Abstract
Ligation of the Fas (CD95) receptor leads to an apoptotic death signal in T cells, B cells, and macrophages. However, human CD34+–derived dendritic cells (DCs) and mouse DCs, regardless of their maturation state, are not susceptible to Fas-induced cell death. This resistance correlates with the constitutive expression of the Fas-associated death domain–like IL-1β–converting enzyme (FLICE)-inhibitory protein (FLIP) ligand. We demonstrate a new role of Fas in DC physiology. Engagement of Fas on immature DCs by Fas ligand (FasL) or by anti-Fas antibodies induces the phenotypical and functional maturation of primary DCs. Fas-activated DCs upregulate the expression of the major histocompatibility complex class II, B7, and DC–lysosome-associated membrane protein (DC-LAMP) molecules and secrete proinflammatory cytokines, in particular interleukin (IL)-1β and tumor necrosis factor α. Mature DCs, if exposed to FasL, produce even higher amounts of IL-1β. Importantly, it is possible to reduce the production of IL-1β and interferon (IFN)-γ during DC–T cell interaction by blocking the coupling of Fas–FasL with a Fas competitor. Finally, during cognate DC–T cell recognition, IL-12 (p70) could not be detected at early or late time points, indicating that Fas-induced, IFN-γ secretion is independent of IL-12.
Keywords: dendritic cells, Fas, interleukin 1β, FLIP, interferon γ
Introduction
The first step in any adaptive immune response is the activation of naive T cells in the LN. Once stimulated by infection, dendritic cells (DCs) migrate to the draining LN where naive T cells circulate and can encounter their specific antigen on the DC surface 1. Because this is a rare event, the prolongation of cell survival and the correct activation state of DCs are of primary importance in order to control and sustain the immune response.
We have previously reported that DCs express the Fas receptor (CD95) 2, but its role on DCs is still a matter of debate 3 4. Stimulation of the Fas receptor normally induces an apoptotic death signal. However, in T cells the interactions between Fas and Fas ligand (FasL) do not always induce a death signal 5. Indeed, depending on the state of T cell activation, Fas–FasL interaction can also lead to T cell activation and proliferation 6 7. Thus, signaling through Fas in the early stages of an immune response might amplify the T cell response, whereas the signaling through Fas could downsize the response via apoptosis of T cells in later stages. Upon Fas activation and trimerization, a set of effector proteins is recruited to this receptor, forming the death inducing signaling complex. Fas-associated death domain (FADD), the first protein that binds to Fas, recruits caspase-8, initiating a cascade of events resulting in efficient cell death 8. Fas-induced apoptosis can be blocked by FADD-like IL-1β–converting enzyme (FLICE)-inhibitory proteins (FLIPs) 9. Furthermore, FLIP mediates the activation of nuclear factor κB and extracellular signal–regulated kinase (Erk), diverting Fas-mediated death signals into signals that lead to proliferation and/or differentiation 10. Both of these signaling pathways are activated by LPS and play important roles in DC maturation and survival 11.
Recently, the disregulation of Fas–FasL interactions has been considered as one of the major causes of exaggerated immune responses, such as organ-specific autoimmune diseases 12. In insulin-dependent diabetes mellitus, β islet cells express Fas on their surface in response to IL-1β and become susceptible to Fas-induced cell death by activated T cells 12.
In this study, we confirm that Fas is unable to induce DC death due to a constitutive FLIP expression and further demonstrate that FasL triggers both a phenotypical and functional maturation of DCs, as well as the secretion of proinflammatory cytokines, in particular IL-1β and TNF-α.
Materials and Methods
Cells and Reagents.
Purification of human CD34+ cells, primary culture with FLT3-L, thrombopoietin, and stem cell factor, and induction of DCs were performed as described previously 13. Two growth factor–dependent DC mice lines, D1 and D8, were used as well as bone marrow–derived fresh DCs 2 14 and were grown as described previously 2 14. Bone marrow cells were grown for 15–20 d and the homogeneity of the DC culture was evaluated by cytofluorimetry. CD4+ T cells were purified from TCR-OVA DO11.10 transgenic mice, bred in BALB/c background, by positive selection with an anti-CD4 antibody coupled to magnetic microbeads, using MiniMacS Separating columns (all from Miltenyi Biotec). LPS (Escherichia coli serotype 026:B6) was purchased from Sigma-Aldrich. FasL and anti-Flag Enhancer were from Alexis, and Fas competitor (Fas-comp) was derived as described 15. Human GM-CSF (Leucomax) was purchased from Essex Chimie & Sandoz and used at 20 ng/ml. Other human cytokines were purchased from Peprotech and used at the following concentrations: FLT3-L at 25 ng/ml, thrombopoietin at 10 U/ml, stem cell factor at 20 ng/ml, IL-4 at 20 ng/ml (100 U/ml), and TNF at 40 ng/ml (200 U/ml).
Cell Stimulation, Antibodies, and Flow Cytometry Analysis.
Human DCs were incubated with TNF-α (or anti-Flag Enhancer) and FasL (Alexis) for 18–24 h at the indicated concentrations. Cultured cells were stained with specific labeled mAbs or appropriate isotypic controls and incubated in 300 μl of saline, 3% FCS containing 10 μg/ml of 7-amino-actinomycin D (7AAD; Sigma-Aldrich) 16. Data were analyzed using WINMDI software by J. Trotter (Scripps Institute, La Jolla, CA). For intracellular staining, cells were incubated with mouse IgM anti-DC–lysosome-associated membrane protein (DC-LAMP, 10 μg/ml) for 30 min on ice in the presence of permeabilization medium (0.3% saponin and 2% BSA). Specific mouse anti–human mAbs were purchased: anti-CD1a (clone OKT6) from Ortho Diagnostic Systems, anti–HLA-DR (clone L243) from Becton Dickinson, anti-CD80 (clone BB1) and anti-CD86 (clone IT2.2) from BD PharMingen. Anti–DC-LAMP antibody (clone 510 F1.65.13) was a gift of Dr. S. Lebecque (Schering-Plough, Dardilly, France). Other reagents used were as follows: polyclonal mouse IgG reagent grade from Sigma-Aldrich and anti-CD34 mIgG-coated M450 Dynabeads from Dynal. LPS-matured or nontreated murine DCs were cultured either with anti-Flag Enhancer and FasL, anti-CD95 (Jo-2), or an isotype-matched control, at the indicated concentrations. After activation, cells were incubated with one of the following mAbs from BD PharMingen: anti–(I-Ad/I-Ed) (2G9), anti-CD86 (B7.2), anti-CD95 (Jo-2), or hamster IgG (G235-2356). Staining was carried out in the presence of 2-4G2 to block Fc receptor binding. For each sample, culture supernatants were collected for the cytokine production assay.
Cytokine Assays.
TNF-α, IL-1β, IL-10, and IFN-γ were measured by using murine DuoSet™ following the manufacturer's instructions (R&D Systems). IL-12 was tested using IL-12 capture (anti–IL-12 p70; 9A5) and detection (anti–IL-12 p40; 5C3). These antibodies and recombinant IL-12 were provided by Dr. D.H. Presky (Hoffman-La Roche, Nutley, NJ). IL-2 was tested by measuring [3H]thymidine incorporation of an IL-2–dependent CTL clone (CTLL).
Assessment of apoptosis.
Apoptosis of DCs was assessed by flow cytometry. For murine cells, the exposure of phosphatidylserine residues on the cells' surface was measured using FITC-conjugated annexin V (BD PharMingen) and dead cells were identified with 1.25 μg/ml propidium iodide (Sigma-Aldrich). Alternatively, human DCs were incubated on ice in 300 ml of saline, 3% FCS containing 10 μg/ml of 7AAD (Sigma-Aldrich). Cells were analyzed using FL-3 for 7AAD staining, which identified living cells as 7AAD low, and dead and apoptotic cells as 7AAD high and medium, respectively 16.
Antigen Presentation Assay.
D8 cells were seeded (104 cells/well) in flat-bottomed microtiter plates (Corning) pulsed with CD4+ T cells (5 × 104 cells/well) purified from TCR-OVA α/β transgenic mice (T/DC, 5:1) in the presence of decreasing concentrations of peptide OVA 327–339 as indicated, with or without 0.5 μg/ml of Fas-comp and in 0.3% normal mouse serum supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine (all from Sigma-Aldrich), and 50 μM 2-mercaptoethanol. Proliferative responses were assessed 48 h later by [3H]thymidine incorporation. Cell culture supernatants were collected at the indicated times for cytokine production measures.
Western Blot Analysis of FLIP.
Human CD34-derived DCs (5 × 105) were lysed in 50 μl of lysis buffer (1% NP-40, 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, and Complete™ protease inhibitor cocktail tablets [Boehringer]). 30 μg of protein per lane was subjected to electrophoresis under reducing conditions and transferred to nitrocellulose (Hybond ECL; Amersham Pharmacia Biotech). Blots were probed with anti-FLIP mAbs (Dave 2; Alexis), visualized using horseradish peroxidase–labeled, anti–rat Abs (Southern Biotechnology Associates, Inc.), and ECL blotting substrate (Amersham Pharmacia Biotech). Quantification on films was performed using the ONE D-Scan program (Scanalytics) as described previously 17.
Results
Fas Is Constitutively Expressed by DCs and Is Upregulated by LPS or TNF-α.
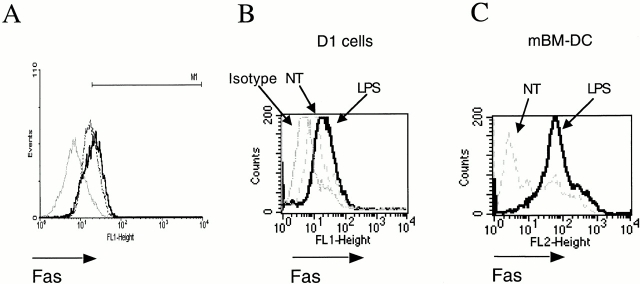
Similar to mouse DCs 2, nonstimulated immature, human CD34+–derived DCs were found to express Fas (Fig. 1 A). Therefore, we studied the regulation of Fas during maturation of human and mouse DCs induced by TNF-α or LPS. Incubation of human DCs with 40 ng/ml of TNF-α (Fig. 1 A) or LPS (data not shown) for 24 h induced a slight upregulation of Fas. We next incubated a well-defined, mouse spleen long-term growth factor–dependent DC line (D1 cells) or primary murine bone marrow–derived DCs (mBM-DCs) with LPS (10 μg/ml), which induced a significant upregulation of Fas that was more pronounced in primary mBM-DCs (compare Fig. 1B and Fig. C). Interestingly, incubation of agonistic anti-Fas antibodies (Jo-2) resulted in a dose-dependent downregulation of Fas (not shown) at early time points and in the upregulation of Fas at late time points (not shown).
Figure 1.
Surface levels of Fas (CD95) in immature versus mature DCs. (A) Human CD34+–derived DCs were treated with 40 ng/ml of TNF-α for 24 h (solid line) or left untreated (dashed line); isotype control (gray line). Results are representative of three independent experiments. (B) Mouse D1 cells or (C) mBM-DCs were treated with 10 μg/ml LPS. Thick line, treated cells; thin line, isotype control; dashed line, untreated cells (NT). Results are representative of four experiments.
DCs Are Resistant to Fas-induced Apoptotic Cell Death.
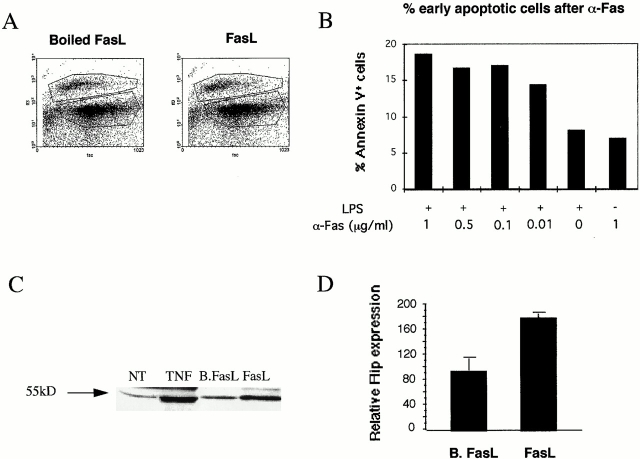
Since the induction of cell death by FasL remains controversial 3 4 18, we have studied the effects of FasL or the Jo-2 antibody on DCs. As shown in Fig. 2A and Fig. B, both human and mouse DCs were resistant to Fas-induced cell death. Human CD34+–derived immature DCs or 72-h, TNF-matured DCs (data not shown) were treated with FasL and enhancer for 24 h and analyzed using 7AAD staining (see Materials and Methods). No significant increase of dead and apoptotic cells was noticed after FasL treatment for 24 h in immature DCs (Fig. 2 A) or TNF-matured DCs (data not shown). D1 cells and mBM-DCs, pretreated or not with LPS for 20 h, were incubated with different concentrations of anti-Fas antibody for 6 h. Cells were double-stained with FITC-conjugated annexin V and with propidium iodide. Cells single positive for annexin V were considered as undergoing early apoptosis. As shown in Fig. 2 B, regardless of the concentration of anti-Fas used, a maximum 10% of the D1 cells underwent apoptosis (when subtracting the amount of apoptotic cells after LPS treatment), and no apoptosis was observed when anti-Fas was tested on immature cells. Similar results were obtained with fresh mBM-DCs (not shown). Finally, no significant cell death occurred in mature or immature DCs during cognate DC–T cell interaction (not shown).
Figure 2.
Fas does not induce apoptotic cell death of immature or mature cells. (A) Flow cytometry of human DCs treated with FasL (right) or with boiled FasL (left) stained with 7AAD (see Materials and Methods). (B) Mouse DCs were either left untreated or treated for 20 h with LPS only, with LPS first and then with anti-Fas Jo-2 antibody for an additional 6 h, or with anti-Fas Jo-2 antibody alone at the indicated concentrations. Cells single positive for annexin V–FITC are considered early apoptotic and are plotted in the graph after subtracting the percentage of untreated cells. (C) Western blot for the expression of FLIPs in cell extracts of human DCs either untreated (NT) or treated with TNF-α, with boiled FasL (B.FasL), or with FasL (FasL) for 24 h. (D) Quantitative measurements of relative FLIP expression of C (based on four independent experiments, ±SEM) as performed using the ONE D-Scan program.
DCs Constitutively Express FLIP.
Fas-induced apoptosis can be blocked during signal transduction by FLIPs 9. We observed by Western blot analysis that FLIP was constitutively expressed by human and mouse DCs. Furthermore, FLIP was upregulated approximately twofold at 24 h of DC incubation with FasL in human DCs (Fig. 2C and Fig. D). A similar upregulation of FLIP was observed after FasL, TNF-α, or LPS treatment in either human or mouse DCs (data not shown).
Fas Induces Phenotypical Maturation of DCs.
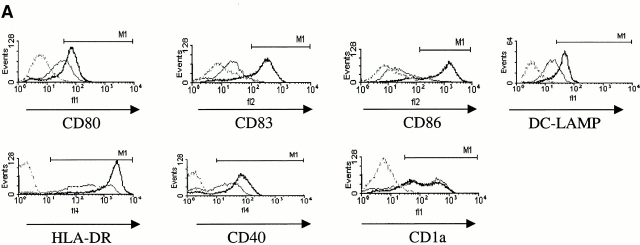
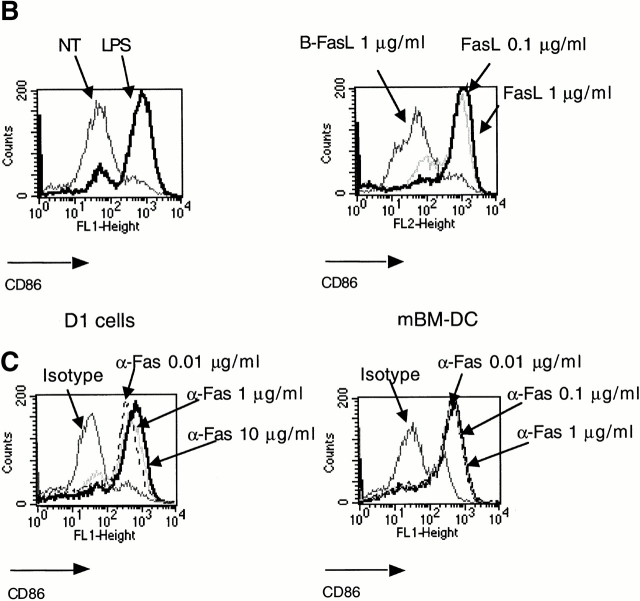
Subsequently, we examined the potential role of FasL in DC maturation (Fig. 3). Human (Fig. 3 A) and mouse (Fig. 3 B) DCs were incubated for 18–24 h with 0.1 μg/ml FasL and with 0.5 μg/ml of enhancer. This stimulus induced a strong upregulation of maturation markers such as MHC class II, CD40, CD80, CD83, and CD86 on the cell surface of human CD34+–derived DCs as well as of intracellular DC-LAMP (Fig. 3 A). The extent of maturation induced by FasL treatment was comparable to that induced by TNF-α or LPS (data not shown). Moreover, human and mouse DCs could be activated by FasL, but not by boiled FasL, excluding a possible endotoxin contamination. The effect of FasL was reproduced by the Jo-2 antibody. As shown in Fig. 3 C, as low as 0.01 μg/ml of Jo-2 antibody was sufficient to induce the maturation of both primary mBM-DCs and the D1 cell line, whereas an isotype control for the Jo-2 antibody had no effect (Fig. 3 C).
Figure 3.
Fas engagement triggers the phenotypical maturation of human and mouse DCs. (A) Surface expression of cellular receptors and intracellular expression of DC-LAMP in human CD34+–derived DCs treated with 0.1 μg/ml FasL plus 0.5 μg/ml Enhancer for 24 h (black line) versus 0.1 μg/ml boiled FasL plus 0.5 μg/ml Enhancer (gray line), or isotype control mAbs (dotted gray line). Results are representative of four independent experiments. (B) Comparison of B7.2 (CD86) upregulation by LPS (left) or FasL plus Enhancer (right) in mouse D1 cells. (C) Treatment of DCs (D1 cells, left; mBM-DCs, right) by anti-CD95 antibody (Jo-2) with concentrations ranging between 0.01 and 10 μg/ml or an isotype control (thin line). Results are representative of three independent experiments.
Fas Induces Functional Activation of Immature and Mature DCs.
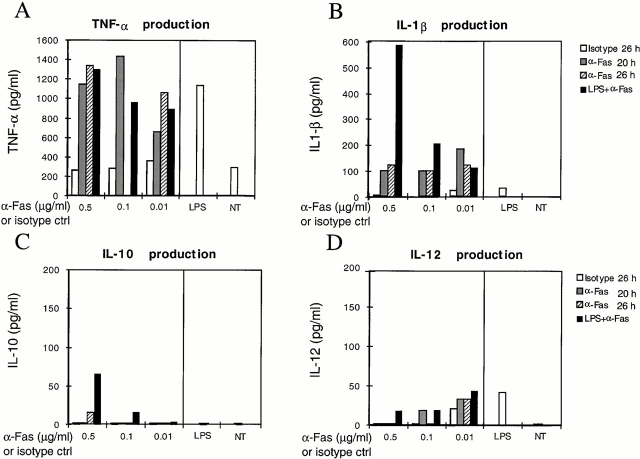
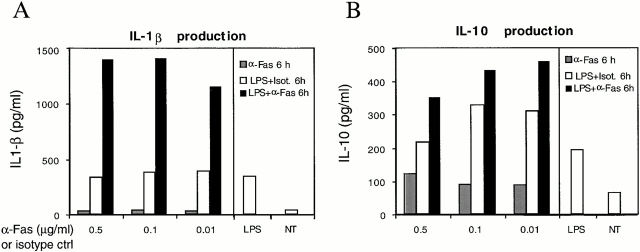
Cytokine secretion by DCs was tested in culture supernatants after Fas engagement and was compared with that induced by LPS (Fig. 4). Although the amount of TNF-α produced was comparable among LPS- and Fas-matured DCs (Fig. 4 A), Fas-matured DCs secreted more IL-1β (Fig. 4 B). If LPS-matured DCs were subsequently exposed to anti-Fas antibody, the production of IL-1β greatly increased (600 vs. 20 pg/ml with LPS alone, Fig. 4 B) as well as the amount of secreted IL-10 (Fig. 4 C). In contrast, the amount of bioactive IL-12 (p70) secreted was very low in Fas-matured DCs (Fig. 4 D). When we analyzed fresh mBM-DCs, similar results were obtained (Fig. 5A and Fig. B).
Figure 4.
Fas-matured D1 cells produce large amounts of TNF-α and IL-1β. Cytokine production was measured by ELISA after 20 h incubation with anti-Fas antibody at the concentrations indicated (α-Fas 20 h); 20 h with 10 μg/ml LPS and then 6 h with anti-Fas (LPS+α-Fas); 26 h with anti-Fas alone (α-Fas 26 h); 26 h with isotype control for Jo-2 antibody alone (Isotype 26 h); 26 h with LPS alone (LPS); or left untreated (NT). Results are representative of three independent experiments.
Figure 5.
Fas-treated fresh mBM-DCs preactivated with LPS produce large amounts of IL-1β. Cytokine production was measured by ELISA after 20 h of treatment with 10 μg/ml LPS and then 6 h with anti-Fas at the indicated concentrations (LPS+α-Fas 6 h); 20 h with 10 μg/ml LPS and then 6 h with isotype control, at the indicated concentrations (LPS+Isot. 6h); 6 h with anti-Fas (α-Fas 6h); 26 h with LPS alone (LPS); or left untreated (NT). Results are representative of three independent experiments.
Blocking Fas–FasL Interaction during Cognate DC–T Cell Interaction Partly Inhibits the Production of IL-1β and IFN-γ.
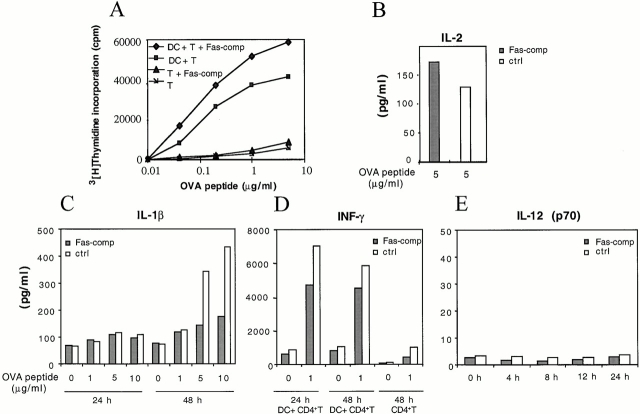
To gain insight on the functional activation of DCs by FasL, we measured cytokine production by DCs during antigen-specific T cell proliferation in the presence or absence of a Fas-comp 15. CD4+ T cells were purified from TCR-OVA DO11.10 mice and incubated with a homogenous mouse bone marrow–derived, long-term DC line (D8; T/DC, 5:1) in the presence of varying concentrations of OVA 327–339 peptide, with or without 0.5 μg/ml Fas-comp. This concentration of competitor completely inhibited Fas-induced activation of DCs (not shown). As shown in Fig. 6 A, T cell proliferation was not reduced in the presence of Fas-comp; rather, an increase in thymidine uptake was observed in the presence of Fas-comp, probably because of a direct interaction with FasL on T cells. Consistently, the amount of IL-2 produced increased in the presence of Fas-comp. In contrast, DCs were impaired in their capacity to secrete IL-1β, as shown by the inhibition of cytokine released 48 h after DC–T cell interaction in the presence of Fas-comp (Fig. 6 C). Although the production of IL-2 by T cells was increased in the presence of Fas-comp, the amount of IFN-γ produced was reduced (Fig. 6 D), suggesting an important role of Fas–FasL interaction for the polarization of T cells. The reduced inhibition of IFN-γ observed at 48 h of antigen presentation could result from a suboptimal amount of Fas-comp when T cells are actively proliferating. Interestingly, the production of IFN-γ was not dependent on IL-12 secretion because bioactive IL-12 (p70) could not be detected in culture supernatants throughout the entire proliferative response (Fig. 6 E).
Figure 6.
IL-1β and IFN-γ release is partly inhibited by Fas-comp during cognate DC–T cell interaction. (A) DO11.10 Tg T cell proliferative response to the OVA 327–339 peptide in the presence (♦) or absence of 0.5 μg/ml Fas-comp (▪). T cells were incubated with or without D8 cells, and T cell proliferation was measured by [3H]thymidine incorporation. (B) IL-2 level in culture supernatants from the experiment in A at 5 μg/ml of peptide with Fas-comp (Fas-comp, gray bars) or without Fas-comp (ctrl, white bars) (0.5 μg/ml). (C) IL-1β was greatly reduced in the presence of Fas-comp at 48 h. (D) IFN-γ secretion was reduced in the presence of Fas-comp at 24 h, and little cytokine production was observed in the absence of DCs (CD4+T). (E) IL-12 was almost undetectable (<5 pg/ml) at all analyzed time points, both with and without Fas-comp. Results are representative of three independent experiments.
Discussion
Unlike B cells and macrophages, which are susceptible to Fas-induced cell death 19 20 21, DCs are largely resistant to FasL. Although it has been reported that murine DCs undergo apoptosis after cognate T cell interaction, partly mediated by Fas–FasL interaction 4, several studies have shown that, in vivo, T cells induce survival of DCs 22, and that in vitro, neither T cells nor FasL induce DC death 3 18. In this report, we confirm that DCs are resistant to Fas-mediated cell death, irrespective of their maturation stage. This resistance correlates with the constitutive expression in DCs of FLIP, an intracellular inhibitor of apoptotsis.
DCs have the unique capacity to prime T cells, and the costimulatory surface molecules B7 and CD40 contribute to this function 1. The counterpart of B7 on T cells, CD28 for instance, increases the duration of TCR signaling which correlates with T cell activation 23. A noncanonical costimulatory molecule expressed on T cells is Fas. The engagement of Fas on naive T cells, in suboptimal stimulation via the TCR, induces the proliferation of T cells 7 24. Conversely, activated CTLs or Th1 cells express high levels of FasL 25; Fas–FasL interaction on T cells has been proposed to promote the differentiation of naive T cells into functional T cells 5 10. In this report, we demonstrate that the engagement of Fas on immature DCs drives their phenotypical maturation and induces the secretion of proinflammatory cytokines such as TNF-α and IL-1β. Moreover, mature DCs exposed to anti-Fas produce increased amounts of IL-1β, indicating a different outcome of Fas engagement of immature and mature DCs. IL-1β was also produced during cognate DC–T cell interaction and its secretion was blocked with Fas-comp, indicating a direct involvement of Fas–FasL for its production. Since FLIP is constitutively expressed in DCs, and as a consequence, caspase-8 and IL-1β–converting enzyme are inhibited, this IL-1β production is likely to be caspase-1 independent. This result concurs with recent data showing that FasL induces caspase-1–independent IL-1β secretion in peritoneal exudate cells 26.
Several reports have shown an important role of IL-1β in the pathogenesis of organ-specific immunity. The exposure of β islet cells to IL-1β induces a selective and functional expression of Fas on β islet cells through the production of nitrous oxide 27. Subsequently, Fas expressed on β islet cells is recognized by FasL on activated T cells causing tissue destruction. Since mature DCs produce large amounts of IL-1β in response to Fas–FasL interaction, we can speculate that during a chronic inflammatory state, such as in the case of organ-specific autoimmunity, mature DCs recruited at the inflammation site are activated by FasL-expressing T cells to produce IL-1β, which in turn can contribute to the progression of the disease. A recent report, in agreement with this hypothesis, shows that transgenic nonobese diabetic mice expressing FasL in β cells are more sensitive to diabetogenic T cells 28 in a FasL-dependant manner. Since transgenic β cells were also expressing higher amounts of Fas, it is possible that these cells, by expressing FasL, were directly activating DCs for IL-1β production, which in turn would be responsible for Fas induction. Accordingly, transplantation of the fetal pancreas from transgenic mice expressing murine CD95L on their β islet cells under the kidney capsules of allogeneic animals resulted in rapid graft rejection 29.
Though murine myeloid DCs did not secrete IL-12 after Fas ligation, T cells did produce IFN-γ in a Fas–FasL-dependant manner. A Fas-comp did not affect T cell proliferation and IL-2 production. Rather, an increased proliferation was observed, suggesting that Fas expressed on DCs may have a dual role as both the activator of T cell proliferation and the director of T cell polarization into a Th1 phenotype, even in the absence of IL-12. Further experiments on the effects of Fas–FasL interaction on T cells will be needed to address this question.
Finally, our results demonstrate that Fas–FasL interaction induces the maturation of DCs, the secretion of large amounts of IL-1β by DCs, and drives the polarization of T cells into a Th1 phenotype. These findings explain, at least in part, the adverse effects obtained by the transgenic expression of FasL in transplanted organs 28 29 and puts clear constraints on the therapeutic use of FasL in autoimmune diseases.
Acknowledgments
We thank Dr. S. Lebecque for providing us with the anti–DC-LAMP antibody, and F. Leuba (Department of Dermatology, University Hospital of Geneva) for his technical assistance.
This work was supported by grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC), the European Community Mucosal Immunization and Vaccine Development (MUCIMM) contract, and a grant from the Leenaards Foundation (to V. Piguet and L. French).
Footnotes
M. Rescigno and V. Piguet contributed equally to this work.
References
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Winzler C., Rovere P., Rescigno M., Granucci F., Penna G., Adorini L., Zimmermann V.S., Davoust J., Ricciardi-Castagnoli P. Maturation stages of mouse dendritic cells in growth factor–dependent long-term cultures. J. Exp. Med. 1997;185:317–328. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashany D., Savir A., Bhardwaj N., Elkon K.B. Dendritic cells are resistant to apoptosis through the Fas (CD95/APO-1) pathway. J. Immunol. 1999;163:5303–5311. [PubMed] [Google Scholar]
- Matsue H., Edelbaum D., Hartmann A.C., Morita A., Bergstresser P.R., Yagita H., Okumura K., Takashima A. Dendritic cells undergo rapid apoptosis in vitro during antigen-specific interaction with CD4+ T cells. J. Immunol. 1999;162:5287–5298. [PubMed] [Google Scholar]
- Lynch D.H., Ramsdell F., Alderson M.R. Fas and FasL in the homeostatic regulation of immune responses. Immunol. Today. 1995;16:569–574. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Tanaka M., Brannan C.I., Jenkins N.A., Copeland N.G., Suda T., Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Kennedy N.J., Kataoka T., Tschopp J., Budd R.C. Caspase activation is required for T cell proliferation. J. Exp. Med. 1999;190:1891–1896. doi: 10.1084/jem.190.12.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J., Irmler M., Thome M. Inhibition of fas death signals by FLIPs. Curr. Opin. Immunol. 1998;10:552–558. doi: 10.1016/s0952-7915(98)80223-9. [DOI] [PubMed] [Google Scholar]
- Irmler M., Thome M., Hahne M., Schneider P., Hofmann K., Steiner V., Bodmer J.L., Schroter M., Burns K., Mattmann C. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Budd R.C., Holler N., Thome M., Martinon F., Irmler M., Burns K., Hahne M., Kennedy N., Kovacsovics M., Tschopp J. The caspase-8 inhibitor FLIP promotes activation of NF-kappaB and Erk signaling pathways. Curr. Biol. 2000;10:640–648. doi: 10.1016/s0960-9822(00)00512-1. [DOI] [PubMed] [Google Scholar]
- Rescigno M., Martino M., Sutherland C.L., Gold M.R., Ricciardi-Castagnoli P. Dendritic cell survival and maturation are regulated by different signaling pathways. J. Exp. Med. 1998;188:2175–2180. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maria R., Testi R. Fas-FasL interactionsa common pathogenetic mechanism in organ-specific autoimmunity. Immunol. Today. 1998;19:121–125. doi: 10.1016/s0167-5699(97)01202-4. [DOI] [PubMed] [Google Scholar]
- Arrighi J.F., Hauser C., Chapuis B., Zubler R.H., Kindler V. Long-term culture of human CD34+ progenitors with FLT3-ligand, thrombopoietin, and stem cell factor induces extensive amplification of a CD34−CD14− and a CD34−CD14+ dendritic cell precursor. Blood. 1999;93:2244–2252. [PubMed] [Google Scholar]
- Citterio S., Rescigno M., Foti M., Granucci F., Aggujaro D., Gasperi C., Matyszak M., Girolomoni G., Ricciardi-Castagnoli P. Dendritic cells as natural adjuvants. Methods. 1999;19:142–147. doi: 10.1006/meth.1999.0839. [DOI] [PubMed] [Google Scholar]
- Holler N., Kataoka T., Bodmer J.L., Romero P., Romero J., Deperthes D., Engel J., Tschopp J., Schneider P. Development of improved soluble inhibitors of FasL and CD40L based on oligomerized receptors. J. Immunol. Methods. 2000;237:159–173. doi: 10.1016/s0022-1759(99)00239-2. [DOI] [PubMed] [Google Scholar]
- Schmid I., Uittenbogaart C.H., Giorgi J.V. Sensitive method for measuring apoptosis and cell surface phenotype in human thymocytes by flow cytometry. Cytometry. 1994;15:12–20. doi: 10.1002/cyto.990150104. [DOI] [PubMed] [Google Scholar]
- Piguet V., Gu F., Foti M., Demaurex N., Gruenberg J., Carpentier J.L., Trono D. Nef-induced CD4 degradationa diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of β-COP in endosomes. Cell. 1999;97:63–73. doi: 10.1016/s0092-8674(00)80715-1. [DOI] [PubMed] [Google Scholar]
- Willems F., Amraoui Z., Vanderheyde N., Verhasselt V., Aksoy E., Scaffidi C., Peter M.E., Krammer P.H., Goldman M. Expression of c-FLIP(L) and resistance to CD95-mediated apoptosis of monocyte-derived dendritic cellsinhibition by bisindolylmaleimide. Blood. 2000;95:3478–3482. [PubMed] [Google Scholar]
- Rothstein T.L., Wang J.K., Panka D.J., Foote L.C., Wang Z., Stanger B., Cui H., Ju S.T., Marshak-Rothstein A. Protection against Fas-dependent Th1-mediated apoptosis by antigen receptor engagement in B cells. Nature. 1995;374:163–165. doi: 10.1038/374163a0. [DOI] [PubMed] [Google Scholar]
- Ashany D., Song X., Lacy E., Nikolic-Zugic J., Friedman S.M., Elkon K.B. Th1 CD4+ lymphocytes delete activated macrophages through the Fas/APO-1 antigen pathway. Proc. Natl. Acad. Sci. USA. 1995;92:11225–11229. doi: 10.1073/pnas.92.24.11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson B.C., Lalwani N.D., Johnson K.J., Marks R.M. Fas ligation triggers apoptosis in macrophages but not endothelial cells. Eur. J. Immunol. 1994;24:2640–2645. doi: 10.1002/eji.1830241111. [DOI] [PubMed] [Google Scholar]
- De Smedt T., Pajak B., Klaus G.G., Noelle R.J., Urbain J., Leo O., Moser M. Antigen-specific T lymphocytes regulate lipopolysaccharide-induced apoptosis of dendritic cells in vivo. J. Immunol. 1998;161:4476–4479. [PubMed] [Google Scholar]
- Iezzi G., Karjalainen K., Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- Alderson M.R., Armitage R.J., Maraskovsky E., Tough T.W., Roux E., Schooley K., Ramsdell F., Lynch D.H. Fas transduces activation signals in normal human T lymphocytes. J. Exp. Med. 1993;178:2231–2235. doi: 10.1084/jem.178.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., Okazaki T., Naito Y., Yokota T., Arai N., Ozaki S., Nakao K., Nagata S. Expression of the Fas ligand in cells of T cell lineage. J. Immunol. 1995;154:3806–3813. [PubMed] [Google Scholar]
- Miwa K., Asano M., Horai R., Iwakura Y., Nagata S., Suda T. Caspase 1-independent IL-1β release and inflammation induced by the apoptosis inducer Fas ligand. Nat. Med. 1998;4:1287–1292. doi: 10.1038/3276. [DOI] [PubMed] [Google Scholar]
- Stassi G., De Maria R., Trucco G., Rudert W., Testi R., Galluzzo A., Giordano C., Trucco M. Nitric oxide primes pancreatic β cells for Fas-mediated destruction in insulin-dependent diabetes mellitus. J. Exp. Med. 1997;186:1193–1200. doi: 10.1084/jem.186.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervonsky A.V., Wang Y., Wong F.S., Visintin I., Flavell R.A., Janeway C.A., Jr., Matis L.A. The role of Fas in autoimmune diabetes. Cell. 1997;89:17–24. doi: 10.1016/s0092-8674(00)80178-6. [DOI] [PubMed] [Google Scholar]
- Allison J., Georgiou H.M., Strasser A., Vaux D.L. Transgenic expression of CD95 ligand on islet β cells induces a granulocytic infiltration but does not confer immune privilege upon islet allografts. Proc. Natl. Acad. Sci. USA. 1997;94:3943–3947. doi: 10.1073/pnas.94.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]