Abstract
Fas ligand (FasL) has been shown to mediate both apoptotic and inflammatory reactions. To rigorously assess the physiological role of different forms of the FasL molecule with regard to these two distinct processes, we isolated stably transfected lymphoma cell lines that expressed either murine wild-type FasL, membrane-only FasL, or functionally distinct forms of soluble FasL. First, the ability of these lines to induce an inflammatory response was assessed in vivo by injecting the transfectants intraperitoneally and measuring subsequent neutrophil extravasation into the peritoneal cavity. Second, lines were assessed by injecting the transfectants subcutaneously and monitoring their growth as solid tumors. Our study clearly demonstrated that the extent of inflammation induced by the transfectants directly correlated with their relative cytotoxic activities. A neutrophil response could only be elicited in mice with intact Fas death domains although Fas expression by the neutrophils was not essential. Lymphoma cells expressing the soluble FasL form corresponding to the natural cleavage product could not trigger apoptosis and did not induce a neutrophil response. In contrast to the other FasL transfectants, these cells survived as tumor transplants. However, expression of soluble FasL was not benign, but actually suppressed the inflammatory response and protected other transfectants from the effector mechanisms elicted by membrane-bound FasL.
Keywords: Fas ligand, apoptosis, neutrophil, chemotaxis, lymphoma
Introduction
Fas ligand (FasL) was originally identified as a 40-kD type II transmembrane protein belonging to the TNF family 1. Mice that inherit the recessive mutation gld lack functional FasL expression and develop a systemic autoimmune disease characterized by massive lymphadenopathy, splenomegaly, and excessive autoantibody production 2 3. FasL expression can be induced on T cells by antigen receptor engagement and confers the ability to kill Fas+ target populations 1 4 5 6. Coexpression of Fas and FasL by activated T cells leads to the phenomenon of activation-induced cell death 7 8 9. Thus, FasL plays an integral role in the regulation of lymphocyte interactions. FasL can also be constitutively expressed by nonlymphoid cells in the eye and testis, where its potent proapoptotic activity is thought to contribute to the immune-privileged status of these organs 10 11 12 13. Furthermore, constitutive FasL expression by certain human and mouse malignant cells may contribute to immune evasion 14 15 16 17 18 19.
Based on the presumed ability of FasL to eliminate activated Fas+ T cells, it was postulated that forced expression of FasL would protect allografts from lymphocyte-mediated effector mechanisms. A protective effect of FasL was observed in studies involving melanoma cells 14, myoblasts cotransplanted with islet cells 20, and allogeneic colon carcinoma cells 21. However, in other cases, forced FasL expression was found to facilitate rejection by triggering a neutrophil-mediated inflammatory response. This neutrophil-dominated response was observed with a variety of cell lines and tissues, namely islets 22 23, myoblasts 24, or malignant cells 25 26 27 28. The circumstances that tilt the FasL reaction toward silent apoptosis as opposed to rampant inflammation remain unclear, but may reflect the extent or form of FasL expression.
Like other members of the TNF family, membrane-bound FasL can be cleaved from the cell surface to generate a 26-kD soluble form (sFasL, 29 30 31 32 33). Soluble TNF-α is known to be an important mediator of inflammation 34 35, whereas membrane TNF-α mediates cellular cytotoxicity 36. The role of soluble FasL has been less clear. The natural cleavage product of human FasL (amino acids 129–279) was shown to have cytotoxic activity when tested on extremely sensitive Fas+ populations. Whether this was physiologically relevant killing is questionable, as human sFasL does not kill Fas-expressing human Jurkat cells or primary mouse hepatocytes 30 37. The equivalent portion of mouse sFasL (amino acids 127–279) does not appear to be cytotoxic under any conditions, although a soluble protein corresponding to the entire extracellular domain of mouse FasL (amino acids 101–279) has been produced experimentally and does induce apoptosis 38. Even though soluble FasL does not efficiently induce apoptosis, it has been shown to bind Fas and specifically block the apoptotic activity of membrane-bound FasL 39 40.
Considerable confusion exists in the literature as to the relative importance of the membrane and soluble forms of FasL on the proinflammatory effects of this molecule. It has been reported that soluble FasL is chemotactic for mouse and human neutrophils, as assessed by in vitro migration assays, consistent with the notion that sFasL can establish a chemoattractive gradient 41 42. However, the soluble FasL reagent used by Ottonello et al. did not represent the natural FasL cleavage product, but rather corresponded to the entire extracellular domain of human FasL 42. Moreover, an IL-1–dependent inflammatory response was observed when fibrosarcoma cells expressing a membrane-only FasL–CD40L chimeric protein were inoculated intraperitoneally, suggesting that the membrane form alone was sufficient to induce neutrophil extravasation 27.
The goal of the current study was to rigorously compare the proinflammatory capacity of mouse wild-type FasL (wtFasL), membrane-only FasL (mFasL), soluble FasL (natural cleavage product, sFasL) and soluble FasL-entire extracellular domain (sFasL.EX) in vivo. We evaluated the ability of the membrane-bound and soluble forms to induce and/or inhibit an inflammatory reaction characterized by neutrophil extravasation into the peritoneum subsequent to intraperitoneal inoculation of FasL transfectants. The effect of the different forms of FasL on long term tumor survival and/or rejection in syngeneic mice was also examined. We found that the ability of the various transfectants to trigger a neutrophil response correlated with their apoptotic activity. Significantly, the natural cleavage product, rather than inducing chemotaxis, actually opposed the activity of mFasL and protected cells from the neutrophil effector mechanisms.
Materials and Methods
Mice.
4–5 wk-old female DBA/2J, MRL-lpr, C3H.MRL-lpr, CBA-lpr cg, and CBA/J mice were purchased from The Jackson Laboratory. MRL-lpr/gld mice were bred and maintained at the Laboratory Animal Science Center, Boston University Medical Center.
Derivation of the Wild-Type, Membrane, Extracellular, and Soluble FasL Constructs.
The full-length wtFasL cDNA was cloned by reverse transcription-PCR from a T cell hybridoma, 12.13, derived from the fusion of MRL × SJL T cells and the BW5147 thymoma cell line. The mouse FasL cDNA was cloned in pcDNA1 and provided by Dr. D. Panka (Harvard Medical School). Nucleotide sequencing revealed the cloned FasL cDNA to be the FasL.1 allotype characteristic of B6, MRL, and SJL mice 43.
The sFasL construct corresponding to the natural FasL cleavage product was generated by fusing the mouse granulocyte CSF (GCSF) signal peptide to the 167–amino acid mouse FasL ectodomain. The mouse FasL ectodomain was derived by PCR amplification from pBL-MFLW4, a mouse FasL cDNA that was cloned from activated C3H splenocytes 2. The pFastBac/mGCSFsig-mFasLext plasmid was generated as follows, and was provided by Steven S. Pullen and Marilyn R. Kehry (Boehringer Ingelheim Pharmaceuticals, Ridgefield, CT). The 123-bp BamHI–EcoRI mouse GCSF signal peptide fragment from pGEMT-mGCSFsig and the 475-bp EcoRI–NotI mFasL ectodomain fragment from pGEMT-mFasLext were coligated into pFastBac1 (GIBCO BRL) digested with EcoRI and BamHI to generate FastBac-mGCSFsig-mFasLext. For cloning purposes the mGCSFsig-mFasLext insert was moved into pcDNA3 and pBluescriptSKII vectors (pcDNA3-sFasL and pSKII-sFasL).
The sFasL.EX was cloned by PCR amplification using the 5′FasL.EX primer (5′-GATTGAATTCCAGCTCTTCCACCTGCAGAA-3′) and 3′FasL.EX primer (5′-AATCGCGGCCGCTCTTTTAAAGCTTATACAAGCGC-3′) with pcDNA1-wtFasL as template. The PCR product was EcoRI–NotI digested and ligated in frame with the mouse GCSF signal peptide of pcDNA1-mGCSF. For cloning purposes the mGCSFsig-sFasL.EX insert was moved into pcDNA3 (Invitrogen) and pSKII vectors (Stratagene) (pcDNA3-sFasL.EX and pSKII-sFasL.EX). The expression plasmids BCMGSNeo-sFasL.EX and BCMGSNeo-sFasL were generated by subcloning the XhoI–NotI fragment from pSKII-sFasL.EX and pSKII-sFasL plasmids, respectively, including the mouse GCSF secretion signal and either amino acids 106–279 or 126–279 from the mouse FasL into the expression vector BCMGSNeo, provided by Dr. H. Karasuyama (Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan). Mouse wtFasL and the deletion mutant mFasL were also subcloned from the pSKII cloning vector into the BCMGSNeo expression vector as XhoI–NotI fragments. The deletion at the FasL metalloproteinase cleavage site was generated by means of recombinant PCR using pcDNA3-FasL as template. The 5′ portion of FasL cDNA was amplified using the T7 sense primer containing sequences from the vector template and the antisense deletion primer asBAH3 (5′-GGGTGTACTGGGGTTGGCCTCACGGAGTTCTGCCAG-3′), a sequence coding for amino acids 109–114 and 130–135 of mouse FasL. The 3′ portion of FasL was amplified using the sense deletion primer sBAH3 (5′-CTGGCAGAACTCCGTGAGGCCAACCCCAGTACACCC-3′), which is complementary to the antisense deletion primer asBAH3 and the SP6 vector primer. The primary PCR products containing the 3′ and 5′ portion of FasL, respectively, were purified by agarose gel electrophoresis, mixed 1:1, and then amplified by secondary PCR with T7 and SP6 flanking primers. The EcoRI–XbaI fragment of the resulting PCR product was cloned into the mammalian expression vector pcDNA3 and subsequently subcloned as BamHI–XbaI fragment into the cloning vector pSKII.
Cell Lines.
The DBA/2 T lymphoma cell line L5178Y-R was obtained from the American Type Culture Collection. L5178Y-R as well as LF+, LF−, LB27.4, and Jurkat E6-1 cells were grown in 10% FCS-RPMI supplemented with 10 μM Hepes, 50 μM 2-ME, 1× penicillin/streptomycin/glutamine (GIBCO BRL). Mouse L5178Y-R T lymphoma cells were transfected by electroporation with different BCGMSNeo expression plasmids using the Gene Pulser II (Bio-Rad) with 320 V and 950 μF in OptiMEM medium (GIBCO BRL). G418-resistant transfectants were selected with 750 μg/ml G418 for 14 d and cloned twice by limiting dilution to ensure clonality. Transfectant clones expressing different forms of mouse FasL were either screened by function (51Cr release of Fas+ target cells), or in the case of L5-sFasL, by Western blot analysis. At least three transformed L5178Y-R clones expressing comparable levels of protein by Western blot or flow cytometry were independently isolated for each construct. Representative clones were chosen for further experiments, and designated L5-wtFasL, L5-mFasL, L5-sFasL.EX, and L5-sFasL. Empty vector–transfected L5178Y-R cells (L5-neo) were used as control throughout the study.
Cytotoxicity Assays.
The cytotoxic activity of the various L5178Y-R transfectants was quantitated by a 51Cr-release assay, essentially as described previously 5. LB27.4 is a mouse B lymphoma hybridoma (American Type Culture Collection) that expresses mouse Fas and is sensitive to FasL-mediated killing. LF+ and LF− are derived from mouse T lymphoma L1210 cells that were either transfected with mouse Fas or mouse antisense Fas such that the former is sensitive and the latter is completely resistant to FasL 44. The transfectants were seeded at different densities to vary the E/T ratios. After a culture period of 6 h, culture supernatants were collected and the radioactivity released to the supernatant was counted with a γ-counter. Labeled cells cultured in medium alone were used as background controls. The radioactivity released by cells cultured in the presence of 1% NP-40 was used as a reference for maximum cell death. The specific activity was determined by the formula: (cpm of experimental sample − cpm of medium control)/(cpm of maximum release − cpm of medium control).
Induction, Isolation, and Characterization of Peritoneal Exudate Cells.
L5178Y-R transfectants (0.6−6 × 106 cells) were washed with serum-free Hank's PBS (HPBS) and injected intraperitoneally into DBA/2 mice, MRL-gld, MRL-lpr/gld, C3H.MRL-lpr, CBA-lpr cg, or CBA/J mice. In some experiments, the L5178Y-R transfectants were preactivated with PMA (300 ng/ml) and ionomycin (400 ng/ml) for 4 h and washed extensively with serum-free HPBS. In some experiments, 5 × 106 L5178Y-R transfectants were coinjected intraperitoneally into MRL-lpr/gld mice together with 6 × 106 peritoneal washout cells (PWCs) from uninduced MRL-+/+ or MRL-lpr/gld mice. 18 h after tumor inoculation, mice were killed by carbon dioxide asphyxiation. Peritoneal exudate cells (PECs) were harvested and washed with HPBS containing 2% FCS. The total number of PECs per mouse was determined by counting exudate cells with a hemocytometer. Cytospins of freshly isolated PECs were stained with HEMA 3 stain set (Wright-Giemsa stain; Fisher Scientific). Other aliquots of fresh PECs were pretreated with 2.4G2, and then stained with FITC-conjugated anti-Gr1 and PE-conjugated anti-Mac1 or biotinylated anti-Thy1.2 followed by streptavidin-PE (PharMingen), and then analyzed on a FACScan™ flow cytometer (Becton Dickinson). Acquired data were plotted using CELLQuest™ software (Becton Dickinson) with contour plot settings of 10% probability, smoothing factor 5, and 0.2% threshold.
5-chloromethylfluorescein Diacetate Labeling of L5178Y-R Cells.
In some experiments, L5-neo and L5-sFasL cells were labeled with 1 μM 5-chloromethylfluorescein diacetate (CMFDA, CellTracker Green; Molecular Probes) before intraperitoneal injection. Cells were labeled by incubating 2 × 106 cells/ml at 37°C for 12 min in prewarmed serum-free medium containing 1 μM CMFDA. The cells were then washed twice, incubated for an additional 1 h in medium at 37°C, and washed two more times.
Analysis of Transfectant Membrane and Soluble FasL Expression.
FasL expression of full-length and soluble forms of FasL was assessed by Western blot analysis. The L5178Y-R transfectants were cultured for 24 h in RPMI 1640 containing 1 or 10% FCS at a concentration of 5 × 105 cells/ml with or without the metalloproteinase inhibitor KB8301 (PharMingen) at a final concentration of 45 μM. For the last 14 h, PMA (Sigma Chemical Co.) and inonomycin (Calbiochem) at final concentrations of 300 and 400 ng/ml, respectively, were added to some of the samples as indicated in the Results. Cells were washed with PBS, resuspended in RIPA buffer that contained a protease inhibitor mix (1× Complete™; Boehringer Mannheim), and incubated on ice for 20 min. Insoluble material was removed by centrifugation for 10 min at 12,000 g and samples were denatured in 1× Laemmli loading buffer at 95°C for 5 min. Culture supernatants were concentrated 10-fold with Microcon concentrators (Amicon) with a 10-kD cutoff membrane (Millipore) and denatured as described above so that cell lysates and supernatants would correspond to equal cell equivalents. Protein preparations were separated with 12% SDS-PAGE gels, transferred onto nitrocellulose membranes (Protran BA83; Schleicher & Schuell Inc.), and developed using a rabbit anti–mouse FasL antibody together with an horseradish peroxidase–conjugated anti–rabbit antibody (Santa Cruz Biotechnology) as secondary antibody. Molecular mass standards (Benchmark protein markers; GIBCO BRL) were fractionated in parallel, and the sizes of the standard proteins are shown in kilodaltons.
The rabbit anti-FasL antibody was generated by immunizing rabbits (Covance) with the peptide C-AminoCaproicAcid-SEKKEPRSVAHLTGNPHSRS (Research Genetics) that corresponds to the previously used immunogenic peptide 136–155 of mouse FasL (Elkon, K.B., Hospital for Special Surgery, New York, NY, personal communication). The peptide was coupled to Imject® maleimide-activated KLH (Pierce Chemical Co.) using the COOH-terminal cysteine.
Subcutaneous Tumor Growth.
L5178Y-R transfectants (2 × 106 cells) were washed with serum-free HPBS and injected subcutaneously into the rear flank of syngeneic DBA/2J mice. Tumor growth was followed for up to three weeks by caliper measurements of perpendicular diameters. Mice were killed at earlier time points if tumor growth exceeded 25 mm.
The effectiveness of bystander rejection was assessed by injecting DBA/2J mice subcutaneously with 2 × 106 L5-neo cells and graded numbers of L5-mFasL cells (0.2–2 × 106) at the same site. The inhibitory effect of soluble FasL on the bystander rejection was evaluated by injecting 6 × 105 L5-mFasL cells subcutaneously together with 2 × 106 L5-neo or 2 × 106 L5-sFasL cells at the same site.
Results
Characterization of FasL-expressing L5178Y-R T Lymphoma Cell Lines.
L5178Y-R T lymphoma cells were transfected with FasL constructs designed to compare the functional properties of wtFasL (L5-wtFasL), mFasL (L5-mFasL), and soluble FasL. Soluble forms of FasL were generated by fusing the mouse GCSF signal peptide with the 187–amino acid sFasL.EX (L5-sFasL.EX) or the 167–amino acid FasL natural cleavage product ectodomain, sFasL (L5-sFasL) (Fig. 1), to facilitate the exclusive production of soluble proteins 30 36. Empty vector (BCMGSneo)-transfected (L5-neo) cells routinely served as controls.
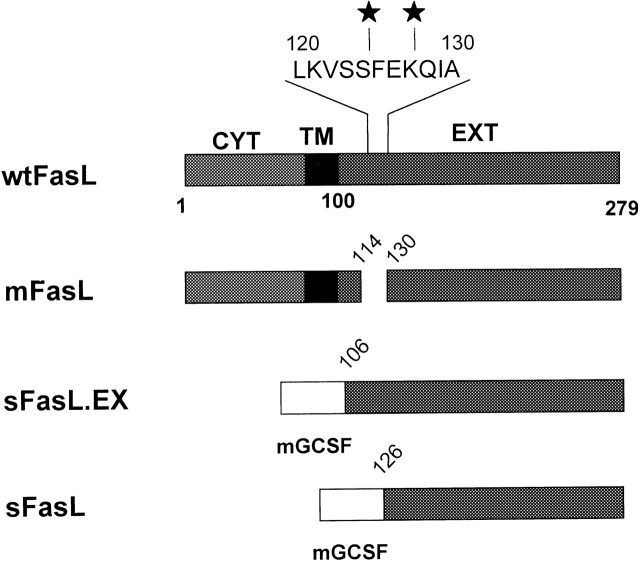
Figure 1.
Constructs used for the analysis of the mouse FasL metalloproteinase cleavage site. The structures of the wtFasL and mFasL are schematically drawn to scale. CYT, TM, and EXT represent the cytoplasmic, transmembrane, and extracellular regions of mouse FasL, respectively. Filled stars indicate the sites that correspond to the metalloproteinase cleavage sites reported for human FasL. The metalloproteinase site mutant was generated by deleting the region around the cleavage site Δ115-129. mGCSF represents the secretion signal of mouse GCSF that was fused in frame with the extracellular region of mouse FasL in case of the sFasL.EX and sFasL constructs.
The cytotoxic activity of the distinct FasL forms expressed by stably transfected L5178Y-R cells was assessed by 51Cr release. Three independently isolated transfected clones were analyzed per group, except for L5-neo where a pool of G418-resistant cells was used. L5-mFasL cells killed Fas+ LB targets (Fig. 2 A) much more effectively then L5-wtFasL. Cytotoxicity in this assay was Fas dependent, as neither the parental L5178Y-R cells nor the L5-neo cells were able to kill the target population. Furthermore, the FasL-transfected cells also killed the Fas+ LF+ line, but not the Fas− cell line LF− (data not shown). Compared with L5-wtFasL and L5-mFasL, the L5-sFasL.EX cells were only weakly cytotoxic in this type of assay, where effector activity during the 6-h assay was compared on a per cell basis. Cells expressing the construct corresponding to the natural cleavage product, L5-sFasL, were not cytotoxic. Activation of the transfected lines with PMA and ionomycin increased the level of expression of the transfected constructs reflected by an increase in the cytotoxic activity of all of the FasL forms except sFasL. Even at high concentrations, the L5-sFasL cells did not kill our most sensitive target population (Fig. 2 B).
Figure 2.
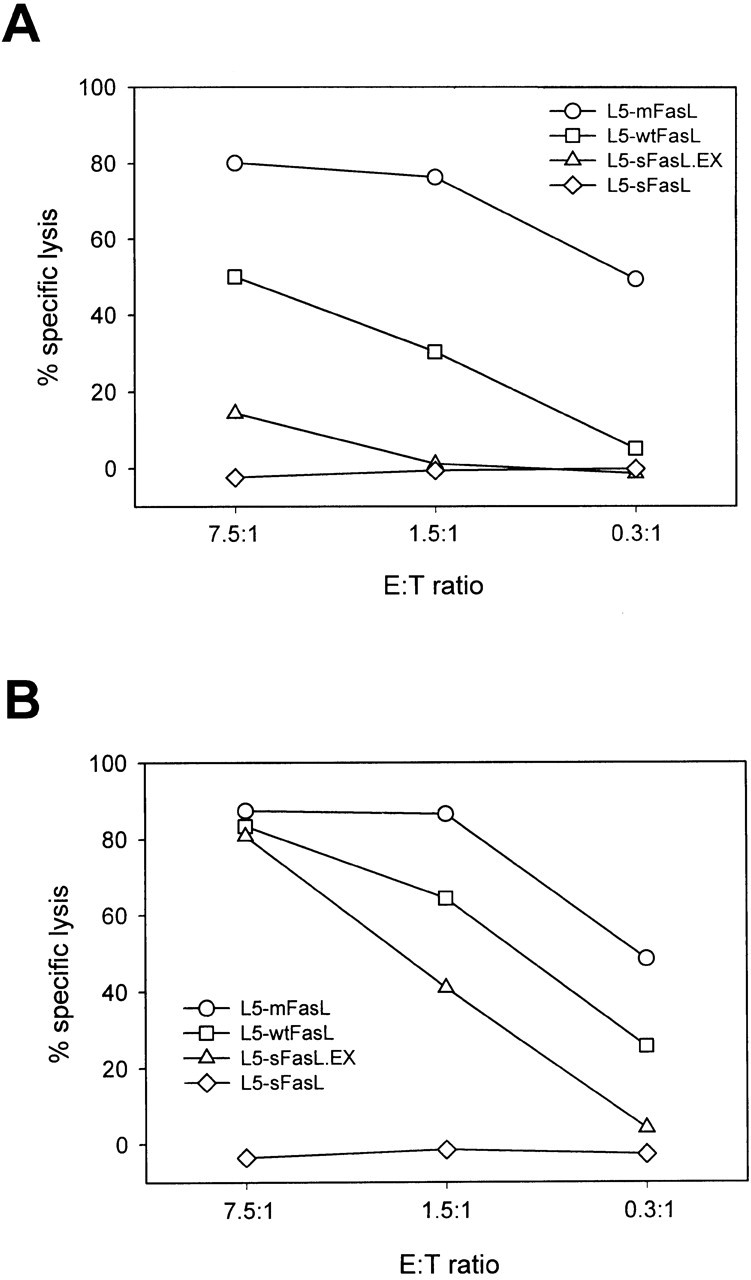
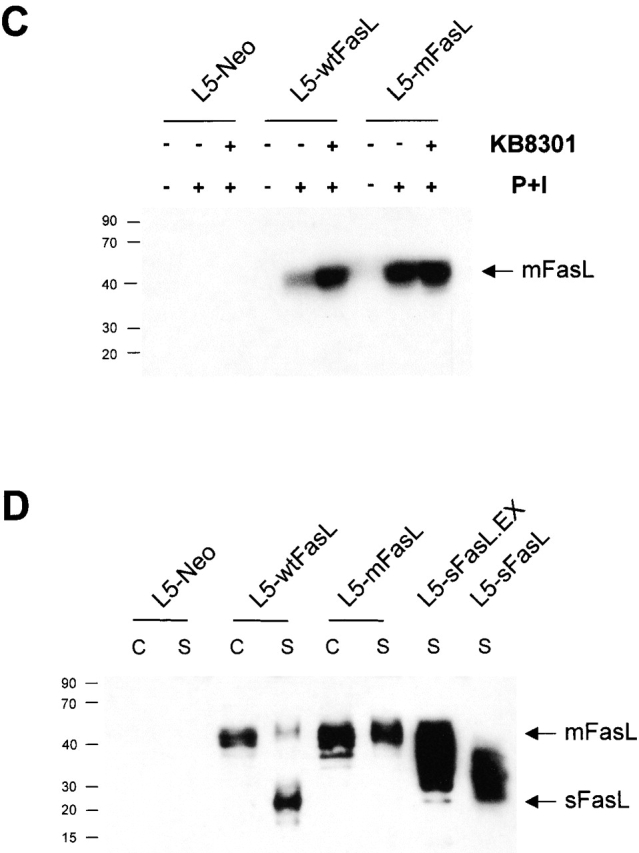
Characterization of FasL-expressing L5178Y-R thymic lymphoma cell lines. (A and B) Cytotoxic activity of FasL constructs. 51Cr-labeled LB targets were mixed with unactivated (A) or PMA plus ionomycin–preactivated (B) L5178Y-R transfectants and cultured at 37°C for 6 h. Spontaneous release was <20%. Values shown represent the mean of triplicate wells; SD was <5%. One representative experiment of three comparable assays is shown. (C) The metalloproteinase inhibitor KB8301 increased cellular FasL expression levels of wtFasL but not mFasL. L5-neo, L5-wtFasL, or L5-mFasL cells were cultured for 24 h in 10% FCS/RPMI medium in the presence or absence of the metalloproteinase inhibitor KB8301 (45 mM) as indicated. 300 ng/ml PMA and 400 ng/ml ionomycin (P+I) were added to some of the samples for the last 14 h of culture. (D) L5-mFasL cannot be cleaved by the metalloproteinase to produce sFasL. Cell lysates (abbreviated as C) and 10-fold concentrated culture supernatants (abbreviated as S) from PMA plus ionmycin–stimulated cells were analyzed by Western blotting using a rabbit anti–mouse FasL antibody.
mFasL Is Not Cleaved from the Cell Surface of L5178Y-R Cells.
The enhanced cytotoxic activity of the L5-mFasL transfectants was consistent with the assumption that these cells expressed a higher level of FasL protein on their surface. To better demonstrate that the Δ115–129 deletion effectively removed the presumed metalloproteinase cleavage site, the L5-wtFasL and L5-mFasL cells were further examined by Western blot analysis. Cell lysates from unactivated and PMA + ionomycin-activated L5-neo, L5-wtFasL, or L5-mFasL cells were obtained. PMA and ionomycin activation induced cellular FasL expression in L5-wtFasL and L5-mFasL cells with an apparently higher steady state level in L5-mFasL cells than in L5-wtFasL cells (Fig. 2 C). A 24-h culture period with the metalloproteinase inhibitor KB8301 had no effect on the expression level of mFasL, but increased the level of cell-associated wtFasL. This indicates that although wtFasL is synthesized at the same rate as mFasL in the selected L5178Y-R clones, constitutive metalloproteinase activity diminished cell surface expression of the wild-type protein. Concentrated culture supernatants from PMA plus ionomycin–stimulated L5-neo, L5-wtFasL, L5-mFasL, L5-sFasL.EX, and L5-sFasL cells were also obtained. Inhibition of cell surface cleavage in the case of mFasL was confirmed by a lack of soluble FasL in the culture supernatant of L5-mFasL clones. The 26 kD soluble FasL fragment could be detected in culture supernatant of L5-wtFasL cells (Fig. 2D) but not when L5-wtFasL cells were cultured in the presence of the metalloproteinase inhibitor KB8301 (data not shown). Soluble products could also be detected in the cell culture supernatants from L5-sFasL.EX and L5-sFasL. The diffuse bands probably result from variable glycosylation of the transfected protein products (Fig. 2 D).
Wild-type, Membrane, and sFasL.EX Are Proinflammatory, but sFasL Is Not Proinflammatory.
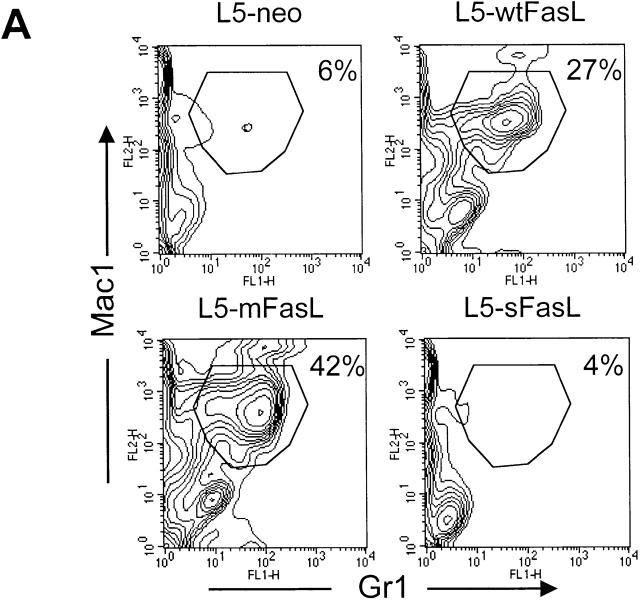
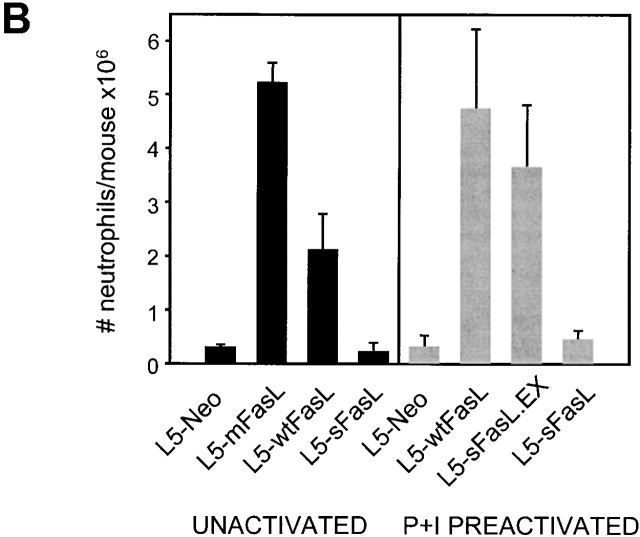
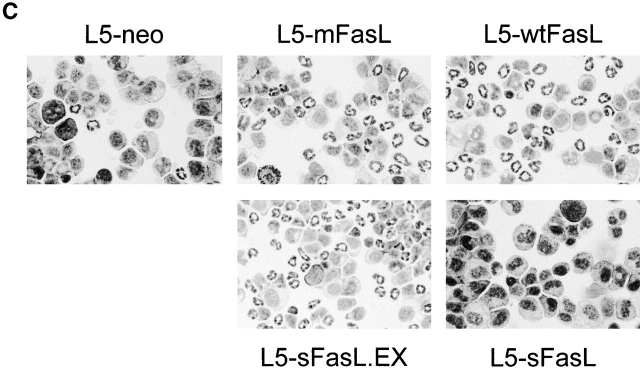
The panel of FasL transfectants allowed us to rigorously compare the proinflammatory properties of membrane and soluble FasL by monitoring the effects of the transfectant inoculation in an in vivo setting. DBA/2 mice were injected intraperitoneally with 6 × 105 syngeneic L5178Y-R lymphoma cells transfected with either mFasL, wtFasL, sFasL, sFasL.EX, or empty vector. PECs were recovered 16 h later. L5-mFasL cells induced a strong inflammatory response characterized by a massive neutrophil infiltration into the peritoneum, as shown by the dramatic increase in the percentage of Mac1/Gr1 double positive cells (Fig. 3 A), as well as in the total number of neutrophils that could be collected from the peritoneal cavity (Fig. 3 B). PECs were also morphologically examined by staining cytospin preparations of the peritoneal washout preparations with a Wright Giemsa stain (Fig. 3 C), and an increased number of polymorphonuclear cells correlated with the flow cytometric results. All of the actively cytotoxic forms of FasL, namely mFasL, wtFasL, and sFasL.EX, induced inflammation; mFasL was the strongest inducer and wtFasL and sFasL.EX were somewhat less effective. Comparable results were also obtained with mFasL, and with empty vector–transfected WR19L lymphoma cells that were inoculated intraperitoneally into syngeneic BALB/c mice (data not shown). Significantly, the cytotoxic inactive natural cleavage product, sFasL, never induced a greater response than the L5-neo control population even when high numbers of cells (2 × 106) were injected and/or when sFasL expression was upregulated by PMA and ionomycin preactivation (Fig. 3 B). These data demonstrate that both the cytotoxic and the proinflammatory effects of FasL are mediated by the transmembrane form of FasL. Nevertheless, membrane expression is not required, as sFasL.EX is able to induce neutrophil inflammation in this system.
Figure 3.
wtFasL, mFasL, and sFasL.EX were proinflammatory; sFasL was not proinflammatory. Sex- and age-matched DBA/2 mice were injected intraperitoneally with either 6 × 105 L5178Y-R unactivated transfectants, or 2 × 106 L5178Y-R transfectants that had been preactivated for 4 h with PMA plus inonomycin as indicated. 16 h later, PECs were recovered from peritoneal cavities. (A) Cells were stained with PE-conjugated anti-Mac1 and FITC-conjugated anti-Gr1 antibodies, and analyzed by flow cytometry. (B) The total number of neutrophils per mouse was calculated by multiplying the percentage of Mac1/Gr1-positive cells with the total number of PECs recovered. Data represent the mean of two to three mice in each group. (C) Cytospin preparations of PECs obtained from mice injected with the indicated transfectants were stained with Wright-Giemsa staining solution to identify polymorphonuclear leukocytes. Experiments were repeated in variation three times with similar results.
Inflammation Depends on Death Domain–intact Recipient Fas Expression.
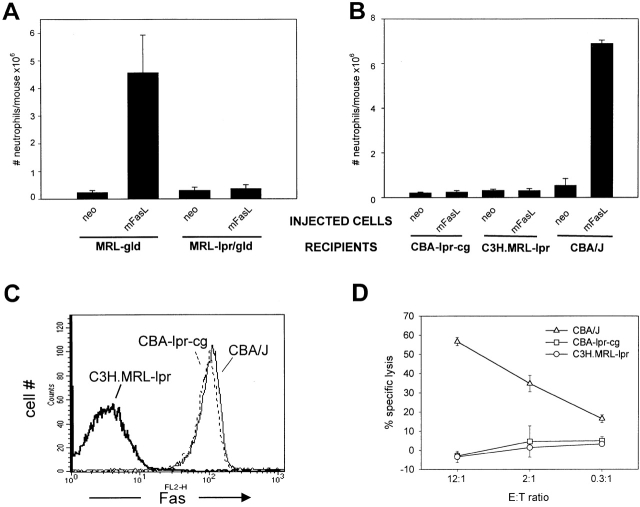
Although L5-neo cells were used as controls throughout the study, it was important to confirm that the observed effect was truly Fas dependent. MRL-gld and double mutant MRL-lpr/gld mice were inoculated intraperitoneally with 5 × 106 L5-neo or L5-mFasL cells. After 16 h, PECs were isolated and analyzed, as described in Fig. 3 C. Only mice with functional Fas expression (MRL-gld) were able to mount a neutrophil response. MRL-lpr/gld mice were completely resistant to high numbers of L5-mFasL cells (Fig. 4 A).
Figure 4.
Inflammation depends on recipient death domain–intact Fas expression. (A) MRL-gld and MRL-lpr/gld mice were injected intraperitoneally with 5 × 106 L5-neo or L5-mFasL cells. After 16 h, PECs were prepared and analyzed as described in the legend to Fig. 3. Shown is one representative experiment out of two; bars represent the mean of three mice per group, and error bars represent the SD. (B) C3H.MRL-lpr, CBA-lpr cg, and CBA/J mice were injected intraperitoneally with 5 × 106 L5-neo or L5-mFasL cells. After 16 h, PECs were prepared and analyzed as described in the legend to Fig. 3. The total number of neutrophils per mouse was compared between different groups. Data represent the mean of two mice per group, and error bars represent the SD. (C) Isolated thymocytes from C3H.MRL-lpr, CBA-lpr cg, and CBA/J mice were stained for Fas surface expression with the mAb Jo-2. (D) The sensitivity of thymocytes from C3H.MRL-lpr, CBA-lpr cg, and CBA/J mice to FasL-mediated killing was assessed. In brief, isolated thymocytes were 51Cr labeled and used as targets in a 6-h cytotoxic assay with L5-mFasL as effector cells. Target thymocytes were mixed with L5-mFasL cells at E/T ratios ranging from 12:1 to 0.3:1 and incubated for 6 h.
Recent studies involving an in vitro migration assay indicated that FasL-mediated chemotaxis was independent of the death domain of the Fas receptor 41. To reexamine this issue in vivo, CBA-lpr cg mice, known to express Fas molecules with a nonfunctional Fas death domain 45, were challenged intraperitoneally with 5 × 106 L5-neo or L5-mFasL cells. CBA/J and C3H.MRL-lpr mice were used as Fas+ and Fas− control recipients. FasL-induced neutrophil inflammation was clearly shown to depend on the intact Fas receptor death domain, as L5-mFasL cells failed to elicit neutrophil extravasation in either C3H.MRL-lpr or CBA-lpr cg mice while the response in CBA/J mice was comparable to that observed in DBA/2J mice (Fig. 4 B). Surface Fas expression was confirmed by staining isolated thymocytes from C3H.MRL-lpr, CBA-lpr cg, and CBA/J mice with the mAb Jo-2 (Fig. 4 C). As expected, CBA-lpr cg and CBA/J showed comparable Fas surface expression, whereas C3H.MRL-lpr mice had greatly diminished levels of Fas. Consistent with previous reports 46, isolated thymocytes from both C3H.MRL-lpr and CBA-lpr cg mice were completely resistant to FasL-mediated apoptosis (Fig. 4 D).
PWCs from MRL-+/+ Mice Restore FasL-mediated Neutrophil Inflammation in MRL-lpr/gld Mice.
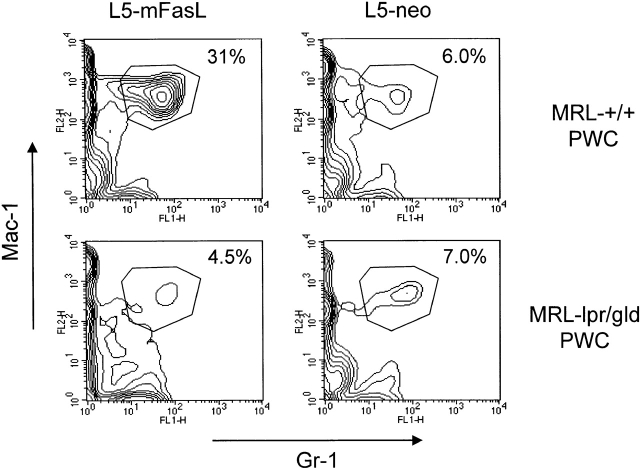
Functional recipient Fas expression is necessary for mounting a neutrophil response to FasL. As a soluble FasL gradient is highly unlikely to establish a neutrophil-chemoattractive gradient in vivo, we hypothesized that the initial apoptotic/proinflammatory event in the peritoneum is Fas dependent and the subsequent neutrophil extravasation is Fas independent. MRL-lpr/gld mice were coinjected with 5 × 106 L5-mFasL or L5-neo cells and 6 × 106 PWCs from uninduced MRL-+/+ or MRL-lpr/gld mice. The percentage of neutrophils in the PWCs from uninduced MRL-+/+ or MRL-lpr/gld mice that were used to reconstitute the response in MRL-lpr/gld mice was <2% (data not shown). MRL-lpr/gld neutrophils extravasated into the peritoneum only in response to L5-mFasL cells plus PWCs from MRL-+/+ mice and did not respond to L5-neo cells plus MRL-+/+ PWCs or L5-mFasL cells plus MRL-lpr/gld PWCs (Fig. 5).
Figure 5.
PWCs from MRL-+/+ mice restore FasL mediated neutrophil inflammation in MRL-lpr/gld mice. PWCs from untreated MRL-+/+ and MRL-lpr/gld mice were prepared as described and coinjected intraperitoneally with L5-mFasL or L5-neo cells into MRL-lpr/gld mice. After 16 h, PECs were prepared and analyzed as described in the legend to Fig. 3 A.
sFasL Inhibits the Proinflammatory Activity of mFasL.
Soluble FasL has been isolated from cell culture supernatants as a stable trimer that can bind to the Fas receptor 40 and at high doses is able to inhibit the cytotoxic activity of mFasL 39. To determine whether sFasL could also block the proinflammatory activity of mFasL, we evaluated the ability of L5-sFasL to protect tumor cells from mFasL-triggered rejection. L5-sFasL and L5-neo cells were labeled in vitro with CMFDA and then inoculated intraperitoneally into DBA/2/J mice either alone or together with L5-mFasL cells. Labeling L5-sFasL and L5-neo cells allowed us to readily monitor their persistence in the adoptive host. From preliminary experiments with unlabeled cells, we had noted that L5-mFasL cells persisted significantly less well than either L5-sFasL or L5-neo cells. When 2 × 106 CMFDA-labeled L5-neo cells were mixed with 6 × 105 L5-mFasL cells, the survival of the L5-neo cells was diminished relative to survival in mice injected with CMFDA-labeled L5-neo cells alone (Fig. 6 A). The decreased survival was presumably due to bystander killing 26. In contrast, the survival of CMFDA-labeled L5-sFasL cells was not compromised in mice coinjected with L5-mFasL cells. This indicates that sFasL has a protective antiinflammatory effect. This interpretation was also supported by a decrease in the percentage of peritoneal neutrophils seen in cytospin preparations from mice that were injected with a combination of L5-mFasL and L5-sFasL, compared with mice that were injected with a combination of L5-mFasL and L5-neo (Fig. 6 B).
Figure 6.
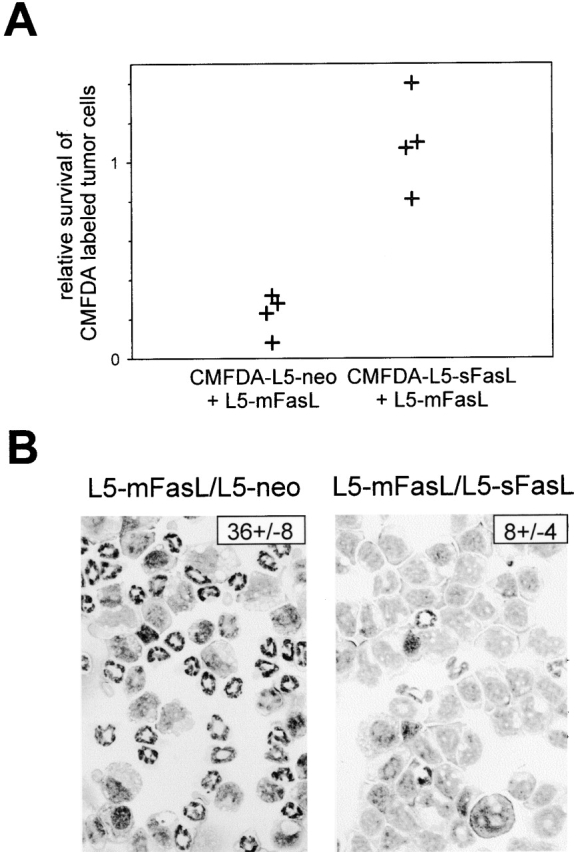
Soluble FasL inhibited the proinflammatory activity of mFasL. (A) DBA/2 mice were injected intraperitoneally with either 2 × 106 PMA plus ionomycin–preactivated or 6 ×106 unactivated, CMFDA-labeled L5-neo, or CMFDA-labeled L5-sFasL cells alone, or as a mixture with 6 × 105 L5-mFasL cells. After 16 h, PECs were isolated and survival of the CMFDA labeled tumor cells was assessed by flow cytometry. The relative rate of tumor cell survival was calculated by dividing the number of cells recovered, when L5-sFasL and L5-neo cells were mixed with L5-mFasL, by the number of cells recovered when L5-sFasL and L5-neo cells were injected alone. Shown are the pooled results from two independent experiments; dots represent relative survival rates of labeled tumor cells per mouse. (B) Neutrophil inflammation was assessed by blind enumeration of the number of neutrophils per 100 PECs in Wright-Giemsa–stained cytospins. A representative field is shown for each group. The number in the top right corner indicates the mean percentage of neutrophils ± SD of three to four mice per group.
To assess the effect of the FasL constructs on long-term L5178Y-R tumor growth, DBA/2 mice were injected subcutaneously in the flank with 2 × 106 L5-neo, L5-mFasL, L5-sFasL.EX, or L5-sFasL cells, and tumor growth was evaluated over a period of 3 wk. As expected with syngeneic transplants, parental L5178Y-R or L5-neo cells grew as solid tumors in DBA/2 mice (Fig. 7 A). However, due to the proinflammatory properties of membrane FasL, L5-mFasL cells were rejected and no tumor growth was observed, even after an extended period of time (2 mo) as has previously been shown for human and mouse wtFasL 26. Tumor progression of the L5-sFasL cells was similar to the parental and L5-neo cells, confirming their inability to trigger an inflammatory response. Interestingly, L5-sFasL.EX cells were not rejected although delayed growth kinetics were observed.
Figure 7.
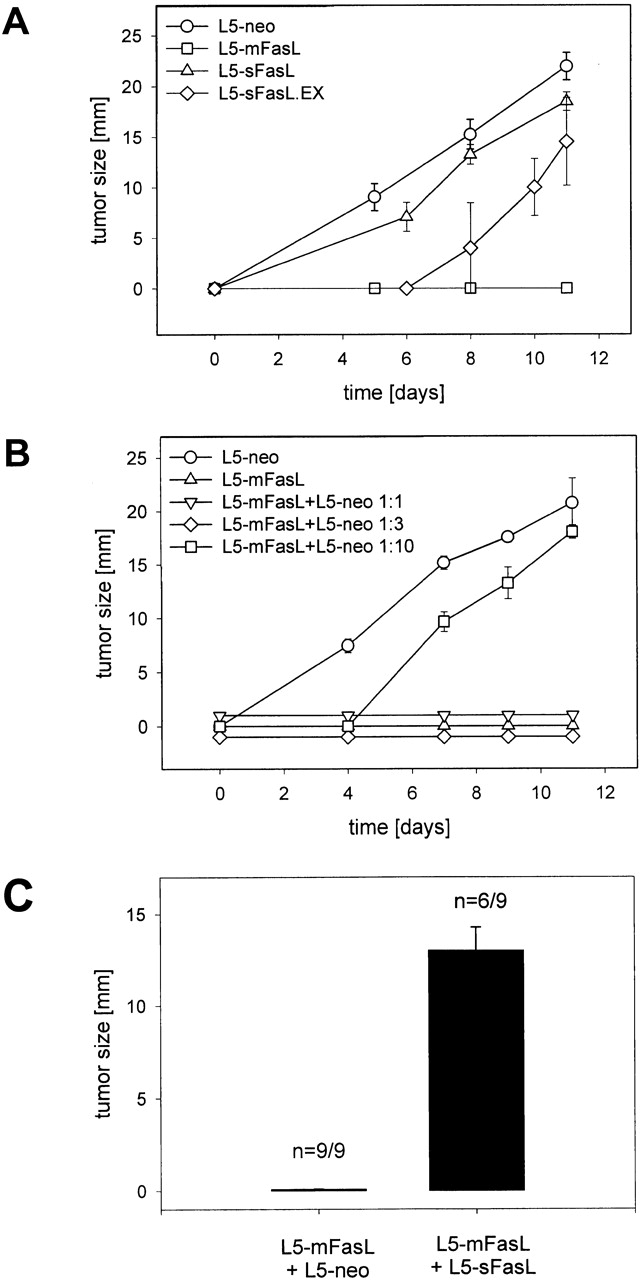
mFasL-induced bystander rejection of subcutaneous tumors was inhibited by sFasL. (A) Subcutaneous tumor growth of L5 transfectants. DBA/2 mice were injected subcutaneously with 2 × 106 L5-neo, L5-mFasL, L5-wtFasL, L5-sFasL.EX, or L5-sFasL cells and tumor size was measured periodically with a caliper. Data from two independent experiments were pooled, bars represent mean tumor size at day 11 of six to eight mice per group, and error bars represent the SD. (B) Bystander rejection of L5-neo but not L5-sFasL cells coinjected with L5-mFasL cells. DBA/2 mice were subcutaneously inoculated with a mixture of L5-neo and L5-mFasL cells. The number of L5-neo cells was kept constant at 2 × 106 cells, and L5-mFasL cells were graded from 2 × 106 to 2 × 105 cells. Tumor size was measured periodically with a caliper for 2 wk. Data represent the mean of two mice per group ± SD. Soluble sFasL inhibits bystander rejection. DBA/2 mice were subcutaneously inoculated with a mixture of 6 × 105 L5-mFasL cells and either 2 × 106 L5-neo or L5-sFasL cells. 6 × 105 L5-mFasL cells, 2 × 106 L5-neo, or 2 × 106 L5-sFasL cells were also inoculated alone as controls. Tumor growth was followed for 3 wk. Data from two independent experiments were pooled, bars represent mean tumor size at day 11 of six to nine mice per group, and error bars represent the SD.
To assess whether the antiinflammatory effect of sFasL observed in the peritoneum would influence long term subcutaneous tumor growth, the bystander rejection of FasL− cells coinjected with FasL-expressing cells was reexamined. DBA/2 mice were subcutaneously inoculated with a mixture of L5-neo and L5-mFasL cells. mFasL effectively mediated bystander killing of FasL− lymphoma cells at ratios of 1:1 and 1:3 (Fig. 7 B) comparable to the bystander rejection observed with human wtFasL 26. To assess whether soluble FasL is able to inhibit bystander killing, a mixture of 2 × 106 L5-sFasL or L5-neo cells was inoculated together with 6 × 105 L5-mFasL cells. The mixture of L5-mFasL and L5-neo cells was again rejected, whereas L5-sFasL inhibited the proinflammatory activity of L5-mFasL and thus allowed for tumor outgrowth (Fig. 7 C).
Discussion
Several of the TNF family members are type II membrane proteins that can be cleaved by metalloproteinases to release soluble protein segments. The soluble form of TNF-α is a potent inflammatory agent 34 35, soluble CD40L 47 induces B cell proliferation and IgE synthesis in conjunction with IL-4 48, and soluble B cell activating factor (BAFF) costimulates B cells 49. Thus, it might have been expected that soluble FasL would also have functional activity. Early reports indicated that the natural cleavage product of human sFasL did have cytotoxic activity 30, albeit relatively weak activity compared with the membrane form 37. The natural cleavage product of mouse FasL was reported to be even less cytotoxic 30, although an artificial construct corresponding to the entire extracellular domain was found to induce significant levels of apoptosis 38. These observations suggested that extracellular residues 101–125 of mouse FasL might facilitate the oligomerization of Fas trimers necessary for efficient activation of the caspase cascade 40. The data also suggested that the most significant outcome of FasL cleavage was to attenuate the potent apoptotic activity of the membrane form. This assumption is at least partially valid, since as shown previously 37, and in this study, cells stably transfected with the membrane-only form of FasL kill much more effectively on a cell for cell basis than cells transfected with the construct corresponding to wild-type protein.
Separate from its apoptotic activity, the forced expression of FasL in several cell types has been shown to induce an effusive neutrophil-mediated inflammatory response, as documented in vivo by either tissue transplant infiltration 22 23 24 25 26 28 50 51 or neutrophil extravasation to the peritoneal cavity 27. In vitro studies involving Boyden chamber migration assays suggested that the neutrophil response could be triggered by the establishment of a sFasL chemoattractant gradient 41 42. We felt it was important to reexamine the role of sFasL in an in vivo system where we could rigorously compare the functional effects of the various FasL products. L5178Y-R T lymphoma cells were stably transfected with constructs that allowed for the discrete expression of wtFasL, mFasL, a secreted form of the natural cleavage product, or the secreted form of the entire extracellular domain. Our data clearly demonstrated that the proinflammatory activity of FasL could be mediated by FasL that was solely membrane-bound and thus neutrophil extravasation did not depend on the establishment of an sFasL chemoattractive gradient. Moreover, the extent of inflammation seemed to correlate with the potential to induce apoptosis, as sFasL.EX is proapoptotic, especially when the level of expression was increased by PMA and ionomycin, and it is also proinflammatory. Previous data indicating that a soluble FasL reagent, corresponding to the sFasL.EX construct, chemoattracted human neutrophils in an in vitro chemotaxis assay might simply reflect direct (or indirect) effects of a proapoptotic sFasL on neutrophil activation 42.
The direct link between apoptosis and inflammation was further supported by the experiments in lpr cg mice, where it was unequivocally shown that an intact Fas death domain had to be expressed by the host for an inflammatory response to ensue. Thus, the initial event leading to an inflammatory response is likely to be the apoptotic demise of a Fas-expressing target population, contrary to the assumption that apoptotic death does not lead to inflammation. We surmise that cytokines or chemokines released by certain target cells (in this case, neutrophils or other resident peritoneal cells) are then the key factor in promoting subsequent inflammation. Previous studies have demonstrated that FasL-mediated apoptosis is not necessarily a passive process, but that dying cells can release functionally significant levels of IL1 27 or IL-10 52.
Although neutrophils have been shown to express Fas, it was not clear whether neutrophils per se must express Fas in order to extravasate in a FasL-triggered inflammatory response. To address this question, we injected Fas-deficient MRL-lpr/gld mice with a combination of L5-mFasL cells and PWCs from unmanipulated mice. The peritoneal washout populations per se contained very few neutrophils. Nevertheless, the combination of L5-mFasL and MRL-+/+ PWCs resulted in significant extravasation to the peritoneal cavity of neutrophils derived from the Fas-deficient host. The combination of L5-mFasL and MRL-lpr/gld PWCs did not induce neutrophil extravasation, indicating that the neutrophil response was not simply due to nonspecific inflammation. These results support a model in which FasL triggering of a target population, not necessarily a neutrophil, can lead to the release of factors chemoattractive for neutrophils, such as IL-1 27. Experiments are in progress to further identify the relevant target population(s). It will be important to determine whether the Fas− neutrophils induced to extravasate by the MRL-+/+ PWCs are functionally equivalent to the Fas+ neutrophils induced in the original protocol. It is possible that activation via Fas may be regulated independently of extravasation.
Activated neutrophils have a powerful arsenal of effector mechanisms, mainly phagocytosis and formation of hydrolytic enzymes and reactive oxygen products, to eradicate FasL-expressing tumor cells 53. In an in vitro cytotoxic assay, it was shown that Fas+ neutrophils preferentially killed FasL-expressing targets over FasL− control cells 26 50. Either Fas–FasL interactions simply facilitate intimate cell contact that enhances the delivery of cytolytic factors, or reverse signaling through FasL somehow contributes to cytolytic events. Our data, demonstrating that L5-sFasL.EX cells induced neutrophil inflammation in the peritoneum even though L5-sFasL.EX cells were not rejected as efficiently as L5-mFasL tumor cells supports the conclusion that Fas/FasL-facilitated cell contact promotes the elimination of the FasL-expressing effector cells.
In contrast to the soluble product corresponding to the entire extracellular domain, the natural cleavage product failed to induce even the slightest indication of an inflammatory response. The exact biochemical characteristics of the two molecules that account for this difference are not clear. Previous studies have shown that sFasL exists as homotrimers, as it was reported that sFasL.EX (WX1) can exist as higher order oligomers 38 40. It may be that the 15 amino acids present in the sFasL.EX were involved in multimerization of FasL in solution or on the target cell surface. Nevertheless, the L5-sFasL cells were functionally distinct from the vector control L5-neo cells, as shown in bystander assays. Although a previous report has shown that neutrophils specifically killed FasL-expressing targets and not control cells 26 50, we found that bystander L5-neo cells, but not L5-sFasL cells, were specifically eliminated by an L5-mFasL–induced neutrophil response. In the latter group, the L5-sFasL antiinflammatory effect was further demonstrated by the reduced number of neutrophils that extravasated to the peritoneum of these mice. The antiinflammatory effect of sFasL was also apparent in the more long term experiments monitoring progression of subcutaneously injected tumor cells. Here, it was found that the proinflammatory effects of the L5-mFasL cells prevented the outgrowth of coinjected L5-neo cells, but that the antiinflammatory sFasL worked in opposition to the mFasL and allowed for the outgrowth of coinjected L5-sFasL cells. Binding of sFasL to the Fas receptor may simply block mFasL engagement or it may provide an antiapoptotic signal. Membrane expression might allow for more extensive oligomerization of FasL trimers or it might retain the Fas receptor complexes on the cell surface and thus allow for more efficient activation of the caspase cascade.
Several examples can now be found in the literature of situations in which FasL-expressing APCs can effectively eliminate antigen-specific T cells without evidence of inflammation 54 55. Whether the outcome of apoptotic death is silent or whether it leads to inflammation must depend on several factors, including the level of metalloproteinase-released sFasL activity as well as the levels of other apoptotically induced chemokines and cytokines.
Acknowledgments
We thank Dr. M. Kehry, Dr. S. Pullen, Dr. D. Panka, Dr. J. Browning, Dr. H. Karasuyama, and Dr. K. Elkon for providing cells and reagents, and Ms. K. Maxwell and Ms. Christine Vogt for outstanding technical support. We also thank Dr. S.-T. Ju and Dr. R. Corley for critical reading of the manuscript.
This work was supported by National Institutes of Health grant GM58724, and by Leukemia Society of America grant 6146-99. A.M. Hohlbaum was supported by a Deutscher Akademischer Austauschdienst (DAAD) postdoctoral fellowship, and National Institutes of Health grant T32-CA64070.
Footnotes
Abbreviations used in this paper: CMFDA, 5-chloromethylfluorescein diacetate; L, ligand; GCSF, granulocyte CSF; HPBS, Hank's PBS; mFasL, membrane-only FasL; PEC, peritoneal exudate cell; PWC, peritoneal washout cell; sFasL, soluble FasL natural cleavage product; sFasL.EX, soluble FasL extracellular domain; wtFasL, wild-type FasL.
References
- Suda T., Takahashi T., Golstein P., Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Tanaka M., Brannan C.I., Jenkins N.A., Copeland N.G., Suda T., Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Lynch D.H., Watson M.L., Alderson M.R., Baum P.R., Miller R.E., Tough T., Gibson M., Davis-Smith T., Smith C.A., Hunter K. The mouse Fas-ligand gene is mutated in gld mice and is part of a TNF family gene cluster. Immunity. 1994;1:131–136. doi: 10.1016/1074-7613(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Kägi D., Vignaux F., Ledermann B., Bürki K., Depraetere V., Nagata S., Hengartner H., Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- Ju S.T., Cui H., Panka D.J., Ettinger R., Marshak-Rothstein A. Participation of target Fas protein in apoptosis pathway induced by CD4+ Th1 and CD8+ cytotoxic T cells. Proc. Natl. Acad. Sci. USA. 1994;91:4185–4189. doi: 10.1073/pnas.91.10.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder T., Hahn S., Erb P. Fas antigen is the major target molecule for CD4+ T cell-mediated cytotoxicity. J. Immunol. 1994;152:1127–1133. [PubMed] [Google Scholar]
- Ju S.T., Panka D.J., Cui H., Ettinger R., el-Khatib M., Sherr D.H., Stanger B.Z., Marshak-Rothstein A. Fas (CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- Brunner T., Mogil R.J., LaFace D., Yoo N.J., Mahboubi A., Echeverri F., Martin S.J., Force W.R., Lynch D.H., Ware C.F. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- Dhein J., Walczak H., Baumler C., Debatin K.M., Krammer P.H. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- Griffith T.S., Brunner T., Fletcher S.M., Green D.R., Ferguson T.A. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- Griffith T.S., Yu X., Herndon J.M., Green D.R., Ferguson T.A. CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity. 1996;5:7–16. doi: 10.1016/s1074-7613(00)80305-2. [DOI] [PubMed] [Google Scholar]
- Bellgrau D., Gold D., Selawry H., Moore J., Franzusoff A., Duke R.C. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377:630–632. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- Stuart P.M., Griffith T.S., Usui N., Pepose J., Yu X., Ferguson T.A. CD95 ligand (FasL)-induced apoptosis is necessary for corneal allograft survival. J. Clin. Invest. 1997;99:396–402. doi: 10.1172/JCI119173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahne M., Rimoldi D., Schroter M., Romero P., Schreier M., French L.E., Schneider P., Bornand T., Fontana A., Lienard D. Melanoma cell expression of Fas(Apo-1/CD95) ligandimplications for tumor immune escape. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- O'Connell J., O'Sullivan G.C., Collins J.K., Shanahan F. The Fas counterattackFas-mediated T cell killing by colon cancer cells expressing Fas ligand. J. Exp. Med. 1996;184:1075–1082. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand S., Hofmann W.J., Hug H., Muller M., Otto G., Strand D., Mariani S.M., Stremmel W., Krammer P.H., Galle P.R. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells—a mechanism of immune evasion? Nat. Med. 1996;2:1361–1366. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- Niehans G.A., Brunner T., Frizelle S.P., Liston J.C., Salerno C.T., Knapp D.J., Green D.R., Kratzke R.A. Human lung carcinomas express Fas ligand. Cancer Res. 1997;57:1007–1012. [PubMed] [Google Scholar]
- Saas P., Walker P.R., Hahne M., Quiquerez A.L., Schnuriger V., Perrin G., French L., Van Meir E.G., de Tribolet N., Tschopp J., Dietrich P.Y. Fas ligand expression by astrocytoma in vivomaintaining immune privilege in the brain? J. Clin. Invest. 1997;99:1173–1178. doi: 10.1172/JCI119273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M.W., O'Connell J., O'Sullivan G.C., Brady C., Roche D., Collins J.K., Shanahan F. The Fas counterattack in vivoapoptotic depletion of tumor-infiltrating lymphocytes associated with Fas ligand expression by human esophageal carcinoma. J. Immunol. 1998;160:5669–5675. [PubMed] [Google Scholar]
- Lau H.T., Yu M., Fontana A., Stoeckert C.J., Jr. Prevention of islet allograft rejection with engineered myoblasts expressing FasL in mice. Science. 1996;273:109–112. doi: 10.1126/science.273.5271.109. [DOI] [PubMed] [Google Scholar]
- Arai H., Chan S.Y., Bishop D.K., Nabel G.J. Inhibition of the alloantibody response by CD95 ligand. Nat. Med. 1997;3:843–848. doi: 10.1038/nm0897-843. [DOI] [PubMed] [Google Scholar]
- Allison J., Georgiou H.M., Strasser A., Vaux D.L. Transgenic expression of CD95 ligand on islet beta cells induces a granulocytic infiltration but does not confer immune privilege upon islet allografts. Proc. Natl. Acad. Sci. USA. 1997;94:3943–3947. doi: 10.1073/pnas.94.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.M., Schneider D.B., Lin Z., Hanahan D., Dichek D.A., Stock P.G., Baekkeskov S. Fas ligand expression in islets of Langerhans does not confer immune privilege and instead targets them for rapid destruction. Nat. Med. 1997;3:738–743. doi: 10.1038/nm0797-738. [DOI] [PubMed] [Google Scholar]
- Kang S.M., Hoffmann A., Le D., Springer M.L., Stock P.G., Blau H.M. Immune response and myoblasts that express Fas ligand. Science. 1997;278:1322–1324. doi: 10.1126/science.278.5341.1322. [DOI] [PubMed] [Google Scholar]
- Arai H., Gordon D., Nabel E.G., Nabel G.J. Gene transfer of Fas ligand induces tumor regression in vivo. Proc. Natl. Acad. Sci. USA. 1997;94:13862–13867. doi: 10.1073/pnas.94.25.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino K., Kayagaki N., Okumura K., Yagita H. Antitumor effect of locally produced CD95 ligand. Nat. Med. 1997;3:165–170. doi: 10.1038/nm0297-165. [DOI] [PubMed] [Google Scholar]
- Miwa K., Asano M., Horai R., Iwakura Y., Nagata S., Suda T. Caspase 1-independent IL-1beta release and inflammation induced by the apoptosis inducer Fas ligand. Nat. Med. 1998;4:1287–1292. doi: 10.1038/3276. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Fontana A., Takeda Y., Yagita H., Yoshimoto T., Matsuzawa A. Induction of antitumor immunity with Fas/APO-1 ligand (CD95L)-transfected neuroblastoma neuro-2a cells. J. Immunol. 1999;162:7350–7357. [PubMed] [Google Scholar]
- Kayagaki N., Kawasaki A., Ebata T., Ohmoto H., Ikeda S., Inoue S., Yoshino K., Okumura K., Yagita H. Metalloproteinase-mediated release of human Fas ligand. J. Exp. Med. 1995;182:1777–1783. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Suda T., Takahashi T., Nagata S. Expression of the functional soluble form of human fas ligand in activated lymphocytes. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:1129–1135. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani S.M., Matiba B., Baumler C., Krammer P.H. Regulation of cell surface APO-1/Fas (CD95) ligand expression by metalloproteases. Eur. J. Immunol. 1995;25:2303–2307. doi: 10.1002/eji.1830250828. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Suda T., Haze K., Nakamura N., Sato K., Kimura F., Motoyoshi K., Mizuki M., Tagawa S., Ohga S. Fas ligand in human serum. Nat. Med. 1996;2:317–322. doi: 10.1038/nm0396-317. [DOI] [PubMed] [Google Scholar]
- Pietravalle F., Lecoanet-Henchoz S., Blasey H., Aubry J.P., Elson G., Edgerton M.D., Bonnefoy J.Y., Gauchat J.F. Human native soluble CD40L is a biologically active trimer, processed inside microsomes. J. Biol. Chem. 1996;271:5965–5967. doi: 10.1074/jbc.271.11.5965. [DOI] [PubMed] [Google Scholar]
- Sherry B., Cerami A. Cachectin/tumor necrosis factor exerts endocrine, paracrine, and autocrine control of inflammatory responses. J. Cell Biol. 1988;107:1269–1277. doi: 10.1083/jcb.107.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzoni F., Beutler B. The tumor necrosis factor ligand and receptor families. N. Engl. J. Med. 1996;334:1717–1725. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- Perez C., Albert I., DeFay K., Zachariades N., Gooding L., Kriegler M. A nonsecretable cell surface mutant of tumor necrosis factor (TNF) kills by cell-to-cell contact. Cell. 1990;63:251–258. doi: 10.1016/0092-8674(90)90158-b. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Itai T., Adachi M., Nagata S. Downregulation of Fas ligand by shedding. Nat. Med. 1998;4:31–36. doi: 10.1038/nm0198-031. [DOI] [PubMed] [Google Scholar]
- Suda T., Tanaka M., Miwa K., Nagata S. Apoptosis of mouse naive T cells induced by recombinant soluble Fas ligand and activation-induced resistance to Fas ligand. J. Immunol. 1996;157:3918–3924. [PubMed] [Google Scholar]
- Suda T., Hashimoto H., Tanaka M., Ochi T., Nagata S. Membrane Fas ligand kills human peripheral blood T lymphocytes, and soluble Fas ligand blocks the killing. J. Exp. Med. 1997;186:2045–2050. doi: 10.1084/jem.186.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P., Holler N., Bodmer J.L., Hahne M., Frei K., Fontana A., Tschopp J. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J. Exp. Med. 1998;187:1205–1213. doi: 10.1084/jem.187.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino K., Iwabuchi K., Kayagaki N., Miyata R., Nagaoka I., Matsuzawa A., Fukao K., Yagita H., Okumura K. Chemotactic activity of soluble Fas ligand against phagocytes. J. Immunol. 1998;161:4484–4488. [PubMed] [Google Scholar]
- Ottonello L., Tortolina G., Amelotti M., Dallegri F. Soluble Fas ligand is chemotactic for human neutrophilic polymorphonuclear leukocytes. J. Immunol. 1999;162:3601–3606. [PubMed] [Google Scholar]
- Kayagaki N., Yamaguchi N., Nagao F., Matsuo S., Maeda H., Okumura K., Yagita H. Polymorphism of murine Fas ligand that affects the biological activity. Proc. Natl. Acad. Sci. USA. 1997;94:3914–3919. doi: 10.1073/pnas.94.8.3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C.M., Glass A.A., Chiu V., Clark W.R. The role of the Fas lytic pathway in a perforin-less CTL hybridoma. J. Immunol. 1994;153:2506–2514. [PubMed] [Google Scholar]
- Matsuzawa A., Moriyama T., Kaneko T., Tanaka M., Kimura M., Ikeda H., Katagiri T. A new allele of the lpr locus, lprcg, that complements the gld gene in induction of lymphadenopathy in the mouse. J. Exp. Med. 1990;171:519–531. doi: 10.1084/jem.171.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C., Copeland N., Jenkins N., Nagata S. Lymphoproliferative disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Graf D., Muller S., Korthauer U., van Kooten C., Weise C., Kroczek R.A. A soluble form of TRAP (CD40 ligand) is rapidly released after T cell activation. Eur. J. Immunol. 1995;25:1749–1754. doi: 10.1002/eji.1830250639. [DOI] [PubMed] [Google Scholar]
- Pietravalle F., Lecoanet-Henchoz S., Aubry J.P., Elson G., Bonnefoy J.Y., Gauchat J.F. Cleavage of membrane-bound CD40 ligand is not required for inducing B cell proliferation and differentiation. Eur. J. Immunol. 1996;26:725–728. doi: 10.1002/eji.1830260333. [DOI] [PubMed] [Google Scholar]
- Schneider P., MacKay F., Steiner V., Hofmann K., Bodmer J.L., Holler N., Ambrose C., Lawton P., Bixler S., Acha-Orbea H. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J. Exp. Med. 1999;189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.J., Sun Y., Nabel G.J. Regulation of the proinflammatory effects of Fas ligand (CD95L) Science. 1998;282:1714–1717. doi: 10.1126/science.282.5394.1714. [DOI] [PubMed] [Google Scholar]
- Takeuchi T., Ueki T., Nishimatsu H., Kajiwara T., Ishida T., Jishage K., Ueda O., Suzuki H., Li B., Moriyama N. Accelerated rejection of Fas ligand-expressing heart grafts. J. Immunol. 1999;162:518–522. [PubMed] [Google Scholar]
- Gao Y., Herndon J.M., Zhang H., Griffith T.S., Ferguson T.A. Antiinflammatory effects of CD95 ligand (FasL)-induced apoptosis. J. Exp. Med. 1998;188:887–896. doi: 10.1084/jem.188.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.A. Neutrophils, host defense, and inflammationa double-edged sword. J. Leukoc. Biol. 1994;56:672–686. doi: 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- Zhang H.G., Su X., Liu D., Liu W., Yang P., Wang Z., Edwards C.K., Bluethmann H., Mountz J.D., Zhou T. Induction of specific T cell tolerance by Fas ligand-expressing antigen-presenting cells. J. Immunol. 1999;162:1423–1430. [PubMed] [Google Scholar]
- Matsue H., Matsue K., Walters M., Okumura K., Yagita H., Takashima A. Induction of antigen-specific immunosuppression by CD95L cDNA-transfected ‘killer’ dendritic cells. Nat. Med. 1999;5:930–937. doi: 10.1038/11375. [DOI] [PubMed] [Google Scholar]