Abstract
Lentiviral vectors have been advocated to be effective vehicles for the delivery and stable expression of genes in nondividing primary cells. However, certain cell types, such as resting T lymphocytes, are resistant to infection with HIV-1. Establishing parameters for stable gene delivery into primary human lymphocytes and approaches to overcome the resistance of resting T cells to HIV infection may permit potential gene therapy applications, genetic studies of primary cells in vitro, and a better understanding of the stages of the lentiviral life cycle. Here we demonstrate that an HIV-1–derived vector can be used for stable delivery of genes into activated human T cells as well as natural killer and dendritic cells. Remarkably, a sizeable fraction of resting T cells was stably transduced with the HIV-1 vector when cultured with the cytokine interleukin (IL)-2, IL-4, IL-7, or IL-15, or, at a lower level, with IL-6, in the absence of any other stimuli. Resting T cells stimulated with these cytokines could also be infected with replication-competent HIV-1. To test the utility of this system for performing structure–function analysis in primary T cells, we introduced wild-type as well as a mutant form of murine CD28 into human T cells and showed a requirement for the CD28 cytoplasmic domain in costimulatory signaling. The ability to stably express genes of interest in primary T cells will be a valuable tool for genetic and structure–function studies that previously have been limited to transformed cell lines. In addition, the finding that cytokine signals are sufficient to permit transduction of resting T cells with HIV may be relevant for understanding mechanism of HIV-1 transmission and pathogenesis.
Keywords: HIV, lentiviral vectors, resting T cells, cytokines, costimulation
Retroviruses have the ability to integrate their genetic material into target cell genomes and thus may be especially useful for delivering genes into primary cells for the purpose of somatic gene therapy (1–3). Retroviral vectors derived from oncoretroviruses such as murine leukemia virus (MLV)1 integrate strictly in cycling cells, due to an apparent inability to translocate the viral genome across an intact nuclear envelope (4–7). In contrast, lentiviruses such as HIV-1 can integrate their genetic material into the genome of nonproliferating cells such as macrophages or cell cycle– arrested transformed cell lines (7–10). The ability of HIV-1 to transduce nonmitotic cells has been ascribed to three karyophilic virion proteins, Integrase, Matrix, and Vpr, each of which can interact with the nuclear import machinery to transport the HIV-1 preintegration complex into the nucleus (11–17). However, resting T cells are not susceptible to productive infection with HIV-1. Although virus can enter into these cells, integration into the host cell genome is not observed (18–25).
Vectors derived from the HIV-1 genome recently have been shown to transduce terminally differentiated nondividing cells, both in vitro and in vivo, enabling long-term expression of transduced gene products (26–33). These vectors were engineered to be replication-incompetent by deletion of the envelope glycoprotein gene (env) and the accessory genes vif, vpr, vpu, and nef. A heterologous envelope glycoprotein expressed in trans is used for production of pseudotyped viral particles. The vesicular stomatitis virus glycoprotein (VSV-G) has been used widely in pseudotyping HIV particles because of its broad host range and the ability of the pseudotyped particles to be concentrated by ultracentrifugation without significant loss of infectivity (34–36).
In addition to their potential applications for therapeutic delivery of genes into primary human cells, lentiviral vectors may have other practical applications. Stable introduction of exogenous wild-type or mutant genes into primary cells such as T lymphocytes would be particularly valuable for dissecting intracellular signaling pathways in these cells, because this area of investigation thus far has been confined to transformed cell lines. Genetic manipulation of resting T cells in vitro could also provide novel approaches for studying their activation and maturation requirements. Moreover, these vector systems would be valuable in dissecting the cellular requirements for the HIV-1 life cycle, such as integration or transcription in primary T cells.
Toward these objectives, we have used a replication- defective, multiply attenuated HIV-1 vector system that can very efficiently and stably transduce preactivated primary T lymphocytes and myeloid lineage cells. Remarkably, resting human T cells of both naive and memory subsets were also stably transduced with HIV when stimulated with IL-2, IL-4, IL-6, IL-7, or IL-15. Moreover, resting T cells treated with cytokines were rendered permissive to infection with replication-competent HIV-1. To demonstrate the feasibility of performing structure–function studies with primary T cells, we have used this vector system to show that wild-type murine CD28, but not the cytoplasmically truncated form, can transmit costimulatory signals in transduced primary human T cells. Our results show that this HIV vector system can be used for genetic manipulation of primary T lymphocytes and suggest that cytokine signals may play an important role in HIV-1 infection of resting T cells in vivo.
Materials and Methods
Plasmids and DNA Constructions.
The modified HIV-1 transduction vector was derived from pHIV-PLAP, which has a backbone of the HIV-1 NL4-3 provirus with a frameshift in env and substitution of the human placental alkaline phosphatase (PLAP) gene in place of the nef gene (37). This vector was further manipulated to remove vif, vpr, vpu, and env genes as previously described (38). Genes of interest were introduced between the 5′ NotI and 3′ XhoI sites within the nef open reading frame, thus replacing the PLAP cDNA. An additional EcoRI site was introduced 3′ of the NotI site to facilitate subcloning of different cDNAs. The murine (m)CD28 cDNA (gift of J. Allison, University of California, Berkeley, CA) was subcloned into pBluescript (Stratagene) and the cytoplasmic mutant version was prepared by site-directed mutagenesis techniques through overlap PCR amplification using mutated oligonucleotides as previously described (39). All constructs were verified by DNA sequencing and were subcloned into the HIV vector. The mCD4 cDNA has been described previously (40). The enhanced green fluorescent protein (EGFP), purchased from Clontech, was subcloned into the HIV vector by introducing 5′ NotI and 3′ XhoI sites through PCR amplification. A bicistronic construct was prepared by ligation of the EGFP to the 3′ end of the encephalomyocarditis virus internal ribosomal entry site (IRES) sequence (41) and was similarly subcloned into the EcoRI-XhoI sites within the HIV vector. A GFP-encoding, replication-competent HIV-1 provirus was constructed by subcloning EGFP in place of the nef gene in the R5-tropic HIV-1 (BAL) backbone. The EGFP was also subcloned into the MLV-derived vector, pMX, provided by Dr. Toshio Kitamura (Tokyo University, Tokyo, Japan).
Preparation of Primary Human T Cell Lines.
PBMCs were separated from buffy coats of healthy donors (New York Blood Bank) through Ficoll-Hypaque (Pharmacia, Sweden). Monocytes were removed by plastic adherence for 2 h at 37°C. To obtain highly purified resting CD4+ and CD8+ T cells, PBMCs depleted of monocytes were incubated with anti-CD4 or anti-CD8 conjugated with Dynabeads (Dynal, Norway) at a 1:4 target/bead ratio. After 30 min of incubation at 4°C and continuous shaking, the bead-bound cells were recovered using a magnet (Dynal). The bead-bound cells were then washed at least four times to remove unbound cells and the CD4+ or CD8+ cells were detached from the beads using Detachabead (Dynal) according to the manufacturer's instructions. These cells were incubated with anti– HLA-DR and anti-CD69 antibodies, and then by Dynabeads conjugated with goat anti–mouse IgG, followed by magnetic removal of bead-bound preactivated cells. This purification protocol typically resulted in 99.5% purity of positively selected cells, as determined by postpurification FACS® analysis. In some experiments, CD4 cells were negatively sorted into CD45RA+RO− and CD45RO+RA− subsets by incubating with anti-CD45RO and anti-CD45RA antibodies, respectively, followed by anti–mouse IgG Dynabead separation as described above. B and NK cells were purified by pre-incubating the cells with purified anti-CD19 (Becton Dickinson) or hybridoma supernatants of anti–human (h)CD16 (American Type Culture Collection [ATCC]), respectively, followed by magnetic separation using Dynabead-conjugated goat anti–mouse antibody. Bead-conjugated cells were then cultured at 37°C for 2 h and separated from released beads by a magnet (Dynal). The culture media used in all experiments was RPMI 1640 (GIBCO BRL) supplemented with 10% FCS (Hyclone), penicillin (50 U/ml; GIBCO BRL), streptomycin (50 μg/ml), sodium pyruvate (1 mM; GIBCO BRL), and glutamine (2 mM; GIBCO BRL). T cell lines were prepared by activation of purified resting T cells with allogeneic PBMCs, treated with 50 μg/ml mitomycin C (Sigma Chemical Co.) for 30 min at 37°C and 5 μg/ml PHA (Sigma Chemical Co.). Alternatively, T cells were activated by cross-linking with plate-bound anti-CD3 antibody (OKT-3) and soluble anti-hCD28 antibody (PharMingen). Cells were split 3 d after activation, expanded, and maintained in culture media supplemented with 200 U/ml recombinant IL-2 (Chiron). Culture of T cells with cytokine combinations has been described previously (42, 43). All cytokines were purchased from Genzyme or R&D Systems. Macrophages were differentiated in vitro after adherence to plastic. Dendritic cells (DCs) were generated as previously described (44). Activation of human B cells was performed by noncognate stimulation with activated T cells as previously described (42). The EBV-transformed B cell line was generated by infection of PBMCs with supernatants from the B95-8 cell line (ATCC) in the presence of 1 μg/ml Cyclosporin A (Sigma Chemical Co.).
Virus Production and Infections.
Replication-incompetent HIV particles pseudotyped with VSV-G were generated by calcium phosphate transfection of HEK-293T cells (ATCC) with 20 μg of proviral HIV vector and 12 μg of pL-VSV-G plasmid (36) per 3 × 106 cells seeded on 10-cm plates. The transfection media was replaced after 8 h with fresh DMEM supplemented with 10% FCS. Supernatants were collected at 48 h after transfection. The virus-containing supernatants were centrifuged for 10 min at 1,200 rpm to remove cells, then passed through 0.4-μm filters to remove fine debris. Supernatants either were used immediately for infections or were frozen in aliquots at −80°C. The viral titers were determined by infection of the human T cell line Hut78 with serially diluted virus supernatant. Typically, viral titers had a range of 3–10 × 106 infectious units (ifu)/ml. T cells were infected at a multiplicity of infection (MOI) of 10–20 in 24-well plates in the presence of 10 μg/ml polybrene (Sigma Chemical Co.). After an additional day of culture with virus supernatants, cells were washed or sedimented through Ficoll and resuspended in fresh culture media. R5-tropic replication-competent viruses were prepared similarly by transfecting 293T cells, and titers of 2–5 × 105 ifu/ml were obtained. VSV-G–pseudotyped MLV-based viruses were similarly prepared by transfecting 293T cells with 12 μg each of pMX.EGFP, pJK3 (expressing MLV gag and pol genes), and pL-VSV-G as well as 3 μg of pCMV-Tat plasmids.
T Cell Costimulation.
Flat-bottomed 96-well microtiter plates were coated for 2 h at 37°C with goat anti–mouse IgG (Caltag) at 20 μg/ml. Plates were washed twice with PBS and coated with anti-CD3 (OKT3, purified from ascites; ATCC) at different concentrations for 2 h at 37°C. Wells were washed an additional two times with PBS, and T cells at 105 cells/well were added to plates. Purified anti-mCD28 or anti-hCD28 antibodies (PharMingen) were added to cultures at 1 μg/ml in a 150 μl final volume. After 48–64 h, cells were pulsed for 16 h with 1 μCi of [3H]thymidine, and [3H]thymidine incorporation was measured with a beta counter (Wallac). To determine IL-2 production, culture supernatants were collected 36 h after initiation of stimulation and IL-2 activity was assayed using the CTLL-2 indicator cell line (obtained from ATCC). The proliferation of CTLL cells were determined by [3H]thymidine incorporation for the last 4 h of a 24-h culture.
Cell Staining and Bromodeoxyuridine Incorporation.
Cells were stained with the relevant antibody on ice for 30 min in PBS buffer with 2% FCS and 0.1% sodium azide. Staining or GFP expression was analyzed on a FACScan® using the CellQuest software (Becton Dickinson). Live cells were gated based on forward and side scatter. Cell sorting was performed at the New York University Medical Center core facility using a Coulter fluorescence-activated cell sorter. The following antibodies with PE, FITC, TC, or PercP conjugations were used for staining: anti-mCD28, anti-mCD4, anti-hCCR5 and anti-hCXCR4 (PharMingen), anti-hCD4 and -hCD45RO (Caltag), anti-hCD3, -hCD8, -hCD14, -hCD16, -hCD19, -hCD25, -hCD56, -hCD69, -hCD45RA, and -hHLA-DR (all from Becton Dickinson). Bromodeoxyuridine (BrdU) incorporation was performed by culturing the cells with 10 μM BrdU (Sigma Chemical Co.) for the indicated time. After washing in staining buffer, the cells were permeabilized using FACS® permeabilizing solution (Becton Dickinson) and stained with anti-BrdU–PE (PharMingen) in the presence of DNAse I (Sigma Chemical Co.). Analyses were performed using a FACScan®.
Results
Stable Gene Transduction into Human Lymphocytes using an HIV-1–derived Vector System.
To achieve stable gene expression in primary human T cells using an HIV-1–derived vector (HDV) system, we used an NL4-3–based HIV-1 provirus that has deletions in the envelope glycoprotein (env) gene and the accessory genes vif, vpr, vpu, and nef (38). The GFP gene was subcloned in place of the nef open reading frame, and this vector was pseudotyped with the VSV-G envelope to produce a high titer, replication-incompetent virus. To determine the efficiency and stability of HIV-1–mediated gene transduction, mitogen-activated T cells were challenged with HDV-EGFP viruses at an MOI of 10–20. After 3 d in IL-2–containing medium, during which time the cells continued to expand, the majority of the T cells (∼65%) expressed GFP (Fig. 1 A). The GFP+ cells were then sorted by a FACS® and subsequently expanded in IL-2 with bimonthly PHA stimulation to determine the stability of expression of the transduced gene. High levels of GFP expression were detected in T cell lines that were expanded in culture for up to 4 mo (Fig. 1 B). The expression of the introduced gene was also stable in individual T cell clones that were established from HDV– transduced T cell lines (data not shown). Furthermore, it was possible to introduce (sequentially or simultaneously) three different genes (GFP, mCD28, and mCD4) into the same cells while maintaining their long-term expression (data not shown). Therefore, it is feasible to exploit the HDV system to express multiple gene products within a given primary T cell population.
Figure 1.
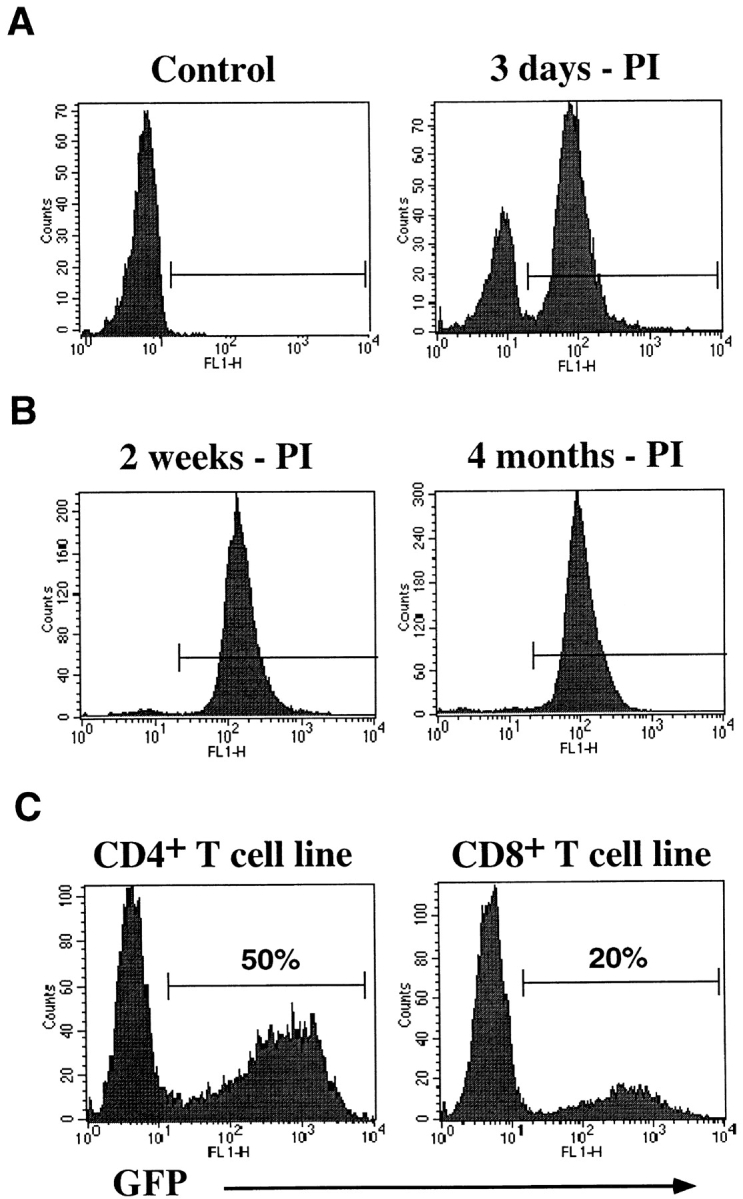
Stable delivery and expression of genes in activated human T cell subsets through the HDV system. Purified T cells were activated with PHA and allogeneic feeder cells for 4 d and transduced with the HDV system. (A) Activated primary hCD4+ T cells were infected with HDV-EGFP viruses and GFP expression was analyzed by a FACS® 3 d after infection. (B) GFP+ T cells were sorted and expanded in culture with repeated mitogen stimulation and expression was assessed 2 wk and 4 mo after infection. (C) GFP expression in CD4+ and CD8+ T cell lines 5 d after HDV-IRES.EGFP infection. PI, post infection.
We next assessed the efficiency of HDV-mediated gene transduction into different subsets of T cells. To increase the sensitivity of detecting transduced cells, we constructed a modified vector, HDV-IRES.EGFP. Both spliced and unspliced transcripts encoded by this vector permit efficient translation of EGFP through the IRES, resulting in up to threefold higher expression levels (data not shown). CD4+ and CD8+ T cell subsets were purified from PBMCs, activated with PHA, and infected with HDV-IRES.EGFP virus. Analyses of GFP expression 5 d after infection consistently demonstrated a higher transduction efficiency (indicated as percentage of GFP expressing cells) of activated CD4+ T cells relative to CD8+ cells (Fig. 1 C). Similar results were observed in separate experiments with other reporter genes and cells from different donors (data not shown).
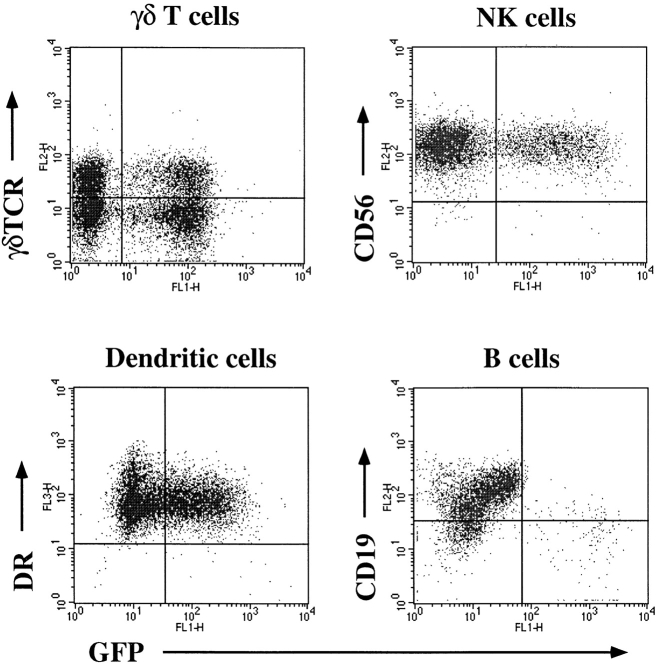
Transduction efficiencies of other subsets of lymphoid and myeloid lineage cells were also examined. Activated γ/δ-TCR+ T cells, IL-2–activated NK cells, and in vitro– derived DCs were efficiently and stably transduced as shown by GFP expression 2–3 wk after infection with HDV-IRES.EGFP virus. However, we were unable to detect transduction of resting B cells, B cells stimulated through noncognate interaction with activated T cells, or B cells cultured with IL-4, IL-2, or IL-6 (Fig. 2 and data not shown). Interestingly, EBV-transformed B cells were susceptible to infection albeit at a lower efficiency than activated T cells (∼20% of cells were transduced at comparable MOI, data not shown). Freshly isolated monocytes were also resistant to transduction using the HDV system but in vitro differentiation into macrophages rendered them susceptible (data not shown).
Figure 2.
Transduction of myeloid cells and lymphocytes other than α/β T cells. To enrich for γ/δ+ T cells, α/β+ T cells were depleted by bead sorting and PHA lines were established as described in Materials and Methods. NK cells and B cells were positively sorted using antibodies to CD16 and CD19 respectively. NK cells were activated with 1,000 U/ml IL-2. B cells were activated as described in Materials and Methods. DC cultures were established as described by Sallusto and Lanzavecchia (44). Lymphocyte infections were done as described in Materials and Methods, whereas macrophages and DCs were infected in the absence of polybrene and at a lower MOI to prevent cytotoxicity induced by the viral supernatants. GFP was analyzed 2 wk after infection for γ/δ T cells and NK cells (gated on CD3−CD56+ cells), and 5 d after infection for B cells. Quadrants were set according to uninfected control stainings. DC infections were assessed 10 d after infection; cells were stained with PE-conjugated anti-CD14 and PercP-conjugated HLA-DR; and GFP expression was determined after gating on CD14− cells.
Cytokine Stimulation of Resting T Cells Permits Infection with HIV.
It is well established that HIV challenge of quiescent T cells is blocked before completion of reverse transcription (22). We have shown that TCR-activated T cells are very efficiently and stably transduced with the HDV system. However, for many experimental applications it would be desirable to transduce resting T cells in the absence of TCR-induced activation, without affecting their differentiation state. To determine the parameters of resting T cell infection, CD4+ and CD8+ T cells were purified from PBMCs that were also negatively sorted for the activation markers HLA-DR and CD69. The purity of sorted cells was 99.5% for CD4+ T cells and 95–98% for CD8+ T cells, as determined by FACS® analyses. The purified cells were cultured overnight and the resting status of these cells was confirmed by the negligible expression levels of activation molecules (HLA-DR and CD69) and the lack of cycling cells (data not shown). These cells were then infected with HDV-IRES.EGFP at an MOI of ∼20. As expected, we found very few T cells expressing GFP 5 d after infection (<0.3%), and concluded that freshly isolated resting T cells are resistant to transduction with the HDV system.
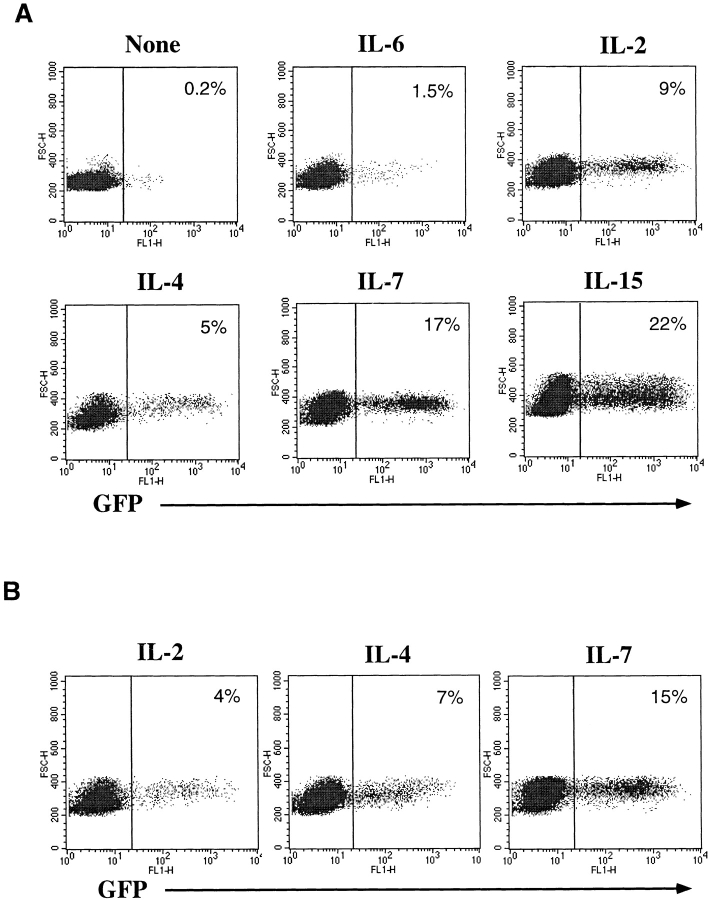
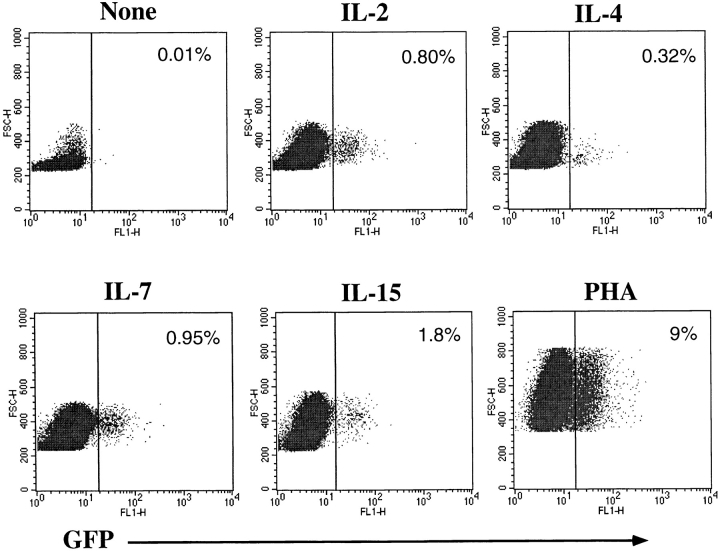
Certain cytokines, such as IL-2, IL-4, IL-7, or proinflammatory cytokines, such as TNF-α or IL-6, have been shown to potentiate HIV replication in vitro (45, 46). Some of these cytokines (IL-2, IL-4, and IL-7) also promote long-term survival of resting T cells in vitro, while maintaining the maturational state of these cells (43, 47). We hypothesized that the signals delivered through cytokine receptors alone may cause resting naive or memory T cells to overcome the resistance to HDV-mediated transduction. To examine this possibility, CD4+ T cells were cultured in the presence of various cytokines (Fig. 3) or in media alone for 4 d before challenge with HDV-IRES-EGFP. 5 d after infection, robust GFP expression was observed in CD4+ cells that were cultured in IL-2, IL-4, IL-7, or IL-15, and a lower but significant enhancement was detected in the presence of IL-6 (Fig. 3 A), but none with TNF-α, IL-12, or IFN-γ (data not shown).
Figure 3.
HDV-mediated transduction of both naive and memory resting T cells cultured in the presence of various cytokines. Purified resting CD4+ T cells were cultured for 4 d in media alone or with the following cytokines: IL-6 (400 U/ml), IL-2 (200 U/ml), IL-4 (20 ng/ml), IL-7 (20 ng/ml), or IL-15 (10 ng/ml). Cells were infected with HDV-IRES.GFP in the presence of cytokines for an additional 5 d and GFP expression was assessed. (A) GFP expression in resting T cells transduced in the presence of cytokines. (B) GFP expression in CD45RA+RO−CD4+ purified naive T cells transduced with HDV-IRES.EGFP.
CD4+ human T cells can be divided into naive and memory subsets based on expression of RA and RO isoforms, respectively, of the CD45 molecule (48). These subsets are phenotypically and functionally distinct and have different activation requirements for antigen-specific stimulation (48). In contrast to memory T cells, naive cells exhibit few effector functions (e.g., cytokine production), are less susceptible to activation induced cell death, and display more robust proliferation in response to TCR-mediated signals. To assess transduction efficiencies of naive versus memory subsets of resting T cells stimulated with cytokines, we purified naive T cells by removal of CD45RO+ T cells using bead selection. The purified population (100% RA+, 98% RO−) was prestimulated with IL-2, IL-4, or IL-7 and challenged with HDV-IRES.EGFP in the presence of the same cytokines. Effective transduction of naive T cells cultured with IL-4, IL-7, and, to a lesser extent, IL-2, was observed (Fig. 3 B). In contrast, memory T cells were more efficiently transduced when stimulated with IL-2 and IL-15 (see below and data not shown). Naive T cells cultured in media alone did not display any GFP expression upon infection (data not shown).
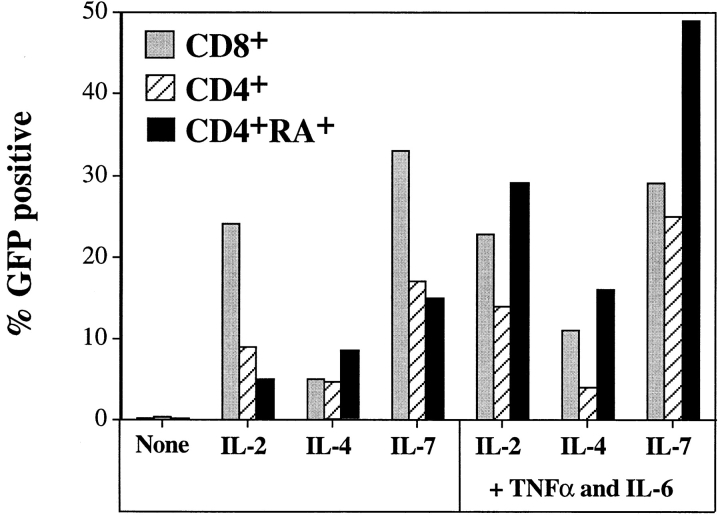
Transduction of resting CD8+ cells was also achieved in the presence of the same cytokines at similar or better efficiencies than that observed with resting CD4+ cells (Fig. 4). Interestingly, this result contrasts those observed with transduction of mitogen-activated CD8+ T cells, which were less efficiently transduced than CD4+ T cell lines (see Fig. 2 C). It has been shown that TNF-α and IL-6, in the presence of IL-2, IL-4, or IL-7, can fully activate resting T cells in an antigen-independent manner (42, 43). These cytokine combinations had a small effect in increasing transduction efficiencies of CD4+ or CD8+ T cells, but greatly boosted infection of the naive subset (Fig. 4).
Figure 4.
Transduction efficiency of CD4+ and CD8+ resting T cells stimulated with individual or combinations of cytokines. Resting T cells were cultured and transduced with HDV-IRES.GFP as described in Fig. 3, with individual cytokines or in cytokine combinations (TNF-α was used at 50 ng/ml). Percentage of GFP+ cells was determined by FACS® analysis 5 d after infection.
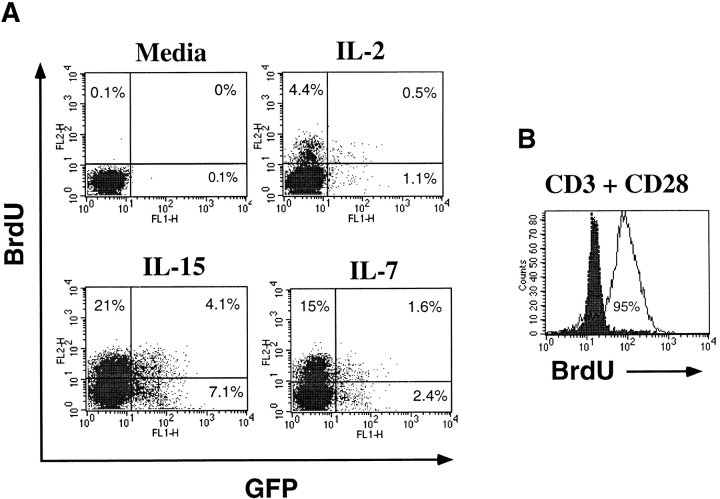
The activation status of resting CD4+ T cells stimulated with individual cytokines was determined by staining infected cells with antibodies specific for activation markers. In each condition, >95% of GFP+ cells were negative for the activation molecules CD69 or HLA-DR (data not shown). Continuous BrdU labeling of cytokine-treated resting cells for 8 d revealed that a total of 5–7% of cells treated with IL-2 or IL-4 and ∼15% cells treated with IL-7 or IL-15 had entered the cell cycle during this period (data not shown). We then wished to determine whether transduction occurs only in the cells that have entered the cell cycle. Therefore, resting T cells were cultured continuously for 8 d with BrdU during cytokine stimulation and HIV infection. Although the presence of BrdU drastically reduced the efficiency of HIV infection (possibly due to adverse effects during reverse transcription), more than half of the cells that expressed GFP had not incorporated BrdU (Fig. 5 A). This result shows that entry into the S phase of the cell cycle is not required for infection of the cytokine-stimulated resting T cells. In contrast to cytokine stimulation, almost all of the T cells activated through the TCR incorporated BrdU after 2 d of culture (Fig. 5 B). Although few cytokine-stimulated T cells enter into the cell cycle, especially in the presence of IL-7 and IL-15, we asked whether MLV-derived vectors could also transduce these cells. Cytokine-stimulated T cells were challenged with a VSV-G–pseudotyped, GFP-expressing MLV-based vector, pMX.EGFP. 4 d after infection, <0.5% of the cytokine-stimulated T cells and ∼1.5% of the TCR-stimulated T cells expressed GFP, whereas nearly 100% of transformed human T cell line, Jurkat, were transduced (data not shown). These results clearly distinguish the efficiency of the HDV in the transduction of primary human T cells.
Figure 5.
Cell cycle entry and HDV transduction of cytokine-stimulated resting T cells. Resting T cells were cocultured in the presence of cytokines and 10 μM BrdU for 4 d and infected with HDV-EGFP for an additional 4 d while being maintained in continuous culture with fresh BrdU. T cells stimulated with anti-CD3 plus anti-CD28 antibodies were cultured, 3 d after activation, with 10 μM BrdU for 2 d. Cells were then permeabilized and stained with a PE-conjugated anti-BrdU antibody. (A) GFP expression and BrdU incorporation in cytokine-stimulated T cells. (B) BrdU staining of TCR-activated T cells.
Another important difference between cytokine-versus TCR-mediated stimulation of resting T cells is that, in contrast to TCR triggering, cytokine signals do not cause differentiation of naive T cells towards the memory phenotype (43, 49). To confirm this, we cultured HDV-IRES.EGFP– infected resting T cells for 1 mo in the presence of IL-2, IL-4, IL-7, or IL-15. These cells were then stained with CD45RO antibody to discriminate memory (RO+) and naive (RO−) T cells. The proportion of naive to memory T cells was comparable to that in the starting population (∼1:1) in all conditions, although some bias was apparent in favor of memory or naive T cells with IL-2 and IL-4, respectively (Fig. 6). The HDV-transduced naive T cells also retained their phenotype and IL-4 stimulation favored infection of these cells (Fig. 6).
Figure 6.
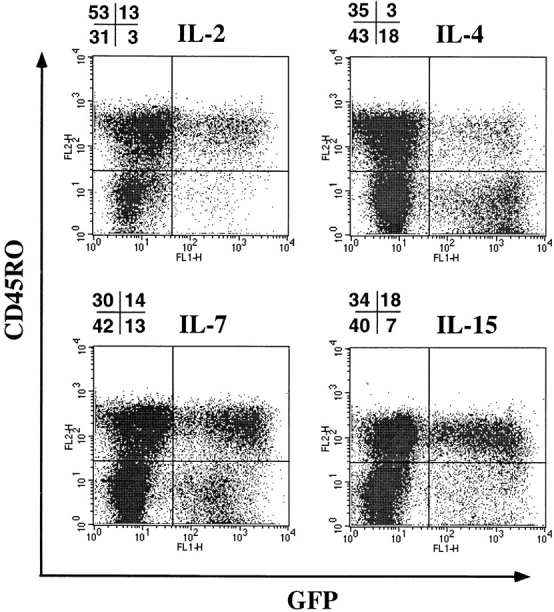
Maintenance of naive phenotype of cytokine-stimulated and HDV-EGFP–transduced resting T cells. HDV-EGFP–infected T cells were cultured for 30 d in the presence of cytokines and stained with PE-conjugated anti-CD45RO antibody. Numbers displayed above FACS® analysis profiles are the percentage of positive cells in each quadrant.
To gain insight as to the level at which cytokines influence HIV infection of T cells, we challenged these cells with HDV-IRES.EGFP in the absence of cytokines. 3 d later cells were extensively washed to remove any remaining virus and were cultured in the presence or absence of cytokines for an additional 4 d. In the absence of cytokines, there were very few GFP+ cells (<0.3%). However, addition of IL-2, IL-4, or IL-7 after infection revealed GFP expression in 1–3% of cells. We also infected resting cells in the presence of IL-2 or IL-4 plus IFN-α, which is known to block HIV infection at a preintegration step (50, 51). Indeed, IFN-α completely blocked the transduction of resting T cells cultured with IL-2 or IL-4 (data not shown). Addition of IFN-α to cultures after 4 d of transduction in the presence of cytokines did not have any effect on GFP expression, even after 10 d of culture (data not shown). Taken together, these results suggest that HIV can enter into a few resting T cells, but is arrested at a preintegration stage of the viral life cycle. Cytokine signals are likely to overcome this preintegration block.
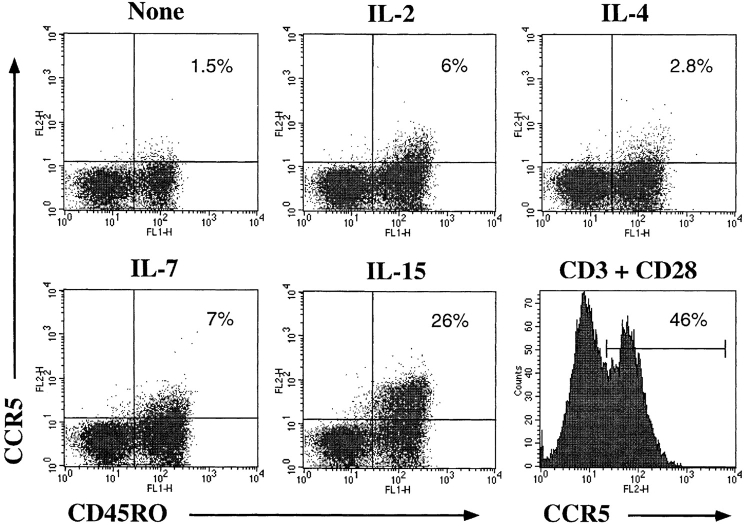
Finally, we asked whether cytokine-activated T cells can also be infected with replication-competent HIV-1, since this may be an important mode of infection of T cells in vivo and in the establishment of latency in resting cells. We used a CCR5-tropic GFP-expressing replication-competent HIV-1 to infect cytokine-stimulated resting T cells under conditions similar to those used for the HDV transduction. Infection of cytokine-stimulated resting T cells was clearly observed 4 d after challenge (Fig. 7). The GFP expression in the cytokine-treated cells remained stable for >2 wk (data not shown). However, the percentage of cells expressing GFP was lower as compared with VSV-G–pseudotyped viruses. This could be due to the relatively low titers of the R5 virus, which limit the infection to an MOI of 1–2 compared with 10–20 for VSV-G viruses. It is also possible that the infection is influenced by the expression of CCR5 on cytokine-stimulated versus TCR-activated T cells. Thus we examined CCR5 expression on resting T cells after culture in the presence of cytokines. The expression of CCR5 was modestly upregulated on IL-2 or IL-7 cultured resting T cells and more strikingly in the presence of IL-15 (Fig. 8). The TCR- mediated stimulation induced CCR5 expression in nearly half of the cells (Fig. 8). The expression of CCR5 was exclusively induced on the memory (CD45RO+) subset of T cells (Fig. 8), consistent with the expression pattern of CCR5 on PBMCs (52). The T-tropic HIV coreceptor CXCR4 is expressed on all the naive T cells and on most of the memory T cells (data not shown and reference 52); however, we also observed an approximately fivefold increase in CXCR4 expression levels in the presence of IL-4 (data not shown), similar to recent reports (53–55).
Figure 7.
Replication-competent CCR5 tropic HIV-1 can infect cytokine-activated resting T cells. Cytokine stimulation and HIV-1 infection was done as described in Fig. 4. Cells were fixed in 4% paraformaldehyde before FACS® analysis.
Figure 8.
Expression of CCR5 on cytokine- or TCR-stimulated T cells. Purified resting CD4+ T cells were cultured with cytokines as described in Fig. 3. Cells were stained with PE-conjugated anti-CCR5 and FITC-conjugated anti-CD45RO antibodies.
Use of the HDV System to Study Signaling Molecules in Primary Human T Cells.
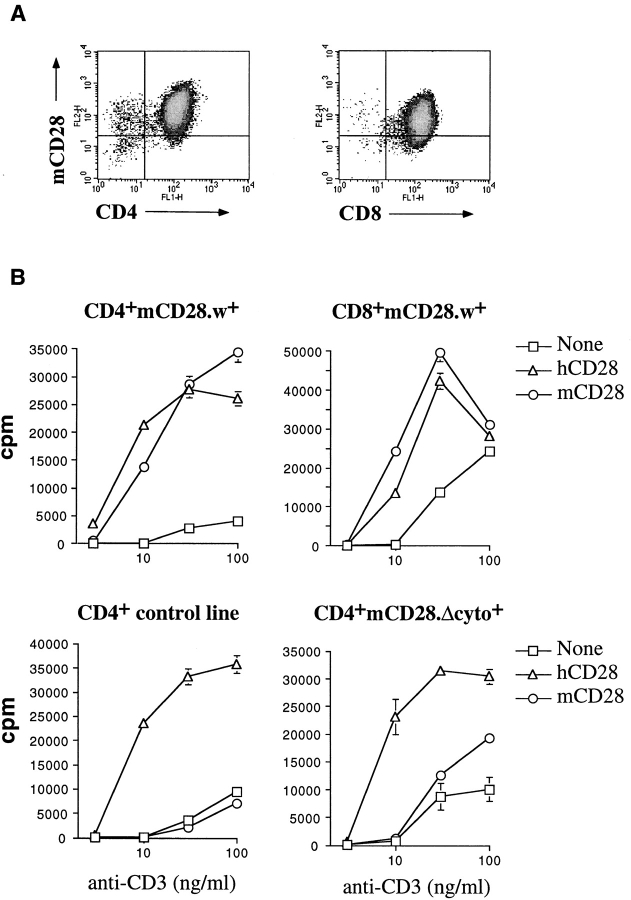
One attractive feature of the retroviral system is its potential use for dissecting signaling pathways in primary cells. Because we are interested in costimulatory signals mediated by the CD28 molecule, we assessed the feasibility of performing a structure–function analysis of this molecule by expressing mCD28 on human T cells. CD4+ or CD8+ human T cells, expressing mCD28 (Fig. 9 A), were stimulated at suboptimal concentrations of plate-bound anti-CD3, in the absence or presence of soluble antibodies to hCD28 or mCD28, and cell proliferation or IL-2 production were measured. T cells that expressed mCD28 proliferated equally well in response to antibodies against mCD28 or hCD28, in the presence of suboptimal TCR cross-linking (Fig. 9 B). As a control, cells that stably expressed a mutant form of mCD28 bearing a frame-shift mutation in its cytoplasmic domain (at Asp188) displayed a severely impaired proliferative response to anti-mCD28 (Fig. 9 B). It should be noted that the increase in proliferation observed with mutant mCD28 at higher concentrations of anti-CD3 was also observed with anti-mCD4 antibody when mCD4-expressing T cells were used, and thus may be the result of antibody-mediated clustering.
Figure 9.
Functional assessment of mCD28 expressed on human T cells. (A) Expression of mCD28 in sorted T cells 2 wk after transduction with HIV. (B) CD4+ and CD8+ human T cell lines transduced with wild-type (w+) or cytoplasmic domain–deleted (Δcyto+) mCD28 were stimulated 2 or 3 wk after the last activation with different concentrations of plate-bound anti-CD3 in the presence or absence of 1 μg/ml soluble anti-hCD28 or -mCD28 antibodies. Proliferation was measured after 2 d by [3H]thymidine incorporation.
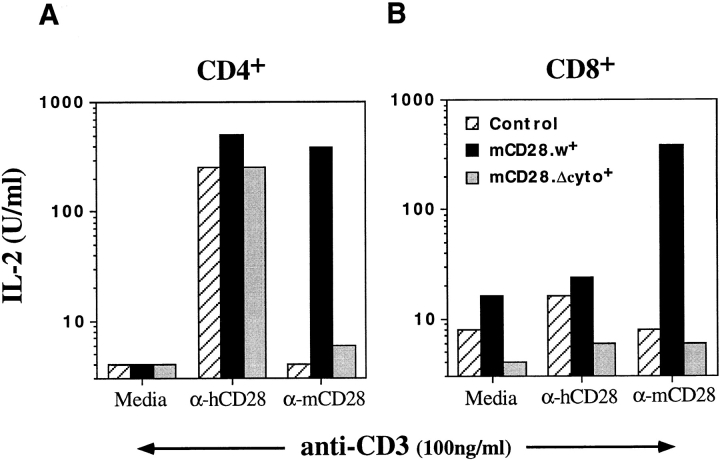
Since one of the hallmarks of CD28 costimulation is upregulation of IL-2 production (56), we also quantitated IL-2 synthesis in response to mCD28 costimulation. IL-2 secretion was increased by up to 50-fold upon treatment of CD4+ T cell lines that expressed mCD28 with anti-mCD28 or -hCD28 (Fig. 10 A). As expected, mCD28 antibodies did not upregulate IL-2 production in mCD28 nonexpressing cells or in cells expressing the cytoplasmic mutant form of mCD28 (Fig. 10). Furthermore, the costimulation through mCD28 was also partially resistant to Cyclosporin A, similar to what is observed upon endogenous CD28 costimulation (data not shown). To exclude the possibility of heterodimer formation between mCD28 and hCD28, we used CD8+ T cell lines that stably express mCD28 but have completely downregulated hCD28 because of repeated stimulations during in vitro culture. These cells produced high levels of IL-2 in response to mCD28 costimulation, but, as expected, did not respond to treatment with anti-hCD28 antibodies (Fig. 10 B). Similar studies were also possible in cytokine-cultured resting T cells expressing mCD28 (data not shown). These experiments clearly demonstrate that it will be feasible to perform a thorough structure–function analysis of CD28 and possibly other signaling molecules in primary human T lymphocytes.
Figure 10.
IL-2 production by costimulation through mCD28 expressed on human T cells. IL-2 production was measured by titrating supernatants from cultures of CD4+ or CD8+ T cell lines that were stimulated with anti-CD3 and anti-CD28. Supernatants were collected after 36 h of stimulation and IL-2 was measured using the CTLL bioassay. 1 U of IL-2 is defined as half-maximal proliferation of CTLL. (A) IL-2 production by CD4+ T cell lines. (B) IL-2 production by CD8+ T cell lines that are negative for hCD28 expression as determined by FACS® analysis.
Discussion
We have shown here, using an HDV system as well as replication-competent HIV-1, that cytokines can stimulate infection of resting T lymphocytes with these viruses. Previous studies have demonstrated a block in reverse transcription of HIV-1 in resting T cells. Cytokine stimulation appears to overcome this block, resulting in proviral integration and expression. This result may have major implications in understanding mechanisms of HIV-1 transmission and pathogenesis.
The HIV-1–based vector system described here can be used for efficient and stable introduction of genes into all subsets of activated human T cells as well as NK cells, macrophages, DCs, and cytokine-stimulated resting T cells. Efficient transduction of both resting and activated cells was achieved despite the deletion of the accessory genes vif, vpr, vpu, and nef in the HIV vector. The removal of these accessory genes avoids many unwanted effects in T cells. Indeed, we did not observe any aberrant function of primary T cells with regards to activation or proliferation after HDV- mediated transduction. The use of a heterologous envelope in pseudotypes and the deletion of accessory proteins important for virulence also makes it very unlikely that replication-competent recombinant virus can be generated. Using a sensitive assay (57), replication-competent viruses were not detected in any of the infected lines.
We found transduction of mitogen-activated T cells with HDV to be highly efficient; however, the transduction of CD4+ T cells was always better than that of CD8+ T cells. This may be due to the secretion of various inhibitory factors such as IFNs by activated CD8+ T cells and/or the lack of factors that promote more efficient integration/ transcription of HIV. In contrast, cytokine-activated resting CD8+ T cells were infected very efficiently, even better than CD4+ T cells stimulated with cytokines or CD8+ T cells activated with mitogens (see Figs. 1 and 4). Additional studies will be needed to identify host factors induced by cytokine- versus TCR-mediated signaling that affect the efficiency of HIV infection.
The Parameters for Infection of Resting T Cells with HIV.
The lentivirus subfamily of retroviruses, which includes HIV-1, can infect nondividing cells because the HIV preintegration complex can exploit the cellular machinery to allow transport into the nucleus (11–17). Efficient infection of nondividing, terminally differentiated cells such as neurons and macrophages or quiescent human hematopoietic stem cells with HDVs has been reported (26, 27, 29, 33). However, infection of quiescent T cells is blocked before integration, probably due to incomplete reverse transcription (18, 58–60) or failure to transport the viral preintegration complex to the nucleus (19, 21, 23, 61). Consistent with these data, the HIV vector system described here does not support expression of ectopic genes in resting human T cells. However, by treating resting T cells with IL-2, IL-4, IL-7, or IL-15 before virus challenge we were able to overcome this block. Remarkably, these cytokines were equally or even more effective in enhancing transduction of CD45RA+ naive human T cells, especially when combined with IL-6 and TNF-α. Although some resting T cells progress through the cell cycle in response to cytokine-mediated signals, this was not a prerequisite for the infection of these cells (Fig. 5). Unlike TCR stimulation, cytokine-mediated signals do not change the differentiation state of the cells, such that naive T cells remain phenotypically naive and there is very little expression of activation markers such as CD69 (with the exception of IL-15 stimulation) and HLA-DR.
Introduction of exogenous genes into purified resting T cells stimulated with cytokines may have applications in clinical gene therapy where it may not be desirable or even possible (for example in some immunodeficiencies), to activate the cells through the TCR. In addition, this system can be used to address questions related to differentiation of naive and memory T cells, by enabling genetic manipulation of these cells in the absence of TCR-mediated activation.
Infection of cytokine-stimulated resting T cells may have important physiological implications for HIV infection in vivo. Cytokines have been reported to have dramatic effects on HIV replication in infected cells (45, 46, 62, 63). In vivo, resting T cells that carry integrated provirus can be detected even after highly active antiretroviral therapy, albeit at a very low frequency (23, 64–66). Interestingly, in a recent study, the cytokine combination of IL-2, TNF-α, and IL-6 was shown to induce expression of HIV in latently infected resting T cells isolated from HIV-infected individuals (67). However, it is not clear how resting T cells in vivo become latently infected. It is generally thought that memory T cells that may have been infected while in an active state become quiescent before the virus replicates and induces cytotoxicity (68). We have shown that an R5 strain of HIV-1, with intact accessory genes, can also infect cytokine-activated resting T cells. Our data and those of others (Fig. 8 and references 52, 69) indicate that CCR5 cell surface expression is upregulated in cytokine-stimulated cells especially with IL-15 and at a lower level with IL-2 or IL-7. Although this may facilitate infection by R5 viruses, the effect of the cytokines clearly extends beyond viral entry in the resting T cells as demonstrated by the HIV (VSV-G)–pseudotyped viruses. Therefore, it is possible that infection with HIV-1 in vivo might occur in resting T cells that are continuously exposed to cytokines at sites of infection or in secondary lymphoid organs (45). This may be important for the establishment of infection and for viral pathogenesis. For example, infection of macrophages or DCs with HIV-1 may induce innate immune responses that result in production of the relevant cytokines that promote infection of resting T cells. It will be necessary to determine the efficiency of viral replication and whether latency can be established in cytokine-stimulated T cells. It is also tempting to speculate that cytokine-mediated bystander activation of resting T cells may facilitate the spread of the virus in vivo, thus contributing to the rapid viral turnover rates (70, 71).
How do cytokine-induced signals allow transduction of resting T cells? Identifying the molecular mechanisms by which these signals enhance proviral establishment in resting T cells will help in understanding which host cofactors are required during early stages of HIV infection. It is interesting to note that, except for IL-6, the cytokines that enhance the transduction of resting T cells all share the γc chain in their receptors (72), suggesting a role for signals delivered through this receptor component. It has been reported that phosphorylation of components of the viral preintegration complex by kinases, such as a virion-associated, mitogen-activated protein kinase, appears to enable efficient transport of this complex to the nucleus (73). Thus, signals delivered from the cytokine receptors may activate host factors that are necessary for the translocation of the preintegration complexes into the nucleus.
Our finding that primary human B cells are not detectably transduced is intriguing. We did not assess whether the block in B cells is at the level of integration or transcription. It may be that primary B cells lack factors required for integration/ transcription and that these are induced in EBV-transformed B cells. It is also possible that other signaling conditions may permit efficient transduction of B cells. The infection of activated primary or transformed mouse T cells also was very inefficient (1–2%), whereas transduction using an MLV-based retroviral system routinely transduced 10–60% of transformed mouse T cells (data not shown). Although we did not attempt to infect other primary mouse cell types, two murine cell lines, NIH 3T3 fibroblasts and, interestingly, the IL-2–dependent T cell line CTLL, were efficiently and stably transduced (data not shown). Elucidating these cell-type specific restrictions or requirements for HDV-mediated transduction may also be helpful in enhancing the susceptibility of murine models of HIV infection (74).
Exploiting the HDV System to Perform Genetic Studies in Primary T Cells.
The ability to introduce genes into primary T lymphocytes should greatly assist the studies of T cell differentiation and signal transduction in vitro. As a proof of this concept we have shown that structure–function analysis of the costimulatory molecule CD28 is feasible in primary lymphocytes and that mCD28 is functional in delivering costimulatory signals in human T cells. Previously, these studies have been limited to transformed cell lines, and it is likely that signaling pathways differ in subtle but critical ways in primary T cells. Indeed, our ongoing mutational analysis of CD28 cytoplasmic domain residues has revealed differences when compared with similar studies performed in the transformed human Jurkat T cell line (39) and identified functionally important regions of CD28 in primary T cells (Unutmaz, D., and S. Marmon, unpublished data). Similar studies can readily be applied to other signaling molecules expressed in primary lymphocytes or myeloid cells. Furthermore, dominant negative or constitutively active forms of intracellular molecules involved in these pathways can be expressed and analyzed using the HDV-IRES. GFP by sorting for GFP+ cells.
In conclusion, the HIV-1–based transduction system described in this study will be a valuable tool in studying many aspects of signal transduction in primary T cells and can have potential applications in somatic gene therapy. Here we have for the first time demonstrated that stimulation of resting T cells with cytokines is sufficient for HIV-1 infection of these cells while preserving their differentiation state. This finding may have major implications in understanding the mechanisms of virus infection of resting T cells in vivo.
Acknowledgments
We thank Michael Emerman (FHCRC, Seattle, WA) for pL-VSV-G and pJK3, Ned Landau (Salk Institute, La Jolla, CA) for HIV-PLAP, Toshio Kitamura for the pMX vector, and Richard Sutton (Baylor University, Dallas, TX) for modifications of the HIV-PLAP vector. We also thank Chris Arendt, Michael Emerman, Wilfried Ellmeier, Isabelle Riviere, Michel Sadelain, and Antonio Lanzavecchia for helpful comments and critical reading of the manuscript, and John Hirst for excellent help in cell sorting.
Abbreviations used in this paper
- BrdU
bromodeoxyuridine
- DC
dendritic cell
- EGFP
enhanced GFP
- GFP
green fluorescent protein
- h
human
- HDV
HIV-1–derived vector
- IRES
internal ribosome entry site
- m
murine
- MLV
murine leukemia virus
- MOI
multiplicity of infection
- PLAP
placental alkaline phosphatase
- VSV-G
vesicular stomatitis virus glycoprotein
Footnotes
V.N. KewalRamani is supported by a postdoctoral fellowship from the Damon Runyon-Walter Winchell Foundation. D.R. Littman is an investigator of the Howard Hughes Medical Institute. This work was supported by National Institutes of Health grants AI33856 and AI36606 to D.R. Littman.
References
- 1.Mulligan RC. The basic science of gene therapy. Science. 1993;260:926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- 2.Verma IM. Gene therapy: hopes, hypes, and hurdles. Mol Med. 1994;1:2–3. [PMC free article] [PubMed] [Google Scholar]
- 3.Miller AD, Miller DG, Garcia JV, Lynch CM. Use of retroviral vectors for gene transfer and expression. Methods Enzymol. 1993;217:581–599. doi: 10.1016/0076-6879(93)17090-r. [DOI] [PubMed] [Google Scholar]
- 4.Humphries EH, Temin HM. Requirement for cell division for initiation of transcription of Rous sarcoma virus RNA. J Virol. 1974;14:531–546. doi: 10.1128/jvi.14.3.531-546.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller DG, Adam MA, Miller AD. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol. 1990;10:4239–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roe T, Reynolds TC, Yu G, Brown PO. Integration of murine leukemia virus DNA depends on mitosis. EMBO (Eur Mol Biol Organ) J. 1993;12:2099–2108. doi: 10.1002/j.1460-2075.1993.tb05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis PF, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinberg JB, Matthews TJ, Cullen BR, Malim MH. Productive human immunodeficiency virus type 1 (HIV-1) infection of nonproliferating human monocytes. J Exp Med. 1991;174:1477–1482. doi: 10.1084/jem.174.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis P, Hensel M, Emerman M. Human immunodeficiency virus infection of cells arrested in the cell cycle. EMBO (Eur Mol Biol Organ) J. 1992;11:3053–3058. doi: 10.1002/j.1460-2075.1992.tb05376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bukrinsky MI, Sharova N, Dempsey MP, Stanwick TL, Bukrinskaya AG, Haggerty S, Stevenson M. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc Natl Acad Sci USA. 1992;89:6580–6584. doi: 10.1073/pnas.89.14.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bukrinsky MI, Haggerty S, Dempsey MP, Sharova N, Adzhubel A, Spitz L, Lewis P, Goldfarb D, Emerman M, Stevenson M. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature. 1993;365:666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Schwedler U, Kornbluth RS, Trono D. The nuclear localization signal of the matrix protein of human immunodeficiency virus type 1 allows the establishment of infection in macrophages and quiescent T lymphocytes. Proc Natl Acad Sci USA. 1994;91:6992–6996. doi: 10.1073/pnas.91.15.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinzinger NK, Bukinsky MI, Haggerty SA, Ragland AM, Kewalramani V, Lee MA, Gendelman HE, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallay P, Swingler S, Aiken C, Trono D. HIV-1 infection of nondividing cells: C-terminal tyrosine phosphorylation of the viral matrix protein is a key regulator. Cell. 1995;80:379–388. doi: 10.1016/0092-8674(95)90488-3. [DOI] [PubMed] [Google Scholar]
- 15.Gallay P, Swingler S, Song J, Bushman F, Trono D. HIV nuclear import is governed by the phosphotyrosine-mediated binding of matrix to the core domain of integrase. Cell. 1995;83:569–576. doi: 10.1016/0092-8674(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 16.Gallay P, Stitt V, Mundy C, Oettinger M, Trono D. Role of the karyopherin pathway in human immunodeficiency virus type 1 nuclear import. J Virol. 1996;70:1027–1032. doi: 10.1128/jvi.70.2.1027-1032.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallay P, Hope T, Chin D, Trono D. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc Natl Acad Sci USA. 1997;94:9825–9830. doi: 10.1073/pnas.94.18.9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zack JA, Arrigo SJ, Weitsman SR, Go AS, Haislip A, Chen IS. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 19.Stevenson M, Stanwick TL, Dempsey MP, Lamonica CA. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO (Eur Mol Biol Organ) J. 1990;9:1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zack JA, Haislip AM, Krogstad P, Chen IS. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J Virol. 1992;66:1717–1725. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spina CA, Guatelli JC, Richman DD. Establishment of a stable, inducible form of human immunodeficiency virus type 1 DNA in quiescent CD4 lymphocytes in vitro. J Virol. 1995;69:2977–2988. doi: 10.1128/jvi.69.5.2977-2988.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zack JA. The role of the cell cycle in HIV-1 infection. Adv Exp Med Biol. 1995;374:27–31. doi: 10.1007/978-1-4615-1995-9_3. [DOI] [PubMed] [Google Scholar]
- 23.Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano RF. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med. 1995;1:1284–1290. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- 24.Sonza S, Maerz A, Deacon N, Meanger J, Mills J, Crowe S. Human immunodeficiency virus type 1 replication is blocked prior to reverse transcription and integration in freshly isolated peripheral blood monocytes. J Virol. 1996;70:3863–3869. doi: 10.1128/jvi.70.6.3863-3869.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chou CS, Ramilo O, Vitetta ES. Highly purified CD25-resting T cells cannot be infected de novo with HIV-1. Proc Natl Acad Sci USA. 1997;94:1361–1365. doi: 10.1073/pnas.94.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 28.Miyoshi H, Takahashi M, Gage FH, Verma IM. Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc Natl Acad Sci USA. 1997;94:10319–10323. doi: 10.1073/pnas.94.19.10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poeschla E, Corbeau P, Wong-Staal F. Development of HIV vectors for anti-HIV gene therapy. Proc Natl Acad Sci USA. 1996;93:11395–11399. doi: 10.1073/pnas.93.21.11395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reiser J, Harmison G, Kluepfel-Stahl S, Brady RO, Karlsson S, Schubert M. Transduction of nondividing cells using pseudotyped defective high-titer HIV type 1 particles. Proc Natl Acad Sci USA. 1996;93:15266–15271. doi: 10.1073/pnas.93.26.15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- 32.Kafri T, Blomer U, Peterson DA, Gage FH, Verma IM. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat Genet. 1997;17:314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- 33.Uchida N, Sutton RE, Friera AM, He D, Reitsma MJ, Chang WC, Veres G, Scollay R, Weissman IL. HIV, but not murine leukemia virus, vectors mediate high efficiency gene transfer into freshly isolated G0/G1 human hematopoietic stem cells. Proc Natl Acad Sci USA. 1998;95:11939–11944. doi: 10.1073/pnas.95.20.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallardo HF, Tan C, Ory D, Sadelain M. Recombinant retroviruses pseudotyped with the vesicular stomatitis virus G glycoprotein mediate both stable gene transfer and pseudotransduction in human peripheral blood lymphocytes. Blood. 1997;90:952–957. [PubMed] [Google Scholar]
- 36.Bartz SR, Vodicka MA. Production of high- titer human immunodeficiency virus type 1 pseudotyped with vesicular stomatitis virus glycoprotein. Methods. 1997;12:337–342. doi: 10.1006/meth.1997.0487. [DOI] [PubMed] [Google Scholar]
- 37.He J, Landau NR. Use of a novel human immunodeficiency virus type 1 reporter virus expressing human placental alkaline phosphatase to detect an alternative viral receptor. J Virol. 1995;69:4587–4592. doi: 10.1128/jvi.69.7.4587-4592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sutton RE, Wu HT, Rigg R, Bohnlein E, Brown PO. Human immunodeficiency virus type 1 vectors efficiently transduce human hematopoietic stem cells. J Virol. 1998;72:5781–5788. doi: 10.1128/jvi.72.7.5781-5788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crooks ME, Littman DR, Carter RH, Fearon DT, Weiss A, Stein PH. CD28-mediated costimulation in the absence of phosphatidylinositol 3-kinase association and activation. Mol Cell Biol. 1995;15:6820–6828. doi: 10.1128/mcb.15.12.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Littman DR, Gettner SN. Unusual intron in the immunoglobulin domain of the newly isolated murine CD4 (L3T4) gene. Nature. 1987;325:453–455. doi: 10.1038/325453a0. [DOI] [PubMed] [Google Scholar]
- 41.Gallardo HF, Tan C, Sadelain M. The internal ribosomal entry site of the encephalomyocarditis virus enables reliable coexpression of two transgenes in human primary T lymphocytes. Gene Ther. 1997;4:1115–1119. doi: 10.1038/sj.gt.3300506. [DOI] [PubMed] [Google Scholar]
- 42.Unutmaz D, Pileri P, Abrignani S. Antigen- independent activation of naive and memory resting T cells by a cytokine combination. J Exp Med. 1994;180:1159–1164. doi: 10.1084/jem.180.3.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Unutmaz D, Baldoni F, Abrignani S. Human naive T cells activated by cytokines differentiate into a split phenotype with functional features intermediate between naive and memory T cells. Int Immunol. 1995;7:1417–1424. doi: 10.1093/intimm/7.9.1417. [DOI] [PubMed] [Google Scholar]
- 44.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fauci AS. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 46.Cohen OJ, Kinter A, Fauci AS. Host factors in the pathogenesis of HIV disease. Immunol Rev. 1997;159:31–48. doi: 10.1111/j.1600-065x.1997.tb01005.x. [DOI] [PubMed] [Google Scholar]
- 47.Boise LH, Minn AJ, June CH, Lindsten T, Thompson CB. Growth factors can enhance lymphocyte survival without committing the cell to undergo cell division. Proc Natl Acad Sci USA. 1995;92:5491–5495. doi: 10.1073/pnas.92.12.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beverley PC. Functional analysis of human T cell subsets defined by CD45 isoform expression. Semin Immunol. 1992;4:35–41. [PubMed] [Google Scholar]
- 49.Soares MV, Borthwick NJ, Maini MK, Janossy G, Salmon M, Akbar AN. IL-7-dependent extrathymic expansion of CD45RA+T cells enables preservation of a naive repertoire. J Immunol. 1998;161:5909–5917. [PubMed] [Google Scholar]
- 50.Shirazi Y, Pitha PM. Alpha interferon inhibits early stages of the human immunodeficiency virus type 1 replication cycle. J Virol. 1992;66:1321–1328. doi: 10.1128/jvi.66.3.1321-1328.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baca-Regen L, Heinzinger N, Stevenson M, Gendelman HE. Alpha interferon-induced antiretroviral activities: restriction of viral nucleic acid synthesis and progeny virion production in human immunodeficiency virus type 1-infected monocytes. J Virol. 1994;68:7559–7565. doi: 10.1128/jvi.68.11.7559-7565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jourdan P, Abbal C, Nora N, Hori T, Uchiyama T, Vendrell JP, Bousquet J, Taylor N, Pene J, Yssel H. IL-4 induces functional cell-surface expression of CXCR4 on human T cells. J Immunol. 1998;160:4153–4157. [PubMed] [Google Scholar]
- 54.Valentin A, Lu W, Rosati M, Schneider R, Albert J, Karlsson A, Pavlakis GN. Dual effect of interleukin 4 on HIV-1 expression: implications for viral phenotypic switch and disease progression. Proc Natl Acad Sci USA. 1998;95:8886–8891. doi: 10.1073/pnas.95.15.8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J, Harada A, Matsushita S, Matsumi S, Zhang Y, Shioda T, Nagai Y, Matsushima K. IL-4 and a glucocorticoid up-regulate CXCR4 expression on human CD4+T lymphocytes and enhance HIV-1 replication. J Leukocyte Biol. 1998;64:642–649. doi: 10.1002/jlb.64.5.642. [DOI] [PubMed] [Google Scholar]
- 56.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 57.Trkola A, Ketas T, KewalRamani VN, Endorf F, Binley JM, Katinger H, Robinson J, Littman DR, Moore JP. Neutralization sensitivity of human immunodeficiency virus type 1 primary isolates to antibodies and CD4-based reagents is independent of coreceptor usage. J Virol. 1998;72:1876–1885. doi: 10.1128/jvi.72.3.1876-1885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krogstad PA, Zack JA, Chen IS. HIV-1 reverse transcription in cord blood lymphocytes: implications for infection of newborns. AIDS Res Hum Retroviruses. 1994;10:143–147. doi: 10.1089/aid.1994.10.143. [DOI] [PubMed] [Google Scholar]
- 59.Tang S, Patterson B, Levy JA. Highly purified quiescent human peripheral blood CD4+T cells are infectible by human immunodeficiency virus but do not release virus after activation. J Virol. 1995;69:5659–5665. doi: 10.1128/jvi.69.9.5659-5665.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Korin YD, Zack JA. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J Virol. 1998;72:3161–3168. doi: 10.1128/jvi.72.4.3161-3168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bukrinsky MI, Stanwick TL, Dempsey MP, Stevenson M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science. 1991;254:423–427. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smithgall MD, Wong JG, Critchett KE, Haffar OK. IL-7 up-regulates HIV-1 replication in naturally infected peripheral blood mononuclear cells. J Immunol. 1996;156:2324–2330. [PubMed] [Google Scholar]
- 63.Al-Harthi L, Roebuck KA, Landay A. Induction of HIV-1 replication by type 1-like cytokines, interleukin (IL)-12 and IL-15: effect on viral transcriptional activation, cellular proliferation, and endogenous cytokine production. J Clin Immunol. 1998;18:124–131. doi: 10.1023/a:1023246800353. [DOI] [PubMed] [Google Scholar]
- 64.Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 65.Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn TC, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 66.Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, Fauci AS. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chun TW, Engel D, Mizell SB, Ehler LA, Fauci AS. Induction of HIV-1 replication in latently infected CD4+T cells using a combination of cytokines. J Exp Med. 1998;188:83–91. doi: 10.1084/jem.188.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weiss RA. How does HIV cause AIDS? . Science. 1993;260:1273–1279. doi: 10.1126/science.8493571. [DOI] [PubMed] [Google Scholar]
- 69.Loetscher P, Seitz M, Baggiolini M, Moser B. Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J Exp Med. 1996;184:569–577. doi: 10.1084/jem.184.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 71.Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 72.Sugamura K, Asao H, Kondo M, Tanaka N, Ishii N, Ohbo K, Nakamura M, Takeshita T. The interleukin-2 receptor gamma chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu Rev Immunol. 1996;14:179–205. doi: 10.1146/annurev.immunol.14.1.179. [DOI] [PubMed] [Google Scholar]
- 73.Jacque JM, Mann A, Enslen H, Sharova N, Brichacek B, Davis RJ, Stevenson M. Modulation of HIV-1 infectivity by MAPK, a virion-associated kinase. EMBO (Eur Mol Biol Organ) J. 1998;17:2607–2618. doi: 10.1093/emboj/17.9.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Unutmaz D, KewalRamani VN, Littman DR. G protein-coupled receptors in HIV and SIV entry: new perspectives on lentivirus-host interactions and on the utility of animal models. Semin Immunol. 1998;10:225–236. doi: 10.1006/smim.1998.0134. [DOI] [PubMed] [Google Scholar]