Abstract
The human tyrosinase-derived peptide YMDGTMSQV is presented on the surface of human histocompatibility leukocyte antigen (HLA)-A*0201+ melanomas and has been suggested to be a tumor antigen despite the fact that tyrosinase is also expressed in melanocytes. To gain information about immunoreactivity and self-tolerance to this antigen, we established a model using the murine tyrosinase-derived homologue of this peptide FMDGTMSQV, together with transgenic mice expressing the HLA-A*0201 recombinant molecule AAD. The murine peptide was processed and presented by AAD similarly to its human counterpart. After immunization with recombinant vaccinia virus encoding murine tyrosinase, we detected a robust AAD-restricted cytotoxic T lymphocyte (CTL) response to FMDGTMSQV in AAD transgenic mice in which the entire tyrosinase gene had been deleted by a radiation-induced mutation. A residual response was observed in the AAD+tyrosinase+ mice after activation under certain conditions. At least some of these residual CTLs in AAD+tyrosinase+ mice were of high avidity and induced vitiligo upon adoptive transfer into AAD+tyrosinase+ hosts. Collectively, these data suggest that FMDGTMSQV is naturally processed and presented in vivo, and that this presentation leads to substantial but incomplete self-tolerance. The relevance of this model to an understanding of the human immune response to tyrosinase is discussed.
Keywords: tyrosinase, self-tolerance, MHC class I, cytotoxic T lymphocytes, immunotherapy
Introduction
CTLs derived from patients with melanoma have been shown to recognize Ags expressed on allogeneic melanoma in addition to autologous tumor 1 2 3 4 5 6 7 8. Characterization of these “shared” melanoma Ags has established that they are peptides restricted by class I MHC molecules and are derived from a variety of source proteins. These include developmentally regulated proteins that are silent in most normal tissues, with the exception of their expression in spermatogonia and primary spermatocytes of the testis, but become activated in several types of tumor cells (MAGE [9, 10], BAGE 11, and GAGE 12). Shared melanoma antigens also include melanocyte differentiation proteins (MDPs) that are normally expressed only in cells of the melanocytic lineage (tyrosinase [13–15], pMEL17/gp100 [2, 3, 16], gp75/tyrosinase-related protein [TRP]-1 17, MART-1/Melan-A [4], and TRP-2 18). CTLs specific for MDP-derived Ags have been found in the metastatic LNs of melanoma patients 19 20, but the presence of these CTLs sometimes correlates with the loss of MDP expression 21. Although MDPs are commonly expressed in primary tumors, they are absent in ∼30% of metastatic melanomas 22 23. Nevertheless, immunotherapies that target MDP+ tumors and utilize MDP-derived Ags induce positive clinical responses 24 25. Taken together, these results suggest that an immune response against MDP-derived Ags may be an important aspect of the patient's ability to control tumor outgrowth.
Several ongoing clinical trials for melanoma immunotherapy are focused on targeting the immune response to MDP-derived Ags 24 25. Since spontaneous regression of melanomas as well as clinical responses after IL-2 therapy correlate with the destruction of normal melanocytes (vitiligo) 26 27, it is important to understand which of the MDP-derived Ags are associated with the development of vitiligo 28. Insight is provided by studies demonstrating that CTLs specific for some MDP-derived peptide Ags recognize human melanocyte cell lines cultured in the presence of growth factors (29 30 31 32; and our unpublished results), suggesting that these Ags may be targets of the immune response that mediates vitiligo. However, the influence of such growth factors on the profile of Ags presented is not clear. In addition, MHC tetramers have been used to show an accumulation of MDP-specific CTLs in lesions of vitiligo patients 33. Although these studies have provided some information about the Ags that may lead to the development of vitiligo, they have not examined the influence of normal melanocyte expression and/or presentation of MDP-derived Ags on an antitumor immune response.
To address these issues, we wished to evaluate whether MDP-derived Ags are presented by normal melanocytes in vivo and to determine how self-tolerance and autoreactivity to these Ags influences the immune response. We have taken advantage of a recently described albino strain in which the entire coding sequence for tyrosinase has been deleted 34. This deletion precludes processing and presentation of Ags derived from tyrosinase. Because no tyrosinase-derived epitopes have been described that are presented by murine class I MHC molecules, we have also used transgenic mice that express a chimeric human MHC class I transgene encoding the α1 and α2 domains from HLA-A*0201 and the α3, transmembrane, and cytoplasmic domains from Dd (AAD) 35. We have previously identified a peptide (YMDGTMSQV) that is derived from the MDP tyrosinase, presented by HLA-A*0201+ human melanomas, and recognized by patient CTLs 15. In this study, we have examined whether FMDGTMSQV, the murine homologue of YMDGTMSQV, is presented in vivo by murine melanocytes and have evaluated the impact of tyrosinase expression on the development of a FMDGTMSQV-specific CTL immune response.
Materials and Methods
Cell Lines and Transfectants.
EL4-A2/Kb (gift of Dr. Linda Sherman, The Scripps Research Institute, La Jolla, CA) is a transfectant of the murine thymoma EL4 (H-2b haplotype) that expresses A2/Kb. The transfectant of the B lymphoblastoid cell line C1R expressing AAD has been described previously 36. AAD is a hybrid MHC class I molecule that contains the α1 and α2 domains from HLA-A*0201 and the α3 domain of the H-2Dd molecule, and has been described previously 37.
Peptides.
Synthetic peptides were made by standard Fmoc chemistry using a peptide synthesizer (model AMS422; Gilson Company, Inc.). All peptides were purified to >98% purity by reverse-phase HPLC on a C-8 column (Vydac). Purity and identity were established using an LCQ Finnigan Mat mass spectrometer with electrospray ionization.
Recombinant Vaccinia Viruses.
Viruses encoding full-length human tyrosinase (rvv-hu tyr) have been described previously 38. The full-length murine tyrosinase recombinant vaccinia virus (rvv-mu tyr) has also been described 39. Virus encoding the full-length matrix protein 1 (M1) from influenza A/PR/8 (rvv-M1) was a gift from Jack Bennink (National Institutes of Health). Purified vaccinia virus stocks were titered and tested for proper expression using specific murine HLA-A*0201–restricted CTLs.
Class I Peptide Binding Affinity Assays.
The relative affinities of peptides for HLA-A*0201 molecules were measured as described 40. In brief, affinity-purified HLA-A*0201 molecules were incubated at room temperature with an iodinated indicator peptide (FLPSDYFPSV) and graded doses of test peptides in PBS, pH 7.0, containing 0.05% NP-40, 1 μM human β2-microglobulin (Calbiochem), 1 mM PMSF, 1.3 mM 1,10-phenanthroline, 73 μM pepstatin A, 8 mM EDTA, and 200 μM N α-p-tosyl-l-lysine chloromethyl ketone (TLCK). After 48 h, class I peptide complexes were separated from free peptides by gel filtration, and the dose of individual test peptides that reduced the binding of indicator peptide by 50% (IC50) was calculated.
Mice.
C57BL/6 mice transgenic for the AAD gene have been described previously 41. Mice carrying the 38R145L neutron radiation–induced deletion at the tyrosinase (c) locus on mouse chromosome 7 (c38R145L/c38R145L) have been described previously 34. Transmission of this deletion in heterozygous form was determined by PCR analysis using MapPair primers that define D7Mit62 and D7Mit301 (Research Genetics). These markers lie <2 cM from either side of the tyrosinase gene 42.
Generation of CTL Lines.
8-wk-old mice were immunized intraperitoneally with 107 PFU of recombinant vaccinia virus expressing murine tyrosinase, human tyrosinase, or influenza M1. After 3 wk, 7.5 × 106 splenocytes from primed mice were cocultured in 24-well plates with 3.5 × 106 irradiated (2,000 rads) autologous splenocytes that had been pulsed with various concentrations of the indicated synthetic peptide antigens. After 6 d of coculture, activity was measured by standard 51Cr-release assay. CTL lines were established from these primary cultures in 12-well plates (Corning Costar) by weekly culture of 5 × 105 CTLs/well with 5 × 106 irradiated (2,000 rads) C57BL/6 AAD+ peptide-pulsed and washed spleen cells. CTL lines specific for FMDGTMSQV are referred to as FMD 10, FMD 1, FMD 0.1, or FMD 0.01 to indicate the concentration of peptide (in μg/ml) used to pulse the stimulators. Murine CTL lines specific for the HLA-A2–restricted peptides YMDGTMSQV (human tyrosinase369–377) or GILGFVFTL (influenza M158–66) 43 are referred to as CJL and M1, respectively. All murine CTL lines were grown in RPMI 1640 supplemented with 2 mM l-glutamine, sodium pyruvate, essential and nonessential amino acids, penicillin/streptomycin, 50 μM β-mercaptoethanol, 10% FBS, 15 mM Hepes, and 10 U/ml of IL-2 in a humidified 8% CO2 atmosphere at 37°C.
In Vitro Cytotoxicity Assay.
Standard 51Cr-release assays were performed to determine CTL recognition of murine and human tyrosinase369–377 peptides. For peptide dose–response assays, 51Cr-labeled EL4-A2/Kb cells were incubated with the indicated concentrations of synthetic peptides for 3 h at room temperature. Con A blasts were generated by incubating 5 × 106 spleen cells/ml in 10 ml of RPMI medium containing 2 μg/ml of Con A for 72 h in a 25-cm2 upright tissue culture flask in a humidified 5% CO2 atmosphere at 37°C. For vaccinia-infected target cells, 10 PFU/cell were added to 106 target cells in 1 ml HBSS supplemented with 0.1% BSA, 1.6 mM MgSO4, and 1.8 mM CaCl2 for 1 h and then 4 ml of RPMI 1640 supplemented with 10% FBS was added for 8 h.
Enrichment of CD8+ T Cells.
CD8+ T cells from spleens of immunized mice were isolated from a StemSep column after incubation with a cocktail of antibodies to enrich for CD8+ cells (StemCell). Preparations were consistently 85–95% CD8+ as assessed by flow cytometry conducted on a FACScan™ using CELLQuest™ software (Becton Dickinson).
Analysis of Intracellular IFN-γ Production.
Peptide-pulsed stimulator cells were incubated with CD8+ T cells for 5 h at a ratio of 1:1 in media supplemented with 50 U/ml IL-2 and 10 μg/ml brefeldin A (Sigma Chemical Co.). Stimulated cells were stained with PE-conjugated anti-CD8 (PharMingen), washed, fixed and permeabilized in Cytofix/Cytoperm (PharMingen), further stained with FITC-conjugated anti–IFN-γ (PharMingen) or isotype-matched controls, and analyzed by flow cytometry. Results are presented as percent positive cells after subtraction of isotype control values.
Adoptive Cell Transfer.
2 d after the last in vitro stimulation, 107 CJL, FMD 1, or M1 CTLs were subcutaneously transferred into irradiated (700 rads) AAD+ or AAD− C57BL/6 mice with or without 5,000 Cetus U of Proleukin (IL-2; Chiron). Mice that received the initial dose of IL-2 received additional IL-2 (5,000 Cetus U) intraperitoneally each day for 4 d after CTL transfer. Mice were examined each week for 4 wk for evidence of coat color changes.
Results
Murine Tyrosinase369–377 Is Homologous to Human Tyrosinase369–377 and Binds to HLA-A*0201.
The amino acid sequences of murine and human tyrosinase proteins are 82% homologous, and almost identical in the region surrounding residues 369–377, from which the HLA-A*0201–restricted melanoma Ag YMDGTMSQV is derived. With the exception of position 369 of this sequence, in which Y is substituted by an F residue, the 11 amino acids upstream and 30 amino acids downstream of the human epitope are identical to those in the murine protein (Fig. 1). As the human epitope undergoes a posttranslational modification in which N371 is converted to a D 15 38, it seemed likely that the homologous murine peptide would also undergo this modification and would be presented by HLA-A*0201 similarly to the human tyrosinase Ag. When the relative affinity of the murine tyrosinase369–377 peptide containing a D at position 371 (FMDGTMSQV) for purified HLA-A*0201 was measured in a competitive binding assay 15, the concentration that inhibited the binding of the iodinated standard peptide to purified HLA-A*0201 by 50% (IC50) was nearly identical to the IC50 value of the human tyrosinase369–377 peptide (YMDGTMSQV) (Table ). Thus, this murine homologue of the human tyrosinase-derived melanoma Ag could potentially be presented by HLA-A*0201+ cells and recognized by CTLs.
Figure 1.
Sequence homology of murine and human tyrosinase in the region surrounding residues 369–377.
Table 1.
Binding Affinities of Human and Murine Tyrosine-derived Peptides for HLA-A*0201
| Origin | Sequence | IC50 |
|---|---|---|
| nM | ||
| Human | YMDGTMSQV | 74 |
| Murine | FMDGTMSQV | 65 |
IC50 values were determined by measuring the binding of the indicated synthetic peptides to purified HLA-A*0201, as described in Materials and Methods.
HLA-A*0201–restricted CTLs Specific for Human Tyrosinase369–377 Cross-react on the Homologous Murine Peptide.
To provide direct evidence for the immunological recognition of FMDGTMSQV, we used CTLs that had been elicited against YMDGTMSQV 38. We found that several such CTL lines recognized EL4-A2/Kb target cells that had been pulsed with similar concentrations of either the human or murine peptides (Fig. 2 A, and data not shown). Similarly, we elicited CTLs from AAD+ mice against FMDGTMSQV (FMD 1) and found that they had a similar affinity for both peptides, as determined from the concentration required to give half-maximal lysis (Fig. 2 B). Collectively, these results confirm that the murine and human tyrosinase-derived peptides bind to HLA-A*0201 and are sufficiently homologous to be recognized by the same CTLs.
Figure 2.
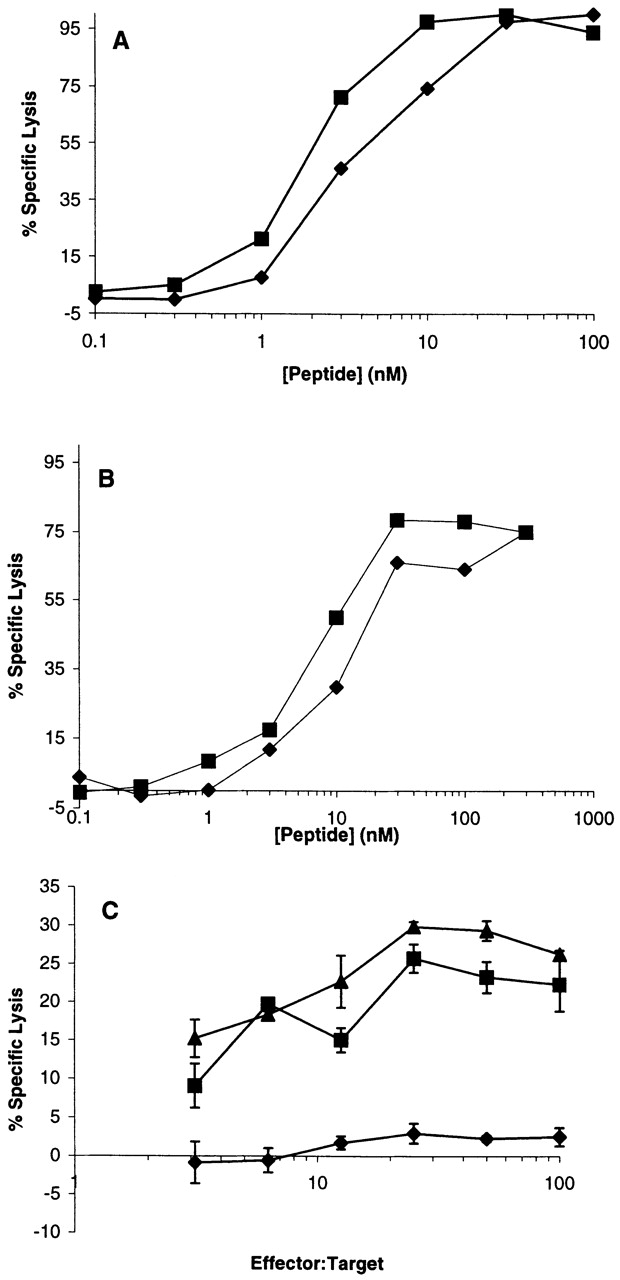
Murine tyrosinase369–377 is naturally processed and presented in vivo. (A) 51Cr-labeled EL4-AAK cells that had been pulsed with the indicated concentration of either murine (♦) or human (▪) tyrosinase peptides were incubated with CJL CTLs stimulated by and reactive with human tyrosinase369–377 at an E/T ratio of 20:1. (B) 51Cr-labeled EL4-AAK cells that had been pulsed with the indicated concentration of either murine (♦) or human (▪) tyrosinase peptides were incubated with FMD 1 CTLs stimulated by and reactive with murine tyrosinase369–377 at an E/T ratio of 20:1. (C) C1R-AAD target cells were infected for 8 h with 10 PFU/cell of either rvv-mu tyr (▪), rvv-hu tyr (▴), or rvv-M1 (♦). Cells were then labeled with 51Cr and incubated with a murine tyrosinase369–377–reactive CTL line.
Murine Tyrosinase369–377 Is Naturally Processed and Presented.
The preceding results demonstrated that a synthetic peptide corresponding to the sequence derived from murine tyrosinase369–377 could be presented by HLA-A*0201 when added to cells. To determine whether this peptide could be naturally processed and presented, we infected cells with rvv-mu tyr. For this purpose we used C1R-AAD, a tyrosinase− B lymphoblastoid cell line that had been transfected with AAD. When infected with either the rvv-mu tyr or rvv-hu tyr, these cells were lysed by FMD 1 CTLs, which recognize murine tyrosinase369–377 (Fig. 2 C). These results confirmed that murine tyrosinase369–377 can be naturally processed and presented in association with HLA-A*0201 molecules.
T Cell Responses to Murine Tyrosinase369–377 in Tyrosinase+ and Tyrosinase− Mice.
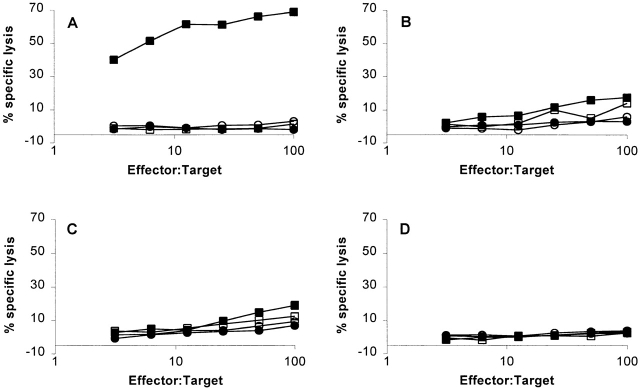
To test the impact of tyrosinase expression on CTL response to murine tyrosinase369–377, we compared AAD+ mice (which are of C57BL/6 origin and tyrosinase+) with 38R145L mice, from which the tyrosinase coding sequence at the c locus has been deleted 34. The latter were crossed to AAD+ mice, and progeny positive for both markers were intercrossed to produce AAD+ mice that were c +/c +, c +/c38R145L, or c38R145L/c38R145L. While the first two genotypes were normally pigmented, animals with the third genotype were albino. After immunization of each type of animal with rvv-mu tyr, spleen cells were cultured with autologous splenocytes pulsed with FMDGTMSQV, and the CTL response was measured 6 d later. CTLs from immunized AAD+ c38R145L/c38R145L mice (hereafter referred to as AAD+ albino) recognized FMDGTMSQV peptide–pulsed EL4-A2/Kb targets (Fig. 3 A). In contrast, T cells from immunized AAD+ mice that were either c +/c38R145L or c +/c + (hereafter referred to as AAD+tyrosinase+) exhibited no significant response to targets presenting FMDGTMSQV (Fig. 3B and Fig. C). These results indicate that expression of tyrosinase is associated with a dramatic reduction in the response to this tyrosinase-derived peptide.
Figure 3.
Cytotoxic T cell responses to murine tyrosinase369–377 in tyrosinase+ and tyrosinase− AAD+ mice. Splenocytes from (A) AAD+ c38R145L/c38R145L mice, (B) AAD+ c +/c38R145L mice, (C) AAD+ c + mice, or (D) AAD− c38R145L/c38R145L mice that had been immunized with rvv-mu tyr were cultured for 6 d in the presence of 1 μM FMDGTMSQV. CTL activity was measured on 51Cr-labeled EL4-AAK (▪, □) or EL4 (•, ○) cells that had been pulsed with 1 μM FMDGTMSQV (filled symbols) or left unpulsed (open symbols). Data are representative of six independent experiments.
Several additional experiments were done to confirm that the CTL response observed in AAD+ albino mice was dependent on the AAD molecule. First, albino mice that did not express AAD also failed to mount a specific CTL response after immunization with rvv-mu tyr and in vitro restimulation with FMDGTMSQV peptide (Fig. 3 D). Second, although FMDGTMSQV-specific CTLs from AAD+ albino mice recognized peptide-pulsed EL4-A2/Kb transfectants, they failed to recognize peptide-pulsed but untransfected EL4 cells (Fig. 3 A). Similarly, these CTLs recognized peptide-pulsed Con A blasts from AAD+, but not AAD− littermates (Fig. 4). These results establish that the CTL response in rvv-mu tyr–immunized AAD+ albino mice is AAD restricted and confirms that FMDGTMSQV cannot be presented by murine restriction elements expressed in the outbred albino strain.
Figure 4.

The T cell response to murine tyrosinase369–377 in tyrosinase− mice is AAD restricted. CTLs from AAD+ albino mice that had been generated as in the legend to Fig. 3 A were assayed for activity using Con A blast targets from either AAD+ albino mice (▪, □) or AAD− albino mice (•, ○) that had been either pulsed with FMDGTMSQV (filled symbols) or left unpulsed (open symbols).
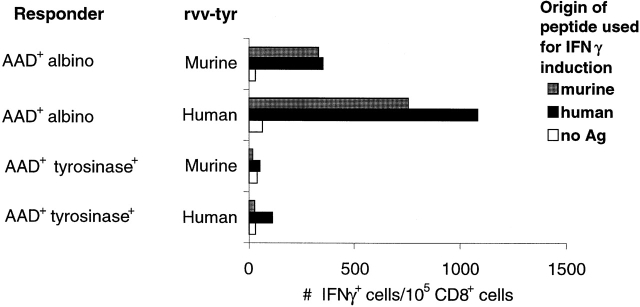
We also examined the impact of tyrosinase expression on the T cell response to FMDGTMSQV by the quantitation of IFN-γ production by CD8+ cells from spleens of rvv-mu tyr–immunized mice. As observed for cytotoxic activity, a significant number of CD8+ cells from AAD+ albino mice produced IFN-γ at the peak of the response to rvv-mu tyr, whereas no IFN-γ production was discernible in CD8+ cells from AAD+tyrosinase+ mice (Fig. 5). Based on the observation that the AAD-restricted murine and human tyrosinase epitopes were recognized in a cross-reactive manner by CTLs, we also examined responses in AAD+ albino mice and AAD+tyrosinase+ mice after immunization with rvv-hu tyr. A strong response was observed in AAD+ albino mice that was largely cross-reactive on the murine tyrosinase-derived peptide. In contrast, a much weaker response after immunization with rvv-hu tyr was observed in AAD+tyrosinase+ mice, and no significant cross-reactivity on the murine tyrosinase epitope was detectable. Taken together, we conclude that in vivo presentation of FMDGTMSQV by the AAD molecule in AAD+tyrosinase+ mice results in a substantially diminished CTL response to both the murine tyrosinase-derived peptide and its human homologue.
Figure 5.
Ex vivo T cell response to murine tyrosinase369–377 in AAD+tyrosinase+ and AAD+ albino mice as measured by IFN-γ production. 7 d after intravenous immunization with 107 rvv-mu tyr or rvv-hu tyr, CD8+ cells were enriched from splenocytes, stimulated for 5 h with synthetic FMDGTMSQV (murine peptide) or YMDGTMSQV (human peptide), and assayed for the intracellular accumulation of IFN-γ as described in Materials and Methods.
A Residual Tyrosinase-specific Response Exists in Tyrosinase+ Mice.
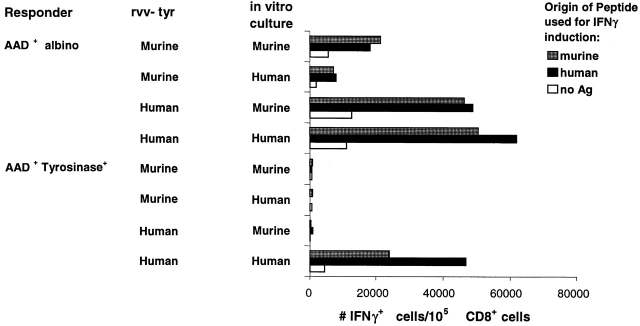
The preceding results demonstrated that there was no detectable FMDGTMSQV-specific CTL response from immunized AAD+tyrosinase+ mice either at the peak of the primary response or after secondary in vitro stimulation. These results appeared at odds with our previous demonstration that CTL lines elicited against the human tyrosinase epitope recognize the murine tyrosinase-derived peptide in a cross-reactive manner (Fig. 2 A). To further investigate the immunogenicity of murine tyrosinase in AAD+tyrosinase+ mice, spleen cells from AAD+tyrosinase+ and AAD+ albino mice immunized with either rvv-mu tyr or rvv-hu tyr were cultured with stimulators pulsed with either the murine or human peptides and analyzed for their ability to make IFN-γ in response to either peptide (Fig. 6). As expected, AAD+ albino mice responded well to both rvv-mu tyr and rvv-hu tyr. The T cells elicited were largely cross-reactive, based on their activation and differentiation in response to either peptide during 7 d of in vitro culture, as well as their subsequent ability to be induced to produce IFN-γ in a 5-h incubation. In contrast, AAD+tyrosinase+ mice failed to respond to rvv-mu tyr based on the lack of any detectable response in spleen cells cultured for 7 d with either the murine or human peptides. Consistent with our previous observations (Fig. 5, and reference 44), spleen cells from AAD+tyrosinase+ mice immunized with rvv-hu tyr and cultured in vitro for 7 d with the human peptide led to their activation and differentiation as measured by their ability to produce IFN-γ when induced with the human peptide for 5 h. Significantly, many of these T cells also recognized the murine tyrosinase-derived peptide as judged by their ability to make IFN-γ when induced with the murine peptide for 5 h. However, in parallel cultures of T cells from these same rvv-hu tyr–immunized AAD+tyrosinase+ mice, this murine tyrosinase-derived peptide did not cause activation and differentiation during a 7-d in vitro culture. These data establish that there are residual murine tyrosinase-specific T cells in AAD+ tyrosinase+ mice but that their ability to respond to the murine peptide is impaired.
Figure 6.
Characteristics of residual tyrosinase-specific response in AAD+tyrosinase+ mice. 3 wk after immunization with either rvv-mu tyr or rvv-hu tyr, splenocytes from AAD+tyrosinase+ mice or AAD+ albino mice were cultured in vitro with an irradiated feeder layer that had been pulsed with 1 μg/ml of either synthetic FMDGTMSQV (murine) or YMDGTMSQV (human) peptides. 7 d later, cultures were harvested, stimulated for 5 h with C1R-AAD cells that had been pulsed with synthetic FMDGTMSQV or YMDGTMSQV, and assayed for the intracellular accumulation of IFN-γ.
Induction of Vitiligo following the Adoptive Transfer of FMDGTMSQV-reactive T Cells into Normal Mice.
The preceding results indicated that the expression of tyrosinase in AAD+ animals led to the development of partial tolerance towards the FMDGTMSQV peptide presented by AAD. However, they did not clearly establish whether this Ag was directly presented by melanocytes. Therefore, we conducted adoptive transfer experiments to determine whether FMDGTMSQV-specific CTLs could mediate melanocyte destruction in vivo (vitiligo). An FMDGTMSQV-specific CTL line that was derived from AAD+ albino mice (FMD 1) was transferred subcutaneously into irradiated AAD+tyrosinase+ recipients together with IL-2. 4 wk after subcutaneous transfer, mice exhibited significant vitiligo as evidenced by depigmentation of the hair in the vicinity of the injection site (Fig. 7 A, and Table ). At 4 wk after treatment, vitiligo was not observed in mice that received IL-2 alone or were irradiated but did not receive either CTLs or IL-2 (Table ). As expected, transfer of the same number of CTLs specific for the HLA-A2–restricted influenza M158–66 epitope in combination with IL-2 failed to cause vitiligo. These results indicate that normal melanocytes in AAD+ tyrosinase+ mice express the tyrosinase Ag in the context of AAD and are targets for CTL-mediated destruction.
Figure 7.
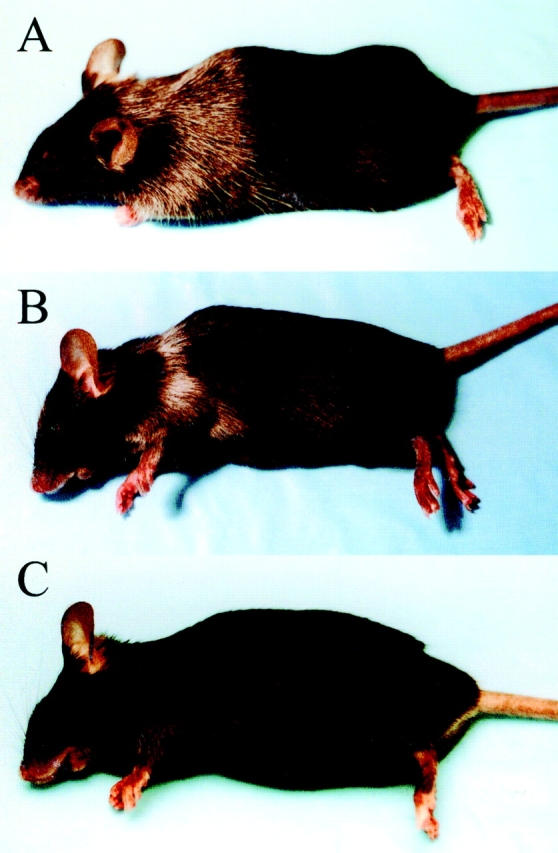
Induction of vitiligo after the adoptive transfer of tyrosinase-specific T cells into AAD+tyrosinase+ mice. FMD 1, a CTL line derived from AAD+ albino mice, was transferred subcutaneously into AAD+ tyrosinase+ mice (A). CJL, a CTL line derived from AAD+tyrosinase+ mice, was transferred subcutaneously into either AAD+tyrosinase+ (B) or AAD−tyrosinase+ (C) mice. Photographs were taken 5 wk after transfer; those shown are representative of multiple mice in each group (for n values, see Table ).
Table 2.
Induction of Vitiligo in AAD+Tyrosinase+ Mice by Adoptive Transfer of Tyrosinase-specific CTLs
| CTLs | Origin | Specificity | Host | IL-2 | Vitiligo | n |
|---|---|---|---|---|---|---|
| FMD 1 | Albino-AAD | Antityrosinase | AAD | + | +++ | 6 |
| None | NA | NA | AAD | + | − | 15 |
| None | NA | NA | AAD | − | − | 6 |
| M1 | B6-AAD | Anti- influenza M1 | AAD | + | − | 12 |
| CJL | B6-AAD | Antityrosinase | AAD | + | +++ | 15 |
| CJL | B6-AAD | Antityrosinase | B6 | + | − | 2 |
| CJL | B6-AAD | Antityrosinase | AAD | − | + | 6 |
All hosts were irradiated (700 rads) and received one of the regimens described above (see Materials and Methods). Vitiligo intensity was scored as the relative number of depigmented follicles within the 1-cm2 area of transfer. Mice were scored 4 wk after transfer as: −, none; +, <30%; +++, >70%. B6, C57BL/6.
We also tested the ability of a tyrosinase-specific CTL line derived from AAD+tyrosinase+ mice to mediate vitiligo. CJL CTL was induced by immunization with rvv hu tyr, but showed equivalent recognition of both human and murine peptides (Fig. 2 A). In addition, the doses of the murine peptide required for half-maximal recognition are comparable to those of intermediate avidity CTLs obtained from albino AAD mice (compare Fig. 2A and Fig. B; see also Table ). Using the same number of transferred CTLs, vitiligo occurred in these mice with a comparable time course and intensity (Fig. 7 B, and Table ). In contrast, these CTLs did not mediate vitiligo in AAD−tyrosinase+ mice, and their induction of vitiligo in the absence of exogenous IL-2 was significantly diminished (Fig. 7 C, and Table ). Interestingly, we have not observed vitiligo in any animals immunized with recombinant vaccinia virus expressing either human or murine tyrosinase (n > 20), nor in mice immunized with either FMDGTMSQV or YMDGTMSQV peptides pulsed onto dendritic cells (n = 8) (data not shown). Collectively, these results demonstrate that at least some of the residual tyrosinase-specific CTLs in AAD+tyrosinase+ mice are capable of mediating vitiligo, but only when appropriately activated.
Table 3.
Avidity of CTL Lines for Murine Tyrosinase Peptide
| CTL line | SD50 |
|---|---|
| μg/ml | |
| CJL | 7.25 |
| FMD 10 | 30 |
| FMD 1 | 10 |
| FMD 0.1 | 8.75 |
| FMD 0.01 | 2 |
CTL lines were generated as described (see Materials and Methods). SD50 values are the average of three (FMD 10) or four (CJL, FMD 1, FMD 0.1, and FMD 0.01) independent determinations.
Discussion
In this report, we have established and characterized a murine model system to further our understanding of the human CTL response to the HLA-A*0201–restricted Ag derived from tyrosinase. Subsequent to the discovery that Ags derived from MDPs, such as tyrosinase, constitute a major target for melanoma-reactive CTLs, it was also established that murine MDPs encode peptide Ags that are presented in the context of murine class I molecules 45. While these models offer the possibility to determine the impact of these Ags on both antitumor immune responses and self-tolerance, the advantage of the system described in this report is that it permits the evaluation of an Ag that is highly homologous in structure, behavior, and expression to a human Ag that is currently in clinical trials for the treatment of melanoma.
The homologous murine peptide derived from tyrosinase differs from the human Ag by a single conservative amino acid change at P1. As a consequence, the binding affinities of the two peptides for HLA-A*0201 are nearly identical, and CTLs raised against one Ag frequently cross-react on the other. Most important, murine tyrosinase is endogenously processed, leading to the presentation of the murine peptide in association with HLA-A*0201. These similarities enabled us to use AAD+ albino mice to evaluate whether the tyrosinase-derived Ag is expressed on melanocytes in vivo and how it affects the development of an HLA-A*0201–restricted Ag-specific CTL response. This is the first model system established for studying self-tolerance to an Ag expressed on melanocytes. Using this unique model, we determined that the murine homologue of tyrosinase is naturally processed and presented in the context of AAD in tyrosinase+ mice in vivo and that this presentation has a tolerizing effect on the immune response.
The expression of tyrosinase protein and/or RNA is confined to neural crest–derived melanocytes 14 46 47, substantia nigra and forebrain 48, and chorioid and retinal pigment epithelium 46 49. Because tyrosinase expression appears limited to a small number of tissues, self-tolerance to the tyrosinase-derived Ag is likely to be mediated by a peripheral rather than a central mechanism. However, one study has demonstrated that tyrosinase transcripts can be detected in a wide range of tissues after 60 cycles of PCR amplification 50, suggesting that very low-level tyrosinase expression is widespread. It was hypothesized that this might be due to the presence of melanocytes in these tissues, resulting from the arrested migration of neural crest cells during development. This observation leaves open the possibility that self-tolerance to tyrosinase might arise through a central mechanism. Studies are currently under way to evaluate the role of the thymus in the mechanism of self-tolerance to tyrosinase and to determine whether tolerance to the tyrosinase-derived Ag can be mediated directly by melanocytes or indirectly through cross-presentation by bone marrow–derived cells 51 52 53.
Regardless of the exact mechanisms responsible, our data show convincingly that self-tolerance is incomplete. Low levels of cytotoxic and IFN-γ+CD8+ T cells specific for FMDGTMSQV are demonstrable in AAD+tyrosinase+ mice after priming and in vitro restimulations, and these T cells induce vitiligo upon adoptive transfer into AAD+ tyrosinase+ mice. In addition, peptide dose–response curves show that the avidity of these CTLs is comparable to that of high avidity CTLs generated in AAD+ albino mice under similar priming conditions. Collectively, these results demonstrate that at least some of the murine tyrosinase-specific T cells in AAD+tyrosinase+ mice are of high avidity. This appears to be at odds with previous observations that peripheral expression of influenza HA under the control of the insulin promoter leads to a selective loss of high avidity CTLs 54. However, it is clear that the site of protein expression, level of protein expression, and the accessibility to the immune system contribute to the mechanism of tolerance to self-Ags expressed in the periphery 55 56. In addition, it is also clear that the detection of murine tyrosinase-specific T cells in AAD+tyrosinase+ mice is not straightforward. These cells do not appear to activate and/or differentiate in response to murine tyrosinase in vivo or in vitro. However, this failure can be circumvented by the use of human tyrosinase, and subsequently, the ability of these cells to recognize murine tyrosinase can be demonstrated either by cytolysis or IFN-γ secretion. These observations are reminiscent of the responses of T cells to altered peptide ligands that function as partial agonists 57 58 59. It is frequently observed that partial agonists fail to induce proliferation, although they do induce cytolysis and the secretion of at least some cytokines 60 61. Further work will be required to fully understand the recognition of the murine tyrosinase peptide by these cells.
The results of this study are also particularly interesting in light of recent work on tolerance to a keratinocyte-specific Ag 62. It was demonstrated that Ag-specific CTL precursors that arise during the first 4–6 wk of life are rendered tolerant due to circulation through the epidermis. Although access to the epidermis ceases after this time, subsequently arising T cells are tolerized by an undefined alternate mechanism. The major sites of expression of tyrosinase, as well as other MDP-derived Ags, are in the melanocytes of the skin, or tissues sequestered behind the blood–brain barrier. It will be of interest to determine if access to the epidermis is necessary for tolerance to these Ags, and whether the activation requirements of residual high avidity FMDGTMSQV-specific CTLs in tyrosinase+ mice are a reflection of this alternate tolerizing mechanism.
In this study, the development of vitiligo in tyrosinase+ mice after the transfer of FMDGTMSQV-reactive CTLs indicates that this murine tyrosinase-derived peptide is expressed in the context of AAD on normal melanocytes. This direct in vivo analysis complements and extends earlier work in which the expression of the homologous human tyrosinase-derived peptide was demonstrated on melanocytes cultured in the presence of growth factors and phorbol esters (32; and our unpublished results). It has previously been demonstrated that vitiligo in mice can be induced as a consequence of antibody responses to TRP-1 63 64 65 or CD8+ CTL responses against an unknown antigen expressed on B16 melanoma 66. Interestingly, it has been demonstrated that vitiligo in both models is associated with tumor regression and protective antitumor immune responses 57 58 59. Because of the similarities between human and mouse tyrosinase, the expression of the tyrosinase-derived peptide in the context of the AAD molecule on murine melanocytes in the present study strongly suggests that the human tyrosinase peptide is also expressed in the context of HLA-A*0201 on normal human melanocytes. This leads us to suggest that immune responses to tyrosinase may also lead to the hypopigmentation that is often observed in association with clinical responses in melanoma patients. In addition, it suggests that the use of this Ag in immunotherapeutic treatment of melanoma may result in vitiligo. Our results offer the possibility of developing a model system in which vitiligo-inducing CTL responses against a known human antigen can be studied for their impact on tumor regression.
The observations made in the murine model system described here allow us to make inferences about the importance of human tyrosinase as a tumor Ag. Our results suggest that the expression of tyrosinase in human melanocytes will lead to a diminished number of activatable CTLs that recognize the peptide YMDGTMSQV in the context of HLA-A*0201. Nonetheless, it remains important to define the conditions for optimal activation and expansion of these cells in vivo to engender an active therapeutic response. The model system described here offers an important and useful approach to this issue.
Acknowledgments
We thank Janet V. Gorman for her enthusiasm and outstanding technical assistance; Drs. Patricia Foley and Sandy Feldman of the University of Virginia Animal Care Facility; and Erin Field and Jennifer Caldwell for the synthesis and purification of peptides used in this study. In addition, we appreciate the thoughtful advice and generosity of Drs. Marcia McDuffie, Kenneth Tung, Kari Irvine, and David J. Kittlesen. Finally, we are grateful to Julie Burns for her excellent administrative support.
This work was supported by U.S. Public Health Service grants AI21393 and CA78400 (to V.H. Engelhard). T.A. Colella was supported by U.S. Public Health Service Training Grants CA09109 and AI07496. R.A. Pierce and D.W. Mullins were supported by U.S. Public Health Service Training Grant AI07496, and C.J. Luckey was supported by U.S. Public Health Service Medical Scientist Training Program Grant GM07267. T.N.J. Bullock is a Fellow of the Cancer Research Institute. L.B. Russell was supported by the U.S. Department of Energy (DE-AC05-960R22464).
Footnotes
Abbreviations used in this paper: IC50, half-maximal inhibitory concentration; M1, matrix protein 1; MDP, melanocyte differentiation protein; rvv-hu tyr, full-length human tyrosinase recombinant vaccinia virus; rvv-M1, full-length matrix protein 1 from influenza A/PR/8; rvv-mu tyr, full-length murine tyrosinase recombinant vaccinia virus; TRP, tyrosinase-related protein.
References
- Slingluff C.L., Jr., Cox A.L., Henderson R.A., Hunt D.F., Engelhard V.H. Recognition of human melanoma cells by HLA-A2.1-restricted cytotoxic T lymphocytes is mediated by at least six shared peptide epitopes. J. Immunol. 1993;150:2955–2963. [PubMed] [Google Scholar]
- Cox A.L., Skipper J.C., Chen Y., Henderson R.A., Darrow T.L., Shabanowitz J., Engelhard V.H., Hunt D.F., Slingluff C.L., Jr. Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994;264:716–719. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- Kawakami Y., Eliyahu S., Jennings C., Sakaguchi K., Kang X., Southwood S., Robbins P.F., Sette A., Appella E., Rosenberg S.A. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J. Immunol. 1995;154:3961–3968. [PubMed] [Google Scholar]
- Castelli C., Storkus W.J., Maeurer M.J., Martin D.M., Huang E.C., Pramanik B.N., Nagabhushan T.L., Parmiani G., Lotze M.T. Mass spectrometric identification of a naturally processed melanoma peptide recognized by CD8+ cytotoxic T lymphocytes. J. Exp. Med. 1995;181:363–368. doi: 10.1084/jem.181.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfel T., Klehmann E., Muller C., Schutt K.H., Meyer zum Büschenfelde K.H., Knuth A. Lysis of human melanoma cells by autologous cytolytic T cell clones. Identification of human histocompatibility antigen A2 as a restriction element for three different antigens. J. Exp. Med. 1989;170:797–810. doi: 10.1084/jem.170.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow T.L., Slingluff C.L., Jr., Seigler H.F. The role of HLA class I antigens in recognition of melanoma cells by tumor-specific cytotoxic T lymphocytes. Evidence for shared tumor antigens. J. Immunol. 1989;142:3329–3335. [PubMed] [Google Scholar]
- Hom S.S., Topalian S.L., Simonis T., Mancini M., Rosenberg S.A. Common expression of melanoma tumor-associated antigens recognized by human tumor infiltrating lymphocytesanalysis by human lymphocyte antigen restriction. J. Immunother. 1991;10:153–164. [PubMed] [Google Scholar]
- Storkus W.J., Zeh H.J., III, Maeurer M.J., Salter R.D., Lotze M.T. Identification of human melanoma peptides recognized by class I restricted tumor infiltrating T lymphocytes. J. Immunol. 1993;151:3719–3727. [PubMed] [Google Scholar]
- Celis E., Tsai V., Crimi C., DeMars R., Wentworth P.A., Chesnut R.W., Grey H.M., Sette A., Serra H.M. Induction of anti-tumor cytotoxic T lymphocytes in normal humans using primary cultures and synthetic peptide epitopes. Proc. Natl. Acad. Sci. USA. 1994;91:2105–2109. doi: 10.1073/pnas.91.6.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bruggen P., Bastin J., Gajewski T., Coulie P.G., Boel P., de Smet C., Traversari C., Townsend A., Boon T. A peptide encoded by human gene MAGE-3 and presented by HLA-A2 induces cytolytic T lymphocytes that recognize tumor cells expressing MAGE-3. Eur. J. Immunol. 1994;24:3038–3043. doi: 10.1002/eji.1830241218. [DOI] [PubMed] [Google Scholar]
- Boel P., Wildmann C., Sensi M.L., Brasseur R., Renauld J.C., Coulie P., Boon T., van der Bruggen P. BAGEa new gene encoding an antigen recognized on human melanomas by cytolytic T lymphocytes. Immunity. 1995;2:167–175. doi: 10.1016/s1074-7613(95)80053-0. [DOI] [PubMed] [Google Scholar]
- Van den Eynde B., Peeters O., De Backer O., Gaugler B., Lucas S., Boon T. A new family of genes coding for an antigen recognized by autologous cytolytic T lymphocytes on a human melanoma. J. Exp. Med. 1995;182:689–698. doi: 10.1084/jem.182.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfel T., Van Pel A., Brichard V., Schneider J., Seliger B., zum Büschenfelde K.H.M., Boon T. Two tyrosinase nonapeptides recognized on HLA-A2 melanomas by autologous cytolytic T lymphocytes. Eur. J. Immunol. 1994;24:759–764. doi: 10.1002/eji.1830240340. [DOI] [PubMed] [Google Scholar]
- Brichard V., Van Pel A., Wolfel T., Wolfel C., De Plaen E., Lethe B., Coulie P., Boon T. The tyrosinase gene codes for an antigen recognized by autologous cytotoxic T lymphocytes on HLA-A2 melanomas. J. Exp. Med. 1993;178:489–495. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper J.C.A., Hendrickson R.C., Gulden P.H., Brichard V., Van Pel A., Chen Y., Shabanowitz J., Wolfel T., Slingluff C.L., Jr., Boon T. An HLA-A2 restricted tyrosinase antigen on melanoma cells results from post-translational modification and suggests a novel processing pathway for membrane proteins. J. Exp. Med. 1996;183:527–534. doi: 10.1084/jem.183.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A.B.H., Schreurs M.W., de Boer A.J., Kawakami Y., Rosenberg S.A., Adema G.J., Figdor C.G. Melanocyte lineage-specific antigen gp100 is recognized by melanoma-derived tumor-infiltrating lymphocytes. J. Exp. Med. 1994;179:1005–1009. doi: 10.1084/jem.179.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.F., Robbins P.F., Kawakami Y., Kang X.Q., Rosenberg S.A. Identification of a gene encoding a melanoma tumor antigen recognized by HLA-A31–restricted tumor-infiltrating lymphocytes. J. Exp. Med. 1995;181:799–804. doi: 10.1084/jem.181.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.F., Appella E., Kawakami Y., Kang X., Rosenberg S.A. Identification of TRP-2 as a human tumor antigen recognized by cytotoxic T lymphocytes. J. Exp. Med. 1996;184:2207–2216. doi: 10.1084/jem.184.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannelli J.R., McConnell S., Parker L., Nishimura M., Robbins P., Yang J., Gamil M.E., Kawakami Y. Melanoma tumor-infiltrating lymphocytes derived from four distinct anatomic sites obtained from a single patientcomparison of functional reactivity and melanoma antigen recognition. J. Immunother. 1996;18:263–271. doi: 10.1097/00002371-199511000-00007. [DOI] [PubMed] [Google Scholar]
- Romero P., Dunbar P.R., Valmori D., Pittet M., Ogg G.S., Rimoldi D., Chen J.L., Lienard D., Cerottini J.C., Cerundolo V. Ex vivo staining of metastatic lymph nodes by class I major histocompatibility complex tetramers reveals high numbers of antigen-experienced tumor-specific cytolytic T lymphocytes. J. Exp. Med. 1998;188:1641–1650. doi: 10.1084/jem.188.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager E., Ringhoffer M., Karbach J., Arand M., Oesch F., Knuth A. Inverse relationship of melanocyte differentiation antigen expression in melanoma tissues and CD8+ cytotoxic-T-cell responsesevidence for immunoselection of antigen-loss variants in vivo. Int. J. Cancer. 1996;66:470–476. doi: 10.1002/(SICI)1097-0215(19960516)66:4<470::AID-IJC10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- de Vries T.J., Fourkour A., Wobbes T., Verkroost G., Ruiter D.J., van Muijen G.N. Heterogeneous expression of immunotherapy candidate proteins gp100, MART-1, and tyrosinase in human melanoma cell lines and in human melanocytic lesions. Cancer Res. 1997;57:3223–3229. [PubMed] [Google Scholar]
- Slingluff C.L., Jr., Colella T.A., Thompson L., Graham D.D., Skipper J.C.A., Caldwell J.A., Brinkerhoff L., Kittlesen D.J., Deacon D.H., Oei C. Melanomas with concordant loss of multiple melanocytic differentiation proteinsimmune escape that may be overcome by targeting unique or undefined antigens. Cancer Immunol. Immunother. 2000;48:661–672. doi: 10.1007/s002620050015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S.A., Yang J.C., Schwartzentruber D.J., Hwu P., Marincola F.M., Topalian S.L., Restifo N.P., Dudley M.E., Schwarz S.L., Spiess P.J. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat. Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle F.O., Alijagic S., Gilliet M., Sun Y., Grabbe S., Dummer R., Burg G., Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat. Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- Duhra P., Ilchyshyn A. Prolonged survival in metastatic malignant melanoma associated with vitiligo. Clin. Exp. Dermatol. 1991;16:303–305. doi: 10.1111/j.1365-2230.1991.tb00383.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg S.A. Cancer vaccines based on the identification of genes encoding cancer regression antigens. Immunol. Today. 1997;18:175–182. doi: 10.1016/s0167-5699(97)84664-6. [DOI] [PubMed] [Google Scholar]
- Song Y.H. Why tyrosinase for treatment of melanoma. Lancet. 1997;350:82–83. doi: 10.1016/S0140-6736(05)61810-7. [DOI] [PubMed] [Google Scholar]
- Kawakami Y., Eliyahu S., Delgado C.H., Robbins P.F., Sakaguchi K., Appella E., Yannelli J.R., Adema G.J., Miki T., Rosenberg S.A. Identification of a human melanoma antigen recognized by tumor infiltrating lymphocytes associated with in vivo tumor rejection. Proc. Natl. Acad. Sci. USA. 1994;91:6458–6462. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anichini A., Maccali C., Mortarini R., Salvi S., Mazzocchi A., Squarcina P., Herlyn M., Parmiani G. Melanoma cells and normal melanocytes share antigens recognized by HLA-A2–restricted cytotoxic T cell clones from melanoma patients. J. Exp. Med. 1993;170:797–810. doi: 10.1084/jem.177.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y., Eliyahu S., Delgaldo C.H., Robbins P.F., Rivoltini L., Topalian S.L., Miki T., Rosenberg S.A. Cloning of the gene for a shared melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc. Natl. Acad. Sci. USA. 1994;91:3515–3519. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anichini A., Mortarini R., Maccali C., Squarcina P., Fleischhauer K., Mascheroni L., Parmiani G. Cytotoxic T cells directed to tumor antigens not expressed on normal melanocytes dominate HLA-A2.1-restricted immune repertoire to melanoma. J. Immunol. 1996;156:208–217. [PubMed] [Google Scholar]
- Ogg G.S., Rod D.P., Romero P., Chen J.L., Cerundolo V. High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. J. Exp. Med. 1998;188:1203–1208. doi: 10.1084/jem.188.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinchik E.M., Stoye J.P., Frankel W.N., Coffin J., Kwon B.S., Russell L.B. Molecular analysis of viable spontaneous and radiation-induced albino (c)-locus mutations in the mouse. Mutat. Res. 1993;286:199–207. doi: 10.1016/0027-5107(93)90184-h. [DOI] [PubMed] [Google Scholar]
- Newberg M.H., Ridge J.P., Vining D.R., Salter R.D., Engelhard V.H. Species specificity in the interaction of CD8 with the α3 domain of MHC class I molecules. J. Immunol. 1992;149:136–142. [PubMed] [Google Scholar]
- Beier D.C., Cox J.H., Vining D.R., Cresswell P., Engelhard V.H. Association of human class I MHC alleles with the adenovirus E3/19K protein. J. Immunol. 1994;152:3862–3872. [PubMed] [Google Scholar]
- Engelhard V.H., Yannelli J.R., Evans G.A., Walk S.F., Holterman M.J. Construction of novel class I histocompatibility antigens by interspecies exon shuffling. J. Immunol. 1985;134:4218–4225. [PubMed] [Google Scholar]
- Mosse C.A., Meadows L., Luckey C.J., Kittlesen D.J., Huczko E.L., Slingluff C.L., Jr., Shabanowitz J., Hunt D.F., Engelhard V.H. The class I antigen processing pathway for the membrane protein tyrosinase involves translation in the endoplasmic reticulum and processing in the cytosol. J. Exp. Med. 1998;187:37–48. doi: 10.1084/jem.187.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwijk W.W., Tsung A., Irvine K.R., Parkhurst M.R., Goletz T.J., Tsung K., Carroll M.W., Liu C., Moss B., Rosenberg S.A., Restifo N.P. gp100/pmel 17 is a murine tumor rejection antigeninduction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J. Exp. Med. 1998;188:277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Sidney J., Southwood S., Cox A.L., Sakaguchi K., Henderson R., Appella E., Hunt D.F., Sette A., Engelhard V.H. Naturally processed peptides longer than nine amino acid residues bind to the class I MHC molecule HLA-A2.1 with high affinity and in different conformations. J. Immunol. 1994;152:2874–2881. [PubMed] [Google Scholar]
- Newberg M.H., Smith D.H., Haertel S.B., Vining D.R., Lacy E., Engelhard V.H. Importance of MHC class I α2 and α3 domains in the recognition of self and non-self MHC molecules. J. Immunol. 1996;156:2473–2480. [PubMed] [Google Scholar]
- Rikke B.A., Johnson D.K., Johnson T.E. Murine albino-deletion complexhigh-resolution microsatellite map and genetically anchored YAC framework map. Genetics. 1997;147:787–799. doi: 10.1093/genetics/147.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhard V.H. Structure of peptides associated with MHC class I molecules. Curr. Opin. Immunol. 1994;6:13–23. doi: 10.1016/0952-7915(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Bullock T.N.J., Colella T.A., Engelhard V.H. The density of peptides displayed by dendritic cells affects immune responses to human tyrosinase and gp100 in HLA-A2 transgenic mice. J. Immunol. 2000;164:2354–2361. doi: 10.4049/jimmunol.164.5.2354. [DOI] [PubMed] [Google Scholar]
- Bloom M.B., Perry-Lalley D., Robbins P.F., Li Y., el-Gamil M., Rosenberg S.A., Yang J.C. Identification of tyrosinase-related protein 2 as a tumor rejection antigen for the B16 melanoma. J. Exp. Med. 1997;185:453–459. doi: 10.1084/jem.185.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluppel M., Beermann F., Ruppert S., Schmid E., Hummler E., Schutz G. The mouse tyrosinase promoter is sufficient for expression in melanocytes and in the pigmented epithelium of the retina. Proc. Natl. Acad. Sci. USA. 1991;88:3777–3781. doi: 10.1073/pnas.88.9.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugler B., Brouwenstijn N., Vantomme V., Szikora J.P., van der Spek C.W., Patard J.J., Boon T., Schrier P., Van den Eynde B. A new gene coding for an antigen recognized by autologous cytolytic T lymphocytes on a human renal carcinoma. Immunogenetics. 1996;44:323–330. doi: 10.1007/BF02602776. [DOI] [PubMed] [Google Scholar]
- Tief K., Schmidt A., Aguzzi A., Beermann F. Tyrosinase is a new marker for cell populations in the mouse neural tube. Dev. Dyn. 1996;205:445–456. doi: 10.1002/(SICI)1097-0177(199604)205:4<445::AID-AJA8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Tief K., Hahne M., Schmidt A., Beerman F. Tyrosinase, the key enzyme in melanin synthesis, is expressed in murine brain. Eur. J. Biochem. 1996;241:12–16. doi: 10.1111/j.1432-1033.1996.0012t.x. [DOI] [PubMed] [Google Scholar]
- Battyani Z., Xerri L., Hassoun J., Bonerandi J.J., Grob J.J. Tyrosinase gene expression in human tissues. Pigment Cell Res. 1993;6:400–405. doi: 10.1111/j.1600-0749.1993.tb00622.x. [DOI] [PubMed] [Google Scholar]
- Heath W.R., Kurts C., Miller J.F.A.P., Carbone F.R. Cross-tolerancea pathway for inducing tolerance to peripheral tissue antigens. J. Exp. Med. 1998;187:1549–1553. doi: 10.1084/jem.187.10.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurts C., Kosaka H., Carbone F.R., Miller J.F.A.P., Heath W.R. Class I–restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+ T cells. J. Exp. Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurts C., Heath W.R., Carbone F.R., Allison J., Miller J.F.A.P., Kosaka H. Constitutive class I–restricted exogenous presentation of self antigens in vivo. J. Exp. Med. 1999;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D.J., Kreuwel H.T., Fleck S., Levitsky H.I., Pardoll D.M., Sherman L.A. Activation of low avidity CTL specific for a self epitope results in tumor rejection but not autoimmunity. J. Immunol. 1998;160:643–651. [PubMed] [Google Scholar]
- Morgan D.J., Kreuwel H.T.C., Sherman L.A. Antigen concentration and precursor frequency determine the rate of CD8+ T cell tolerance to peripherally expressed antigens. J. Immunol. 1999;163:723–727. [PubMed] [Google Scholar]
- Schonrich G., Momburg F., Malissen M., Schmitt-Verhulst A.M., Malissen B., Hammerling G.J., Arnold B. Distinct mechanisms of extrathymic T cell tolerance due to differential expression of self antigen. Int. Immunol. 1992;4:581–590. doi: 10.1093/intimm/4.5.581. [DOI] [PubMed] [Google Scholar]
- Evavold B.D., Sloan-Lancaster J., Hsu B.L., Allen P.M. Separation of IL-4 production from Th cell proliferation by an altered T cell receptor ligand. J. Immunol. 1993;150:3131–3140. [Google Scholar]
- Jameson S.C., Bevan M.J. T cell receptor antagonists and partial agonists. Immunity. 1995;2:1–11. doi: 10.1016/1074-7613(95)90074-8. [DOI] [PubMed] [Google Scholar]
- Hemmer B., Stefanova I., Vergelli M., Germain R.N., Martin R. Relationships among TCR ligand potency, thresholds for effector function elicitation, and the quality of early signaling events in human T cells. J. Immunol. 1998;160:5807–5814. [PubMed] [Google Scholar]
- Rogers P.R., Grey H.M., Croft M. Modulation of naive T cell activation with altered peptide ligandsthe nature of the peptide and presentation in the context of costimulation are critical for a sustained response. J. Immunol. 1998;160:3698–3704. [PubMed] [Google Scholar]
- Hollsberg P., Weber W.E., Dangond F., Batra V., Sette A., Hafler D.A. Differential activation of proliferation and cytotoxicity in human T-cell lymphotropic virus type I Tax-specific CD8 T cells by an altered peptide ligand. Proc. Natl. Acad. Sci. USA. 1995;92:4036–4040. doi: 10.1073/pnas.92.9.4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alferink J., Tafuri A., Vestweber D., Hallmann R., Hammerling G.J., Arnold B. Control of neonatal tolerance to tissue antigens by peripheral T cell trafficking. Science. 1998;282:1338–1341. doi: 10.1126/science.282.5392.1338. [DOI] [PubMed] [Google Scholar]
- Hara I., Takechi Y., Houghton A.N. Implicating a role for immune recognition of self in tumor rejectionpassive immunization against the brown locus protein. J. Exp. Med. 1995;182:1609–1614. doi: 10.1084/jem.182.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftzger C., Takechi Y., Kohda H., Hara I., Vijayasaradhi S., Houghton A.N. Immune response to a differentiation antigen induced by altered antigena study of tumor rejection and autoimmunity. Proc. Natl. Acad. Sci. USA. 1996;93:14809–14814. doi: 10.1073/pnas.93.25.14809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwijk W.W., Lee D.S., Surman D.R., Irvine K.R., Touloukian C.E., Chan C.C., Carroll M.W., Moss R., Rosenberg S.A., Restifo N.P. Vaccination with a recombinant vaccinia virus encoding a “self” antigen induces autoimmune vitiligo and tumor cell destruction in micerequirement for CD4+ T lymphocytes. Proc. Natl. Acad. Sci. USA. 1999;96:2982–2987. doi: 10.1073/pnas.96.6.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elsas A.H.A.A., Allison J.P. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]