Abstract
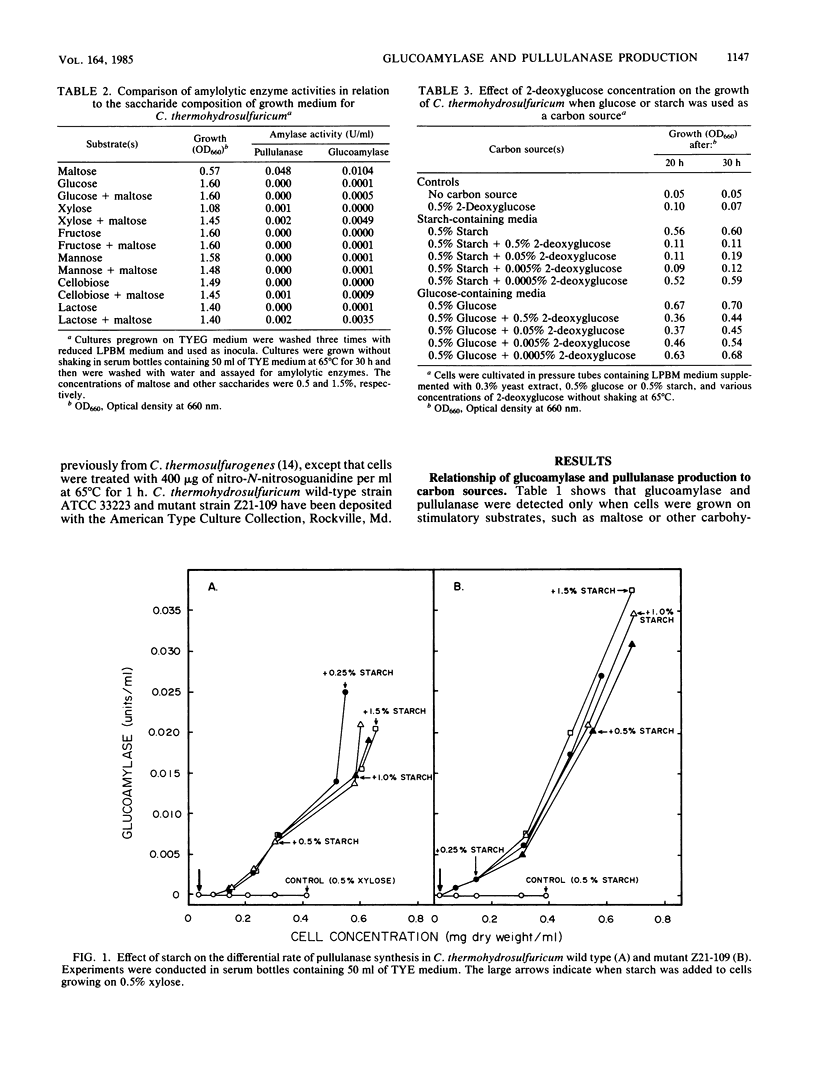
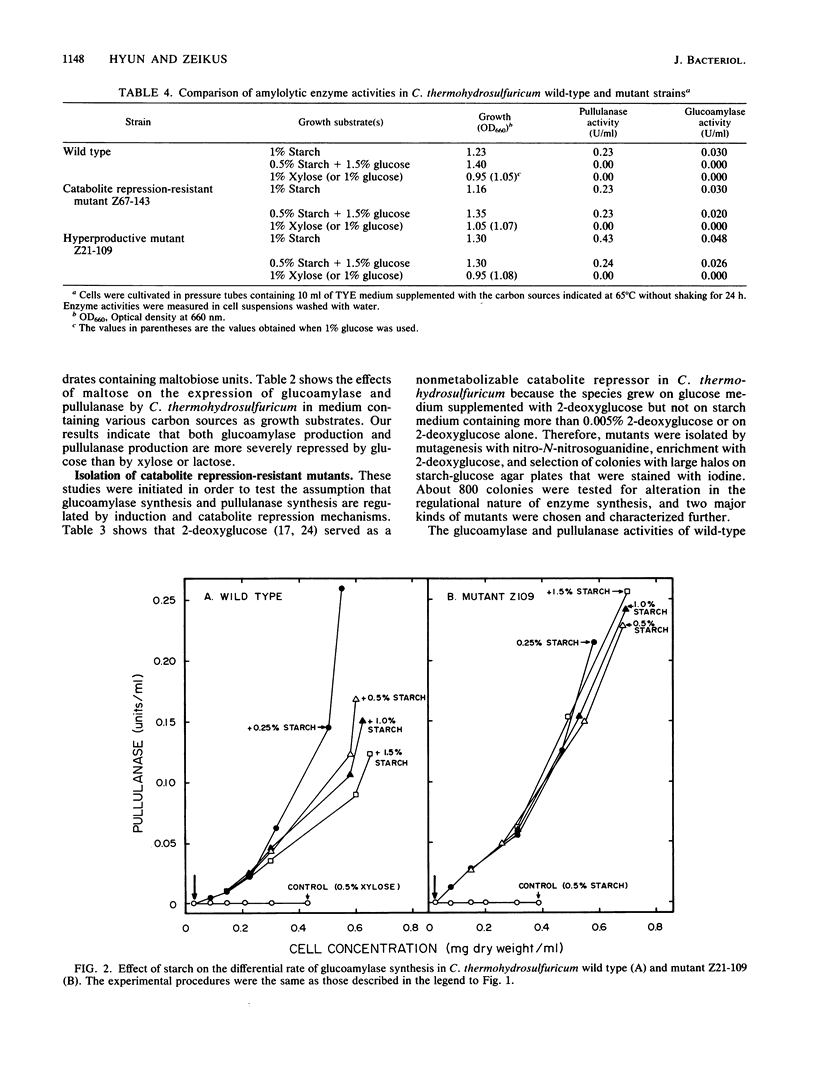
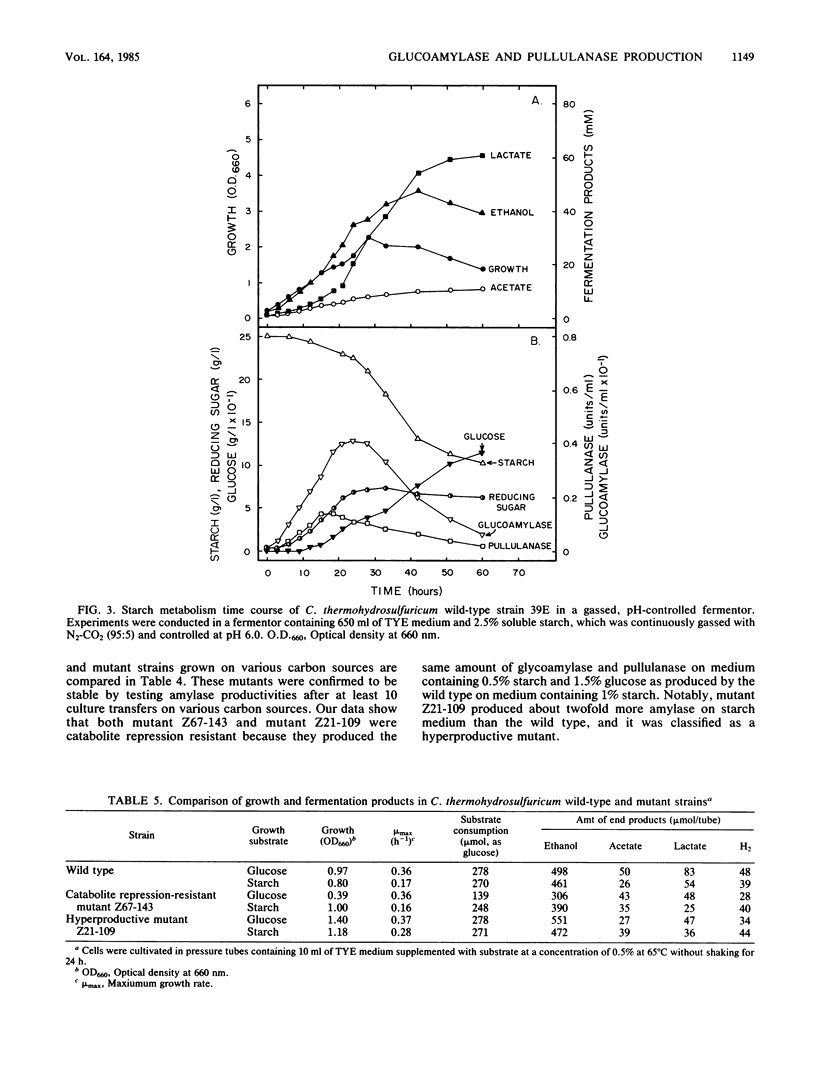
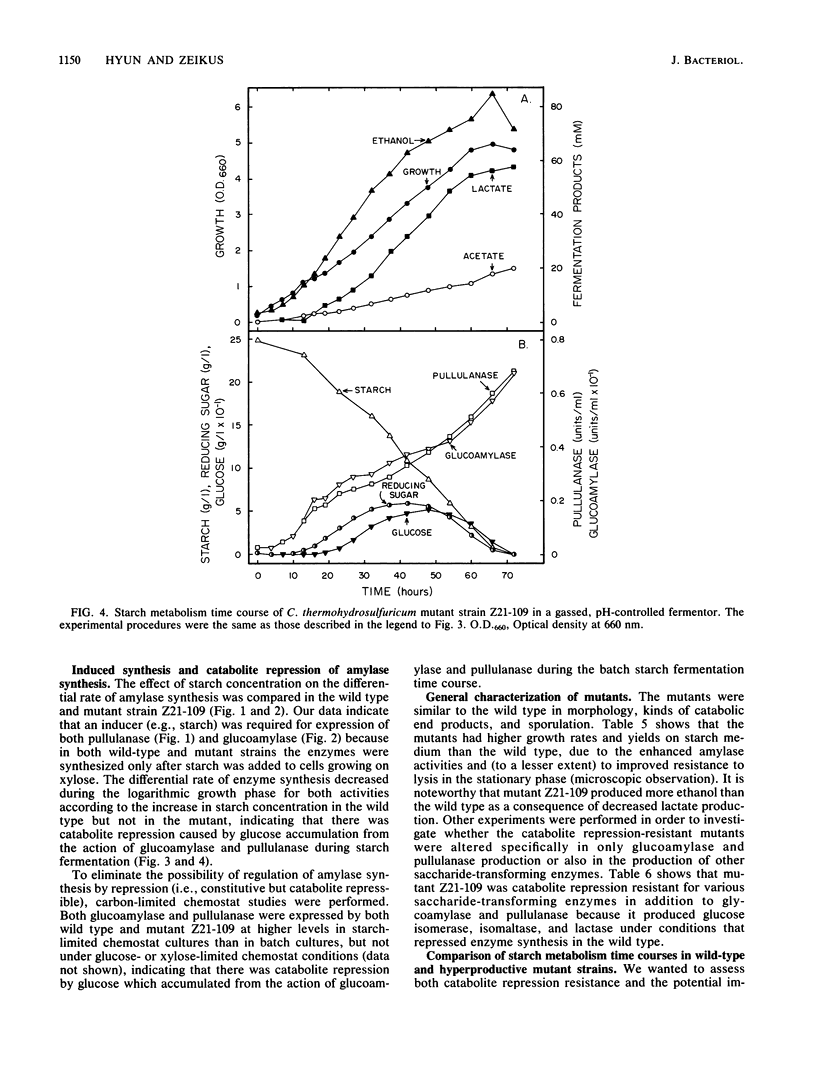
We studied the general mechanism for regulation of glucoamylase and pullulanase synthesis in Clostridium thermohydrosulfuricum. These amylases were expressed only when the organism was grown on maltose or other carbohydrates containing maltose units. Amylase synthesis was more severely repressed by glucose than by xylose. Catabolite repression-resistant mutants were isolated by using nitrosoguanidine treatment, enrichment on 2-deoxyglucose, and selection of colonies with large clear zones on iodine-stained glucose-starch agar plates. Amylases were produced in both wild-type and mutant strains when starch was added to cells growing on xylose but not when starch was added to cells growing on glucose. In both wild-type and mutant strains, glucoamylase and pullulanase were produced at high levels in starch-limited chemostats but not in glucose- or xylose-limited chemostats. Therefore, we concluded that amylase synthesis in C. thermohydrosulfuricum was inducible and subject to catabolite repression. The mutants produced about twofold more glucoamylase and pullulanase, and they were catabolite repression resistant for production of glucose isomerase, lactase, and isomaltase. The mutants displayed improved starch metabolism features in terms of enhanced rates of growth, ethanol production, and starch consumption.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attia R. M., Ali S. A. Utilization of agricultural wastes of Aspergillus awamori for the production of glucoamylase. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. 1977;132(4):322–325. doi: 10.1016/s0044-4057(77)80021-x. [DOI] [PubMed] [Google Scholar]
- Barton L. L., Georgi C. E., Lineback D. R. Effect of maltose on glucoamylase formation by Aspergillus niger. J Bacteriol. 1972 Sep;111(3):771–777. doi: 10.1128/jb.111.3.771-777.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero A. A., Gomez R. F. Development of ethanol tolerance in Clostridium thermocellum: effect of growth temperature. Appl Environ Microbiol. 1980 Sep;40(3):571–577. doi: 10.1128/aem.40.3.571-577.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope G. C., Dean A. C. Pullulanase synthesis in klebsiella (aerobacter) aerogenes strains growing in continuous culture. Biochem J. 1974 Nov;144(2):403–411. doi: 10.1042/bj1440403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Shen G. J., Zeikus J. G. Differential amylosaccharide metabolism of Clostridium thermosulfurogenes and Clostridium thermohydrosulfuricum. J Bacteriol. 1985 Dec;164(3):1153–1161. doi: 10.1128/jb.164.3.1153-1161.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G. General Biochemical Characterization of Thermostable Extracellular beta-Amylase from Clostridium thermosulfurogenes. Appl Environ Microbiol. 1985 May;49(5):1162–1167. doi: 10.1128/aem.49.5.1162-1167.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G. General Biochemical Characterization of Thermostable Pullulanase and Glucoamylase from Clostridium thermohydrosulfuricum. Appl Environ Microbiol. 1985 May;49(5):1168–1173. doi: 10.1128/aem.49.5.1168-1173.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G. Regulation and genetic enhancement of beta-amylase production in Clostridium thermosulfurogenes. J Bacteriol. 1985 Dec;164(3):1162–1170. doi: 10.1128/jb.164.3.1162-1170.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G. Simultaneous and Enhanced Production of Thermostable Amylases and Ethanol from Starch by Cocultures of Clostridium thermosulfurogenes and Clostridium thermohydrosulfuricum. Appl Environ Microbiol. 1985 May;49(5):1174–1181. doi: 10.1128/aem.49.5.1174-1181.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovitt R. W., Longin R., Zeikus J. G. Ethanol Production by Thermophilic Bacteria: Physiological Comparison of Solvent Effects on Parent and Alcohol-Tolerant Strains of Clostridium thermohydrosulfuricum. Appl Environ Microbiol. 1984 Jul;48(1):171–177. doi: 10.1128/aem.48.1.171-177.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito N., Yamamoto K. Regulatory factors affecting alpha-amylase production in bacillus licheniformis. J Bacteriol. 1975 Mar;121(3):848–856. doi: 10.1128/jb.121.3.848-856.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton R., Jr, Amelunxen R. E. Proteins from thermophilic microorganisms. Bacteriol Rev. 1973 Sep;37(3):320–342. doi: 10.1128/br.37.3.320-342.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELKER N. E., CAMPBELL L. L. INDUCTION OF ALPHA-AMYLASE OF BACILLUS STEAROTHERMOPHILUS BY MALTODEXTRINS. J Bacteriol. 1963 Oct;86:687–691. doi: 10.1128/jb.86.4.687-691.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]


