Abstract
Dendritic cells (DCs) express several receptors for the Fc portion of immunoglobulin (Ig)G (FcγR), which mediate internalization of antigen–IgG complexes (immune complexes, ICs) and promote efficient major histocompatibility complex (MHC) class II–restricted antigen presentation. We now show that FcγRs have two additional specific attributes in murine DCs: the induction of DC maturation and the promotion of efficient MHC class I–restricted presentation of peptides from exogenous, IgG-complexed antigens. Both FcγR functions require the FcγR-associated γ chain. FcγR-mediated MHC class I–restricted antigen presentation is extremely sensitive and specific to immature DCs. It requires proteasomal degradation and is dependent on functional peptide transporter associated with antigen processing, TAP1-TAP2. By promoting DC maturation and presentation on both MHC class I and II molecules, ICs should efficiently sensitize DCs for priming of both CD4+ helper and CD8+ cytotoxic T lymphocytes in vivo.
Keywords: Fc receptors, dendritic cells, antigen presentation, immune complexes, cross-priming
ajor histocompatibility complex (MHC) class I molecules are generally complexed exclusively with peptides derived from cytosolic antigens (1). However, this picture is too restrictive to explain the priming of naive CD8+ T cells by bone marrow (BM)1-derived APCs (2): APCs also internalize exogenous antigens for processing and presentation on MHC class I molecules. The induction of CTL response due to exogenous antigen transfer was first examined in response to minor histocompatibility antigens, and was referred to as cross-priming (3). Recent results suggest that DCs may play a critical role in this process (4).
Indeed, dendritic cells (DCs) are the most potent APCs for inducing differentiation of naive CD4+ and CD8+ T cells into helper and cytotoxic T cells, respectively, and for initiating primary and secondary immune responses (5, 6). To prime T cell responses, DCs require several separate signals. The first is provided by antigens themselves, which are processed into peptides and loaded intracellularly onto MHC molecules. Efficient T cell priming also requires a cell activation signal, delivered by either inflammatory cytokines (TNF-α or IL-1) or bacterial components (such as LPS). This signal induces expression of MHC and T cell costimulatory molecules at the DC surface and causes migration from peripheral tissues to secondary lymphoid organs, where T cell priming occurs. Cognate CD4+ T cell help is also required for efficient CD8+ T cell priming, with antigen recognition by both CD4+ and CD8+ T cells on the same DC (7–10). Therefore, this DC requires the simultaneous presentation of peptides from exogenous antigens on both MHC class I and II molecules.
Presentation of peptides derived from exogenous antigens on MHC class I molecules may occur through two different pathways (11). First, internalized antigens may exit endocytic compartments and, once in the cytosol, be processed by the proteasome into peptides which then reach the conventional transporter associated with antigen processing (TAP)1/2- dependent MHC class I antigen presentation pathway. Alternatively, internalized antigens may be processed in endocytic compartments, generating peptides which associate to preexisting MHC class I molecules, either in endosomes or at the cell surface after peptide regurgitation.
Regardless of the pathway, cross-priming in vitro after fluid phase antigen internalization is generally very inefficient, since it requires very high antigen concentrations—in the mg/ml range (11). Antigen coupling to or mixing with latex beads forces internalization by phagocytosis and strongly enhances the efficiency of MHC class I–restricted antigen presentation in macrophages or DCs (12, 13). Phagocytosis of bacteria (14, 15) or of apoptotic cells (4) also results in efficient MHC class I–restricted antigen presentation in macrophages and/or DCs. Thus, the pathway by which antigens are internalized appears to influence the efficiency of presentation on both MHC class I and II molecules.
In the case of MHC class II–restricted presentation, a major breakthrough came from the observation that antigens internalized through specific membrane receptors are more efficiently presented to CD4+ T cells than they are after fluid phase internalization (16). FcγRs, which bind antigen–IgG complexes (immune complexes, ICs [17]), represent a privileged antigen internalization route for efficient MHC class II–restricted antigen presentation in DCs (18). Human DCs express several types of FcγRs, including type I (FcγRI, CD64 [19]) and type II (FcγRII, CD32 [18]). FcγR expression by murine DCs has not been fully examined. Importantly, in addition to IC internalization, FcγRI and FcγRIII trigger cell activation (17) through the associated γ chain, which bears a motif called immunoreceptor tyrosine-based activation motif (ITAM), also required for IC internalization (20, 21).
Here, we examined the role of FcγRs in DC activation and in MHC class I–restricted presentation of peptides derived from internalized IgG-complexed antigens. We found that FcγR engagement in DCs triggers maturation and induces efficient MHC class I and II–restricted antigen presentation. These results suggest the existence of unknown connections between humoral and cytotoxic components of immune responses.
Materials and Methods
Mice.
γ chain−/− mice were obtained on a B6 × 129 background (22) and TAP−/− mice from Centre National de la Recherche Scientifique (Orleans, France). TAP−/− mice were on a B6 × 129 background (23).
DCs and Culture Medium.
Immature DCs were prepared as described (24). C57BL/6 and γ chain−/− BM cells were incubated 3 wk in IMDM (Sigma Chemical Co.) containing 10% heat-inactivated FBS (GIBCO BRL), 100 IU/ml penicillin, 100 mg/ml streptomycin, 2 mM l-glutamine (Sigma), and 50 mM 2-βME with 30% conditioned medium from GM-CSF–producing NIH/3T3 cells (R1 medium). D1 long-term cultured cell line is an H-2b splenic DC cell line described by Winzler et al. (24) and was also cultured in the same medium with 30% R1 medium. The cells expressed MHC class I and II molecules, CD40, CD80, CD86, and 24G.2+ FcγR, but not Gr1. The surface expression of all of these molecules, except FcγRs, was increased after LPS treatment for 24 h, indicating effective DC maturation.
Antibodies, Cell Surface Staining, and Immunofluorescence.
The following antibodies were purchased from PharMingen: CD80/ B7.1 (1G10), CD40 (HM40-3), CD86/B7.2 (GL1), CD107a/ Lamp-1. Before labeling experiments, FcR blocking was performed by incubating cells with 2.4G2 supernatant. Staining was carried out according to standard techniques, and flow cytometry analysis was performed with a FACScan® (using CellQuest software; Becton Dickinson). For intracellular immunofluorescence, cells were fixed for 20 min in 3% paraformaldehyde and then permeabilized for 30 min in PBS containing 1% saponin, 5% BSA, and then stained in PBS containing 1% saponin, 5% BSA.
Antigen Presentation Assay.
OVA batches from different companies were screened for the absence of presentation to B3Z cells with fixed cells. The selected batch (from Worthington) did not induce DC activation by immunofluorescence and flow cytometry. Presentation of OVA epitope 257–264 on Kb was detected using the T cell hybridoma B3Z, which carries a β-galactosidase construct driven by NF-AT elements from the IL-2 promoter (25). For antigen presentation assays, DCs were exposed to OVA, at the concentration and for the periods of time specified, in the presence of the T cell hybridoma B3Z. After exposure to OVA, cells were washed with PBS, and a colorimetric assay using ONPG (Sigma Chemical Co.) as a substrate was used to detect LacZ activity in B3Z lysates. Where indicated, the following inhibitors were included in the antigen presentation assays.
MHC class II–restricted response to ovalbumin was detected using two T cell hybridomas, BO97.10, specific for OVA 323– 339 on I-Ab (26) on DCs, and 54.8, specific for the same peptide presented on I-Ad. 20 h after antigen pulsing, DCs were fixed with glutaraldehyde 0.001%, and BO97.10 cells were added for 24 h. After 24 h, 50 ml of supernatant was harvested and the IL-2 production by BO97.10 was measured with [3H]thymidine incorporation by IL-2–dependent CTL L2 cell line.
Lactacystin and the peptide aldehyde N-acetyl-Leu-l-Leu- l-norleucinal (LLnL) were stored in DMSO as 1 and 2.5 mM stock solutions, respectively, and they were diluted in culture media for use. 5 × 104 D1 cells/well were incubated with the inhibitors for 1 h before antigen pulsing and during the antigen pulse. OVA-containing ICs (OVA-ICs) were prepared by incubation at 37°C, 15 min of soluble OVA at a final concentration of 0.4 and 20 μg/ ml of anti-OVA IgGs purified from rabbit sera (Sigma Chemical Co.). As a control for inhibitor toxicity, we used the OVA 257– 264 peptide at a final concentration of 10 ng/ml. After a chase of 12 h, D1 cells were fixed with glutaraldehyde 0.001%, overlaid with 5 × 104 B3Z cells/well, and incubated for 24 h.
Activation Induced by ICs.
5 × 104 D1 cells/well were incubated with soluble OVA alone or in the presence of hen egg lysozyme (HEL)-ICs (at final concentrations: HEL 30 μg/ml, and mAbs anti-HEL, HyHEL5, and 5253C7, 15 μg/ml each [27]) and were overlaid with 5 × 104 B3Z cells/well and incubated for 24 h.
H-2Kb Transfection of B Lymphomas.
IIA1.6 B lymphoma cells expressing FcγR-ctγ chimeric receptors (21) were supertransfected with cDNA encoding H-2Kb molecule (28) under the control of the SRα promoter. After selection with puromycin, clones obtained by limited dilution were tested by FACScan® using the H-2Kb–specific mAb SF1-1.1. Two independent H-2Kb– expressing clones were used for MHC class I–restricted antigen presentation, with similar results.
Results
ICs Induce DC Activation.
To analyze the expression and function of FcγR in murine DCs, we first used a well-characterized, growth factor–dependent, spleen-derived DC line called D1 (24). D1 cells display all of the phenotypic characteristics of immature DCs: low levels of surface MHC and costimulatory molecules, and abundant endocytic MHC class II–containing compartments. Upon treatment with LPS or TNF-α, D1 cells show all of the phenotypical changes characteristic of DC maturation (24).
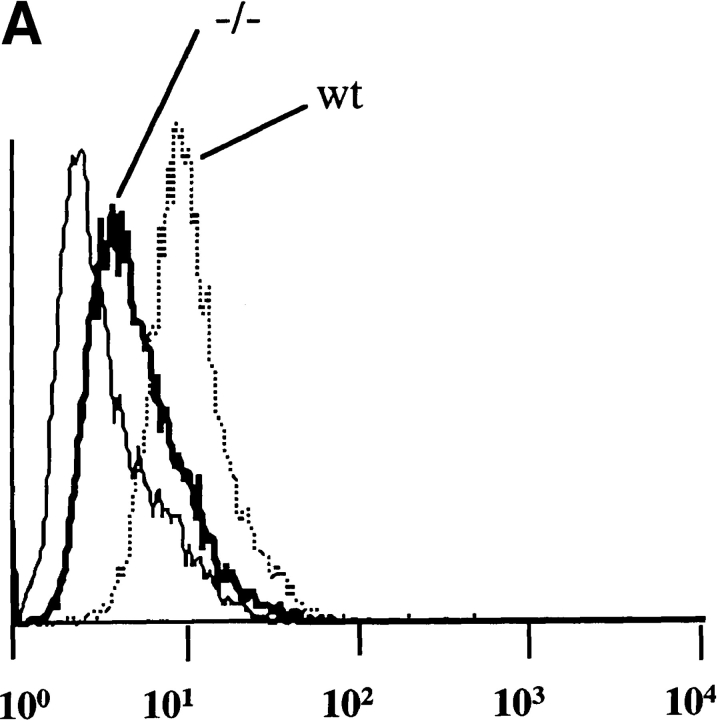
We first analyzed the nature of the FcγRs expressed by D1 cells. Western blot analysis after immunoprecipitation with the anti-FcγRII and FcγRIII 2.4G2 antibody showed that D1 cells expressed FcγRIIb1, FcγRIIb2, and FcγRIII (Fig. 1 A). The two intermediate bands between FcγRIIb1 and FcγRIIb2 most likely represent FcγRIIb1′ and an unidentified spliced variant (29). FcγRI are also expressed in murine DCs, since mRNA encoding this receptor was readily detected by reverse transcription PCR (not shown). These results indicate that murine DCs express FcγRI, II, and III.
Figure 1.
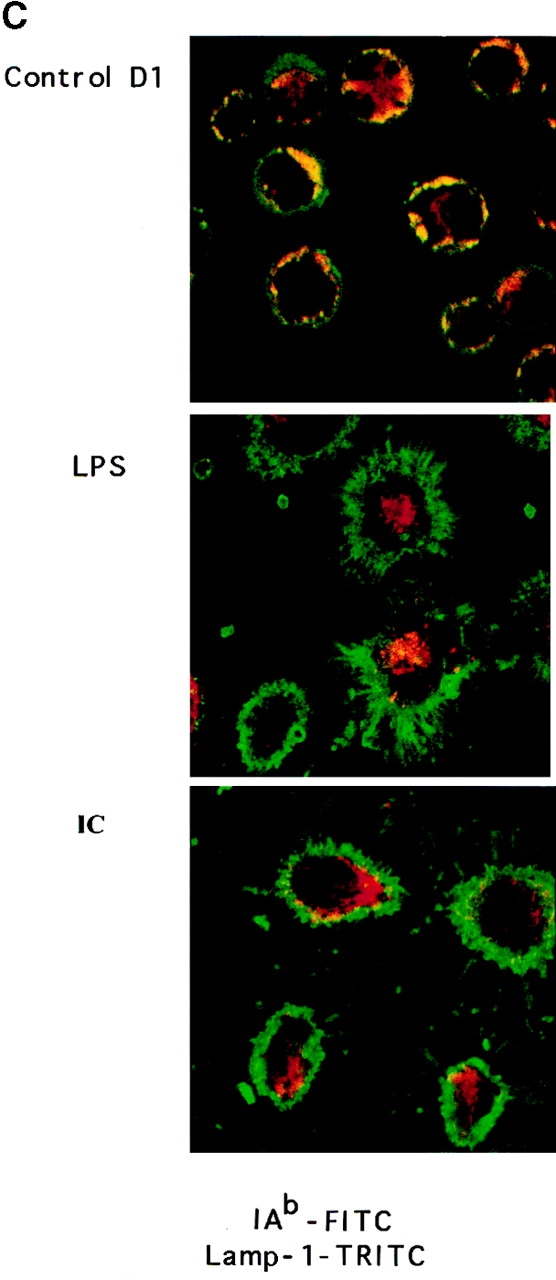
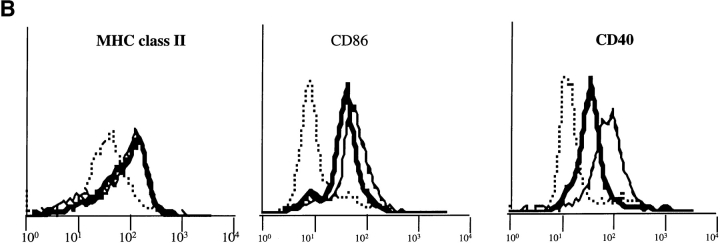
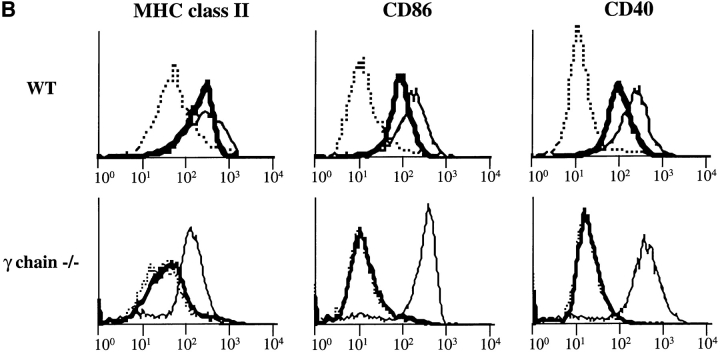
(A) FcγR expression in D1 cells. FcγRII and FcγRIII α chain molecules were immunoprecipitated with 2.4G2 mAb, and FcγRIIb1, b2, and FcγRIII were revealed by Western blot with (+) or without (−) deglycosylation. (B) ICs induce DC maturation. D1 cells were incubated for 24 h alone (dotted line) or in the presence of LPS (solid line) or OVA-ICs (0.4 μg/ml of OVA and 20 μg/ml of rabbit anti-OVA IgGs; bold line). The cells were then stained for MHC class II (Y3P), CD40 (HM40-3), and CD86/B7.2 (GL1) and analyzed by FACscan.® IC- and LPS-treated D1 cells expressed increased surface levels of all three molecules, indicating effective DC maturation. (C) Subcellular distribution of MHC class II molecules. D1 cells were incubated for 24 h in the absence (top) or presence of LPS (middle) or OVA-ICs (bottom). The cells were then fixed, permeabilized, stained, and analyzed by confocal microscopy. MHC class II molecules were visualized (green) using the mAb Y3P and FITC-conjugated secondary antibodies, and lysosomal membrane protein Lamp-1 was visualized (red) using the mAb CD107a and TRITC-conjugated secondary antibody. Yellow, codistribution of the two markers.
Engagement of FcγRI or III in macrophages triggers cell activation, causing the production of various cytokines and chemokines, as well as changes in expression of cell surface proteins involved in antigen presentation (17, 30). To evaluate the ability of ICs to induce DC activation, D1 cells were incubated for 24 h in the presence of OVA complexed to specific polyclonal anti-OVA IgG antibodies (OVA-ICs) or LPS, which induces D1 maturation.
As shown in Fig. 1 B, OVA-ICs, like LPS, induced a marked increase in the surface expression of MHC class II, CD86, and CD40 molecules (Fig. 1 B), phenotypic changes characteristic of DC maturation (6, 24). Immunofluorescence and confocal microscopy analysis showed that both LPS (Fig. 1 C, middle) and OVA-ICs (bottom) also induced MHC class II redistribution to the plasma membrane (compared with Fig. 1 C, top), as lysosomes became devoid of MHC class II molecules. Incubation with OVA alone, or with the antibodies in the absence of OVA, induced no changes in the surface expression of any of the markers analyzed or in DC morphology and MHC class II localization (not shown). Similar results were obtained with fresh BM-derived DCs (BM-DCs, see below). Like other maturation stimuli (31, 32), OVA-ICs induced an increase in MHC class II synthesis and a strong decrease in their rate of degradation (MHC class II half-life raised from 3–5 to >40 h; not shown). Therefore, like LPS or TNF-α, FcγR engagement by ICs induces murine DC maturation in vitro.
IC Internalization Results in Efficient MHC Class I–restricted Antigen Presentation.
The other main consequence of FcγR engagement is IC internalization, which induces potent MHC class II–restricted presentation in various cell types, including DCs (18, 33). However, during cross-priming, DCs also need to present exogenous antigens on MHC class I molecules to initiate CTL responses. To determine if ICs may participate in the acquisition of antigens by DCs for MHC class I–restricted presentation, we next examined presentation of an OVA-derived peptide to a CD8+ T cell hybrid after FcγR-mediated internalization of OVA-ICs by murine D1 cells.
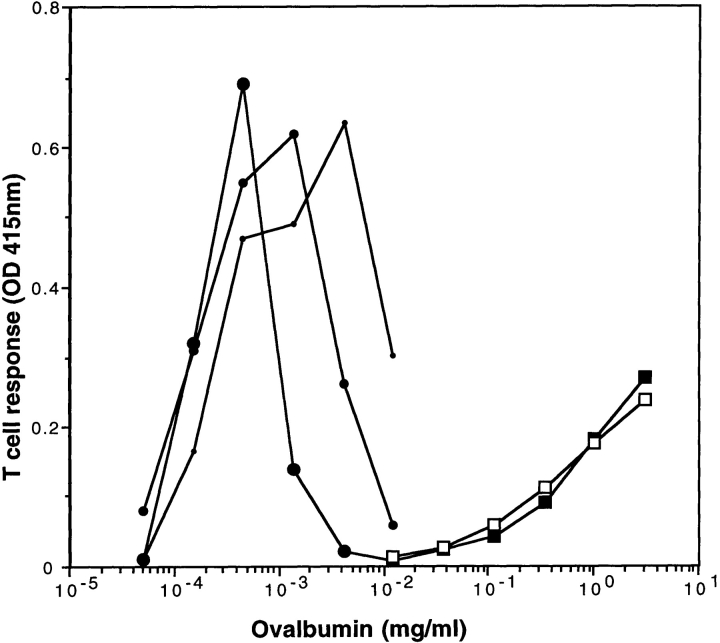
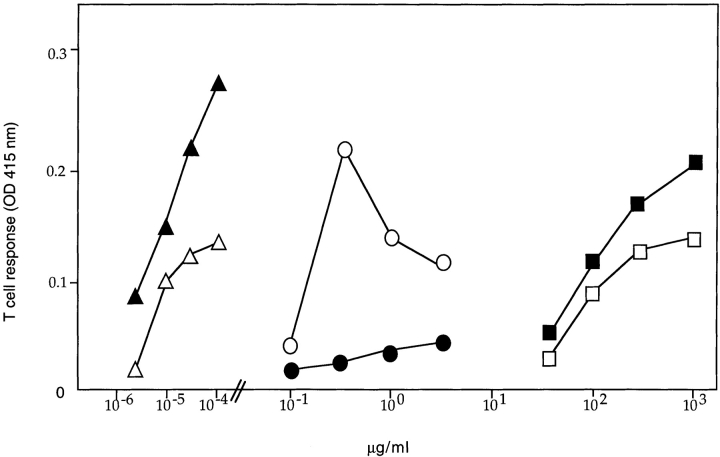
As shown previously (34–36), presentation of OVA 257–264/H-2Kb epitope to B3Z T cells (25) after OVA uptake by fluid phase was only observed at very high, nonphysiological antigen concentrations of 1–10 mg/ml (Fig. 2). In contrast, presentation of the same epitope after internalization of OVA-ICs was observed at OVA concentrations ranging between 0.1 and 1 μg/ml (Fig. 2), i.e., three to four orders of magnitude lower antigen concentrations than uncomplexed OVA. As expected for ICs, the optimal antigen to antibody ratio was achieved at lower antigen concentrations as the antibody concentrations decreased (Fig. 2). The highly efficient OVA presentation observed after OVA-IC internalization was not due to FcγR engagement per se, since presentation of soluble OVA to B3Z T cells was not modified by the presence of irrelevant HEL-ICs (Fig. 2). HEL-ICs induced D1 maturation, as detected by surface immunostaining of MHC and costimulation molecules (not shown). Therefore, formation of ICs allows efficient acquisition of antigens for peptide presentation to CTLs on MHC class I molecules.
Figure 2.
MHC class I presentation after IC internalization. (A) D1 cells (5 × 104 cells/well) were incubated at 37°C with OVA-ICs, prepared with the indicated concentrations of soluble OVA, and incubated for 15 min at 37°C with 50 (small circles), 25 (medium circles), or 12.5 (large circles) μg/ml of rabbit anti-OVA purified IgGs. In parallel, D1 cells were incubated with soluble OVA (open squares) or soluble OVA and irrelevant ICs (HEL 30 μg/ml, mAbs anti-HEL, HyHEL5, and 5253C7, at 15 μg/ml each; filled squares). D1 cells were then incubated for 24 h in the presence of B3Z cells, a T cell hybridoma specific for OVA-Kb, which carries a β-galactosidase construct driven by NF-AT elements from the IL-2 promoter. T cell activation was measured using a colorimetric assay for LacZ activity with ONPG as a substrate (one representative experiment out of five is shown).
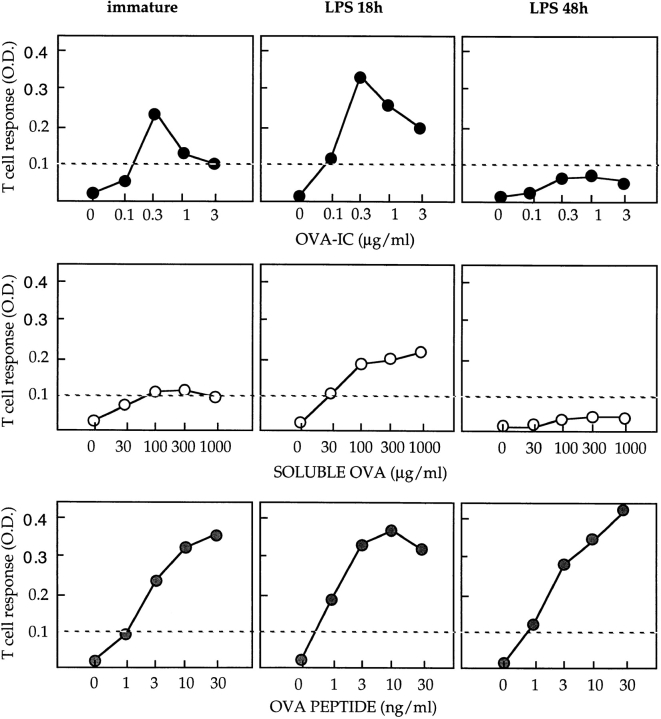
The capacity of DCs to ingest and process exogenous antigens for presentation by MHC class II molecules is downmodulated upon DC maturation (37). Therefore, it was important to determine whether MHC class I–restricted presentation after IC internalization was also modulated during DC maturation. Like D1 cells, immature (untreated) BM-DCs presented OVA epitope 257–264 to B3Z cells more efficiently after OVA-IC internalization than after uncomplexed OVA uptake (Fig. 3, top left and middle left panels). In cells treated with LPS for 18 h, presentation after both OVA-IC and soluble OVA internalization was transiently and reproducibly enhanced (Fig. 3, top center and middle center panels). In contrast, MHC class I–restricted presentation after OVA-IC and soluble OVA internalization was almost completely abrogated in DCs treated with LPS for 48 h (Fig. 3, top right and middle right panels). Direct presentation of the 257–264 OVA peptide was not significantly modified by LPS treatment (Fig. 3, bottom panels). Therefore, as shown previously for MHC class II (31, 32), MHC class I–restricted presentation of exogenous antigens is first transiently upregulated and then shut down during DC maturation. Downregulation of MHC class I–restricted presentation by DCs upon maturation may prevent presentation of IgG-complexed antigens encountered after DC migration to secondary lymphoid organs.
Figure 3.
MHC class I–restricted presentation is downmodulated upon LPS-induced DC maturation. C57BL/6 BM-DCs were cultured in the absence (left panels) or presence of LPS (20 μg/ml) for 18 h (center panels) or 48 h (right panels), washed, and incubated for an additional 18-h period with increasing concentrations of OVA-ICs (top panels), soluble OVA (middle panels), or OVA 257–264 peptide (bottom panels) in the presence of B3Z cells. One representative experiment out of three is shown. T cell stimulation was then tested as described above. The efficiency of cross-priming is transiently upregulated and then downmodulated upon DC maturation.
MHC Class I Presentation after IC Internalization Is TAP Dependent and Lactacystin Sensitive.
Two pathways for MHC class I–restricted presentation of exogenous antigens have been described (11). One reaches the so-called “conventional” pathway after exogenous antigen transfer from endocytic compartments to the cytosol. This pathway requires proteasomal degradation, TAP-dependent transport of the peptides into the lumen of the endoplasmic reticulum, and association to newly synthesized MHC class I molecules (11). The second pathway is TAP and proteasome independent; it is inhibited by NH4Cl (which neutralizes lysosomal pH) but not by cycloheximide (CHX; an inhibitor of protein synthesis), suggesting that the generation of peptides and their association to MHC class I may occur in endosomes (11). We next determined which of these two pathways was used for MHC class I–restricted presentation after IC internalization.
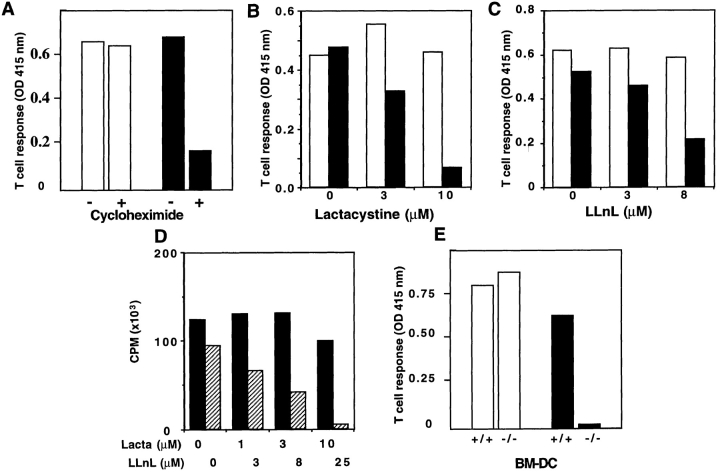
Antigen presentation by D1 cells after OVA-IC internalization was strongly inhibited by CHX (Fig. 4 A), lactacystin (a specific proteasome inhibitor [38]; Fig. 4 B), and the peptide aldehyde N-acetyl-Leu-l-Leu-l-norleucinal (LLnL; Fig. 4 C), which inhibits both lysosomal proteases and the proteasome (38). Direct presentation of the 257–264 OVA peptide was not affected by any of these drugs (Fig. 4, A–C). To assess the specificity of the two protease inhibitors in DCs, lactacystin and LLnL were tested in parallel on the MHC class II–restricted presentation after OVA-IC internalization. Only LLnL, and not lactacystin, blocked MHC class II–restricted presentation of OVA peptide 323–339 on I-Ab to BO97.10-specific T cells (Fig. 4 D), indicating that lactacystin specifically inhibits proteasomal degradation at the concentrations used. MHC class I–restricted presentation after OVA-IC internalization was also sensitive to brefeldin A (which blocks protein transport from the endoplasmic reticulum, not shown).
Figure 4.
Antigen presentation after IC internalization reaches the conventional MHC class I antigen presentation pathway. (A–C) D1 cells were preincubated alone or in the presence of CHX (3 μg/ml, A) for 3 h or lactacystin (10 μM, B) or LLnL (8 μM, C) for 1 h. Pretreated D1 cells were then incubated for an additional 7-h period with OVA-ICs (0.4 μg/ml of OVA and 20 μg/ml of rabbit anti-OVA IgGs; black bars) or with the 257–264 synthetic peptide (10 ng/ml; white bars) in the presence of the same concentrations of inhibitors. D1 cells were glutaraldehyde fixed before coculture with B3Z cells. (D) MHC class II–restricted presentation after OVA-IC internalization was tested in the presence of lactacystin (black bars) and LLnL (hatched bars) using the T cell hybridoma BO97.10 (specific for OVA 323–339 on I-Ab) on DCs fixed with glutaraldehyde after antigen pulsing. IL-2 production by BO97.10 was measured with [3H]thymidine incorporation by IL-2– dependent CTL.L2 cell line. (E) BM-DCs were generated as described (reference 33) from C57BL/6 or TAP-1–deficient mice and were incubated with 257–264 (SIINFKEL) peptide (10 ng/ml; white bars) or with OVA-ICs (0.4 μg/ml of OVA and 20 μg/ml of anti-OVA rabbit IgGs; black bars) in the presence of B3Z cells for 24 h. Cultures were then treated as described above (one representative experiment out of three is shown).
MHC class I–restricted presentation after OVA-IC internalization also required the TAP1-TAP2 peptide transporter. BM-DCs derived from TAP1-deficient mice did not present the OVA epitope after internalization of OVA-ICs, whereas direct presentation of the 257–264 OVA peptide to B3Z T cells was as efficient as with DCs derived from wild-type (wt) C57BL/6 mice (Fig. 4 E). Therefore, MHC class I–restricted presentation after IC internalization reaches the conventional MHC class I presentation pathway.
MHC Class I–restricted Presentation after IC Internalization Is DC Specific.
However, efficient IC internalization is not restricted to DCs. In B lymphoma cells, we showed that expression of endocytic FcγRs induces efficient MHC class II–restricted presentation after IC internalization (20, 39). To determine whether FcγR-mediated MHC class I–restricted presentation of exogenous antigens is DC specific, we next examined antigen presentation after OVA-IC internalization in B lymphoma cells. IIA1.6 B lymphoma cells are an FcγR clone derived from A20 B lymphoma cells, one of the cell lines most widely used to analyze MHC class II–restricted antigen presentation (20, 39). We have shown previously that IIA1.6 cells expressing recombinant FcγRIIb2 or FcγRIII efficiently internalize IC and strongly promote MHC class II–restricted antigen presentation (20, 39). In addition, a chimeric receptor composed of the lumenal and transmembrane domains of FcγRII and the cytoplasmic tail of the γ chain (FcγR-ctγ) presents all of the functional characteristics of FcγRIII in terms of internalization and antigen presentation (20, 33). I-Ad– expressing B lymphoma IIA1.6 cells expressing recombinant FcγRIIb2 or FcγR-ctγ chimeras were supertransfected with H-2Kb, and compared with D1 cells for MHC class I and II presentation after OVA-IC internalization.
In FcγR-ctγ/H-2Kb–expressing B lymphoma cells, MHC class II–restricted presentation of the 323–339 OVA peptide on I-Ad to 54.8 T cell hybridomas was strongly enhanced (three to four orders of magnitude) after OVA-IC internalization (Fig. 5 A). In D1 cells, presentation of the same peptide on I-Ab to BO97.10 T cell hybridomas was also enhanced by three to four orders of magnitude after OVA-IC internalization (Fig. 5 B). Similar results were obtained with cells expressing FcγRIIb2 (not shown). Therefore, internalization of ICs results in efficient MHC class II–restricted presentation in both B lymphoma cells and DCs.
Figure 5.
MHC class I–restricted presentation after OVA-IC internalization is DC specific. D1 cells were tested concurrently with a B cell line expressing recombinant internalization-competent FcγR-ctγ chimeric receptors and supertransfected with cDNA encoding the Kb MHC class I molecule. B lymphoma cells (A and C) and D1 cells (B and D) were incubated with the indicated concentrations of OVA (squares) or OVA complexed to 20 μg/ ml of anti-OVA rabbit polyclonal IgG (OVA-IC; circles). D1 cells were fixed with glutaraldehyde before coculture with T cells. MHC class II–restricted presentation of OVA was tested using the T cell hybridomas BO97.10 (for I-Ab on D1 cells; B) or 54.8 (for I-Ad on B lymphoma cells; A), which both recognize the 323–339 epitope, in association with I-Ab and I-Ad, respectively. MHC class I presentation by B lymphoma cells (C) or D1 cells (D) was tested using B3Z T cell hybridoma as described in the legend to Fig. 2. The 323–339 and 257–264 synthetic peptides (10 μg/ml) were used as positive controls (histograms).
In D1 cells, as shown previously, soluble MHC class I–restricted presentation was observed after soluble OVA at high concentrations and after OVA-IC internalization at low antigen concentrations (Fig. 5 D). In contrast, B lymphoma cells expressing FcγR-ctγ (Fig. 5 C) or FcγRIIb2 (not shown) were completely incompetent for MHC class I–restricted presentation of OVA-derived peptide 257– 264, after internalization of OVA-ICs or uncomplexed OVA at high concentrations. In contrast, when incubated with the OVA 257–264 synthetic peptide, both D1 cells and B lymphoma cells activated B3Z cells (Fig. 5, C and D). Like B lymphoma cells, IFN-γ–treated peritoneal macrophages were capable of directly presenting OVA peptide 257– 264 to B3Z cells, but not after OVA-IC or uncomplexed OVA internalization (not shown). Therefore, in contrast to MHC class II–restricted presentation, the FcγR-mediated pathway for presentation of exogenous antigens by MHC class I molecules is restricted to DCs.
Role of the FcR-associated γ Chain in IC-induced DC Maturation and MHC Class I–restricted Antigen Presentation.
The results presented thus far suggest that FcγRs are involved in both the induction of DC maturation and antigen uptake for efficient MHC class I–restricted presentation of exogenous antigens. Importantly, FcγRI and FcγRIII trigger cell activation in a variety of cell types through an ITAM found in the associated γ chain (17). To determine the nature of the FcγRs involved in the triggering of DC maturation by ICs, we prepared BM-DCs from wt mice and from mice deficient for the FcγRI- and FcγRIII-associated γ chain (22). Surface expression of FcγRII and III (as detected by the mAb 2.4G2) was decreased but not abolished in BM-DCs from the γ chain−/− mice (Fig. 6 A), confirming that BM-DCs from the γ chain−/− mice still express FcγRII and suggesting that BM-DCs from wt C57BL/6 mice expressed both FcγRII and III.
Figure 6.
The γ subunit of FcγRs is required for IC-induced DC maturation. (A) 2.4G2 staining in BM-DCs from wt C57BL/6 or γ chain−/− mice. BM-DCs from wt and γ chain−/− mice were stained for FcγRII and III with 2.4G2 mAb before analysis by FACscan®. BM-DCs from wt mice showed stronger surface staining with 2.4G2 than BM-DCs from γ chain−/− mice, suggesting that BM-DCs from wt C57BL/6 mice expressed both FcγRII and III. (B) BM-DCs from γ chain−/− mice are not activated by ICs. BM-DCs from wt and γ chain−/− mice were incubated for 24 h alone (dotted line) or in the presence of LPS (solid line) or OVA-ICs (0.4 μg/ml of OVA and 20 μg/ml of rabbit anti-OVA IgGs; bold line) and stained for MHC class II (Y3P), CD40 (HM40-3), and CD86/B7.2 (GL1) before analysis by FACscan®. BM-DCs from wt mice were activated by both LPS and ICs. In contrast to LPS, OVA-ICs did not induce effective DC maturation in γ chain−/− mice.
To determine the requirement for γ chain in DC activation, the ability of LPS and OVA-ICs to induce maturation was analyzed. LPS induced a marked increase of the surface expression of MHC class II, CD86, and CD40 molecules on DCs from wt and γ chain−/− mice (Fig. 6 B), as well as all of the morphological modifications characteristic of DC maturation (not shown). In contrast, OVA-ICs did not induce any detectable maturation in BM-DCs from γ−/− mice, whereas they induced maturation of BM-DCs from wt mice (Fig. 6 B). Therefore, DCs from γ chain−/− mice presented a selective defect in IC-induced maturation.
The involvement of the γ chain in MHC class I–restricted presentation was tested next. As expected, BM-DCs from both wt and γ chain−/− mice presented soluble OVA and the 257–264 OVA peptide to the B3Z T cell hybridomas with similar efficiencies (Fig. 7). BM-DCs from wt mice also presented the OVA epitope after OVA-IC internalization at low antigen concentrations (Fig. 7). In contrast, DCs from γ−/− mice did not show any significant MHC class I–restricted presentation after incubation with OVA-ICs (Fig. 7). These DCs had also lost the ability to present OVA peptide 323–339 in association to I-Ab MHC class II molecules (not shown). In addition, FcγR-mediated IC internalization in BM-DCs from γ chain−/− mice was decreased, as measured both biochemically and by immunofluorescence and confocal microscopy (not shown), suggesting that the absence of antigen presentation by BM-DCs from γ chain−/− mice results from inefficient IC internalization.
Figure 7.
BM-DCs derived from γ chain−/− mice fail to present peptides derived from OVA-ICs on MHC class I molecules. C57BL/6 (open symbols) or γ chain−/− BM-DCs (filled symbols) were incubated in the presence of the indicated concentrations of soluble OVA (squares), OVA-ICs (20 μg/ml rabbit IgG anti-OVA; circles), or 257–264 peptide (triangles) for 18 h in the presence of B3Z T cells. Antigen presentation was quantified as described above. One representative of three experiments is shown.
Therefore, the FcγR-associated γ chain is required for both induction of DC maturation by ICs and promotion of MHC class I–restricted presentation, indicating that FcγRI and/or FcγRIII (the two γ chain–associated FcγRs) are required for the functions of ICs in DCs.
Discussion
Our results evidence a novel receptor-mediated pathway for antigen acquisition for MHC class I–restricted presentation. This FcγR-mediated cross-priming pathway is DC specific and is inactive in mature DCs. It requires the TAP1-TAP2 transporter and is sensitive to lactacystin, a proteasome inhibitor, indicating that after internalization, IgG-complexed antigens are transferred into the cytosol and reach the conventional MHC class I antigen presentation pathway. FcγR engagement also induces full DC activation, reflected by increased levels of MHC and costimulatory molecules (CD40, CD80, and CD86) at the cell surface. Simultaneous induction of maturation and MHC class I and class II–restricted presentation by a single receptor–ligand interaction should result in efficient T cell priming in vivo.
This pathway is not the first described for cross-priming in vitro. Fluid phase internalization can result in MHC class I–restricted presentation, but only at very high antigen concentrations (3–10 mg/ml in the OVA system), in both macrophages and DCs (34, 36). Interestingly, induction of macropinocytosis was shown to result in cross-priming in vitro (35). In addition, phagocytosis somehow favors MHC class I–restricted presentation of exogenous antigens in macrophages and DCs. Indeed, internalization of bacteria by macrophages or DCs (12, 14, 15) and phagocytosis of apoptotic bodies by DCs (4) also result in cross-priming. Likewise, antigen coupling to (12), or in some case mixing with (13), synthetic beads forces antigen phagocytosis and remarkably increases the efficiency of MHC class I antigen presentation (peptides derived from OVA may then be presented at 1–3 μg OVA/ml). Thus, the mode of antigen internalization influences cross-priming in vitro.
The mode of IC internalization (endocytosis or phagocytosis) in DCs is still unclear. However, our results exclude the possibility that the efficient cross-priming observed with ICs is due to FcγR-independent phagocytosis. Indeed, in DCs from γ chain−/− mice, MHC class I–restricted presentation was not observed, demonstrating that the DCs (and the FcγRs they express) and not the eventual particulate form of the antigen are determinant for cross-priming with ICs.
FcγR-mediated cross-priming is TAP dependent and sensitive to the proteasome inhibitor lactacystin, suggesting that IgG-complexed antigens are transferred from endocytic compartments into the cytosol. Although the mechanism of this transfer is still unclear, it was shown previously that macropinocytosis results in increased antigen delivery to the cytosol (40). After IC internalization at low antigen concentrations, we observed antigen transfer to the cytosol by immunofluorescence and confocal microscopy (Rodriguez, A., unpublished results). However, the efficient cross-priming observed in DCs after IC internalization was not due to an overall effect of FcγR engagement, since simultaneous engagement of FcγRs by irrelevant ICs did not increase the efficiency of cross-priming with soluble OVA internalized by fluid phase (Fig. 2) or its transfer to the cytosol (not shown). This observation also suggests that, to be transferred into the cytosol after internalization, antigens need to be targeted by FcγRs to a particular population of endosomes or lysosomes.
In contrast, IC internalization in other cell types expressing endocytosis-competent FcγRs, like macrophages or transfected B lymphoma cells, did not result in MHC class I–restricted presentation. The molecular bases of this DC specificity are still unclear. They are certainly not related to the ability of FcγRs to mediate IC internalization or cell activation, which are both efficient in macrophages or transfected B lymphoma cells. In contrast, we found that antigen transfer to the cytosol is inefficient in these two cell types compared with DCs (Rodriguez, A., unpublished results). Therefore, the specificity of DCs for cross-priming might be related to a selective ability of DCs to deliver antigen from endosomes or lysosomes into the cytosol.
The other major effect of FcγR engagement in DCs is induction of maturation. Indeed, all of the phenotypical, morphological, and functional modifications caused by inflammatory cytokines or LPS were also induced by ICs. In addition, none of these modifications were observed with DCs from γ chain−/− mice, demonstrating the implication of the γ chain in the induction of DC maturation by ICs. We found here that mouse D1 cells (as well as BM-DCs) express the two γ chain–dependent FcγRs, FcγRI (CD64) and FcγRIII (CD16). It is not clear to date which of these two receptors is required for DC maturation, but it will be directly addressed using FcγRI and/or FcγRIII−/− mice.
Whether FcγRI or FcγRIII is used, the involvement of the FcγR-associated γ chain in DC maturation indicates that an ITAM-bearing receptor triggers DC activation. FcγR cross-linking causes activation of protein tyrosine kinases (PTKs) from the src family (17). These PTKs phosphorylate tyrosine residues in the ITAM, thus inducing association to syk PTK, leading to Ca2+ release from intracellular stocks and to a wide variety of biological responses. Our observation represents the first evidence of induction of DC activation and maturation through an ITAM-containing receptor. Interestingly, the γ chain ITAM also bears an internalization signal that mediates endocytosis of FcγRIII (20), and more recently, we showed that the ITAM also determines γ chain–mediated transport from endosomes to lysosomes (41). Thus, FcγRs may initiate DC maturation and simultaneously target antigen to the appropriate endocytic compartment, where peptides are loaded onto MHC class II and from where antigens are transferred into the cytosol.
Indeed, CD4+ T cells play an important role in antiinfectious CD8+ T cell–mediated responses, even if they are dispensable in some of them (42–44). The antigen presentation on both MHC class I and class II molecules that we observed after IC internalization by DCs would ensure an optimal stimulation of both CD4+ and CD8+ T cells. At the end of primary responses and in the course of secondary immune responses, the production of specific antibodies induces formation of complexes between antigens derived from infected cells or tumor cells and specific IgGs. These complexes could be taken up by FcγRs on DCs. After internalizing ICs, DCs would then present the antigen to specific CD4 T cells, which activate them through interactions implicating costimulatory molecules like CD40-CD40L and convert them into DCs capable of priming CD8+ T cells (7–10). However, since FcγR engagement also induces efficient maturation, IC-activated DCs could potentially prime CD8+ T cells directly, bypassing cognate CD4+ T cell help. This mechanism could operate in certain antiviral and/or antitumoral immune responses. However, it could also induce inappropriate CTL responses, since the absence of CD4+ T cells would not allow a control of specificity, i.e., a “double check.”
In what physiological situation might ICs trigger CTL responses? Specific antibodies, which may potentially form ICs, are produced during most immune responses, including those where the final effectors are CTLs. ICs may participate in DC-mediated CD8+ T cell priming in the case of secondary immune responses, when specific IgGs may be produced very rapidly. In the case of ongoing immune responses, which in many cases correspond to situations of immunosuppression, such as chronic infections or tumors, cross-priming through ICs may contribute to the establishment of specific tolerance. ICs have also been reported to play critical roles in several autoimmune diseases. Amplification of anti-self CTL responses by DCs that have acquired autoantigens from ICs may contribute to the triggering or development of autoimmune pathologies.
Acknowledgments
We thank S. Viel for technical assistance; N. Shastri (Berkeley, CA) and C. Watts (Dundee, UK) for the anti-OVA T cell hybridoma B3Z, and C. Watts for providing us with OVA; J.-C. Guery (Toulouse, France) for the anti-OVA T cell hybridoma BO97.10; and Dr. A. Sarukhan (Necker Institute), Dr. P. Pereira (Institut Pasteur), and Dr. P. Benaroch (Institut Curie) for helpful discussions and critical comments on the manuscript.
This work was supported by grants from Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique, and Ligue Nationale Contre le Cancer. A. Regnault is funded by the Ligue Nationale Contre le Cancer, C. Théry by the Société de Secours des Amis des Sciences, and A. Rodriguez by the TMR Fellowship from the EEC.
Abbreviations used in this paper
- BM-DC
bone marrow–derived DC
- CHX
cycloheximide
- DC
dendritic cell
- FcγR-ctγ
chimeric receptor composed of the lumenal and transmembrane domains of FcγRII and the cytoplasmic tail of the γ chain
- HEL
hen egg lysozyme
- IC
immune complex
- ITAM
immunoreceptor tyrosine-based activation motif
- LLnL
aldehyde N-acetyl-Leu-l-Leu-l-norleucinal
- OVA-IC
OVA-containing IC
- PTK
protein tyrosine kinase
- TAP
transporter associated with antigen processing
- wt
wild-type
References
- 1.Townsend A, Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- 2.Huang AYC, Golumbeck P, Ahmadzadeh M, Jaffee A, Pardoll DM, Levitsky HI. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 3.Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in cytotoxic assay. J Exp Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 5.Hart DNJ. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–3287. [PubMed] [Google Scholar]
- 6.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 7.Bennett SRM, Carbone FR, Karamalis F, Miller JFAP, Heath WR. Induction of a CD8+ cytotoxic lymphocyte response by cross-priming requires cognate CD4+T cell help. J Exp Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett SRM, Carbone FR, Karamalis F, Flavell RA, Miller JFAP, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 9.Ridge JP, DiRosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 10.Schoenberger SP, Toes REM, van der Voort EIH, Offringa R, Melief CJM. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 11.Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol. 1997;15:821–850. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- 12.Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 13.Reis e Sousa C, Germain RN. Major histocompatibility complex class I presentation of peptides derived from soluble exogenous antigen by a subset of cells engaged in phagocytosis. J Exp Med. 1995;182:841–851. doi: 10.1084/jem.182.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfeifer JD, Wick MJ, Roberts RL, Finlay K, Normark SJ, Harding CV. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 15.Rescigno M, Citterio S, Théry C, Rittig M, Medaglini D, Pozzi G, Amigorena S, Ricciardi-Castagnoli P. Bacteria-induced neo-biosynthesis, stabilization, and surface expression of functional class I molecules in mouse dendritic cells. Proc Natl Acad Sci USA. 1998;95:5229–5234. doi: 10.1073/pnas.95.9.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lanzavecchia A. Mechanisms of antigen uptake for presentation. Curr Opin Immunol. 1996;8:348–354. doi: 10.1016/s0952-7915(96)80124-5. [DOI] [PubMed] [Google Scholar]
- 17.Ravetch JV. Fc receptors: rubor redux. Cell. 1994;78:553–560. doi: 10.1016/0092-8674(94)90521-5. [DOI] [PubMed] [Google Scholar]
- 18.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin-4 and downregulated by tumor necrosis factor α. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fanger NA, Voigtlaender D, Liu C, Swink S, Wardwell K, Fisher J, Graziano RF, Pfefferkorn LC, Guyre PM. Characterization of expression, cytokine regulation, and effector function of the high affinity IgG receptor Fc gamma RI (CD64) expressed on human blood dendritic cells. J Immunol. 1997;158:3090–3098. [PubMed] [Google Scholar]
- 20.Amigorena S, Salamero J, Davoust J, Fridman WH, Bonnerot C. Tyrosine-containing motif that transduces cell activation signals also determines internalization and antigen presentation via type III receptors for IgG. Nature. 1992;358:337–341. doi: 10.1038/358337a0. [DOI] [PubMed] [Google Scholar]
- 21.Bonnerot C, Amigorena S, Choquet D, Pavlovich R, Choukroun V, Fridman WH. Role of associated gamma-chain in tyrosine kinase activation via murine Fc gamma RIII. EMBO (Eur Mol Biol Organ) J. 1992;11:2747–2757. doi: 10.1002/j.1460-2075.1992.tb05340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Vugt MJ, Heijnen AF, Capel PJ, Park SY, Ra C, Saito T, Verbeek JS, van de Winkel JG. FcR gamma-chain is essential for both surface expression and function of human Fc gamma RI (CD64) in vivo. Blood. 1996;87:3593–3599. [PubMed] [Google Scholar]
- 23.Van Kaer L, Ashton-Rickardt PG, Ploegh HL, Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules and CD4-8+ T cells. Cell. 1992;71:1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 24.Winzler C, Rovere P, Rescigno M, Granucci F, Penna G, Adorini L, Zimmermann VS, Davoust J, Ricciardi-Castagnoli P. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J Exp Med. 1997;185:317–328. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanderson S, Shastri N. Lac Z inducible, antigen/MHC-specific T cell hybrids. Int Immunol. 1994;6:369–376. doi: 10.1093/intimm/6.3.369. [DOI] [PubMed] [Google Scholar]
- 26.Guery JC, Ria F, Adorini L. Dendritic cells but not B cells present complexes to MHC class II–restricted T cells after administration of protein in adjuvant. J Exp Med. 1996;183:751–757. doi: 10.1084/jem.183.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harper M, Lema F, Boulot G, Poljack RJ. Antigen specificity and cross-reactivity of monoclonal anti-lysozyme antibodies. Mol Immunol. 1987;2:97–108. doi: 10.1016/0161-5890(87)90081-2. [DOI] [PubMed] [Google Scholar]
- 28.Wang M, Stepkowski SM, Hebert JS, Tian L, Yu J, Kahan BD. Nucleotide sequences of three H-2K and three H-2D cDNA clones coding mouse class I MHC heavy chain proteins. Ann Transplant. 1996;1:26–31. [PubMed] [Google Scholar]
- 29.Latour S, Fridman WH, Daeron M. Identification, molecular cloning, biologic properties, and tissue distribution of a novel isoform of murine low-affinity IgG receptor homologous to human Fc gamma RIIB1. J Immunol. 1996;157:189–197. [PubMed] [Google Scholar]
- 30.Ravetch JV, Kinet J-P. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 31.Pierre P, Turley SJ, Gatti E, Hull M, Meltzer J, Mirza A, Inaba K, Steinman RM, Mellman I. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 32.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 33.Amigorena S, Bonnerot C. Role of B-cell and Fc receptors in the selection of T-cell epitopes. Curr Opin Immunol. 1998;10:88–92. doi: 10.1016/s0952-7915(98)80037-x. [DOI] [PubMed] [Google Scholar]
- 34.Rock KL, Gamble S, Rothstein L. Presentation of exogenous antigen with class I major histocompatibility complex molecules. Science. 1990;249:918–921. doi: 10.1126/science.2392683. [DOI] [PubMed] [Google Scholar]
- 35.Norbury CC, Chambers BJ, Prescott AR, Ljunggren H-G, Watts C. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur J Immunol. 1997;27:280–288. doi: 10.1002/eji.1830270141. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell DA, Nair SK, Gilboa E. Dendritic cell/macrophage precursors capture exogenous antigen for MHC class I presentation by dendritic cells. Eur J Immunol. 1998;28:1923–1933. doi: 10.1002/(SICI)1521-4141(199806)28:06<1923::AID-IMMU1923>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 37.Steinman RM, Swanson J. The endocytic activity of dendritic cells. J Exp Med. 1995;182:283–288. doi: 10.1084/jem.182.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ, Schreiber SL. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 39.Amigorena S, Bonnerot C, Drake JR, Choquet D, Hunziker W, Guillet JG, Webster P, Sautes C, Mellman I, Fridman WH. Cytoplasmic domain heterogeneity and functions of IgG Fc receptors in B lymphocytes. Science. 1992;256:1808–1812. doi: 10.1126/science.1535455. [DOI] [PubMed] [Google Scholar]
- 40.Norbury CC, Hewlett LJ, Prescott AR, Shastri N, Watts C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity. 1995;3:783–791. doi: 10.1016/1074-7613(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 41.Bonnerot C, Briken V, Brachet V, Lankar D, Cassard S, Jabri B, Amigorena S. Syk protein tyrosine kinase regulates Fc receptor γ chain transport to lysosomes. EMBO (Eur Mol Biol Organ) J. 1998;17:101–111. doi: 10.1093/emboj/17.16.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keene JA, Forman J. Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J Exp Med. 1982;155:768–782. doi: 10.1084/jem.155.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. Progressive loss of CD8+ T cell–mediated control of γ-herpesvirus in the absence of CD4+T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Herrath MG, Yokoyama M, Dockter J, Oldstone MBA, Whitton JL. CD4-deficient mice have reduced levels of memory cytotoxic T lymphocytes after immunization and show diminished resistance to subsequent virus challenge. J Virol. 1996;70:1072–1079. doi: 10.1128/jvi.70.2.1072-1079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]