1004. doi: 10.1084/jem.182.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SH, Roark JH, Bendelac A. Tissue-specific recognition of mouse CD1 molecules. J Immunol. 1998;160:3128–3134. [PubMed] [Google Scholar]
- 22.Pamer E, Cresswell P. Mechanisms of MHC class I–restricted antigen processing. Annu Rev Immunol. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- 23.Aldrich CJ, Ljunggren HG, Van Kaer L, Ashton-Rickardt PG, Tonegawa S, Forman J. Positive selection of self- and alloreactive CD8+T cells in Tap-1 mutant mice. Proc Natl Acad Sci USA. 1994;91:6525–6528. doi: 10.1073/pnas.91.14.6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanau D, Fricker D, Bieber T, Esposito-Farese ME, Bausinger H, Cazenave JP, Donato L, Tongio MM, de la Salle H. CD1 expression is not affected by human peptide transporter deficiency. Hum Immunol. 1994;41:61–68. doi: 10.1016/0198-8859(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 25.Behar SM, Roy C, Lederer J, Fraser P, Brenner MB. Clonally expanded Vα12+ (AV12S1),CD8+T cells from a patient with rheumatoid arthritis are autoreactive. Arthritis Rheum. 1998;41:498–506. doi: 10.1002/1529-0131(199803)41:3<498::AID-ART16>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 26.Van Kaer L, Ashton-Rickardt PG, Ploegh HL, Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4−8+T cells. Cell. 1992;71:1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 27.Chen YH, Chiu NM, Mandal M, Wang N, Wang CR. Impaired NK1+T cell development and early IL-4 production in CD1-deficient mice. Immunity. 1997;6:459–467. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- 28.Smiley ST, Kaplan MH, Grusby MJ. Immunoglobulin E production in the absence of interleukin-4–secreting CD1-dependent cells. Science. 1997;275:977–979. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- 29.Fite GL, Cambre PJ, Turner MH. Procedure for demonstrating lepra bacilli in paraffin sections. Arch Pathol. 1947;43:624–625. [PubMed] [Google Scholar]
- 30.Ljunggren HG, Van Kaer L, Ploegh HL, Tonegawa S. Altered natural killer cell repertoire in Tap-1 mutant mice. Proc Natl Acad Sci USA. 1994;91:6520–6524. doi: 10.1073/pnas.91.14.6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pamer EG, Wang CR, Flaherty L, Lindahl KF, Bevan MJ. H-2M3 presents a Listeria monocytogenespeptide to cytotoxic T lymphocytes. Cell. 1992;70:215–223. doi: 10.1016/0092-8674(92)90097-v. [DOI] [PubMed] [Google Scholar]
- 32.Stenger S, Mazzaccaro RJ, Uyemura K, Cho S, Barnes PF, Rosat JP, Sette A, Brenner MB, Porcelli SA, Bloom BR, Modlin RL. Differential effects of cytolytic T cell subsets on intracellular infection. Science. 1997;276:1684–1687. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- 33.Stenger S, Hanson DA, Teitelbaum R, Dewan P, Niazi KR, Froelich CJ, Ganz T, Thoma-Uszynski S, Melian A, Bogdan C, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–125. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 34.Cheroutre H, Holcombe HR, Tangri S, Castano AR, Teitell M, Miller JE, Cardell S, Benoist C, Mathis D, Huse WD, et al. Antigen-presenting function of the TL antigen and mouse CD1 molecules. Immunol Rev. 1995;147:31–52. doi: 10.1111/j.1600-065x.1995.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 35.Lindahl KF, Byers DE, Dabhi VM, Hovik R, Jones EP, Smith GP, Wang CR, Xiao H, Yoshino M. H2-M3, a full-service class Ib histocompatibility antigen. Annu Rev Immunol. 1997;15:851–879. doi: 10.1146/annurev.immunol.15.1.851. [DOI] [PubMed] [Google Scholar]
- 36.Wilson SB, Kent SC, Patton KT, Orban T, Jackson RA, Exley M, Porcelli S, Schatz DA, Atkinson MA, Balk SP, et al. Extreme Th1 bias of invariant Vα24JαQ T cells in type 1 diabetes. Nature. 1998;391:177–181. doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]
- 37.Tompkins SM, Kraft JR, Dao CT, Soloski MJ, Jensen PE. Transporters associated with antigen processing (TAP)-independent presentation of soluble insulin to α/β T cells by the class Ib gene product, Qa-1(b) J Exp Med. 1998;188:961–971. doi: 10.1084/jem.188.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodgers JR, Mehta V, Cook RG. Surface expression of beta 2-microglobulin–associated thymus-leukemia antigen is independent of TAP2. Eur J Immunol. 1995;25:1001–1007. doi: 10.1002/eji.1830250421. [DOI] [PubMed] [Google Scholar]
- 39.Lenz LL, Dere B, Bevan MJ. Identification of an H2-M3–restricted Listeriaepitope: implications for antigen presentation by M3. Immunity. 1996;5:63–72. doi: 10.1016/s1074-7613(00)80310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bouwer HG, Seaman MS, Forman J, Hinrichs DJ. MHC class Ib–restricted cells contribute to antilisterial immunity: evidence for Qa-1b as a key restricting element for Listeria-specific CTLs. J Immunol. 1997;159:2795–2801. [PubMed] [Google Scholar]
- 41.Tabaczewski P, Stroynowski I. Expression of secreted and glycosylphosphatidylinositol-bound Qa-2 molecules is dependent on functional TAP-2 peptide transporter. J Immunol. 1994;152:5268–5274. [PubMed] [Google Scholar]
- 42.Joyce S, Negishi I, Boesteanu A, DeSilva AD, Sharma P, Chorney MJ, Loh DY, Van Kaer L. Expansion of natural (NK1+) T cells that express α/β T cell receptors in transporters associated with antigen presentation 1–null and thymus leukemia antigen–positive mice. J Exp Med. 1996;184:1579–1584. doi: 10.1084/jem.184.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laochumroonvorapong P, Wang J, Liu CC, Ye W, Moreira AL, Elkon KB, Freedman VH, Kaplan G. Perforin, a cytotoxic molecule which mediates cell necrosis, is not required for the early control of mycobacterial infection in mice. Infect Immun. 1997;65:127–132. doi: 10.1128/iai.65.1.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cooper AM, D'Souza C, Frank AA, Orme IM. The course of Mycobacterium tuberculosisinfection in the lungs of mice lacking expression of either perforin- or granzyme-mediated cytolytic mechanisms. Infect Immun. 1997;65:1317–1320. doi: 10.1128/iai.65.4.1317-1320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tascon RE, Stavropoulos E, Lukacs KV, Colston MJ. Protection against Mycobacterium tuberculosis infection by CD8+T cells requires the production of gamma interferon. Infect Immun. 1998;66:830–834. doi: 10.1128/iai.66.2.830-834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]