Abstract
Murine intestinal intraepithelial lymphocytes (iIELs) are made up of a heterogeneous mix of T cells with unique phenotypes. Whereas CD8+ T cells in peripheral lymphoid organs use CD8α/β and are selected on MHC class Ia molecules, a majority of iIELs use CD8α/α. Here, we report that the presence of CD8α/α TCR-α/β cells in iIELs is independent of classical MHC class I molecules Kb and Db, as illustrated by their presence in Kb/Db double-knockout mice and in mice lacking a nonclassical MHC class I molecule, CD1d. Most strikingly, their presence is decreased by ∼70% in mice lacking transporter associated with antigen processing (TAP). The TAP-dependent nonclassical MHC class I molecule Qa-2 is strongly implicated in the presence of these cells, as inferred from the low numbers of CD8α/α TCR-α/β T cells in mice deficient in Qa-2 genes. Second, a Qa-2–transgenic mouse made in a Qa-2− strain showed an increase in the numbers of CD8α/α cells among its iIELs. Thus, the presence of CD8α/α TCR-α/β cells in iIELs is mainly dependent on the nonclassical MHC class I molecule Qa-2.
Keywords: CD8α/α TCR-α/β cells, MHC class I–deficient mice, Qa-2–transgenic mice, Qa-2–deficient mice, intestinal intraepithelial lymphocyte
Introduction
In contrast to conventional thymus-derived α/β TCR cells, intestinal intraepithelial lymphocytes (iIELs) are a distinct population having several unique features. The vast majority of such cells are CD8+, the frequency of α/β and γ/δ T cells is roughly equal, and most γ/δ T cells express a CD8α/α homodimer and may develop extrathymically 1 2 3 4 5 6 7 8. TCR-α/β cells in the iIEL compartment are generally heterogeneous in terms of their expression of CD8α/α and CD8α/β. CD8α/β cells are reported to govern conventional immune responses against pathogens and environmental antigens 9 10 11 12 13 14. Information regarding the CD8α/α TCR-α/β subset of iIELs is still lacking information about the MHC molecules that they recognize and that present antigen to them. They appear to be a resident subset, do not participate in antigen-specific immune responses, and may play a role(s) in localized immune regulation 15 16. Mice aged 6–8 wk and raised in specific pathogen–free conditions contain an equal number of α/β and γ/δ T cells among their iIELs, which are primarily, but not solely, CD8α/α 17. Previously, it had been shown that the classical MHC class I molecules, Kb and Db, are required for the presence of CD8α/β TCR-α/β cells in peripheral lymphoid organs as well as in iIELs. On the other hand, CD8α/α TCR-α/β cells were found only in intestinal epithelium and do not require the classical MHC class I molecules Kb and Db 17 18 19. However, the presence of such cells is dependent on β2-microglobulin (β2m), suggesting a requirement for an MHC-related protein 20.
Here, we report that while the maintenance of CD8+ TCR-α/β iIELs is dependent on β2m and transporter associated with antigen processing (TAP) function, the absence of the Kb and Db molecules does not alter the development or maintenance of such cells. This observation allowed us to study the involvement of TAP-dependent nonclassical MHC molecules on the maintenance of TCR-α/β cells among the iIELs. All of the MHC class I molecules and the well studied, nonclassical MHC class Ib molecules Qa-2 proteins are known to be expressed in a TAP-dependent fashion 21. These proteins are encoded by four genes (Q6, Q7, Q8, and Q9) in C57BL/6 mice 22, contributing to a Qa-2high phenotype 23. BALB/cJ mice carry only two functional Qa-2 genes (Q6 and Q7; reference 24) and express Qa-2 at an intermediate level (Qa-2dull; reference 25). We find that BALB/cJ mice have a corresponding decrease in the percentage of CD8α/α TCR-α/β cells in their iIEL compartments. BALB/cByJ and C3H/HeJ mice, which lack functional Qa-2 genes altogether and are therefore deficient in Qa-2 expression 23 25 26, have a severe deficit in the number of CD8α/α TCR-α/β+ iIELs. To further confirm the apparent importance of Qa-2 on the presence of CD8α/α TCR-α/β T cells in iIELs, we examined their abundance in Qa-2–transgenic mice. Indeed, the Qa-2–transgenic mice produce higher numbers of CD8α/α TCR-α/β iIELs. Thus, Qa-2 by itself can account for most of the TAP-dependent presence of CD8α/α TCR-α/β cells among iIELs.
Materials and Methods
Mice and Antibodies.
C57BL/6, BALB/c, BALB/cByJ, C3H/HeJ, B6C3F1/J, CByB6F1/J, and B6-β2m−/− mice were purchased from The Jackson Laboratory. TAP−/− mice, which had been backcrossed with C57BL/6 mice for 12 generations, were a gift from Dr. K.A. Hogquist (University of Minnesota, Minneapolis, MN). The KbDb−/− mice were described previously 27. CD1−/−TAP−/− mice were generated by an F1 brother and sister mating of CD1−/− and TAP−/− mice. CD1d−/− mice were described previously 28, as were Qa-2–transgenic mice 29. In brief, these mice were produced in (C57BL/6 × BALB/AnN) F1 and backcrossed to BALB/cAnN for 10 generations. 6–8-wk-old mice, irrespective of their sex, were used throughout the study. Mice were maintained in a pathogen-free colony and fed sterile food prepared at the Yale Animal Research Center. Anti–TCR-α/β (H-57), anti–TCR-γ/δ (GL-3), anti-CD8β (Ly-3.2), and anti–Qa-2 1 2, anti-Vβ2 (B20), -Vβ3 (KJ25), -Vβ4 (KT4), -Vβ5.1,5.2 (MR9-4), -Vβ6 (RR4-7), -Vβ7 (TR310), -Vβ8.1,8.1 (MR5-2), -Vβ9 (MR10-2), -Vβ10b (B21.5), -Vβ11 (RR3-15), -Vβ12 (MR12-3), -Vβ14 (14-2), and anti-CD1D (1B1) antibodies were purchased from PharMingen. Anti-CD8α (53-6.7) was purchased from Sigma-Aldrich. Anti-MHC class I antibody (Kb and Db; HB-51) was purified from the culture supernatant.
Preparation of iIELs.
iIELs were prepared as described earlier 30 with a minor modification. In brief, small intestines were harvested and washed by swirling in PBS. Mesentery and Peyer's patches were carefully removed. The intestines were cut longitudinally and then into ∼0.5-cm pieces. Intestinal pieces were agitated in 25 ml of extraction buffer (PBS, 3% FCS, 1 mM dithiothreitol, 1 mM EDTA) for 30 min at 37°C. This slurry was passed through a loosely packed nylon wool column to remove the aggregates. The follow-through was layered on a discontinuous Percoll gradient (Amersham Pharmacia Biotech). This gradient was then centrifuged at 900 g for 20 min. Cells at the interface of the 40/70% layer were collected and washed in staining buffer.
FACS® Staining and Analysis.
Cells were suspended in staining buffer at a concentration of 107 cells/ml. 100 μl of suspension was incubated either with directly conjugated antibodies or biotinylated antibodies for 30 min on ice. For the latter, streptavidin–PE was used as the secondary reagent for an additional incubation of 30 min on ice. Cells were washed twice with staining buffer and fixed with 1% paraformaldehyde. Fluorescence intensities were measured with FACScan™ (Becton Dickinson). Cells used for sorting were stained in Click's Earls Hanks amino acids, 5% FCS media. Cells were sorted by using a FACStarPLUS™ (Becton Dickinson).
Results and Discussion
Mice deficient in MHC class I expression lack peripheral CD8α/β T cells 31 32. To test the fate of intestinal T cells, we analyzed the composition of iIELs in β2m−/− mice. Compared with wild-type animals, TCR-α/β iIELs in these mice disappear almost completely. There was no significant difference in the number of γ/δ T cells among the iIELs of β2m−/− mice (Fig. 1 and reference 18). Hence, γ/δ T cells in the gut wall develop independently of MHC class I molecules in β2m−/− animals. In contrast, the development of TCR-α/β iIELs requires the presence of β2m-dependent MHC class I molecules. As β2m is involved in the assembly of both classical (Ia) and nonclassical (Ib) MHC class I products, the above experiment does not differentiate between the roles of MHC class Ia and Ib molecules in the development and persistence of CD8 TCR-α/β iIELs. The availability of KbDb−/− mice 27 allowed us to separate genetically the functional contribution of MHC class Ib molecules from those of MHC class Ia molecules.
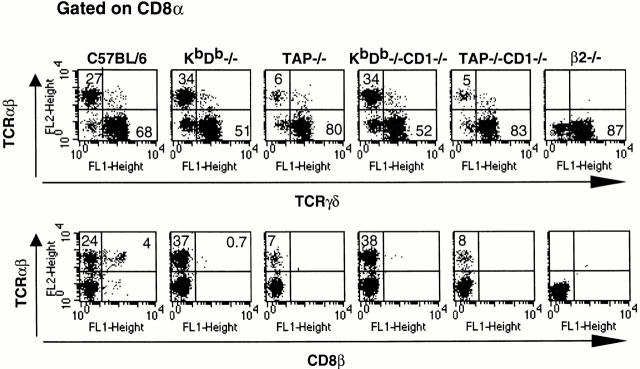
Figure 1.
CD8α/α TCR-α/β iIELs are present in Kb/Db double-knockout mice but absent in TAP- and/or β2m−/− mice. Comparison of iIELs from C57BL/6, KbDb−/−, and KbDb−/− CD1−/− mice showed almost equal numbers of CD8α/α TCR-α/β cells, whereas TAP−/−, TAP−/−CD1−/−, and β2m−/− mice had only a few of these cells. TCR-γ/δ cell numbers are not affected in any of these mice. The data shown represents six independent experiments.
In H-2b mice, most of the thymus-derived peripheral CD8 T cells develop on the products of either the Kb or Db gene; the deletion of these two genes severely reduces the number of CD8 T cells in peripheral lymphoid organs 27. In contrast, the composition of iIELs in KbDb−/− mice showed no detectable reduction in CD8α/α TCR-α/β cell numbers (Fig. 1).
In peripheral lymphoid organs, most of the CD8 T cells bear the CD8α/β heterodimer, whereas TCR-α/β cells in iIELs bear either the CD8α/β or CD8α/α homodimer. Previously, it was reported that the appearance of CD8α/β cells among iIELs increases with age 33 and environmental pathogens 9 10 11 12 13 14 34. Recently, it was found that the presence of CD8α/β T cells in peripheral lymphoid organs is mainly dependent on Kb and Db molecules 17. Thus, we analyzed the content of α/β TCR iIELs in relatively young mice, where we had previously detected mainly CD8α/α cells 17. The population of CD8α/α TCR-α/β cells in the KbDb−/− mouse might bear a diverse repertoire or might result from expansion of a clonal population of T cells. To address this question, we examined the usage of Vβ genes by the CD8α/α TCR-α/β iIELs from several individual mice by FACS® staining. We observed representation of all commonly used Vβ chains without marked skewing of Vβ usage. This implies the presence of a diverse T cell receptor repertoire in CD8α/α TCR-α/β cells (Table ) and argues against selective clonal expansion of iIELs in KbDb−/− animals.
Table 1.
Vβ Chain Usage by FACS® in iIELs of C57BL/6 and KbDb−/−CD1−/− Mice
| Vβ usage | C57BL/6 | KbDb−/−CD1−/− |
|---|---|---|
| 2 | 5.8 ± 0.13 | 4.21 ± 0.3 |
| 3 | 5.08 ± 1.4 | 4.5 ± 0.25 |
| 4 | 3.07 ± 1.62 | 4.04 ± 1.08 |
| 5 | 13.04 ± 3.8 | 11.5 ± 6 |
| 6 | 5.62 ± 0.6 | 5.4 ± 1.4 |
| 7 | 2.7 ± 0.24 | 1.5 ± 0.3 |
| 8.12 | 16.44 ± 3.5 | 14.7 ± 3.3 |
| 8.3 | 8 ± 1.5 | 8 ± 0.95 |
| 9 | 1.44 ± 0.12 | 1.53 ± 0.25 |
| 10 | 4.03 ± 0.9 | 3.8 ± 1.14 |
| 11 | 11.33 ± 1.5 | 11.32 ± 1.95 |
| 12 | 3.43 ± 0.3 | 2.76 ± 0.3 |
| 13 | 4.1 ± 1 | 4.64 ± 1 |
| 14 | 3.97 ± 0.9 | 2.4 ± 0.20 |
| 17 | 1.57 ± 0.15 | 1.98 ± 0.3 |
In H-2b mice, TCR-α/β iIELs do not require Kb and Db for their development and maintenance, nor are they the result of a clonally expanded population. As shown, there was no remarkable skewing of TCR usage.
Because β2m−/− mice are highly deficient in CD8α/α TCR-α/β iIELs and mice lacking the classical MHC class Ia molecules have normal numbers of these cells, it follows that the development of iIELs is dependent on MHC class Ib molecules. To determine if selection of CD8α/α TCR-α/β iIELs involves a TAP-dependent MHC class Ib product, we performed FACS® analysis on iIELs from TAP1−/− mice. iIELs from TAP1−/− mice contain only one-third of the TCR-α/β cells found in wild-type mice and thus demonstrate a requirement for the MHC class Ib products that are TAP dependent (Fig. 1). This observation supports previously reported TAP-dependent selection and maintenance of TCR-α/β iIELs 20 35. Considering data from both TAP−/− and KbDb−/− mice, we conclude that the development and maintenance of CD8α/α TCR-α/β iIELs is dependent, at least partially, on one or more TAP-dependent nonclassical MHC class Ib molecule(s).
Several reports indicate that intestinal epithelial cells express a restricted set of MHC class Ib molecules, including CD1d 36. To investigate the possible involvement of CD1d molecules in the selection and maintenance of CD8 TCR-α/β iIELs, we performed FACS® analysis of iIEL populations in CD1d−/− and TAP−/−CD1d−/− mice, and we found no difference in TCR-α/β T cell content when compared with normal or TAP−/− mice, respectively (Fig. 1). Thus, CD1d does not significantly contribute to the development of α/β T cells in the iIEL compartment. This is consistent with a CD8α/β T cell phenotype independent of TAP and CD1d expression 37.
Among the well characterized nonclassical MHC class Ib molecules, Qa-2 has been shown to be expressed in a TAP-dependent fashion 21. In contrast to most of the nonclassical MHC class Ib molecules, Qa-2 binds a wide variety of endogenous peptides 38. Therefore, it was expected that T cells that recognize Qa-2 would use a wide repertoire of TCRs. In fact, when we analyzed the TCRs used by the CD8α/α TCR-α/β cells in KbDb−/− mice, we found that these cells use a diverse set of TCR β chains similar to those in C57BL/6 mice (Table ).
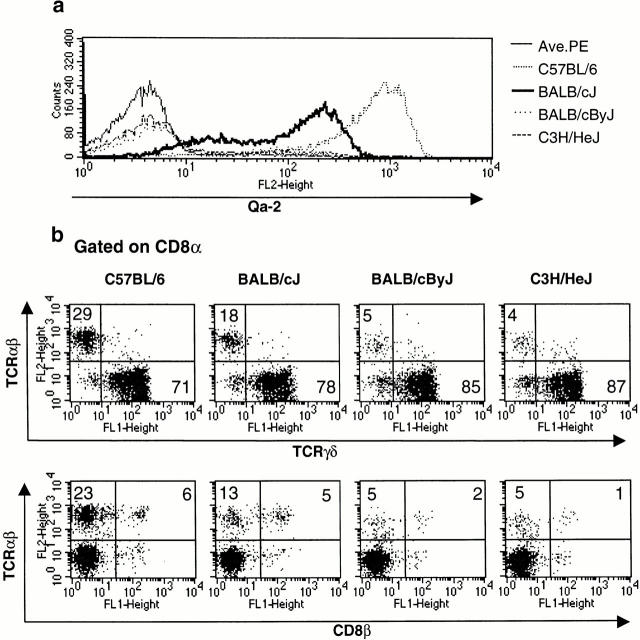
Qa-2 proteins are expressed in various cell types, including epithelial cell lines 39. Their expression status in enterocytes is currently unknown, although Qa-2 transcription was detected in gut tissue (Chiang, E.Y. and I. Stroynowski, unpublished data). Qa-2–deficient mice are susceptible to Taenia crassiceps infection, whereas the presence of Qa-2 in host mice confers resistance to this parasite 29. These characteristics suggest that Qa-2 may play a role in the selection and/or maintenance of iIELs. We therefore examined the iIEL population present in two closely related strains of BALB/c mice, BALB/cJ (Qa-2dull) and BALB/cByJ (Qa-2null). These two strains were genetically separated around 60 years ago and differ at very few loci 40, including one known mutation of MHC class I genes. This mutation involved internal deletion of functional Qa-2 genes, which led to silencing of the Qa-2 locus 25. Our results indicate that BALB/cJ mice produce a lower number of CD8α/α TCR-α/β iIELs than the C57BL/6 mice, whereas BALB/cByJ mice produce even fewer TCR-α/β iIELs when compared with C57BL/6 and BALB/cJ mice (Fig. 2). To confirm these results, we examined the iIEL populations present in naturally Qa-2–deficient C3H/HeJ mice, which lack Qa-2 genes altogether 26. We observed that these mice resemble both BALB/cByJ and TAP−/− mice in that only a small number of CD8α/α TCR-α/β iIELs are present.
Figure 2.
Qa-2 is implicated in the presence of CD8α/α TCR-α/β iIELs. (a) Expression of Qa-2 on iIELs was measured by FACS®. BALB/cByJ and C3H/HeJ mice were deficient in Qa-2 protein, BALB/cJ mice show an intermediate level of Qa-2 expression, and C57BL/6 mice show the highest level of Qa-2 expression. (b) The level of CD8α/α TCR-α/β iIELs was proportional to the level of Qa-2 expression. The highest numbers of these cells appear in C57BL/6 mice, an intermediate number in BALB/cJ mice, and the lowest numbers in BALB/cByJ and C3H/HeJ mice, which lack Qa-2 genes altogether. Data shown is representative of four independent experiments. Ave. PE, streptavidin R–PE.
To further confirm the apparent importance of Qa-2 in the presence of CD8α/α TCR-α/β T cells in iIELs, we examined their abundance in Qa-2–transgenic mice. Our data show that Qa-2–transgenic mice produce higher numbers of CD8α/α TCR-α/β iIELs than do those of their Qa-2− littermates (Fig. 3). Thus, Qa-2 by itself can account for most of the TAP dependence of CD8α/α TCR-α/β cells among iIELs.
Figure 3.
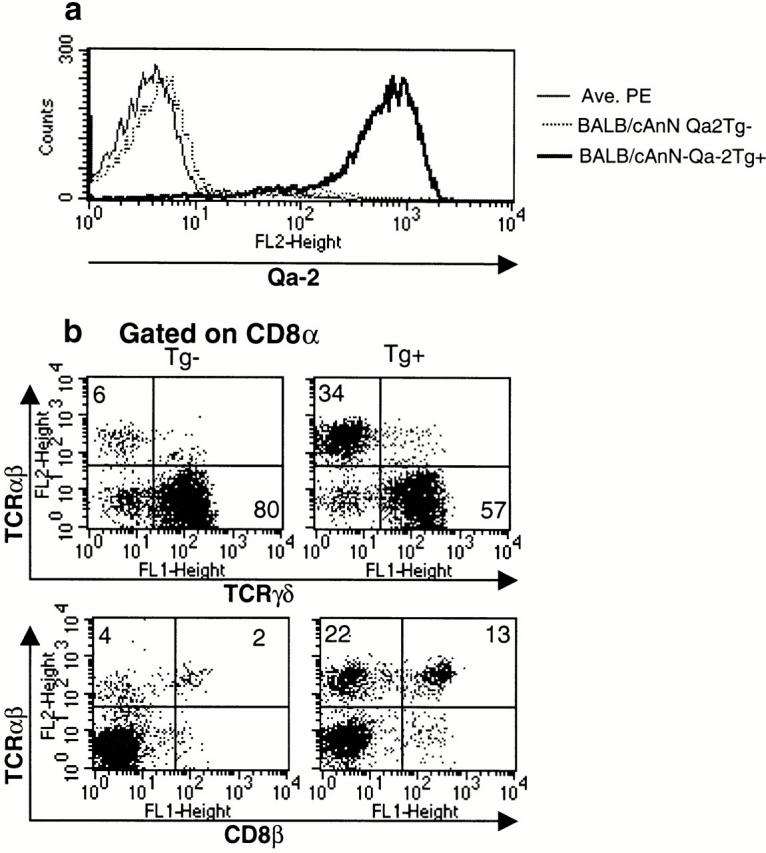
CD8α/β TCR-α/β iIELs in Qa-2–transgenic mice. iIELs were separated from Qa-2–transgenic and nontransgenic mice. (a) Expression of Qa-2 is high in transgenic mice and virtually absent in BALB/cAnN mice. (b) FACS® analysis shows that mice that express Qa-2 from a transgene (Tg) have elevated numbers of CD8α/α TCR-α/β cells that are comparable to the numbers found in wild-type mice (compare to Fig. 1). Results are compiled from four individual transgenic and three individual control mice analyzed on three separate days.
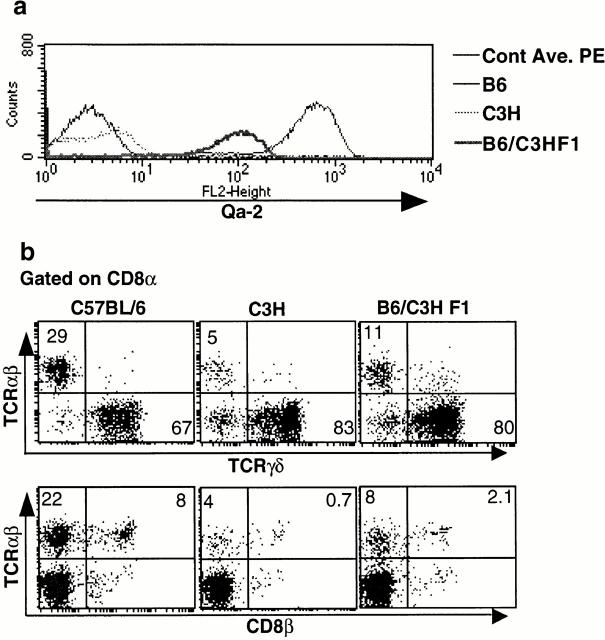
Interestingly, it was found that decreased Qa-2 expression not only led to the reduction of CD8α/α TCR-α/β cells but also resulted in a reduced number of CD8α/β TCR-α/β cells (Fig. 2 and Fig. 3). Previously, it had been shown that CD8α/β TCR-α/β cells are dependent on classical MHC class I molecules 17 18 19. Therefore, the reduction in the number of these cells in Qa-2–deficient mice is certainly not due to their dependence on Qa-2. This reduction could result from (a) CD8α/β TCR-α/β cells being vulnerable targets of NK cells in the absence of Qa-2 or (b) a dependency of CD8α/β cells on survival factors that are produced by CD8α/α TCR-α/β cells. To address the former issue, we examined the F1 animals of a cross between Qa-2+ (C57BL/6) and Qa-2− (C3H/He) parents, where the level of Qa-2 expression is intermediate. In these mice, we also found a partial decrease in both the numbers of CD8α/β TCR-α/β cells and CD8α/α TCR-α/β cells (Fig. 4). We further confirmed this data by analyzing an F1 littermate of Qa-2+ (C57BL/6) and Qa-2− (BALB/cByJ) parents (not shown). If Qa-2 was important in conferring NK cell inhibitory function, intermediate levels of Qa-2 expression would have been sufficient to protect the CD8α/β cells, or if the level of Qa-2 was too low to protect from NK cells, then all of the CD8α/β cells should have been killed. Therefore, this effect is most likely not due to targeting of CD8α/β TCR-α/β cells by NK cells in the absence of Qa-2. It is of a potential future importance to explore how CD8α/β cells depend on CDα/α cells for their presence in the gut. It is worth mentioning that an earlier report has shown dependency between two subsets of T cells with regard to their functional maturity 41. Therefore, the presence of CD8α/β cells in the gut could be dependent on CD8α/α TCR-α/β cells.
Figure 4.
Intermediate level of Qa-2–expressing F1 mice from Qa-2+ and Qa-2− parents show intermediate numbers of both CD8α/α TCR-α/β and CD8α/β TCR-α/β iIELs. iIELs were separated from Qa-2+ C57BL/6, Qa-2− C3H, and Qa-2+/− F1 mice and stained for Qa-2, CD8α, CD8β, TCR-α/β, and TCR-γ/δ. (a) iIELs from F1 mice showed an intermediate level of Qa-2 expression. (b) F1 mice derived from Qa-2+ and Qa-2− parents showed an intermediate number of CD8α/α TCR-α/β and CD8α/β TCR-α/β cells. Cont. Ave. PE, control streptavidin R–PE.
In summary, CD8α/α TCR-α/β lymphocytes in the intestinal epithelium develop in a manner distinct from that of CD8+ T cells in the peripheral lymphoid organs. In particular, in H-2b mice, CD8α/α TCR-α/β iIEL selection is independent of the classical MHC class I molecules Kb and Db. CD1d MHC class Ib products are not involved in this selection process, as shown by the lack of effect of deletion of the CD1d gene. The presence of CD8α/α TCR-α/β iIELs also requires the expression of TAP and β2m, indicating that these cells may be selected on TAP- and β2m-dependent class Ib MHC products. This MHC class Ib molecule was shown to be Qa-2, which is the product of up to four MHC class Ib genes in the Q region of the mouse genome. Variation in Qa-2 levels correlated with the level of CD8α/α TCR-α/β iIELs, and a Qa-2− mouse carrying a Qa-2 transgene had high levels of surface expression of Qa-2 and an excess of CD8α/α TCR-α/β iIELs.
Acknowledgments
We gratefully acknowledge Mr. Grigoriy Losyev for timely supplying antibodies and Ms. Jennifer Boucher-Reid for excellent secretarial assistance.
G. Das is a fellow of the Howard Hughes Medical Institute (HHMI), which supported this work. D.S. Gould is supported by an HHMI Predoctoral Fellowship. M.M. Augustine is a Medical Student Research Fellow of the HHMI. D.J. Schust is supported by a Reproductive Scientist Development Award and the Society for Gynecologic Investigation. L. Van Kaer is an associate investigator of HHMI. H. Ploegh and this research were funded in part by a grant from the National Institutes of Health (NIH) entitled, “Regulation of the T-Cell Response to Antigen.” C.A. Janeway, Jr. is an investigator of HHMI. I. Stroynowski is supported in part by NIH grants AI19624 and AI37818.
References
- Guy-Grand D., Cerf-Bensussan N., Malissen B., Malassis-Seris M., Briottet C., Vassalli P. Two gut intraepithelial CD8+ lymphocyte populations with different T cell receptorsa role for the gut epithelium in T cell differentiation. J. Exp. Med. 1991;173:471–481. doi: 10.1084/jem.173.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley R.L., Styre D., Klein J.R. Differentiation and functional maturation of bone marrow-derived intestinal epithelial T cells expressing membrane T cell receptor in athymic radiation chimeras. J. Immunol. 1990;145:1369–1375. [PubMed] [Google Scholar]
- Lefrancois L. Extrathymic differentiation of intraepithelial lymphocytesgeneration of a separate and unequal T-cell repertoire? Immunol. Today. 1991;12:436–438. doi: 10.1016/0167-5699(91)90015-L. [DOI] [PubMed] [Google Scholar]
- Saito H., Kanamori Y., Takemori T., Nariuchi H., Kubota E., Takahashi-Iwanaga H., Iwanaga T., Ishikawa H. Generation of intestinal T cells from progenitors residing in gut cryptopatches. Science. 1998;280:275–278. doi: 10.1126/science.280.5361.275. [DOI] [PubMed] [Google Scholar]
- Rocha B., Vassalli P., Guy-Grand D. The extrathymic T-cell development pathway. Immunol. Today. 1992;13:449–454. doi: 10.1016/0167-5699(92)90074-H. [DOI] [PubMed] [Google Scholar]
- Lefrancois L., LeCorre R., Mayo J., Bluestone J.A., Goodman T. Extrathymic selection of TCR gamma delta + T cells by class II major histocompatibility complex molecules. Cell. 1990;63:333–340. doi: 10.1016/0092-8674(90)90166-c. [DOI] [PubMed] [Google Scholar]
- Poussier P., Edouard P., Lee C., Binnie M., Julius M. Thymus-independent development and negative selection of T cells expressing T cell receptor alpha/beta in the intestinal epitheliumevidence for distinct circulation patterns of gut- and thymus-derived T lymphocytes. J. Exp. Med. 1992;176:187–199. doi: 10.1084/jem.176.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeira A., Itohara S., Bonneville M., Burlen-Defranoux O., Mota-Santos T., Coutinho A., Tonegawa S. Extrathymic origin of intestinal intraepithelial lymphocytes bearing T-cell antigen receptor gamma delta. Proc. Natl. Acad. Sci. USA. 1991;88:43–47. doi: 10.1073/pnas.88.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London S.D., Cebra J.J., Rubin D.H. Intraepithelial lymphocytes contain virus-specific, MHC-restricted cytotoxic cell precursors after gut mucosal immunization with reovirus serotype 1/Lang. Reg. Immunol. 1989;2:98–102. [PubMed] [Google Scholar]
- Sydora B.C., Jamieson B.D., Ahmed R., Kronenberg M. Intestinal intraepithelial lymphocytes respond to systemic lymphocytic choriomeningitis virus infection. Cell. Immunol. 1996;167:161–169. doi: 10.1006/cimm.1996.0023. [DOI] [PubMed] [Google Scholar]
- Chardes T., Buzoni-Gatel D., Lepage A., Bernard F., Bout D. Toxoplasma gondii oral infection induces specific cytotoxic CD8 alpha/beta+ Thy-1+ gut intraepithelial lymphocytes, lytic for parasite-infected enterocytes. J. Immunol. 1994;153:4596–4603. [PubMed] [Google Scholar]
- Roberts S.J., Smith A.L., West A.B., Wen L., Findly R.C., Owen M.J., Hayday A.C. T-cell alpha beta + and gamma delta + deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc. Natl. Acad. Sci. USA. 1996;93:11774–11779. doi: 10.1073/pnas.93.21.11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka Y., Yamashiro Y., Maeda M., Oguchi S., Shimizu T., Nagata S., Yagita H., Yabuta K., Okumura K. Food antigen activates intraepithelial and lamina propria lymphocytes in food-sensitive enteropathy in mice. Pediatr. Res. 1996;39:862–866. doi: 10.1203/00006450-199605000-00020. [DOI] [PubMed] [Google Scholar]
- Buzoni-Gatel D., Lepage A.C., Dimier-Poisson I.H., Bout D.T., Kasper L.H. Adoptive transfer of gut intraepithelial lymphocytes protects against murine infection with Toxoplasma gondii . J. Immunol. 1997;158:5883–5889. [PubMed] [Google Scholar]
- Guy-Grand D., Cuenod-Jabri B., Malassis-Seris M., Selz F., Vassalli P. Complexity of the mouse gut T cell immune systemidentification of two distinct natural killer T cell intraepithelial lineages. Eur. J. Immunol. 1996;26:2248–2256. doi: 10.1002/eji.1830260942. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., DiSanto J.P., Henchoz P., Malassis-Seris M., Vassalli P. Small bowel enteropathyrole of intraepithelial lymphocytes and of cytokines (IL-12, IFN-gamma, TNF) in the induction of epithelial cell death and renewal. Eur. J. Immunol. 1998;28:730–744. doi: 10.1002/(SICI)1521-4141(199802)28:02<730::AID-IMMU730>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Das G., Janeway C.A., Jr. Development of CD8α/α and CD8α/β T cells in major histocompatibility complex class I–deficient mice. J. Exp. Med. 1999;190:881–884. doi: 10.1084/jem.190.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.H., Guy-Grand D., Lemonnier F.A., Wang C.R., Bendelac A., Jabri B. Selection and expansion of CD8alpha/alpha(1) T cell receptor alpha/beta(1) intestinal intraepithelial lymphocytes in the absence of both classical major histocompatibility complex class I and nonclassical CD1 molecules. J. Exp. Med. 1999;190:885–890. doi: 10.1084/jem.190.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapin L., Cheroutre H., Kronenberg M. Cutting edgeTCR alpha beta+ CD8 alpha alpha+ T cells are found in intestinal intraepithelial lymphocytes of mice that lack classical MHC class I molecules. J. Immunol. 1999;163:4100–4104. [PubMed] [Google Scholar]
- Fujiura Y., Kawaguchi M., Kondo Y., Obana S., Yamamoto H., Nanno M., Ishikawa H. Development of CD8 alpha alpha+ intestinal intraepithelial T cells in beta 2-microglobulin- and/or TAP1-deficient mice. J. Immunol. 1996;156:2710–2715. [PubMed] [Google Scholar]
- Tabaczewski P., Stroynowski I. Expression of secreted and glycosylphosphatidylinositol-bound Qa-2 molecules is dependent on functional TAP-2 peptide transporter. J. Immunol. 1994;152:5268–5274. [PubMed] [Google Scholar]
- Devlin J.J., Weiss E.H., Paulson M., Flavell R.A. Duplicated gene pairs and alleles of class I genes in the Qa2 region of the murine major histocompatibility complexa comparison. EMBO (Eur. Mol. Biol. Organ.) J. 1985;4:3203–3207. doi: 10.1002/j.1460-2075.1985.tb04066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson J., Flaherty L., Bushkin Y., Yudkowitz H. Further biochemical data on Qa-2. Immunogenetics. 1981;14:129–140. doi: 10.1007/BF00344306. [DOI] [PubMed] [Google Scholar]
- Winoto A., Steinmetz M., Hood L. Genetic mapping in the major histocompatibility complex by restriction enzyme site polymorphismsmost mouse class I genes map to the Tla complex. Proc. Natl. Acad. Sci. USA. 1983;80:3425–3429. doi: 10.1073/pnas.80.11.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor A.L., Antoniou J., Robinson P.J. Structure and expression of genes encoding murine Qa-2 class I antigens. Proc. Natl. Acad. Sci. USA. 1985;82:5920–5924. doi: 10.1073/pnas.82.17.5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts S., Davis A.C., Gaut B., Wheeler C., Hill L., Goodenow R.S. Organization and structure of the Qa genes of the major histocompatibility complex of the C3H mouseimplications for Qa function and class I evolution. EMBO (Eur. Mol. Biol. Organ.) J. 1989;8:1749–1759. doi: 10.1002/j.1460-2075.1989.tb03568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vugmeyster Y., Glas R., Perarnau B., Lemonnier F.A., Eisen H., Ploegh H. Major histocompatibility complex (MHC) class I KbDb−/− deficient mice possess functional CD8+ T cells and natural killer cells. Proc. Natl. Acad. Sci. USA. 1998;95:12492–12497. doi: 10.1073/pnas.95.21.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiratta S.K., Martin W.D., Hong S., Boesteanu A., Joyce S., Van Kaer L. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL–4. Immunity. 1997;6:469–477. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- Fragoso G., Lamoyi E., Mellor A., Lomeli C., Hernandez M., Sciutto E. Increased resistance to Taenia crassiceps murine cysticercosis in Qa-2 transgenic mice. Infect. Immun. 1998;66:760–764. doi: 10.1128/iai.66.2.760-764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Heijden P.J., Stok W. Improved procedure for the isolation of functionally active lymphoid cells from the murine intestine. J. Immunol. Methods. 1987;103:161–167. doi: 10.1016/0022-1759(87)90285-7. [DOI] [PubMed] [Google Scholar]
- Van Kaer L., Ashton-Rickardt P.G., Ploegh H.L., Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4-8+ T cells. Cell. 1992;71:1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- Zijlstra M., Bix M., Simister N.E., Loring J.M., Raulet D.H., Jaenisch R. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
- Ibraghimov A.R., Lynch R.G. Heterogeneity and biased T cell receptor alpha/beta repertoire of mucosal CD8+ cells from murine large intestineimplications for functional state. J. Exp. Med. 1994;180:433–444. doi: 10.1084/jem.180.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeira A., Mota-Santos T., Itohara S., Degermann S., Heusser C., Tonegawa S., Coutinho A. Localization of gamma/delta T cells to the intestinal epithelium is independent of normal microbial colonization. J. Exp. Med. 1990;172:239–244. doi: 10.1084/jem.172.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydora B.C., Brossay L., Hagenbaugh A., Kronenberg M., Cheroutre H. TAP-independent selection of CD8+ intestinal intraepithelial lymphocytes. J. Immunol. 1996;156:4209–4216. [PubMed] [Google Scholar]
- Bleicher P.A., Balk S.P., Hagen S.J., Blumberg R.S., Flotte T.J., Terhorst C. Expression of murine CD1 on gastrointestinal epithelium. Science. 1990;250:679–682. doi: 10.1126/science.1700477. [DOI] [PubMed] [Google Scholar]
- Brutkiewicz R.R., Bennink J.R., Yewdell J.W., Bendelac A. TAP-independent, beta 2-microglobulin–dependent surface expression of functional mouse CD1.1. J. Exp. Med. 1995;182:1913–1919. doi: 10.1084/jem.182.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce S., Tabaczewski P., Angeletti R.H., Nathenson S.G., Stroynowski I. A nonpolymorphic major histocompatibility complex class Ib molecule binds a large array of diverse self-peptides. J. Exp. Med. 1994;179:579–588. doi: 10.1084/jem.179.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederkorn J.Y., Chiang E.Y., Ungchusri T., Stroynowski I. Expression of a nonclassical MHC class Ib molecule in the eye. Transplantation. 1999;68:1790–1799. doi: 10.1097/00007890-199912150-00025. [DOI] [PubMed] [Google Scholar]
- Potter M. History of the BALB/c family. Curr. Top. Microbiol. Immunol. 1985;122:1–5. doi: 10.1007/978-3-642-70740-7_1. [DOI] [PubMed] [Google Scholar]
- Kohyama M., Nanno M., Kawaguchi-Miyashita M., Shimada S., Watanabe M., Hibi T., Kaminogawa S., Ishikawa H. Cytolytic and IFN-gamma-producing activities of gamma delta T cells in the mouse intestinal epithelium are T cell receptor-beta-chain dependent. Proc. Natl. Acad. Sci. USA. 1999;96:7451–7455. doi: 10.1073/pnas.96.13.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]