Abstract
During their final differentiation or maturation, dendritic cells (DCs) redistribute their major histocompatibility complex (MHC) class II products from intracellular compartments to the plasma membrane. Using cells arrested in the immature state, we now find that DCs also regulate the initial intracellular formation of immunogenic MHC class II–peptide complexes. Immature DCs internalize the protein antigen, hen egg lysozyme (HEL), into late endosomes and lysosomes rich in MHC class II molecules. There, despite extensive colocalization of HEL protein and MHC class II products, MHC class II–peptide complexes do not form unless the DCs are exposed to inflammatory mediators such as tumor necrosis factor α, CD40 ligand, or lipoplolysaccharide. The control of T cell receptor (TCR) ligand formation was observed using the C4H3 monoclonal antibody to detect MHC class II–HEL peptide complexes by flow cytometry and confocal microscopy, and with HEL-specific 3A9 transgenic T cells to detect downregulation of the TCR upon MHC–peptide encounter. Even the binding of preprocessed HEL peptide to MHC class II is blocked in immature DCs, including the formation of C4H3 epitope in MHC class II compartments, suggesting an arrest to antigen presentation at the peptide-loading step, rather than an enhanced degradation of MHC class II–peptide complexes at the cell surface, as described in previous work. Therefore, the capacity of late endosomes and lysosomes to produce MHC class II–peptide complexes can be strictly controlled during DC differentiation, helping to coordinate antigen acquisition and inflammatory stimuli with formation of TCR ligands. The increased ability of maturing DCs to load MHC class II molecules with antigenic cargo contributes to the >100-fold enhancement of the subsequent primary immune response observed when immature and mature DCs are compared as immune adjuvants in culture and in mice.
Keywords: dendritic cell, maturation, MHC class II–peptide complex, lysosome, inflammation
Introduction
Dendritic cells (DCs), perhaps the most potent and versatile of APCs, capture foreign antigens encountered in peripheral tissues, process the antigens into peptides bound to MHC molecules, and migrate to lymphoid organs for presentation of the MHC–peptide complexes to T lymphocytes 1 2. In keeping with this critical role in stimulating antigenically naive T cells, the immunostimulatory activity of DCs is carefully regulated, with the cells exhibiting at least two functionally distinct states. Resting or immature DCs, corresponding to those found in the periphery, generally have a high capacity for endocytosis and thus for antigen uptake 3 4 5 6, but a low capacity for binding and stimulating T cells. Mature DCs, on the other hand, are generated from immature cells by exposure to proinflammatory agents (TNF, LPS), physical trauma, or T cell surface molecules (CD40 ligand [CD40L]; references 7–10). Mature DCs exhibit a limited capacity for endocytosis, but they are exceptional at stimulating T cells 3 4 5 6 7 8 9 10, typically presenting immunogenic peptides derived from previously encountered antigens. The capacity of mature DCs to activate naive T cells reflects several developmentally regulated changes. These include upregulation of CD40, CD80, and CD86 costimulatory molecules 11 12, release of cytokines like IL-12 13 14, resistance to IL-10–mediated immunosuppression 15, and expression of new chemokine receptors like CCR7 that guide DCs to the T cell areas 16 17 18 19. Importantly, mature DCs upregulate surface MHC class II and functional MHC class II–peptide complexes that engage the TCR 20 21.
This increased expression of MHC class II molecules is known to reflect at least two alterations 20 21. First, newly synthesized MHC class II molecules become rerouted intracellularly. Instead of being transported to lysosomes for sequestration or degradation, the MHC class II is targeted to the plasma membrane. One underlying mechanism involves the activation of the APC-restricted protease cathepsin S, which in turn mediates the post–Golgi complex cleavage of the MHC class II–associated invariant (Ii) chain. These events facilitate the early release of Ii chain from MHC class II α/β dimers, allowing surface transport rather than intracellular retention due to lysosomal targeting/endocytosis signals found in the Ii chain cytoplasmic domain 22. The cleavage of Ii could also free the MHC class II binding site for antigen binding 23 24, but this level of regulation has not yet been demonstrated in maturing DCs. Second, because of the downregulation of endocytosis that occurs concomitant with maturation, there is an increase in the surface residence time, half-life, and thus overall expression of any MHC class II–peptide complexes that do reach the plasma membrane 20 21.
To further delineate the mechanism underlying the critical conversion of DCs from immature sentinels to mature immunostimulatory cells, we made use of a monoclonal antibody, C4H3, that allows the direct visualization of a peptide–MHC class II complex formed from a hen egg lysozyme (HEL) peptide and an I-Ak α/β dimer 25 26. We also identified conditions that allow the in vitro maintenance and rapid manipulation of immature bone marrow–derived DCs. Together, these approaches revealed an additional and more fundamental level at which the development of antigen presentation capacity is controlled during DC maturation, the actual formation of MHC class II–peptide complexes within MHC class II–rich lysosomal compartments. We show that this maturation is a major control for T cell priming by DCs in vivo, a new finding that impacts upon their optimal use for immunotherapy in humans 27 28 29 30.
Materials and Methods
Mice.
CBA/J and lipid A–unresponsive C3H/HeJ female mice were purchased from The Jackson Laboratory or from Japan SLC, and were used at 6–8 wk of age.
Dendritic Cells.
DCs were grown in RPMI 1640 containing 5% FCS from bone marrow progenitors with mouse rGM-CSF 31 at 10 ng/ml (gift of Dr. Y. Yamaguchi, Kirin, Maebashi, Japan), or the supernatant (3% vol/vol) from J558L cells transduced with murine (m)GM-CSF (from Dr. A. Lanzavecchia, Basel Institute, Basel, Switzerland). At day 6, the cultures contained many aggregates of immature DCs loosely attached to the monolayer. To mature the DCs, we added either TNF (100–500 U/ml; Dainippon Pharmaceutical Co.), CD40L (a baculovirus preparation provided by Drs. M. Nussenzweig and Y. Choi, The Rockefeller University, New York, NY), or LPS (1–10 ng/ml, Escherichia coli type 0111.B4; Sigma Chemical Co.), or we simply transferred the cells to a fresh vessel at <5 × 106 cells/ml 31.
Antigen Administration.
HEL (Sigma Chemical Co.) was added to immature bone marrow DCs at 30–3,000 μg/ml for 0.5–24 h. Also, explants of ear skin (epidermis and dermis) were bathed in 3,000 μg/ml, after which the DCs were examined within epidermal sheets or as cells that had emigrated from the explants 32 33. The dominant HEL 46-61 peptide for I-Ak was synthesized at Yale University Medical School. OVA was used as control protein. HEL protein uptake was visualized with 1B12 monoclonal IgG2b anti-HEL antibody, provided by Dr. P. Allen (Washington University, St. Louis, MO), and MHC class II–HEL peptide complexes visualized with C4H3 rat IgG2b monoclonal antibody 25 26. Antibody staining, including that with isotype controls (PharMingen), was assessed on a FACScan™ (Becton Dickinson) or by immunofluorescence confocal microscopy. DCs were identified by labeling for the I-E MHC II product (14-4-4S antibody), and mature DCs by high expression of CD86 (GL-1; PharMingen).
Removal of Endotoxin Activity.
To deplete much of the endotoxin activity (limulus amebocyte assay; BioWhittaker) in HEL preparations, HEL at 10 mg/ml was adsorbed with tachyplesin III–conjugated Sepharose CL (34; Kuttsuclean™; Maruha Corp.) according to the manufacturer's instructions.
Antigen Presentation Assays.
Presentation of HEL to T cells was monitored using purified CD4+ T cells from 3A9 TCR transgenic mice 35 provided by Dr. M. Davis (Stanford University, Palo Alto, CA). These T cells are specific for the same MHC class II–peptide complex recognized by the C4H3 antibody. Graded doses of DCs that were exposed to HEL minus or plus a maturation stimulus were applied to 250,000 CD4+ transgenic T cells in 96-well flat-bottomed microtest plates in RPMI 1640 containing 5% FCS. The DCs were fixed beforehand in 0.75% paraformaldehyde for 30 min on ice. CD4+ T cells were enriched by negative selection from spleen and lymph node suspensions by coating other cells with antibodies (TIB 120 anti–MHC class II, TIB 207 anti-CD8, HB198 F4/80 anti-macrophage, 6B2 anti-B220, and NK1.1) and depleting them with sheep anti–rat Ig Dynabeads® M-450 (No. 110.08; Dynal). T cell responses were monitored at 5 h by a decrease in TCR (Vβ8) or increase in CD69 (PharMingen antibodies), or at 30–42 h by [3H]thymidine (3H-TdR) uptake at 1 μCi/ml. Data are from triplicate cultures with SE <10% of the mean. For presentation studies in vivo with adoptively transferred DCs, immature cells were cultured with graded doses of HEL overnight with or without CD40L or LPS as a maturation stimulus. The DCs were harvested, washed, and injected subcutaneously at a dose of 200,000 DCs per paw of nontransgenic mice. 5 d later, the draining lymph nodes were removed, dissociated into single cell suspensions, and cultured at 300,000 cells per flat-bottomed microtest well in Click's medium with 0.75% mouse serum and graded doses of HEL. 3H-TdR uptake was measured at 52–64 h to document the extent of CD4+ T cell priming.
Results
Synergistic Effects between the Exposure to Antigen and a Maturation Stimulus in the Formation of MHC Class II–Peptide Complexes.
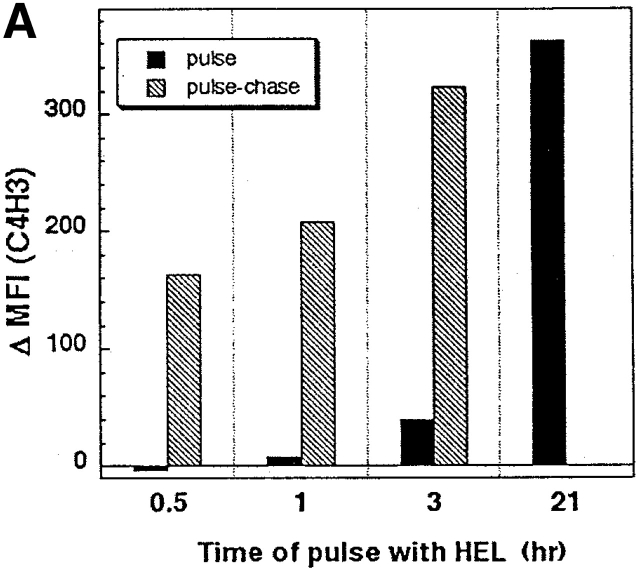
Immature DCs from day 6 GM-CSF–stimulated marrow cultures 31 were exposed for various periods of time to increasing concentrations of HEL. The formation of the C4H3 epitope, i.e., MHC class II–HEL peptide complexes, was monitored by FACS®. Strong signals were not seen at the DC surface after a 3-h exposure to HEL, but were present after 18–24 h (pulse data; Fig. 1 A). The formation of C4H3 was proportional to the added dose of HEL; signals became detectable at 30 μg/ml (not shown). Immature DCs only had to be pulsed a short time with HEL to develop strong C4H3 signals 1 d later (pulse–chase data; Fig. 1 A). For example, a 3-h pulse of 3,000 μg/ml HEL followed by an 18-h chase in its absence gave comparable C4H3 staining to continuous culture in HEL.
Figure 1.
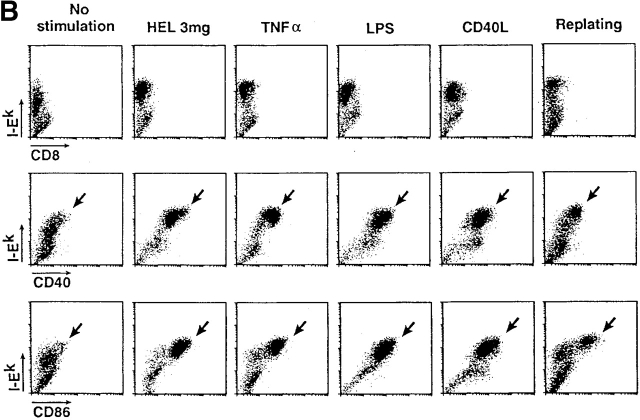
Formation of MHC–peptide complexes by bone marrow–derived DCs. (A) DCs in bone marrow cultures were pulsed with 3,000 μg/ml of HEL for the times indicated on the x-axis, washed, and assayed immediately for C4H3 staining (pulse) or chased until 21 h of total culture (pulse–chase). The y-axis displays increases in mean fluorescence index (MFI) for I-E+ DCs relative to cultures in the absence of HEL protein. (B) A maturation stimulus is present in commercial preparations of HEL. Immature 6-d marrow cultures were maintained for one additional day in the absence of another stimulus or in the presence of the stimuli listed on the top, including transfer to a new vessel (right). Maturation was monitored at the level of surface MHC class II expression (y-axis) and surface CD40 and CD86 (x-axis), with anti-CD8 antibody used as a nonreactive isotype control.
However, the addition of HEL to immature DCs not only led to the appearance of C4H3 epitope, but also to maturation. Most MHC class II–positive DCs began to express high levels of CD40 and CD86 and higher levels of surface MHC class II compared with cultures not incubated with HEL (Fig. 1 B). Although initially surprising, HEL-induced maturation could be explained by the fact that 1 mg/ml HEL contained high endotoxin activity, 1–10 ng/ml in the limulus endotoxin assay, as reported 36. Likewise, simple transfer of the immature DCs to a fresh culture dish, a procedure that is used to enhance the expansion of residual proliferating cells in the culture, enhanced maturation (Fig. 1 B; references 21 and 31). Therefore, in all subsequent experiments, a new invertebrate adsorbent termed Kuttsuclean™ was used to remove most endotoxin 34 without decreasing HEL content, and we compared immature and maturing cells in cultures that were not subcultured from their initial wells.
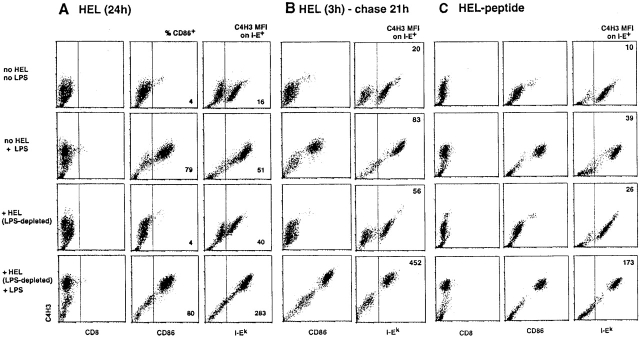
The addition of Kuttsuclean™-treated HEL did not induce DC maturation, as indicated by a lack of generation of CD86+ cells or the increased expression of surface MHC class II (I-E and I-A). Importantly, only small amounts of C4H3 epitope appeared at the plasma membrane (Fig. 2 A). DCs incubated under these conditions could be kept in the immature state for at least 3–4 d. In prior work 20 21, immature DCs exhibited a slow but spontaneous rate of maturation, and some formation of the SDS stable dimers suggestive of MHC–peptide complex formation. The maturation stimuli (LPS, TNF, CD40L, and transfer to a new culture vessel) induced an increase in CD86+ cells, and some increase in background C4H3 staining (Fig. 2 A), presumably because the higher levels of I-A on mature DCs presented self-peptides that cross-reacted with HEL peptide 25 26. Nevertheless, the combination of HEL and a maturation stimulus was markedly synergistic, with very high C4H3 levels being observed on most DCs in >10 similar experiments (Fig. 2 A).
Figure 2.
Antigen and a maturation stimulus synergize to form MHC–peptide complexes. (A) Day 6 bone marrow cultures from CBA/J mice were cultured for 24 h in the absence of HEL or LPS, with either HEL (1,000 μg/ml) or LPS (5 ng/ml), or with both. The HEL had been passed over a Kuttsuclean™ adsorbent. Left: anti-CD8 isotype control. Middle: CD86 marker for DC maturation, with the percentage of mature CD86-rich cells indicated. Right: mean fluorescent index (MFI) of the C4H3 signal on I-E+ DCs. Similar results were obtained using C3H/HeJ DCs matured with CD40L in three experiments. (B) Cells were pulsed with HEL protein for 3 h, and then washed before adding the maturation stimulus where indicated for a chase period of 21 h. (C) Cells were pulsed with preprocessed HEL peptide for 3 h, and then washed before adding LPS where indicated for a chase period of 21 h.
Mechanisms Underlying the Synergism of Antigen and a Maturation Stimulus in Controlling MHC Class II–Peptide Complex Formation.
To establish that the maturation stimulus was not merely increasing uptake of HEL, we applied the antigen as a pulse for 3 h, and then washed the cells before the addition of LPS or CD40L. The synergistic effect of the maturation stimulus remained apparent in all three experiments (Fig. 2 B), indicating that maturation functioned to enhance HEL processing and/or formation of C4H3-reactive MHC class II–HEL peptide complexes. In a companion study 37, we found that the HEL-pulsed DCs can be cultured for at least 48 h before adding a maturation stimulus, but the cells still display C4H3 at levels >50% of that seen when the addition of LPS or CD40L is not delayed. Therefore, maturation stimuli increase the formation of TCR ligands, and do not simply reduce their degradation.
To determine if maturation was acting only at the level of antigen processing/proteolysis, we pulsed DCs for 3 h with preprocessed HEL peptide (that is able to bind directly to I-Ak), washed the cells, and then cultured without or with a maturation stimulus. The latter was again required for MHC–peptide complex formation (Fig. 2 C), indicating that peptide access to the MHC class II binding site was controlled by the maturation process.
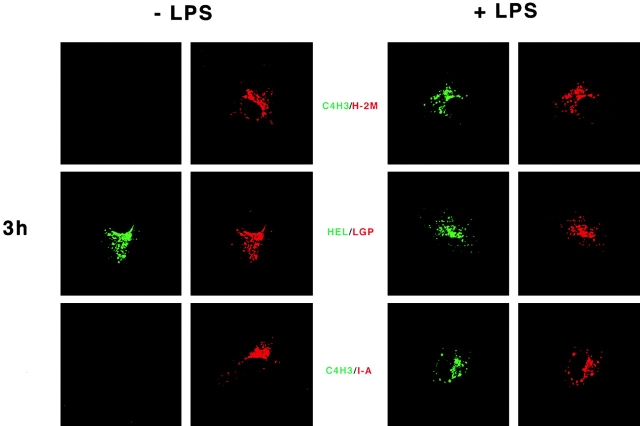
Conceivably, maturation might control C4H3 epitope expression by controlling the delivery of HEL to the MHC class II compartments (MIICs) that are thought to be critical for MHC–peptide complex formation. Therefore, we performed immunofluorescence confocal microscopy to localize C4H3 in the absence or presence of a maturation stimulus. As shown in Fig. 3 (left), immature DCs accumulated endotoxin-free HEL protein in intracellular compartments, as detected with 1B12 antibody to intact HEL. These structures were positive for MHC class II, Ii chain, and the peptide editing H-2M product, and were judged to be late endosomes and lysosomes by staining for the lysosomal membrane glycoprotein, lgp-B (or lysosomal-associated membrane protein [LAMP]-2; Fig. 3). Despite the fact that HEL was delivered to MIICs, no C4H3 staining was observed even after incubations of up to 24 h in HEL (Fig. 3) or preprocessed HEL peptide (not shown). Also, no C4H3 staining was found when only a maturation stimulus was given (LPS or CD40L; not shown), in spite of the weak signals on the FACS® (Fig. 2).
Figure 3.
Localization of HEL protein and MHC class II–peptide complexes in DCs. The procedure was the same as described in the legend to Fig. 2, but here the formation of MHC class II–peptide was monitored at 3 and 24 h by immunofluorescence confocal microscopy. On the left are CBA DCs cultured in HEL only, and on the right are cells cultured with HEL + LPS. Representative cells are shown following double labeling in green for the HEL antigen, either HEL protein with 1B12 antibody or I-Ak/HEL MHC-peptide complexes with C4H3. Red stain identifies the lysosomal membrane glycoprotein lysosomal-associated membrane protein 2. Identical red staining is seen with H-2M or MHC class II. The results are representative of >10 experiments, and of experiments with both HEL protein and preprocessed HEL peptide. The maturation-induced C4H3 signal at 24 h is shown for a well-spread cell, but in optical sections through thicker cells, C4H3 stain is almost entirely along the cell perimeter.
Quite a different result was obtained in cells exposed to HEL together with a maturation stimulus (Fig. 3, right). As before, HEL protein was found in MIICs, but in all the DCs, abundant C4H3 stain could also be observed within MIICs. After 3 h or less in HEL, C4H3 staining was only observed within MIICs (Fig. 3). At 24 h, most of the MHC–peptide complexes were on the cell surface (Fig. 3), as described previously for the distribution of MHC class II products in maturing DCs 20 21. Similar findings were made in pulse–chase experiments, carried out as in Fig. 2 B; when a maturation stimulus was applied after the pulse with HEL antigen, MHC–peptide complexes began to form in MIICs within 3 h, and were later found on the cell surface.
MIICs as a Site for MHC–Peptide Complex Formation in the Epidermis.
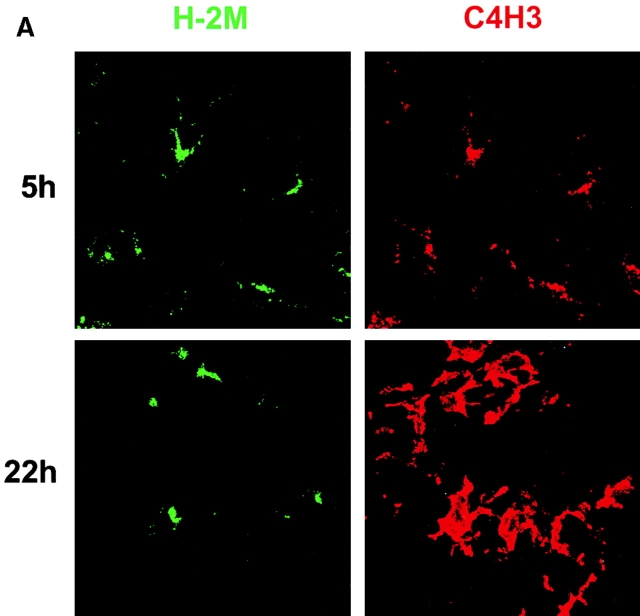
To visualize the intracellular sites for MHC class II–peptide formation in DCs in intact tissue, we studied epidermal DCs that mature when they migrate from skin 32 33 via afferent lymphatics 38 39. Endotoxin-containing HEL was injected into the ear dermis or added to skin explants in vitro. Epidermal sheets and the emigrated DCs were then examined. 5 h after injection, clear cut colabeling for C4H3 and the lysosomal marker H-2M was observed within all DCs in the epidermal sheets (Fig. 4 A), but not in DCs given control OVA protein. At 22 h, the C4H3 was on the surface of most DCs (Fig. 4 A). DCs that emigrated from explants into the medium during 2 d of culture had a mature CD86++ phenotype (Fig. 4 B) and stained strongly for C4H3 if HEL had been added to the explant. If HEL was added to mature DCs after emigration, C4H3 staining was not seen (Fig. 4 B). Thus, immature endocytic epidermal DCs form MHC–peptide complexes in MIICs in vivo, but mature DCs are no longer able to do so.
Figure 4.
Formation of MHC–peptide complexes in immature DCs in situ. (A) Explants of CBA ear skin were bathed in 3,000 μg/ml LPS containing HEL for 5 or 22 h. At each time point, epidermal sheets were prepared and double labeled for the H-2M marker of MIICs (green) and for C4H3 epitope (red). The sheets were examined by two-color immunofluoresence confocal microscopy. The effect of a maturation stimulus could not be examined in vivo, because simple injection of PBS could induce some DCs to mature. (B) Explants of ear skin were cultured in the absence or presence of HEL for 48 h, and then the emigrated cells were doubled labeled for CD86 to identify the DCs (arrows) and C4H3. Some of the cells that emigrated in the absence of HEL were then cultured for 2 d in the presence of HEL before similar FACS® studies. The MFI for CD86+ cells is given in each panel.
Functional Consequences of the Synergism between Antigen and a Maturation Stimulus: In Vitro Studies of Antigen Presentation to Naive TCR Transgenic T Cells.
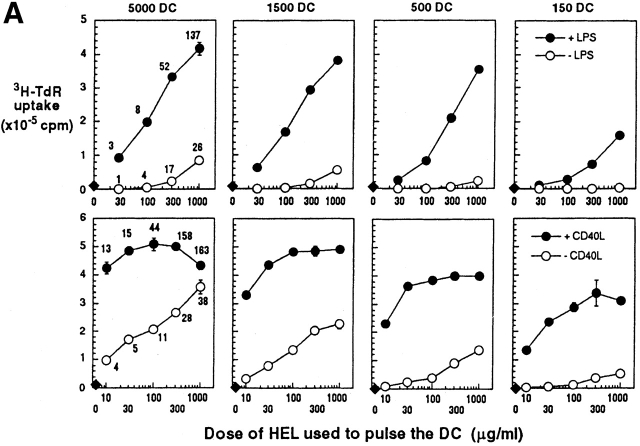
In vitro antigen presentation assays were used to assess the functional consequences of DC maturation and increased amounts of MHC–peptide complexes. We used naive 3A9 TCR transgenic T cells 35 specific for the same I-Ak–HEL peptide complex recognized by C4H3 antibody. Immature, marrow-derived DCs were exposed for 1 d to HEL in graded doses, to CD40L or LPS, or to both HEL and the maturation stimulus. The cells were washed, fixed in paraformaldehyde, and added in graded doses to TCR transgenic T cells (Fig. 5 A). Fixation ensured that the DCs did not mature during the culture with T cells. HEL-pulsed immature DCs only weakly stimulated the TCR transgenic T cells, but when the DCs had been exposed to both HEL and CD40L or LPS, strong T cell stimulation ensued (Fig. 5 A). With mature DCs, the DNA synthesis (3H-TdR uptake) that we observed early in the response (day 2) with low DC to T cell ratios (1:60–1:2,000) was >2 × 105 cpm per well under standard assay conditions, and it was proportional to the level of MHC class II–peptide on the DCs (Fig. 5 A).
Figure 5.
Antigen presenting activity of DCs in vitro after culture with graded doses of HEL, minus or plus LPS or CD40L as a maturation stimulus. (A) Day 6 bone marrow DCs from CBA mice (top) or C3H/HeJ mice (bottom) were cultured for 20 h with graded doses of HEL (x-axis) that had been endotoxin depleted with Kuttsuclean™. One group of cultures was matured by simultaneous addition of LPS or CD40L (closed symbols), and the other was unstimulated (open symbols). After 20 h, the cells were harvested, washed, and fixed in paraformaldehyde to block further processing and maturation. MHC–peptide complexes were quantified in terms of C4H3 staining, and are shown as mean fluorescence indices on the left. The fixed DCs were added in graded doses (top) to 250,000 CD4+ T cells from 3A9 TCR transgenic mice (specific for the same complex of I-Ak + HEL peptide as C4H3 antibody). 3H-TdR uptake was measured at 30–42 h. One of three similar experiments. (B) The display of TCR ligands by fixed DCs was monitored at 5 h on T cells (gated away from DCs by light scattering) by the criteria of TCR downregulation (anti-Vβ8, y-axis) and CD69 upregulation (x-axis). The DC/T cell ratio was 1:3 in the data that are shown, and the percentage of CD69+ cells is indicated in each dot plot (upper right).
The poor T cell–stimulatory activity of immature DCs could reflect a lack of either TCR ligand (“signal one”) or costimulatory molecules such as CD86 (“signal two”). The use of the C4H3 antibody for the detection of signal one was likely to be insensitive relative to that of the TCR. For example, an exposure of DCs to 30 μg/ml of HEL was necessary to detect MHC–peptide complex formation with C4H3 antibody, whereas an exposure to 10 μg/ml was sufficient to saturate T cell stimulation at a DC/T cell ratio of 1:60 (Fig. 5 A, left). To assess MHC class II–peptide complexes at the level of the TCR, we used the criterion of TCR downregulation. Prior studies have shown that the TCR is cleared from the cell surface upon encounter of MHC–peptide complexes; this downregulation appears not to require a special type of APC or the presence of costimulatory molecules on that APC 40 41. Using staining with anti-Vβ8 antibody, we monitored expression of the TCR on 3A9 cells cultured for 5 h with mature or immature HEL-pulsed DCs, prepared exactly as described in the legend to Fig. 5 A. At all doses of HEL studied (10–1,000 μg/ml), mature DCs quickly activated a sizable fraction of the T cells, as indicated by de novo expression of the CD69 T cell activation marker. TCR downregulation was evident on all CD69+ cells. In contrast, immature DCs induced little increase in CD69 or decrease in TCR (Fig. 5a). We conclude that a maturation stimulus is needed for DCs to form MHC class II–peptide complexes, as assessed with B cell (C4H3 antibody) or T cell (the 3A9 TCR) receptors.
Functional Consequences of the Synergism between Antigen and a Maturation Stimulus: In Vivo Studies of Antigen Presentation to Naive Mice.
The above data establish a relationship between maturation and the formation of immunogenic MHC–peptide complexes in vitro, but the relevance of DC maturation to immunogenicity in vivo has yet to be tested. To this end, immature DCs were incubated in graded doses of endotoxin-free HEL with or without a maturation stimulus, CD40L. Unfixed DCs were then injected subcutaneously into naive syngeneic mice. The mature cells were far more powerful immunogens compared with immature cells, and priming was proportional to the dose of HEL and MHC peptide on the DCs (Fig. 6). When we tested T cells isolated from lymph nodes of mice injected with mature HEL-loaded DCs, the proliferation was >100-fold more sensitive to a given dose of antigen. If 25 μg of HEL was injected without DCs, T cell priming was not seen (not shown). Therefore, maturation markedly increases the adjuvant capacity of adoptively transferred DCs that have been previously pulsed with antigens ex vivo.
Figure 6.
Antigen presenting activity of DCs in vivo after culture with graded doses of HEL, minus (open symbols) or plus (closed symbols) CD40L as a maturation stimulus. As described in the legend to Fig. 5, graded doses of antigen (top) were used to pulse C3H/He DCs overnight. The washed, unfixed cells were injected subcutaneously into syngeneic mice, 2 × 105 per paw. 5 d later, cultured draining lymph node cells were challenged with different doses of HEL protein (x-axis), and 3H-TdR uptake measured at 42–48 h (y-axis). Data are shown as stimulation relative to the background cpm in lymph nodes cultured without antigen. One of three similar experiments.
Discussion
As introduced above, maturation changes DCs in many ways that explain their potency in initiating immunity. Maturing cells express surface molecules like CD40 and CD86 required for T cell expansion and cytokine synthesis 11 12, produce high levels of IL-12 13 14, resist suppression by IL-10 15, and upregulate chemokine receptors 16 17 18 19 that recognize chemokines produced in lymphoid organs 42 43. Here, a new feature of maturation has emerged, an intensified capacity to convert endocytosed antigens to MHC class II–peptide complexes.
There is evidence with human monocyte–derived DCs that maturation can prolong the life span of MHC class II–peptide complexes on the cell surface 20. However, direct visualization of these complexes now shows that the maturation process can regulate an earlier and more critical step, the formation of TCR ligands in MIICs. The results in a companion study 37, in which antigen-pulsed DCs can be cultured for 48 h before forming MHC–peptide complexes in response to a maturation stimulus, nullifies the possibility that immature DCs are simply degrading these complexes.
MHC class II products are designed to capture antigenic peptides from pathogens internalized by endocytosis 44 45. Through the chaperone function of the Ii chain 46 47 or by dileucine motifs within the cytoplasmic domain of the class II β chain 48, MHC class II molecules are targeted to acidic compartments termed MIICs 49 50 51. These lysosomes contain proteases for antigen processing, as well as HLA-DM or H-2M molecules for enhancing peptide loading to MHC class II 52 53. Nevertheless, there has been little direct visualization of newly formed MHC–peptide complexes in physiological APCs. A key control is likely to be the uptake and delivery of antigens into MIICs. DCs are actively endocytic at the immature stage of development 3 4, when they also contain numerous MIICs 21 54 55. Both endocytosis and the number of MIICs are decreased, and even extinguished, upon further differentiation or maturation in response to bacteria and TNF family members. Here, we have studied DCs frozen at the immature stage (Materials and Methods). These DCs internalize antigens into MIICs, but surprisingly, MHC class II–peptide complexes fail to form efficiently until an inflammatory stimulus is applied.
Because cathepsin S controls Ii chain proteolysis 56 and the availability of MHC class II molecules for peptide loading, one of the possible mechanisms explaining these data is that maturation downregulates the cathepsin S inhibitor, cystatin C 22. Consistent with this, we observed a striking inability to load the MHC class II products of immature DCs with peptide, until a maturation stimulus was applied. Similar events could take place in vivo, because when the maturation stimulus LPS in HEL preparations is given to mice, there is an accumulation of DCs with high levels of C4H3 in T cell areas 36. The control of MHC class II–peptide formation could also be exerted at other levels, e.g., the acidity of the endocytic system and the function of additional proteases.
In companion studies, we show that MHC class II–peptide complexes, newly formed in the late endosomes or lysosomes of DCs, selectively accumulate, together with CD86 costimulators, into specialized vesicular carriers before their delivery to the plasma membrane 37. We term these MHC class II–positive lysosome-negative vesicles “CIIVs”, for class II vesicles. Remarkably, the MHC class II–peptide complexes and CD86 costimulators remain in clusters at the cell surface. This juxtaposition may explain the potent naive T cell stimulation of DCs that we now observe, even when the cells are fixed in a low dose of formaldehyde (Fig. 5).
Our findings, especially the in vivo priming results in Fig. 6, are relevant to the optimal use of DCs for immunotherapy in humans 27 28 29 30. It is not only valuable to deliver antigens to DCs, but also to mature the cells to increase successful antigen processing and to provide other valuable physiological features cited above. The data also relate to the potential of DCs to control the alternative outcome of antigen deposition, tolerance rather than immunity. Antigen-bearing immature DCs may induce nonresponsiveness, according to views that tolerance develops if T cells recognize antigen in the absence of costimulation 57 58. We would question whether immature DCs, at least the cells that we have generated here from bone marrow progenitors, can directly tolerize, since requisite MHC class II–peptides do not form efficiently. Instead, the recently described transfer of antigen 59 from immature peripheral DCs to resident lymph node–processing DCs may generate the MHC class II peptide complexes needed by tolerizing APCs that are found in lymph nodes 60. The mature cells that we generate ex vivo and that efficiently prime T cells in vivo (Fig. 6) express high levels of CD86 costimulators. We are intrigued by the fact that in the apparent absence of maturation stimuli, lymph node DCs that are implicated in tolerance 60 can express high levels of MHC–peptide but relatively low levels of CD86 61.
Acknowledgments
We are grateful to many colleagues who provided vital reagents for this work: Dr. Mark Davis for 3A9 TCR transgenic T cells, Dr. E. Unanue for 1G12 anti-clonotype antibody, and Dr. P. Allen for 1B12 anti-HEL antibody.
This work was supported by grants to R.M. Steinman from the National Institute of Allergy and Infectious Diseases (AI-13013 and AI-39672) and the Yamanouchi Fund, to I. Mellman from the National Institutes of Health (AI-34098 and GM-33904), and to K. Inaba from the Ministry of Education, Science and Culture of Japan (Nos. 08282104, 10153226, and 10044268).
Footnotes
C. Reis e Sousa's present address is Immunobiology Laboratory, Imperial Cancer Research Fund, London WC2A 3PX, United Kingdom.
Abbreviations used in this paper: CD40L, CD40 ligand; DC, dendritic cell; HEL, hen egg lysozyme; 3H-TdR, [3H]thymidine; Ii chain, MHC class II–associated invariant chain; MIICs, MHC class II compartments.
References
- Hart D.N. Dendritic cellsunique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–3287. [PubMed] [Google Scholar]
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Inaba K., Inaba M., Naito M., Steinman R.M. Dendritic cell progenitors phagocytose particulates, including bacillus Calmette-Guerin organisms, and sensitize mice to mycobacterial antigens in vivo. J. Exp. Med. 1993;178:479–488. doi: 10.1084/jem.178.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis e Sousa C., Stahl P.D., Austyn J.M. Phagocytosis of antigens by Langerhans cells in vitro. J. Exp. Med. 1993;178:509–519. doi: 10.1084/jem.178.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani N., Koide S., Crowley M., Witmer-Pack M., Livingstone A.M., Fathman C.G., Inaba K., Steinman R.M. Presentation of exogenous protein antigens by dendritic cells to T cell clonesintact protein is presented best by immature, epidermal Langerhans cells. J. Exp. Med. 1989;169:1169–1178. doi: 10.1084/jem.169.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pure E., Inaba K., Crowley M.T., Tardelli L., Witmer-Pack M.D., Ruberti G., Fathman G., Steinman R.M. Antigen processing by epidermal Langerhans cells correlates with the level of biosynthesis of major histocompatibility complex class II molecules and expression of invariant chain. J. Exp. Med. 1990;172:1459–1469. doi: 10.1084/jem.172.5.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smedt T., Pajak B., Muraille E., Lespagnard L., Heinen E., De Baetselier P., Urbain J., Leo O., Moser M. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J. Exp. Med. 1996;184:1413–1424. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Sallusto F., Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr. Opin. Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- Caux C., Massacrier C., Vanbervliet B., Dubois B., Van Kooten C., Durand I., Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J. Exp. Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzler C., Rovere P., Rescigno M., Granucci F., Penna G., Adorini L., Zimmermann V.S., Davoust J., Ricciardi-Castagnoli P. Maturation stages of mouse dendritic cells in growth factor–dependent long-term cultures. J. Exp. Med. 1997;185:317–328. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Witmer-Pack M., Inaba M., Hathcock K.S., Sakuta H., Azuma M., Yagita H., Okumura K., Linsley P.S., Ikehara S. The tissue distribution of the B7-2 costimulator in miceabundant expression on dendritic cells in situ and during maturation in vitro. J. Exp. Med. 1994;180:1849–1860. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caux C., Vanbervliet B., Massacrier C., Azuma M., Okumura K., Lanier L.L., Banchereau J. B70/B7-2 is identical to CD86 and is the major functional ligand for CD28 expressed on human dendritic cells. J. Exp. Med. 1994;180:1841–1847. doi: 10.1084/jem.180.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Scheidegger D., Palmer-Lehmann K., Lane P., Lanzavecchia A., Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacityT-T help via APC activation. J. Exp. Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch F., Stanzl U., Jennewien P., Janke K., Heufler C., Kampgen E., Romani N., Schuler G. High level IL-12 production by murine dendritic cellsupregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J. Exp. Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurner B., Roder C., Dieckmann D., Heuer M., Kruse M., Glaser A., Keikavoussi P., Kampgen E., Bender A., Schuler G. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J. Immunol. Methods. 1999;223:1–15. doi: 10.1016/s0022-1759(98)00208-7. [DOI] [PubMed] [Google Scholar]
- Dieu M.-C., Vanbervliet B., Vicari A., Bridon J.-M., Oldham E., Ait-Yahia S., Briere F., Zlotnik A., Lebecque S., Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara S., Komura E., Nagafune J., Watarai H., Yamaguchi Y. EB11/CCR7 is a new member of dendritic cell chemokine receptor that is upregulated upon maturation. J. Immunol. 1998;161:3096–3102. [PubMed] [Google Scholar]
- Sallusto F., Schaerli P., Loetscher P., Schaniel C., Lenig D., Mackay C.R., Qin S., Lanzavecchia A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 1998;28:2760–2769. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Tang H.L., Cyster J.G. Chemokine up-regulation and activated T cell attraction by maturing dendritic cells. Science. 1999;284:819–822. doi: 10.1126/science.284.5415.819. [DOI] [PubMed] [Google Scholar]
- Cella M., Engering A., Pinet V., Pieters J., Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- Pierre P., Turley S.J., Gatti E., Hull M., Meltzer J., Mirza A., Inaba K., Steinman R.M., Mellman I. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- Pierre P., Mellman I. Developmental regulation of invariant chain proteolysis controls MHC class II trafficking in mouse dendritic cells. Cell. 1998;93:1135–1145. doi: 10.1016/s0092-8674(00)81458-0. [DOI] [PubMed] [Google Scholar]
- Roche P.A., Cresswell P. Invariant chain association with HLA-DR molecules inhibits immunogenic peptide binding. Nature. 1990;345:615–618. doi: 10.1038/345615a0. [DOI] [PubMed] [Google Scholar]
- Romagnoli P., Germain R.N. The CLIP region of invariant chain plays a critical role in regulating major histocompatibility complex class II folding, transport, and peptide occupancy. J. Exp. Med. 1994;180:1107–1113. doi: 10.1084/jem.180.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G., Reis e Sousa C., Germain R.N. Production, specificity, and functionality of monoclonal antibodies to specific peptide-major histocompatibility complex class II complexes formed by processing of exogenous protein. Proc. Natl. Acad. Sci. USA. 1997;94:13856–13861. doi: 10.1073/pnas.94.25.13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G., Reis e Sousa C., Germain R.N. Antigen-unspecific B cells and lymphoid dendritic cells both show extensive surface expression of processed antigen–major histocompatibility class II complexes after soluble protein exposure in vivo or in vitro. J. Exp. Med. 1997;186:673–682. doi: 10.1084/jem.186.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler G., Steinman R.M. Dendritic cells as adjuvants for immune-mediated resistance to tumors. J. Exp. Med. 1997;186:1183–1187. doi: 10.1084/jem.186.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle F.O., Alijagic S., Gilliet M., Sun Y., Grabbe S., Dummer R., Burg G., Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat. Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- Dhodopkar M., Steinman R.M., Sapp M., Desai H., Fossella C., Krasovsky J., Donahoe S.M., Dunbar P.R., Cerundolo V., Nixon D.F., Bhardwaj N. Rapid generation of broad T-cell immunity in humans after single injection of mature dendritic cells. J. Clin. Invest. 1999;104:173–180. doi: 10.1172/JCI6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurner B., Haendle I., Roder C., Dieckmann D., Keikavoussi P., Jonuleit H., Bender A., Maczek C., Schreiner D., von den Driesch P. Vaccination with Mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J. Exp. Med. 1999;190:1669–1678. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Inaba M., Romani N., Aya H., Deguchi M., Ikehara S., Muramatsu S., Steinman R.M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C.P., Steinman R.M., Witmer-Pack M., Hankins D.F., Morris P.J., Austyn J.M. Migration and maturation of Langerhans cells in skin transplants and explants. J. Exp. Med. 1990;172:1483–1493. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope M., Betjes M.G., Hirmand H., Hoffman L., Steinman R.M. Both dendritic cells and memory T lymphocytes emigrate from organ cultures of human skin and form distinctive dendritic-T-cell conjugates. J. Invest. Dermatol. 1995;104:11–17. doi: 10.1111/1523-1747.ep12613452. [DOI] [PubMed] [Google Scholar]
- Muta T., Fugimoto T., Nakajima H., Iwanaga S. Tachyplesins isolated from hemocytes of Southeast Asian horseshoe crabs (Carcinoscorpius rotundicauda and Tachypleus gigas)identification of a new tachyplesin, tachyplesin III, and a processing intermediate of its precursor. J. Biochem. 1990;108:261–266. doi: 10.1093/oxfordjournals.jbchem.a123191. [DOI] [PubMed] [Google Scholar]
- Ho W.Y., Cooke M.P., Goodnow C.C., Davis M.M. Resting and anergic B cells are defective in CD28-dependent costimulation of naive CD4+ T cells. J. Exp. Med. 1994;179:1539–1549. doi: 10.1084/jem.179.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis e Sousa C., Germain R.N. Analysis of adjuvant function by direct visualization of antigen presentation in vivoendotoxin promotes accumulation of antigen-bearing dendritic cells in the T cell areas of lymphoid tissue. J. Immunol. 1999;162:6552–6561. [PubMed] [Google Scholar]
- Turley S.J., Inaba K., Garrett W.S., Jr., Ebersold M., Unternaehrer J., Steinman R.M., Mellman I. Transport of peptide-MHC class II complexes in developing dendritic cells. Science. 2000;In press doi: 10.1126/science.288.5465.522. [DOI] [PubMed] [Google Scholar]
- Ortner U., Inaba K., Koch F., Heine M., Miwa M., Schuler G., Romani N. An improved isolation method for murine migratory cutaneous dendritic cells. J. Immunol. Methods. 1996;193:71–79. doi: 10.1016/0022-1759(96)00058-0. [DOI] [PubMed] [Google Scholar]
- Weinlich G., Heine M., Stossel H., Zanella M., Stoitzner P., Ortner U., Smolle J., Koch F., Sepp N.T., Schuler G., Romani N. Entry into afferent lymphatics and maturation in situ of migrating murine cutaneous dendritic cells. J. Invest. Dermatol. 1998;110:441–448. doi: 10.1046/j.1523-1747.1998.00161.x. [DOI] [PubMed] [Google Scholar]
- Cai Z., Kishimoto H., Brunmark A., Jackson M.R., Peterson P.A., Sprent J. Requirements for peptide-induced T cell receptor downregulation on naive CD8+ T cells. J. Exp. Med. 1997;185:641–651. doi: 10.1084/jem.185.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iezzi G., Karjalainen K., Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- Willimann K., Legler D.F., Loetscher M., Roos R.S., Delgado M.B., Clark-Lewis I., Baggiolini M., Moser B. The chemokine SLC is expressed in T cell areas of lymph nodes amd mucosal lymphoid tissues and attracts activated T cells via CCR7. Eur. J. Immunol. 1998;28:2025–2034. doi: 10.1002/(SICI)1521-4141(199806)28:06<2025::AID-IMMU2025>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Gunn M.D., Kyuwa S., Tam C., Kakiuchi T., Matsuzawa A., Williams L.T., Nakano H. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 1999;189:451–460. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf P.R., Ploegh H.L. How MHC class II molecules acquire peptide cargobiosynthesis and trafficking through the endocytic pathway. Annu. Rev. Cell Dev. Biol. 1995;11:267–306. doi: 10.1146/annurev.cb.11.110195.001411. [DOI] [PubMed] [Google Scholar]
- Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu. Rev. Immunol. 1997;15:821–850. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- Bakke O., Dobberstein B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 1990;63:707–716. doi: 10.1016/0092-8674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- Elliot E.A., Drake J.R., Amigorena S., Elsemore J., Webster P., Mellman I., Flavell R.A. The invariant chain is required for intracellular transport and function of major histocompatibility complex class II molecules. J. Exp. Med. 1994;179:681–694. doi: 10.1084/jem.179.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G., Romagnoli P., Germain R.N. Related leucine-based cytoplasmic targeting signals in invariant chain and major histocompatibility complex class II molecules control endocytic presentation of distinct determinants in a single protein. J. Exp. Med. 1997;185:429–438. doi: 10.1084/jem.185.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guagliardi L.E., Koppelman B., Blum J.S., Marks M.S., Cresswell P., Brodsky F.M. Co-localization of molecules involved in antigen processing and presentation in an early endocytic compartment. Nature. 1990;343:133–139. doi: 10.1038/343133a0. [DOI] [PubMed] [Google Scholar]
- Tulp A., Verwoerd D., Dobberstein B., Ploegh H.L., Pieters J. Isolation and characterization of the intracellular MHC class II compartment. Nature. 1994;369:120–126. doi: 10.1038/369120a0. [DOI] [PubMed] [Google Scholar]
- Amigorena S., Drake J.R., Webster P., Mellman I. Transient accumulation of new class II MHC molecules in a novel endocytic compartment in B lymphocytes. Nature. 1994;369:113–120. doi: 10.1038/369113a0. [DOI] [PubMed] [Google Scholar]
- Sanderson F., Kleijmeer M.J., Kelly A., Verwoerd D., Tulp A., Neefjes J.J., Geuze H.J., Trowsdale J. Accumulation of HLA-DM, a regulator of antigen presentation, in MHC class II compartments. Science. 1994;266:1566–1569. doi: 10.1126/science.7985027. [DOI] [PubMed] [Google Scholar]
- Pierre P., Denzin L.K., Hammond C., Drake J.R., Amigorena S., Cresswell P., Mellman I. HLA-DM is localized to conventional and unconventional MHC class II-containing endocytic compartments. Immunity. 1996;4:229–239. doi: 10.1016/s1074-7613(00)80431-8. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Cella M., Danieli C., Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate antigen in the major histocompatibility class II compartment. Downregulation by cytokines and bacterial products. J. Exp. Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijman H.W., Kleijmeer M.J., Ossevoort M.A., Oorschot V.M., Vierboom M.P., van de Keur M., Kenemans P., Kast W.M., Geuze H.J., Melief C.J. Antigen capture and MHC class II compartments of freshly isolated and cultured human blood dendritic cells. J. Exp. Med. 1995;182:163–174. doi: 10.1084/jem.182.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riese R.J., Wolf P.R., Bromme D., Natkin L.R., Villadangos J.A., Ploegh H.L., Chapman H.A. Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity. 1996;4:357–366. doi: 10.1016/s1074-7613(00)80249-6. [DOI] [PubMed] [Google Scholar]
- Schwartz R.H. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Boussiotis A., Freeman G.J., Gray G., Gribben J., Nadler L.M. B7 but not intercellular adhesion molecule-1 costimulation prevents the induction of human alloantigen–specific tolerance. J. Exp. Med. 1993;178:1753–1763. doi: 10.1084/jem.178.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Turley S., Yamaide F., Iyoda T., Mahnke K., Inaba M., Pack M., Subklewe M., Sauter B., Sheff D. Efficient presentation of phagocytosed cellular fragments on the MHC class II products of dendritic cells. J. Exp. Med. 1998;188:2163–2173. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurts C., Kosaka H., Carbone F.R., Miller J.F., Heath W.R. Class I–restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+ T cells. J. Exp. Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Pack M., Inaba M., Sakuta H., Isdell F., Steinman R.M. High levels of a major histocompatibility complex II–self-peptide complex on dendritic cells from the T cell areas of lymph nodes. J. Exp. Med. 1997;186:665–672. doi: 10.1084/jem.186.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]