Abstract
To analyze the antiviral protective capacities of CD4+ T helper (Th) cell subsets, we used transgenic T cells expressing an I-Ab–restricted T cell receptor specific for an epitope of vesicular stomatitis virus glycoprotein (VSV-G). After polarization into Th1 or Th2 effectors and adoptive transfer into T cell–deficient recipients, protective capacities were assessed after infection with different types of viruses expressing the VSV-G. Both Th1 and Th2 CD4+ T cells could transfer protection against systemic VSV infection, by stimulating the production of neutralizing immunoglobulin G antibodies. However, only Th1 CD4+ T cells were able to mediate protection against infection with recombinant vaccinia virus expressing the VSV-G (Vacc-IND-G). Similarly, only Th1 CD4+ T cells were able to rapidly eradicate Vacc-IND-G from peripheral organs, to mediate delayed-type hypersensitivity responses against VSV-G and to protect against lethal intranasal infection with VSV. Protective capacity correlated with the ability of Th1 CD4+ T cells to rapidly migrate to peripheral inflammatory sites in vivo and to respond to inflammatory chemokines that were induced after virus infection of peripheral tissues. Therefore, the antiviral protective capacity of a given CD4+ T cell is governed by the effector cytokines it produces and by its migratory capability.
Keywords: Th1/Th2 cells, vesicular stomatitis virus, vaccinia, chemokines, migration
Introduction
In the case of cytopathic viruses, T cell–dependent cytokines together with neutralizing antibodies are usually essential for viral eradication and protection against reinfection 1 2. CD4+ T cells play a crucial role in many antiviral immune responses. As well as their direct antiviral effects via the production of cytokines such as IFN-γ and TNF-α 3 4, they provide the cognate signals that induce the production of protective virus-neutralizing IgG by specific B cells 5 and enhance the magnitude of antiviral CTL responses 6 7 8. Several experimental models of viral infection have emphasized the important role CD4+ T cells may play in the eradication of viruses by both humoral and cell-mediated mechanisms. Mice deficient in CD8+ CTLs can clear influenza A virus, and adoptively transferred CD4+ T cell clones have been shown to be able to promote recovery from lethal infection 9 10 11. In addition, CD8+ CTL–deficient mice can also effectively control vaccinia virus infection 12 13. Furthermore, poliovirus-specific CD4+ T cell clones can confer protection against lethal infection by stimulating neutralizing antibody production 14.
Effector CD4+ T cells can be subdivided, on the basis of the cytokines that they secrete, into distinct populations that direct different types of immune response. Two main subsets of effector CD4+ T cells have been described: Th1 cells produce inflammatory cytokines such as IFN-γ and TNF and participate in cell-mediated immune responses, whereas Th2 cells secreting IL-4, IL-5, IL-6, IL-10, and IL-13 primarily help B cells produce antibodies and also mediate immunity against intestinal nematodes 15 16 17. Although the roles of Th1 and Th2 CD4+ T cells in protection against some parasitic infections such as Leishmania and Nippostrongylus have been well described 18 19 20, thus far little is known about the ability of these cells to protect against different types of virus infection. Vesicular stomatitis virus (VSV) infection of immunocompetent mice induces a rapid T cell–independent neutralizing IgM response, followed by production of neutralizing IgG antibodies that are strictly dependent on CD4+ T cell help 5. CD4+ T cells seem to be crucial for recovery from primary infections and for eliciting neutralizing IgG antibodies required for protection against reinfection 21 22. To analyze the antiviral protective capacities of CD4+ Th cell subsets, we used transgenic mice (designated tg7) expressing an MHC class II (I-Ab)–restricted TCR specific for a peptide derived from the glycoprotein of VSV (VSV-G) on 50% of CD4+ T cells 23. Naive tg7 transgenic CD4+ T cells facilitated protective VSV-neutralizing IgG production after adoptive transfer into T cell–deficient recipients, but were unable to confer cell-mediated antiviral protection against recombinant vaccinia virus expressing the VSV-G 23. In contrast, in vitro–primed tg7 CD4+ T cells rapidly eliminated recombinant vaccinia virus from peripheral tissues 23. Here, we analyzed the protective capacities of distinct Th1 and Th2 effector populations of CD4+ T cells in different types of antiviral responses, namely, the induction of VSV-neutralizing IgG antibodies and the cell-mediated clearance of recombinant vaccinia virus expressing VSV-G.
Materials and Methods
Mice.
C57BL/6 (H-2b) mice, TCR transgenic tg7 mice 23, and SMARTA mice 24 were obtained from the breeding colonies of the Institut für Zuchthygiene, Zürich, Switzerland. T cell–deficient mice (TCR-β−/−δ−/−) 25 on a C57BL/6 (H-2b) background were obtained from The Jackson Laboratory.
Cytofluorimetric Analysis of Intracellular Cytokines.
The following mAbs were used: FITC-conjugated anti–IFN-γ, PE-conjugated anti–IL-4, FITC-conjugated anti–TNF-α, PE-conjugated anti–IFN-γ, and FITC-conjugated IL-10 (all from BD PharMingen). Staining was performed as described previously 26. In brief, aliquots of 5 × 105 CD4+ T cells were stimulated in vitro at 37°C for 4 h in RPMI 1640/10% FCS containing PMA (10 ng/ml), ionomycin (100 ng/ml), and monensin (2 mM; all from Sigma-Aldrich). Samples were then stained for 30 min at 4°C with Tricolor–anti-CD4 (Caltag). Surface staining was fixed by incubation in 100 μl of PBS/4% paraformaldehyde for 10 min, and the cells were permeabilized by addition of 2 ml permeabilization buffer (PB: PBS/1% saponin/0.05% sodium azide, both from Sigma-Aldrich) for 10 min. Samples were stained for 30 min at 4°C in PB containing the appropriate anticytokine antibodies. After washing twice with PB, samples were resuspended in FACS buffer and analyzed using a FACScan™. To control for nonspecific intracellular staining, parallel samples of stimulated and permeabilized CD4+ T cells were stained with PE-conjugated isotype-matched mAbs of irrelevant specificity, which did not result in any staining signal.
Viruses and Inactivation of VSV.
VSV serotype Indiana (VSV-IND, Mudd-Summers isolate) and VSV serotype New Jersey (VSV-NJ, Pringle isolate) were grown on BHK 21 cells and plaqued on Vero cells 27. UV light inactivation of VSV was performed under a 15 W UV lamp for 4 min and verified by plaquing on Vero cells 28. Recombinant vaccinia viruses expressing the VSV-IND glycoprotein (Vacc-IND-G [29]) or lymphocytic choriomeningitis virus nucleoprotein (LCMV-NP) (Vacc-LCMV-NP 30) were grown and plaqued on BSC 40 cells. LCMV isolate WE 31 was grown on L929 cells. Recombinant baculoviruses expressing VSV-IND-G or VSV-IND-NP were obtained and grown as described previously 32.
Immunizations and Assessment of Antiviral Immunity.
Mice were immunized intravenously or intranasally with 2 × 106 PFU of live or inactivated VSV-IND or VSV-NJ. Sera were collected by bleeding from the retroorbital plexus at different time points after immunization for determination of VSV-specific neutralizing antibody titers using a plaque assay as described previously 33. To determine IgG titers, undiluted serum was pretreated with an equal volume of 0.1 M 2-ME in saline 34.
Alternatively, mice were infected intraperitoneally with 5 × 106 PFU of Vacc-IND-G or Vacc-LCMV-NP. Organs were harvested at the indicated time points, and the vaccinia titers were determined on BSC 40 monolayers as described previously 35.
For footpad delayed-type hypersensitivity (DTH) responses, mice received 10 μg of baculovirus-derived recombinant VSV-G (Bac-G) or recombinant VSV-NP (Bac-NP), or 500 PFU LCMV-WE, in a total of 50 μl into the right hind footpad. Footpad swelling was monitored daily using calipers (Kroeplin).
T Cell Proliferative Assays and Cytokine Production.
Proliferative responses and cytokine secretion by tg7 Th1 and Th2 effector cells were assayed after in vitro restimulation with irradiated C57BL/6 spleen cells and VSV-G peptide p8 (36; VSV-G amino acids 415–433), using [3H]thymidine incorporation and ELISA, respectively, as described previously 23.
Polarization of Transgenic CD4+ T Cells into Th1 and Th2 Cells.
Effector Th1 and Th2 cells were generated by in vitro polarization of naive transgenic tg7 CD4+ cells as described previously 37. Naive tg7 CD4+ spleen cells were obtained at a purity of 98% by magnetic cell sorting (MACS) with anti-CD4 microbeads (Miltenyi Biotec). Aliquots of 5 × 105 CD4+ T cells were cultured in 24-well tissue culture plates (TPP) in 2 ml RPMI 1640 medium containing 10% FCS, penicillin, streptomycin, l-glutamine, and 5 × 10−5 M 2-ME (proliferation medium), in the presence of 5 × 106 irradiated C57BL/6 spleen cells plus p8 (1 μg/ml). Th1 cultures were supplemented with 50 U/ml recombinant murine IL-2 (rmIL-2; BD PharMingen), whereas Th2 cultures contained rmIL-2 plus rmIL-4 (500 U/ml; BD PharMingen) and polyclonal sheep anti–IFN-γ antibodies (1:100 38). After 4 d of culture at 37°C in 5% CO2, cells were washed and expanded for a further 4–5 d in 6-well tissue culture plates (TPP) with 5 ml proliferation medium containing rmIL-2 (Th1 cultures) or rmIL-2 plus rmIL-4 (Th2 cultures). To obtain irreversibly polarized effector populations, three identical rounds of the above in vitro stimulation were performed 39. Effector cells were washed twice in HBSS before use.
Adoptive Transfer of Antiviral Immunity.
TCR-β−/−δ−/− or C57BL/6 mice were adoptively transfused intravenously with the indicated numbers of transgenic Th1 or Th2 CD4+ T cells and challenged 24 h later with virus or recombinant viral proteins as described in the figure legends.
Immunohistochemistry.
Immunohistochemical analysis was performed as described previously 40. In brief, organs were immersed in HBSS, snap frozen in liquid N2, and 5-μm-thick cryostat sections were cut and fixed in acetone. Sections were stained with rat antibodies against CD4 or CD8, followed by alkaline phosphatase–labeled goat anti–rat Ig (TAGO) and then alkaline phosphatase–labeled donkey anti–goat Ig (Jackson ImmunoResearch Laboratories). Alkaline phosphatase was detected with naphthol A-BI phosphate and New Fuchsin (Fluka), resulting in a red color reaction. Sections were counterstained with Mayer's hemalum.
Analysis of Chemokine and Chemokine Receptor Expression by Reverse Transcription PCR.
RNA was extracted with 10 μg carrier yeast tRNA using the TRIZOL reagent (GIBCO BRL). 1 μg RNA extracted from ovaries isolated from C57BL/6 mice 48 h after infection with 2 × 106 PFU Vacc-IND-G was used in the reverse transcription (RT) reaction. Alternatively, RNA extracted from 2 × 106 polarized Th1 or Th2 cells was digested with DNase (Roche) in 20 mM Tris-HCl (pH 8.0) containing 100 mM MgCl2. RT was carried out using Superscript II reverse transcriptase and random hexamer primers (GIBCO BRL). Conventional PCR was performed in 50-μl volumes using the following primer pairs: JE (monocyte chemotactic protein [MCP]-1), 5′-CCCACTCACCTGCTGCTACT-3′ and 5′-GTCTGGACCCATTCCTTCTT-3′; macrophage-derived chemokine (MDC), 5′-GCCAGGACTACATCCGTCAC-3′ and 5′-GAAGAATAGGGCTTGCTGAG-3′; macrophage inflammatory protein (MIP)-1α, 5′-ACTGCCCTTGCTGTTCTTCT-3′ and 5′-CCCAGGTCTCTTTGGAGTCA-3′; MIP-1β, 5′-CTCTGCGTGTCTGCCCTC-3′; regulated on activation normal T cell expressed and secreted protein (RANTES), 5′-CGAAGGAACCGCCAAGTGTG-3′; monokine induced by IFN-γ (Mig), 5′-TCTTTTCCTTTTGGGCATCA-3′ and 5′-GACGACGACTTTGGGGTGTT-3′; IP-10, 5′-GTGCTGCCGTCATTTTCTGC-3′ and 5′-AGGCTCTCTGCTGTCCATCA-3′; stromal cell–derived factor (SDF)-1α, 5′-TAAAGACACTCCGCCATAGC-3′ and 5′-ACTCAGGACAAGGCATCTGT-3′; CC chemokine receptor (CCR)2, 5′-TCAGTTCATCCACGGCATAC-3′ and 5′-GAGCAGGAAGAGCAGGTCAG-3′; CCR4, 5′-CAGGTCTACTCGGCTGACAT-3′ and 5′-TCCACTCAAGGGCTCATTGT-3′; CCR5, 5′-GAGTGGGTCTTTGGGAACAT-3′; CXC chemokine receptor (CXCR)3, 5′-TATGCCTTTGTGGGAGTGAA-3′ and 5′-AGGGATGGCTGAG-TTCTACT-3′; and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), 5′-CATCAAGAAGGTGGTGAAGC-3′ and 5′-CCTGTTGCTGTAGCCGTATT-3′. PCR conditions were: 94°C for 30 s, 55°C for 45 s, and 72°C for 30 s, repeated for 35 cycles. cDNA templates for the GAPDH reference PCR were diluted in 10-fold steps to avoid PCR signal saturation. After electrophoresis in an ethidium bromide–stained 1.5% agarose gel, the luminescence of nonsaturated amplicons was densitometrically quantitated using the Image Station 440 CF and the 1D software (Kodak Digital Science, Eastman Kodak Co.). Band fluorescence intensities were expressed in arbitrary units, calculated as a ratio of the reference GAPDH signal.
Chemokine Migration Assays.
Migration of polarized Th1 and Th2 cells was assayed with Transwell filters (3-μm high density pores; Becton Dickinson) using 5 × 106 cells/well in RPMI 1640/2% FCS in the upper chamber with chemokine placed in the lower chamber. After 1 h at 37°C, the upper chamber was removed and the cells in the lower chamber were quantified with FACS® by counting the total number of cells per minute. Results were expressed in migration indices: [(cells migrating in response to test chemokine/cells migrating spontaneously) − 1] × 100. Spontaneous migration was assessed without chemokine in the lower chamber.
Results
Characterization of Polarized Transgenic Th1 and Th2 Cells.
Transgenic (tg7) CD4+ T cells expressing a TCR that recognizes a peptide (p8) derived from the VSV-G in association with I-Ab 23 were polarized by three rounds of in vitro stimulation with p8-bearing APCs 37. Polarized Th1 effector cells secreted high amounts of IFN-γ and no IL-4, whereas Th2 effectors produced high levels of IL-4 and no IFN-γ (Fig. 1 A). Intracellular cytokine staining showed that some heterogeneity existed within these effector populations in that Th1 effectors contained cells producing TNF-α and/or IFN-γ, whereas Th2 effectors contained cells producing IL-4 and/or IL-10 (Fig. 1 B). Th1 and Th2 effector cells mounted similar proliferative responses when cultured with p8 in vitro (Fig. 1 C).
Figure 1.
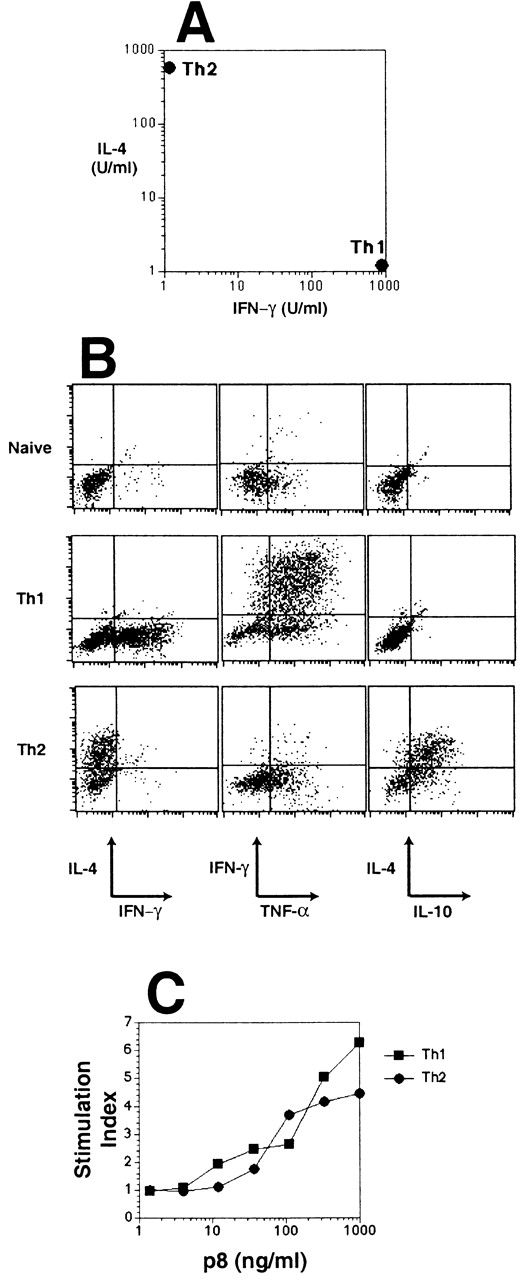
Characterization of polarized Th1 and Th2 effector cells. Polarized Th1 and Th2 tg7 CD4+ effectors were restimulated in vitro with irradiated splenic APCs plus p8 for 72 h. Secretion of IL-4 and IFN-γ was assayed by ELISA (A) and proliferation of the cells was measured using [3H]thymidine incorporation (C). Intracellular cytokine staining of Th1 and Th2 effectors was performed after in vitro restimulation with PMA and ionomycin (B). Results shown are representative of three similar experiments.
Both Th1 and Th2 Cells Provide Help for VSV-neutralizing Protective IgG Responses.
Although T cell–deficient TCR-β−/−δ−/− mice mount normal T cell–independent neutralizing IgM responses to VSV, they do not produce CD4+ T cell–dependent neutralizing IgG antibodies 5 41. Indeed, despite producing neutralizing IgM antibodies, VSV-infected TCR-β−/−δ−/− mice succumbed to infection on around day 14 after infection (Fig. 2 A), confirming previous findings with nude mice 22. In contrast, TCR-β−/−δ−/− mice adoptively transfused with 106 VSV-specific Th1 or Th2 CD4+ T cells produced high titers of VSV-neutralizing IgG and were completely protected against disease (Fig. 2).
Figure 2.
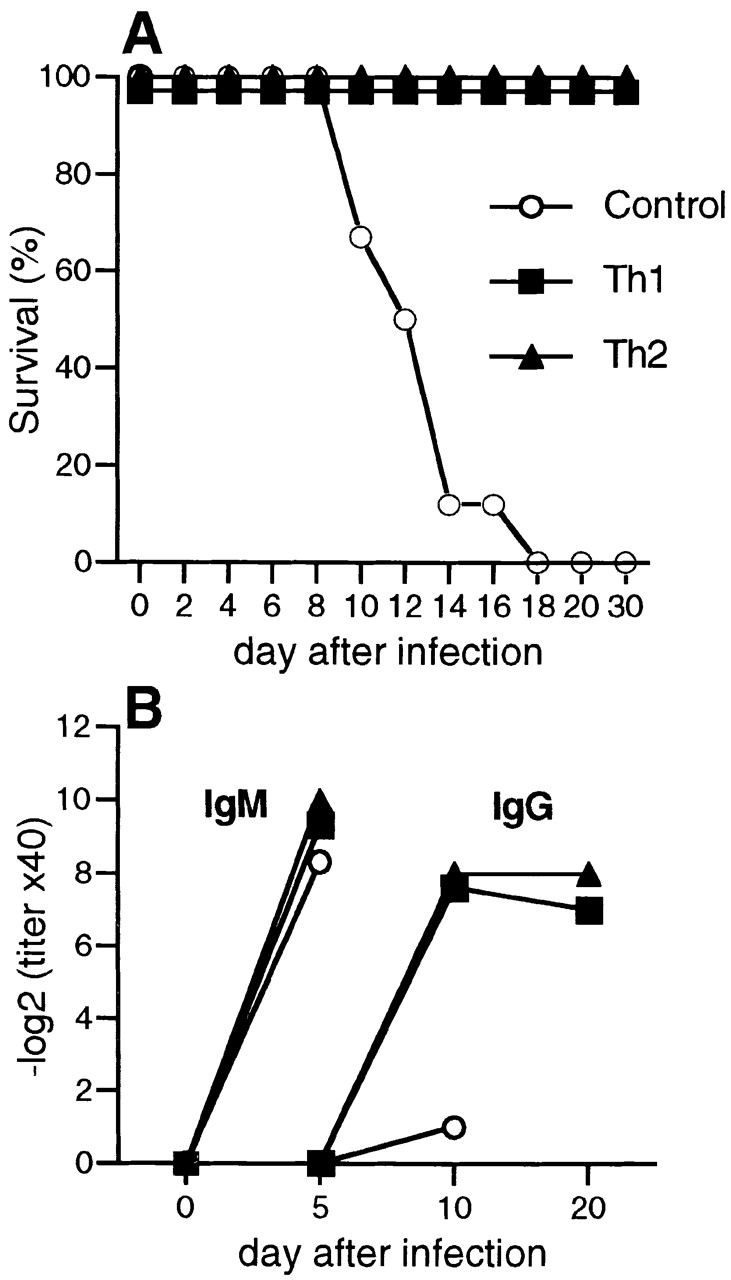
Both Th1 and Th2 cells can protect T cell–deficient mice against VSV infection, by inducing the production of neutralizing IgG antibodies. Groups of 6 TCR-β−/−δ−/− mice were adoptively transfused with 107 Th1 or Th2 tg7 CD4+ T cells, and 24 h later recipients were infected with 2 × 106 PFU of live VSV-IND intravenously. (A) Mice were monitored daily for hind limb paralysis and killed when seriously ill. (B) On indicated days, blood was taken and the serum was analyzed for the presence of VSV-IND–neutralizing IgM and IgG. Mean titers from groups of five to six mice are shown, and intragroup variations were ≤2 titer steps. One of two similar experiments is shown.
To evaluate the sensitivity of Th1 and Th2 cells to facilitate neutralizing antibody responses, graded numbers of VSV-specific Th1 or Th2 cells were adoptively transferred into naive C57BL/6 mice and the recipients were subsequently challenged with UV-inactivated VSV (UVi-VSV). Nonreplicating UVi-VSV efficiently triggers T cell–independent IgM responses, but does not induce isotype switching to IgG unless excess VSV-specific Th cells are present 23 28. C57BL/6 mice adoptively transferred with Th1 or Th2 CD4+ T cells produced high titers of VSV-neutralizing IgG after challenge with UVi-VSV, and in both cases at least 105 effector CD4+ T cells were required to support isotype switching (Fig. 3 A). Th1 cells induced the production of IgG2a and IgG2b, whereas Th2 cells promoted IgG1 and IgG2b secretion (Fig. 3 B).
Figure 3.
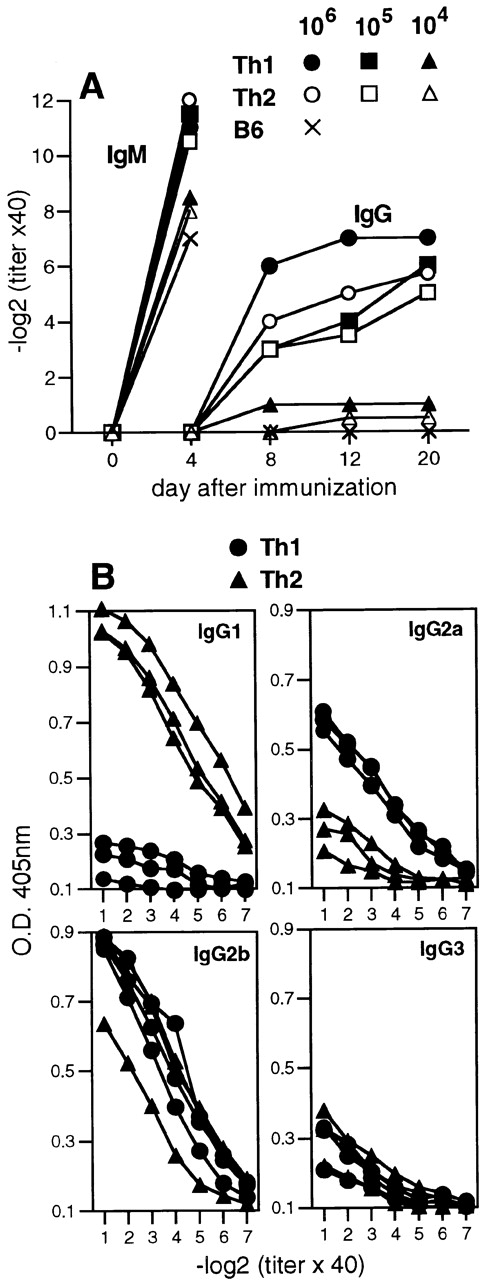
Adoptively transferred Th1 and Th2 cells mediate quantitatively similar, but qualitatively different, Ig class switching in vivo. Graded numbers of Th1 or Th2 tg7 CD4+ T cells were adoptively transferred intravenously into syngeneic TCR-β−/−δ−/− mice, and recipients were immunized 24 h later with 2 × 106 PFU UVi-VSV intravenously. (A) On indicated days, blood was taken and the serum was analyzed for the presence of VSV-IND–neutralizing IgM and IgG. Mean titers from groups of two to three mice are shown, and intragroup variations were ≤2 titer steps. (B) VSV-specific IgG isotypes present in sera isolated on day 20 after immunization from mice receiving 107 Th1 or Th2 cells were assayed by ELISA. Symbols represent individual sera. One of two similar experiments is shown.
Th1 CD4+ T Cells, but Not Th2 CD4+ T Cells, Can Adoptively Transfer Cell-mediated Protection against Recombinant Vaccinia Virus.
Previous work has shown that C57BL/6 mice primed with VSV-IND were resistant against challenge with a recombinant vaccinia virus expressing the VSV-IND glycoprotein (Vacc-IND-G) and that this protection was mediated by CD4+ T cells 42. Therefore, we examined whether the antivaccinia protection conferred by VSV-G–specific CD4+ T cells was dependent on their effector phenotype. T cell–deficient TCR-β−/− δ−/− mice were unable to control infection with Vacc-IND-G, developing a progressive wasting disease that culminated in death ∼3–4 wk after infection (Fig. 4 A). Adoptive transfer of 107 Th1 tg7 CD4+ T cells completely protected the TCR-β−/−δ−/− mice, whereas transfer of 107 Th2 tg7 CD4+ T cells failed to protect TCR-β−/−δ−/− mice against Vacc-IND-G (Fig. 4 A). Death correlated with the presence of vaccinia virus in peripheral organs in mice that had received Th2 tg7 CD4+ T cells, whereas those receiving Th1 tg7 CD4+ T cells remained healthy and had no detectable vaccinia virus (Fig. 4 B). The inability of Th2 cells to protect against lethal vaccinia virus infection was not due to inefficient inactivation of these cells, since the TCR-β−/−δ−/− mice that received Th2 cells mounted strong VSV-G–specific neutralizing antibody responses (Fig. 4 C). However, as vaccinia virus may spread from cell to cell by using actin filaments to form enveloped particles which are resistant to antibody 43 44 and because the VSV-G is not expressed on the surface of the Vacc-IND-G 29, neutralizing antibodies are unable to clear the infection.
Figure 4.
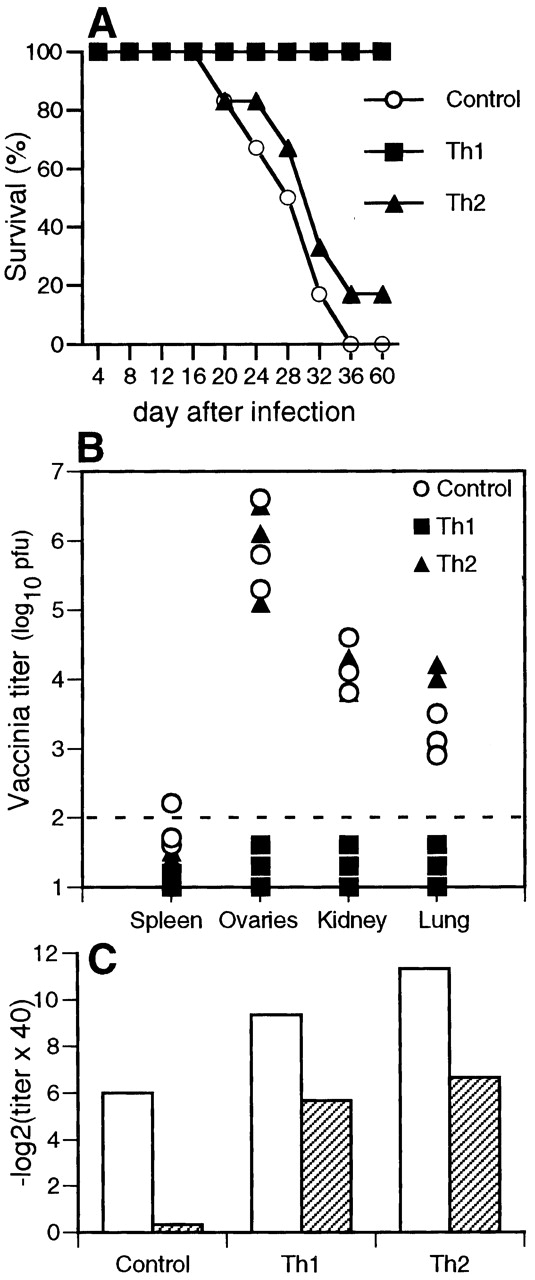
Th1, but not Th2, cells can protect T cell–deficient mice from vaccinia virus infection. Groups of 6 TCR-β−/−δ−/− mice were adoptively transfused with 107 Th1 or Th2 tg7 CD4+ T cells, and 24 h later recipients were infected with 107 PFU of Vacc-IND-G intraperitoneally. (A) Mice were monitored daily for signs of disease and were killed when seriously ill. (B) Vacc-IND-G titers in organs from sick (Control or Th2 recipients) or healthy (Th1 recipients) mice were determined by plaquing. Representative results from three mice per group are shown, and symbols represent individual mice. (C) Sera from the mice shown in A were analyzed for the presence of VSV-IND–neutralizing IgM (day 4 after infection, white bars) and IgG (day 20 after infection, striped bars). Mean titers from groups of five to six mice are shown, and intragroup variations were ≤2 titer steps. One of two similar experiments is shown.
Protection against Vaccinia Correlates with Enhanced Migration of Th1 CD4+ T Cells to Peripheral Tissues.
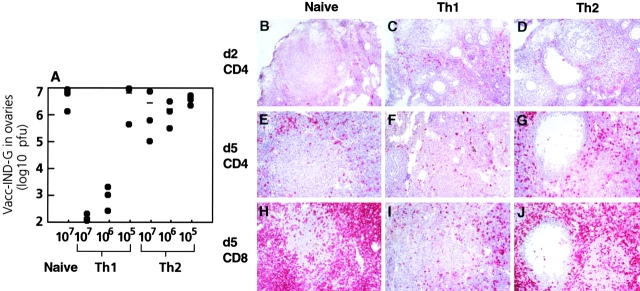
In a second approach, we examined the ability of Th1 and Th2 cells to rapidly eradicate Vacc-IND-G from peripheral solid organs. Thus, normal C57BL/6 mice were adoptively transfused with VSV-G–specific CD4+ T cells and challenged with Vacc-IND-G. In naive female C57BL/6 mice, the Vacc-IND-G grew well in ovaries, with titers detectable on day 2 and peaking around day 5 (Fig. 5 A). Adoptive transfer of 107 naive tg7 CD4+ or Th2 tg7 CD4+ T cells had no influence on the vaccinia virus titers in ovaries, whereas mice receiving ≥106 Th1 tg7 CD4+ T cells rapidly eradicated the virus (Fig. 5 A). Histological analysis of ovaries showed that the transferred Th1 cells were present in the ovaries on day 2 after infection (Fig. 5 C), whereas naive or Th2 tg7 CD4+ T cells did not migrate into the ovaries at this time point (Fig. 5B and Fig. D). By day 5 after infection, extensive inflammatory infiltrates were present in ovaries of mice receiving naive or Th2 tg7 CD4+ T cells, comprising both CD4 and CD8 T cells (Fig. 5E–J). In contrast, much smaller infiltrates were present in ovaries of mice that received Th1 tg7 CD4+ T cells (Fig. 5F and Fig. I). Thus, earlier migration of Th1 cells into peripheral organs correlated with a rapid elimination of vaccinia virus and a decreased late inflammatory reaction.
Figure 5.
Th1, but not Th2, cells can protect against peripheral vaccinia virus infection, and this correlates with enhanced migration of Th1 cells to peripheral organs. (A) Graded numbers of naive or Th1 or Th2 tg7 CD4+ T cells were adoptively transferred intravenously into syngeneic naive C57BL/6 female mice. 1 d later, recipients were challenged with 5 × 106 PFU Vacc-IND-G intraperitoneally. After 5 d, ovaries were removed and Vacc-IND-G titers were determined. Symbols represent individual mice, and one representative experiment of three is shown. (B–J) Immunohistological analysis of T cell infiltration into the ovary. C57BL/6 mice that had received 107 naive (B, E, and H), Th1 (C, F, and I), or Th2 (D, G, and H) tg7 CD4+ T cells were killed 2 d (B–D) or 5 d (E–J) after Vacc-IND-G challenge, and frozen sections of ovaries were analyzed for infiltration by CD4+ (B–G) or CD8+ (H–J) T cells using immunohistochemistry. Positively stained cells exhibit a bright red color. Original magnifications: ×125.
Induction of Virus-specific DTH Responses Confirms That Th1 Cells, but Not Th2 Cells, Effectively Respond to Peripheral Antigen Challenge.
We next examined Th1 and Th2 cells for their ability to mediate virus-specific DTH responses. Thus, normal C57BL/6 mice were adoptively transfused with VSV-G–specific CD4+ T cells and challenged in the hind footpad with recombinant baculovirus–derived VSV-G (Bac-G). As shown in Fig. 6 A, control C57BL/6 mice exhibited a small transient increase in footpad thickness, peaking 24 h after injection and declining 48–72 h after injection. C57BL/6 mice adoptively transfused with Th2 tg7 CD4+ T cells had identical responses to control mice (Fig. 6 A). In contrast, mice given Th1 tg7 CD4+ T cells mounted strong DTH responses after challenge with Bac-G, which peaked 72–96 h after injection and slowly declined thereafter (Fig. 6 A). These responses were antigen specific, since they were not provoked by challenge with baculovirus-derived VSV-NP (Bac-NP) (Fig. 6 A). Histological analysis of footpads showed that the ability of Th1 cells to mediate DTH responses correlated with enhanced migration and accumulation of CD4+ T cells in the footpad, which was evident as early as 48 h after injection (Fig. 6B–E).
Figure 6.
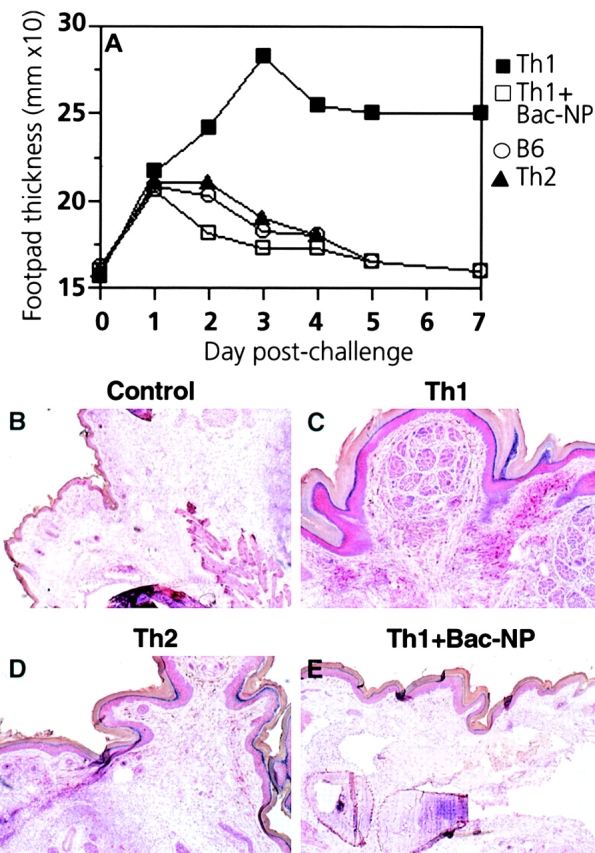
Th1, but not Th2, cells rapidly migrate to inflammatory sites and mediate DTH responses after peripheral antigen challenge. (A) 107 Th1 or Th2 tg7 CD4+ T cells were adoptively transfused intravenously into syngeneic naive C57BL/6 mice. 1 d later, recipients were challenged in the right hind footpad with 10 μg Bac-VSV-G or Bac-VSV-NP. Footpad swelling was monitored daily using calipers. Symbols represent mean values from groups of four to six mice, and intragroup variations were ≤0.4 mm. One representative experiment of three is shown. (B–E) Immunohistological analysis of CD4+ T cell infiltration into the footpad. Two mice from each group described in A were killed 2 d after antigen challenge, and frozen sections of footpads were analyzed for infiltration by CD4+ T cells using immunohistochemistry. Positively stained cells exhibit a bright red color. Original magnifications: ×50.
To examine whether enhanced responsiveness to peripheral antigen challenge was a general property of Th1 CD4+ T cells, we used SMARTA transgenic CD4+ T cells, which recognize a peptide from LCMV-GP in the context of I-Ab 24. While control C57BL/6 mice or those that received Th2 SMARTA CD4+ T cells showed no increase in footpad swelling after infection with LCMV-WE, mice that received Th1 SMARTA CD4+ T cells mounted a DTH response 72–96 h after infection (Fig. 7 A). This again correlated with greatly enhanced migration of Th1 CD4+ T cells into the footpad (Fig. 7B–D).
Figure 7.
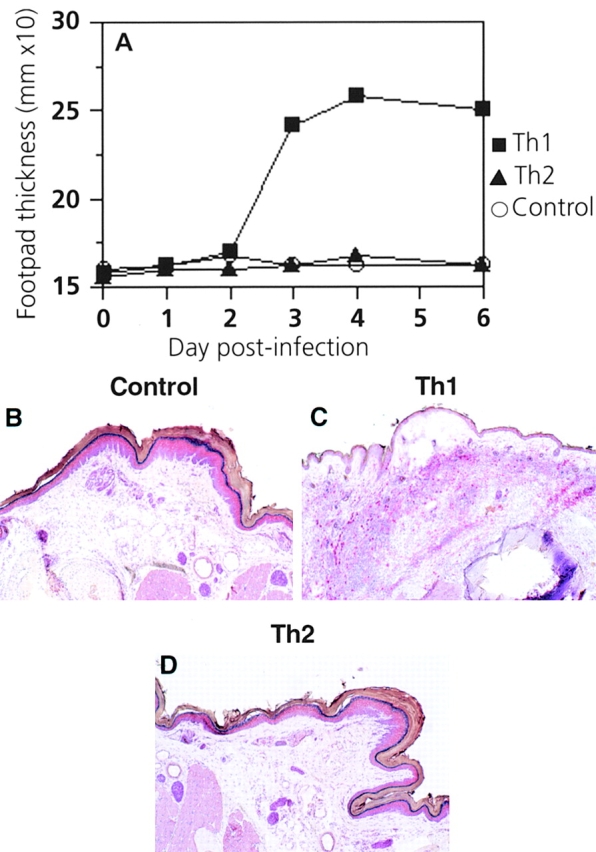
Th1, but not Th2, cells rapidly migrate to inflammatory sites and mediate DTH responses after peripheral challenge with live virus. (A) 107 Th1 or Th2 SMARTA CD4+ T cells were adoptively transferred intravenously into syngeneic naive C57BL/6 mice. 1 d later, recipients were challenged in the right hind footpad with 500 PFU LCMV-WE. Footpad swelling was monitored daily using calipers. Symbols represent mean values from groups of four to six mice, and intragroup variations were ≤0.4 mm. One representative experiment of three is shown. (B–D) Immunohistological analysis of CD4+ T cell infiltration into the footpad. Two mice from each group described in A were killed 3 d after LCMV-WE challenge, and frozen sections of footpads were analyzed for infiltration by CD4+ T cells using immunohistochemistry. Positively stained cells exhibit a bright red color. Original magnifications: ×50.
Th1 Cells Exhibit Enhanced Migratory Capacity toward Chemokines That Are Induced after Virus Infection.
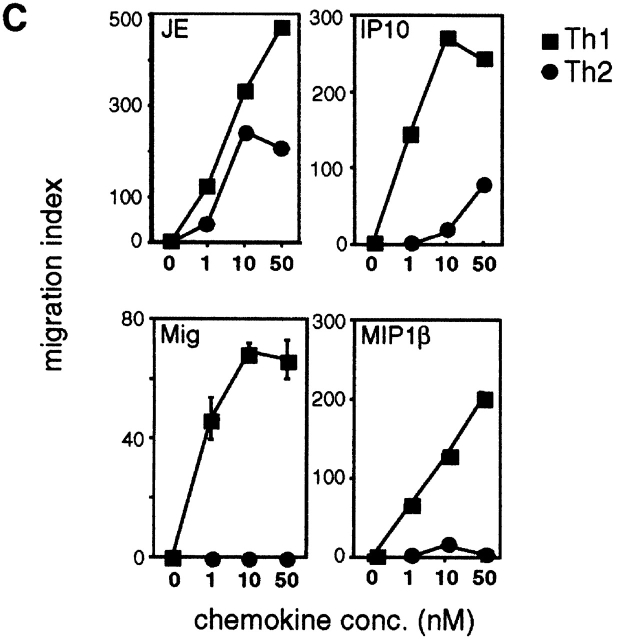
Lymphocyte migration involves a complex series of adhesive interactions between T cells and endothelium 45 46. Both Th1 and Th2 cells had a phenotype characteristic of activated T cells, expressing low levels of CD62L and high levels of CD25, CD69, intracellular adhesion molecule 1 (ICAM-1), and CD49d (very late antigen 4 [VLA-4]), but there were no readily detectable differences in surface phenotype (Maloy, K., unpublished observations). As evidence is increasing that Th1 and Th2 cells show differential expression of chemokine receptors 47 48 49, we examined whether chemokines may play a role in the preferential migration of Th1 cells into virus-infected tissues. Using semiquantitative RT-PCR analysis, we found that Th1 cells expressed higher levels of CCR2, CCR5, and CXCR3 than Th2 cells, whereas CCR4 was expressed at higher levels by Th2 cells (Fig. 8 A). We then assayed the expression of inflammatory chemokines in the ovaries of normal C57BL/6 mice 48 h after intraperitoneal injection of Vacc-IND-G. As shown in Fig. 8 B, several chemokines were upregulated in the ovaries of vaccinia-infected mice, including MIP-1α, MIP-1β, RANTES, MDC, and SDF-1α. Most striking, however, were our observations that the chemokines JE (MCP-1), Mig, and IP-10, which were undetectable in uninfected ovaries, were strongly induced after vaccinia infection (Fig. 8 B). Using in vitro migration assays, we found that Th1 cells showed strong migratory responses to these newly induced chemokines JE, Mig, and IP-10, as well as to the CCR5-binding chemokine MIP-1β, whereas Th2 cells migrated either poorly (JE) or not at all (Mig, IP-10, and MIP-1β) (Fig. 8 C). These results suggest that virus infection of peripheral tissues leads to the production of inflammatory chemokines that preferentially attract Th1 cells.
Figure 8.
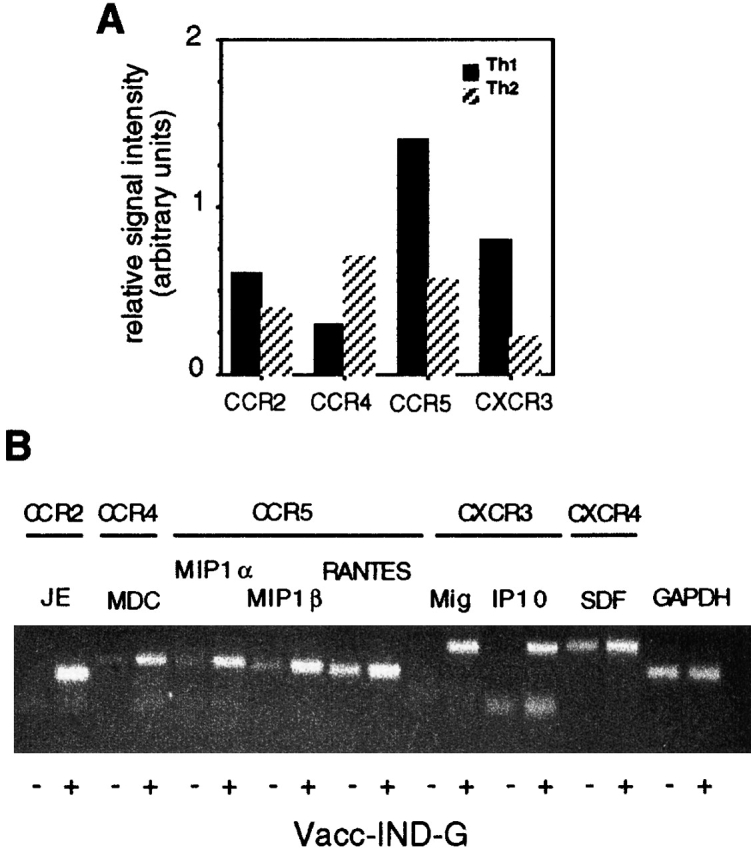
Th1 cells exhibit enhanced migratory responses toward chemokines that are induced after virus infection. (A) Chemokine receptor expression by Th1 or Th2 tg7 CD4+ T cells. RNA extracted from 2 × 106 Th1 or Th2 cells was analyzed using RT-PCR. Signal intensity was calculated as a ratio of the reference GAPDH signal as detailed in Materials and Methods. (B) Inflammatory chemokine expression in virus-infected ovaries. RNA was extracted from ovaries of C57BL/6 mice isolated 48 h after intraperitoneal injection of 2 × 106 PFU Vacc-IND-G, and chemokine expression was analyzed using RT-PCR. The receptors for the various chemokines are also indicated. (C) Migration of Th1 and Th2 tg7 CD4+ T cells in response to chemokines. Migration assays were performed using an in vitro transwell system.
Th1 Cells, but Not Th2 Cells, Can Protect against Intranasal Challenge with VSV.
Although neutralizing antibodies are sufficient in mediating protection against VSV administered systemically, intranasal infection with VSV facilitates rapid infection of the central nervous system (CNS) via the olfactory nerve, which can be rapidly lethal even in immunocompetent mice 50 51. Thus, C57BL/6 mice were adoptively transfused with Th1 or Th2 tg7 CD4+ T cells 24 h before intranasal challenge with 2 × 106 PFU VSV. As shown in Table , mice that received Th1 cells were completely protected against intranasal infection with VSV-IND, whereas those that received Th2 cells succumbed to infection with similar kinetics to control C57BL/6 mice. Protection was again antigen specific, since Th1 SMARTA CD4+ T cells were unable to provide protection against intranasal infection with VSV-IND and tg7 CD4+ T cells were unable to protect against VSV-NJ challenge (Table ). Protection by Th1 cells correlated with complete eradication of VSV from the brain, whereas brains from control mice or those that received Th2 cells harbored high titers of VSV (Table ).
Table 1.
Protection against Lethal Intranasal VSV Infection by Antigen-specific Th1 Cells
| Adoptive transfer | Challenge (2 × 106 PFU) | No. of mice surviving | VSV titer in brain |
|---|---|---|---|
| log10 PFU | |||
| None | VSV-IND | 0/6 | 6.3 ± 1.3 |
| 107 tg7 Th1 | VSV-IND | 6/6 | <1.2 |
| 107 tg7 Th2 | VSV-IND | 0/6 | 5.8 ± 1.8 |
| 107 SMARTA Th1 | VSV-IND | 0/6 | 5.6 ± 1.5 |
| None | VSV-NJ | 0/6 | 4.9 ± 0.8 |
| 107 tg7 Th1 | VSV-NJ | 0/6 | 4.5 ± 1.2 |
Naive C57BL/6 mice were adoptively transferred intravenously with transgenic Th1 or Th2 CD4+ T cells. 1 d later, recipients were challenged with 2 × 106 PFU VSV intranasally. Mice were monitored daily for hind limb paralysis and killed when seriously ill. Brains were isolated from killed mice or from surviving mice (on day 21 after challenge), and VSV titers were measured by plaquing on Vero cells. Mean VSV titers are shown. One of two similar experiments is shown.
Virus Infection Preferentially Induces Differentiation of Th1 Cells In Vivo.
To examine whether virus infection preferentially induces the development of Th1 effector cells, naive tg7 CD4+ T cells were adoptively transferred into TCR-β−/−δ−/− mice that were subsequently challenged with VSV or Vacc-IND-G. In the absence of viral challenge, the adoptively transferred CD4+ T cells remained largely undifferentiated as judged by the low numbers producing IFN-γ after restimulation (Table ). In contrast, infection with VSV-IND, or with Vacc-IND-G, led to the differentiation of high numbers of IFN-γ–secreting CD4+ T cells, whereas very few IL-4–producing cells could be detected (Table ).
Table 2.
Virus-induced Differentiation of Th1 Cells In Vivo
| Percentage of CD4+ T cells producing: | |||
|---|---|---|---|
| Challenge | IFN-γ | TNF-α | IL-4 |
| None | 16 | 21 | 1 |
| UVi-VSV | 35 | 52 | 8 |
| VSV | 51 | 71 | 4 |
| Vacc-IND-G | 56 | 76 | 0 |
TCR-β2/−δ2/− mice were adoptively transferred with 5 × 106 naive tg7 CD4+ T cells, and 24 h later recipients were injected intravenously with 2 × 106 PFU of live or UV-inactivated VSV-IND or with 2 × 106 PFU Vacc-IND-G. 1 wk after challenge, spleen cells were isolated, and the production of cytokines by CD4+ T cells was assessed using intracellular cytokine staining after in vitro stimulation with PMA and ionomycin. Mean values from groups of three mice from one of two similar experiments are shown.
Discussion
Our results show that several factors contribute to whether a given type of CD4+ effector T cell can efficiently mediate antiviral protection, including viral attributes such as route of infection and protective mechanisms, as well as T cell characteristics such as cytokine production and migratory patterns. Furthermore, our findings clearly demonstrate that Th1 CD4+ effector T cells possess much greater antiviral protective capacity than their Th2 counterparts. Th1 cells were able to mediate protection against viruses controlled by antibodies (VSV) or cytokines (vaccinia), regardless of the route of virus administration. In contrast, although Th2 cells protected against systemic infection with VSV by inducing neutralizing antibodies, they were unable to eradicate vaccinia virus and were also unable to efficiently respond to peripheral virus challenge in several different infection models.
Th1 and Th2 cells showed similar capabilities in helping B cells produce protective, VSV-neutralizing IgG antibodies, with Th1 cells inducing higher amounts of IgG2a and Th2 cells inducing IgG1 production. Previous studies with VSV have shown that antibody concentration, rather than IgG isotype, is the primary factor in determining protection 52. There were no quantitative differences in the levels of neutralizing antibodies induced by Th1 or Th2 cells, or in the number of effector T cells required to mediate class switching. It is not surprising that Th1 cells are as capable as Th2 cells in this respect, since the antibody response against most viruses is dominated by IgG2a, an isotype that is associated with IFN-γ production and Th1 responses 53 54.
The successful control of vaccinia infection depends on the production of T cell–derived cytokines, most notably IFN-γ and TNF 2. We found that adoptively transferred Th1, but not Th2, CD4+ T cell effectors could protect T cell–deficient mice from lethal Vacc-IND-G infection, confirming the important role of Th1-type cytokines in the eradication of vaccinia virus. This difference did not appear to be due to less efficient activation of the adoptively transferred Th2 cells, since the mice that received Th2 cells produced even higher titers of VSV-G–neutralizing IgG antibodies than those receiving Th1 cells. The preferential ability of Th1 cells to mediate antivaccinia protection was confirmed by our experiments examining the clearance of Vacc-IND-G from peripheral tissues of normal mice. We previously found that although naive tg7 CD4+ T cells were unable to adoptively transfer protection against peripheral Vacc-IND-G challenge, in vitro–primed tg7 CD4+ T cells quickly eradicated the vaccinia from ovaries 23. Here, we have extended these findings by showing that only Th1 CD4+ T cells are capable of such antiviral protection and that rapid clearance of vaccinia correlated with enhanced migration of the Th1 cells into ovaries as early as 48 h after infection. In contrast, Th2 CD4+ effector T cells were unable to clear vaccinia from peripheral tissues and did not efficiently migrate into infected organs. The ability of Th1 cells to efficiently respond to peripheral antigen challenge was confirmed by their induction of footpad DTH responses in response to either nonreplicating virus antigen (tg7 Th1 cells to Bac-VSV-G) or live virus (SMARTA Th1 cells to LCMV). Histological analysis showed that the enhanced responsiveness of Th1 cells again correlated with their rapid migration into peripheral tissues. The observed differences in homing did not appear to be due to preferential survival or expansion of the Th1 cells in vivo, since we observed that similar numbers of Th1 and Th2 cells homed to the spleen and peripheral lymph nodes after adoptive transfer (Maloy, K., unpublished observations).
Lymphocyte migration into peripheral tissues is a multistep process, involving adhesion and extravasation via selectins and integrins and chemotactic attraction by chemokines 45 46. The recirculation patterns of T cells are dependent on their differentiation state; naive T cells are restricted to the lymphoid tissues, whereas primed T cells may patrol through peripheral tissues 55 56. Selective recruitment of Th1 cells to sites of peripheral inflammation has previously been observed using protein antigens and involves interactions with P- and E-selectin molecules expressed on vascular endothelium 57. Furthermore, evidence is growing that effector CD4+ T cell subsets differ in terms of chemokine receptor expression, which may also influence their migratory patterns 47 48 49. We found similar differences in chemokine receptor expression, with Th1 effectors expressing higher levels of CCR2, CCR5, and CXCR3, whereas Th2 cells had higher levels of CCR4. Importantly, we found that several inflammatory chemokines were upregulated in virus-infected tissues, including MIP-1α, MIP-1β, and RANTES (all of which bind CCR5) as well as MDC (a CCR4 ligand). Most significantly, we observed that the chemokines JE (MCP-1), Mig, and IP-10 were newly induced after vaccinia virus infection and these chemokines all bind to receptors which were expressed at higher levels by Th1 cells (CCR2 and CXCR3). An almost identical pattern of chemokine upregulation and induction was observed after LCMV infection of the footpad (Junt, T.M., unpublished observations) or brain 58. In vitro migration assays showed that Th1 cells migrated well in response to these newly induced chemokines, as well as to the upregulated CCR5 ligand MIP-1β, whereas Th2 cells did not. Taken together, our results suggest that virus infection induces the production of chemokines that preferentially attract Th1 cells. Furthermore, they demonstrate positive correlations among protection against peripheral virus infections, migration into virus-infected tissues in vivo, and migration in response to chemokines that are induced after virus infection, all properties exhibited by Th1 effectors but not by Th2 cells. Interestingly, the antiviral capacities of effector CD8+ T cells may similarly correlate with their migratory properties. Effector CD8+ T cells can mediate protection against peripheral vaccinia infection, and this is dependent on recent antigenic stimulation, which enhances extravasation of effector T cells 59 60. In addition, recent studies with hemagglutinin-specific CD8+ effector T cells found that Tc1 effectors (analogous to Th1 cells) were much more effective than Tc2 effectors (analogous to Th2 cells) in protecting against pulmonary infection with influenza virus, despite the fact that both populations were equally cytolytic 61. The greater protective capacity of Tc1 effectors correlated with more rapid migration of these cells into the airway epithelium and with differences in chemokine receptor expression between the Tc1 and Tc2 cells 61. Together with our findings, these results demonstrate that the antiviral protective capabilities of both CD4+ T cells and CD8+ T cells are governed not only by the effector molecules they produce, but also by their migratory characteristics.
Intranasal inoculation of VSV leads to infection of local olfactory receptor neurons, which is quickly followed by acute infection of the CNS 50. Recovery from CNS infection is T cell dependent and is associated with increased T cell infiltration of the CNS and with enhanced production of cytokines that are characteristically present during Th1 responses 51 62. We observed that Th1 cells, but not Th2 cells, were able to protect mice against the lethal consequences of intranasal challenge with VSV. This again is likely to have been due to enhanced migration of the Th1 cells and/or increased production of cytokines such as IFN-γ, which may play a crucial role during viral infection of the CNS 63.
The primary role of Th1 responses in antiviral protection was confirmed by our finding that virus infection predominantly promoted differentiation of Th1 effectors from naive CD4+ T cells in vivo. Our results are consistent with other reports showing that CD4+ T cells with a Th1 cytokine phenotype contribute to protection against a variety of viral infections, including influenza virus 10 11, poliovirus 14, murine cytomegalovirus 64, herpes simplex virus 65, and measles virus 66. Viral infection is known to stimulate the production of IL-12, which has beneficial effects on many viral infections and is a potent inducer of Th1 responses 62 67 68 69, although it is worth noting that viruses can induce Th1 responses in the absence of IL-12 70. Thus, adjuvants that promote Th1 responses may be the best for vaccinating against cytopathic viruses, which are controlled by neutralizing antibodies and T cell cytokines 1. However, in the case of noncytopathic viruses, CD8+ CTLs play a central role in protection 71; thus, caution must be used, since pathological consequences of excessive virus-specific CD4+ T cell activation have been observed 72.
Acknowledgments
We thank Lenka Vlk for assistance with the immunohistology and Norbert Wey for the photographs. This manuscript is dedicated to the memory of Mary McDermid.
This work was supported by grants from the Swiss National Science Foundation and the Kanton Zürich. C. Burkhart was a fellow of the Studienstiftung des Deutschen Volkes, Bonn, Germany.
Footnotes
Abbreviations used in this paper: Bac-G, baculovirus-derived recombinant VSV-G; Bac-NP, baculovirus-derived recombinant VSV-NP; CCR, CC chemokine receptor; CNS, central nervous system; CXCR, CXC chemokine receptor; DTH, delayed-type hypersensitivity; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; LCMV, lymphocytic choriomeningitis virus; MCP, monocyte chemotactic protein; MDC, macrophage-derived chemokine; Mig, monokine induced by IFN-γ; MIP, macrophage inflammatory protein; RANTES, regulated upon activation, normal T cell expressed and secreted protein; RT, reverse transcription; SDF, stromal cell–derived factor; VSV, vesicular stomatitis virus; VSV-IND, VSV serotype Indiana; Vacc-IND-G, recombinant vaccinia virus expressing VSV-IND glycoprotein; VSV-NJ, VSV serotype New Jersey; UVi-VSV, UV-inactivated VSV.
C. Burkhart and T.M. Junt contributed equally to this work.
C. Burkhart's present address is Department of Rheumatology and Clinical Immunology/Allergology, Inselsspital, CH-3010 Bern, Switzerland.
References
- Zinkernagel R.M. Immunology taught by viruses. Science. 1996;271:173–178. doi: 10.1126/science.271.5246.173. [DOI] [PubMed] [Google Scholar]
- Ramshaw I.A., Ramsay A.J., Karupiah G., Rolph M.S., Mahalingam S., Ruby J.C. Cytokines and immunity to viral infections. Immunol. Rev. 1997;159:119–135. doi: 10.1111/j.1600-065x.1997.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Czarniecki C.W., Fennie C.W., Powers D.B., Estell D.A. Synergistic antiviral and antiproliferative activities of Escherichia coli-derived human alpha, beta and gamma interferons. J. Virol. 1984;49:490–496. doi: 10.1128/jvi.49.2.490-496.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G.H.W., Goeddel D. Tumour necrosis factors α and β inhibit virus replication and synergize with interferons. Nature. 1986;323:819–822. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]
- Leist T.P., Cobbold S.P., Waldmann H., Aguet M., Zinkernagel R.M. Functional analysis of T lymphocyte subsets in antiviral host defense. J. Immunol. 1987;138:2278–2281. [PubMed] [Google Scholar]
- Kasaian M.T., Leite M.K., Biron C.A. The role of CD4+ cells in sustaining lymphocyte proliferation during lymphocytic choriomeningitis virus infection. J. Immunol. 1991;146:1955–1963. [PubMed] [Google Scholar]
- Leist T.P., Kohler M., Zinkernagel R.M. Impaired generation of antiviral cytotoxicity against lymphocytic choriomeningitis and vaccinia virus in mice treated with CD4-specific monoclonal antibody. Scand. J. Immunol. 1989;30:679–686. doi: 10.1111/j.1365-3083.1989.tb02476.x. [DOI] [PubMed] [Google Scholar]
- Matloubian M., Conception R.J., Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T cell responses during chronic viral infection. J. Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelberger M., Allan W., Zijlstra M., Jaenisch R., Doherty P.C. Clearance of influenza virus respiratory infection in mice lacking class I major histocompatibility complex–restricted CD8+ T cells. J. Exp. Med. 1991;174:875–880. doi: 10.1084/jem.174.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherle P.A., Palladino G., Gerhard W. Mice can recover from pulmonary influenza virus infection in the absence of class I-restricted cytotoxic T cells. J. Immunol. 1992;148:212–217. [PubMed] [Google Scholar]
- Graham M.B., Braciale V.L., Braciale T.J. Influenza virus–specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J. Exp. Med. 1994;180:1273–1282. doi: 10.1084/jem.180.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs M.K., Koller B.H., Sato T., Morrissey P.J., Fanslow W.C., Smithies O., Voice R.F., Widmer M.B., Maliszewski C.R. β2-microglobulin-, CD8+ T-cell-deficient mice survive inoculation with high doses of vaccinia virus and exhibit altered IgG responses. Proc. Natl. Acad. Sci. USA. 1992;89:6070–6074. doi: 10.1073/pnas.89.13.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kägi D., Seiler P., Pavlovic J., Ledermann B., Bürki K., Zinkernagel R.M., Hengartner H. The roles of perforin and Fas-dependent cytotoxicity in protection against cytopathic and non-cytopathic viruses. Eur. J. Immunol. 1995;25:3256–3262. doi: 10.1002/eji.1830251209. [DOI] [PubMed] [Google Scholar]
- Mahon B.P., Katrak K., Nomoto A., Macadam A., Minor P.D., Mills K.H.G. Poliovirus-specific CD4+ T cell clones with both cytotoxic and helper activity mediate protective humoral immunity against a lethal poliovirus infection in transgenic mice expressing the human poliovirus receptor. J. Exp. Med. 1995;181:1285–1292. doi: 10.1084/jem.181.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T.R., Coffman R.L. TH1 and TH2 cellsdifferent patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Abbas A.K., Murphy K.M., Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- O'Garra A. Cytokines induce the development of functionally heterogeneous T helper subsets. Immunity. 1998;8:275–283. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- Reiner S.L., Wang Z.E., Hatam F., Scott P., Locksley R.M. Th1 and Th2 cell antigen receptors in experimental leishmaniasis. Science. 1993;259:1457–1460. doi: 10.1126/science.8451641. [DOI] [PubMed] [Google Scholar]
- Else K.J., Finkelman F.D., Maliszewski C.R., Grencis R.K. Cytokine-mediated regulation of chronic intestinal helminth infection. J. Exp. Med. 1994;179:347–351. doi: 10.1084/jem.179.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J.F., Jr., Noben-Trauth N., Donaldson D.D., Madden K.B., Morris S.C., Collins M., Finkelman F.D. IL-13, IL-4Rα and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis . Immunity. 1998;8:255–264. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- Lefrancois L. Protection against lethal viral infection by neutralizing and nonneutralizing monoclonal antibodiesdistinct mechanisms of action in vivo. J. Virol. 1984;51:208–214. doi: 10.1128/jvi.51.1.208-214.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen A.R., Nansen A., Anderson C., Johansen J., Marker O., Christensen J.P. Cooperation of B and T cells is required for survival of mice infected with vesicular stomatitis virus. Int. Immunol. 1997;9:1757–1766. doi: 10.1093/intimm/9.11.1757. [DOI] [PubMed] [Google Scholar]
- Maloy K.J., Burkhart C., Freer G., Rülicke T., Pircher H.P., Kono D.H., Theofilopoulos A.N., Ludewig B., Hoffman-Rohrer U., Zinkernagel R.M., Hengartner H. Qualitative and quantitative requirements for CD4+ T cell-mediated antiviral protection. J. Immunol. 1999;162:2867–2874. [PubMed] [Google Scholar]
- Oxenius A., Bachmann M.F., Zinkernagel R.M., Hengartner H. Virus-specific MHC class II-restricted TCR-transgenic miceeffects on humoral and cellular immune responses after viral infection. Eur. J. Immunol. 1998;28:390–400. doi: 10.1002/(SICI)1521-4141(199801)28:01<390::AID-IMMU390>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Mizoguchi E., Ljunggren H.G., Iacomini J., Ishikawa H., Wang L., Grusby M.J., Glimcher L.H., Winn H.J., Bhan A.K., Tonegawa S. Peripheral lymphoid development and function in TCR mutant mice. Int. Immunol. 1994;6:1061–1070. doi: 10.1093/intimm/6.7.1061. [DOI] [PubMed] [Google Scholar]
- Assenmacher M., Schmitz J., Radbruch A. Flow cytometric determination of cytokines in activated murine T helper lymphocytesexpression of interleukin-10 in interferon-gamma and in interleukin-4-expressing cells. Eur. J. Immunol. 1994;24:1097–1101. doi: 10.1002/eji.1830240513. [DOI] [PubMed] [Google Scholar]
- Charan S., Zinkernagel R.M. Antibody mediated suppression of secondary IgM response in nude mice against vesicular stomatitis virus. J. Immunol. 1986;136:3057–3061. [PubMed] [Google Scholar]
- Bachmann M.F., Kündig T.M., Kalberer C., Hengartner H., Zinkernagel R.M. Formalin inactivation of vesicular stomatitis virus impairs T-cell- but not T-help-independent B cell responses. J. Virol. 1993;67:3917–3922. doi: 10.1128/jvi.67.7.3917-3922.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Yilma T., Rose J.K., Moss B. Vaccinia virus recombinantsexpression of VSV genes and protective immunization of mice and cattle. Science. 1985;227:433–435. doi: 10.1126/science.2981435. [DOI] [PubMed] [Google Scholar]
- Schulz M., Aichele P., Vollenweider M., Bobe F.W., Cardinaux F., Hengartner H., Zinkernagel R.M. MHC dependent T cell epitopes of LCMV nucleoprotein and their protective capacity against viral disease. Eur. J. Immunol. 1989;19:1657–1667. doi: 10.1002/eji.1830190921. [DOI] [PubMed] [Google Scholar]
- Kimmig W., Lehmann G.F. The immune response of the mouse to lymphocytic choriomeningitis virus. I. Circulating antibodies. J. Gen. Virol. 1979;45:703–710. doi: 10.1099/0022-1317-45-3-703. [DOI] [PubMed] [Google Scholar]
- Burkhart C., Freer G., Steinhoff R.U., Li Y., Bishop D.H., Zinkernagel R.M., Hengartner H. Localization of T helper cell epitopes in the vesicular stomatitis virusthe nucleoprotein is responsible for serotype cross-reactive help. Viral Immunol. 1994;7:103–111. doi: 10.1089/vim.1994.7.103. [DOI] [PubMed] [Google Scholar]
- Wagner R.R., Snyder R.M., Yamazaki S. Proteins of vesicular stomatitis viruskinetics and cellular sites of synthesis. J. Virol. 1970;5:548–558. doi: 10.1128/jvi.5.5.548-558.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D.W., Gershon R.K. Determination of total and mercaptoethanol-resistant antibody in the same serum sample. Clin. Exp. Immunol. 1970;6:313–317. [PMC free article] [PubMed] [Google Scholar]
- Binder D., Kündig T.M. Antiviral protection by CD8+ versus CD4+ T cellsCD8+ T cells correlating with cytotoxic activity in vitro are more efficient in antivaccinia virus protection than CD4-dependent interleukins. J. Immunol. 1991;146:4301–4307. [PubMed] [Google Scholar]
- Burkhart C., Freer G., Castro R., Adorini L., Wiesmüller K.H., Zinkernagel R.M., Hengartner H. Characterization of T helper epitopes of the glycoprotein of vesicular stomatitis virus. J. Virol. 1994;68:1573–1580. doi: 10.1128/jvi.68.3.1573-1580.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Openshaw P., Murphy E.E., Hosken N.A., Maino V., Davis K., Murphy K., O'Garra A. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J. Exp. Med. 1995;182:1357–1367. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist T.P., Eppler M., Zinkernagel R.M. Enhanced virus replication and inhibition of lymphocytic choriomeningitis virus disease in anti-IFN gamma-treated mice. J. Virol. 1989;63:2813–2819. doi: 10.1128/jvi.63.6.2813-2819.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E., Shibuya K., Hosken N., Openshaw P., Maino V., Davis K., Murphy K., O'Garra A. Reversibility of T helper 1 and 2 populations is lost after long-term stimulation. J. Exp. Med. 1996;183:901–913. doi: 10.1084/jem.183.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M.F., Odermatt B., Hengartner H., Zinkernagel R.M. Induction of long-lived germinal centers associated with persisting antigen after viral infection. J. Exp. Med. 1996;183:2259–2269. doi: 10.1084/jem.183.5.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy K.J., Odermatt B., Hengartner H., Zinkernagel R.M. Interferon γ-producing γδ T cell-dependent antibody isotype switching in the absence of germinal center formation during virus infection. Proc. Natl. Acad. Sci. USA. 1998;95:1160–1165. doi: 10.1073/pnas.95.3.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kündig T., Castelmur I., Bachmann M.F., Abraham D., Binder D., Hengartner H., Zinkernagel R.M. Fewer protective cytotoxic T cell epitopes than T helper cell epitopes on vesicular stomatitis virus. J. Virol. 1993;67:3680–3683. doi: 10.1128/jvi.67.6.3680-3683.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudmore S., Cossart P., Griffiths G., Way M. Actin-based motility of vaccinia virus. Nature. 1995;378:636–638. doi: 10.1038/378636a0. [DOI] [PubMed] [Google Scholar]
- Smith G.L. Vaccinia virus immune evasion. Immunol. Lett. 1999;65:55–62. doi: 10.1016/s0165-2478(98)00125-4. [DOI] [PubMed] [Google Scholar]
- Springer T.A. Traffic signals for lymphocyte recirculation and leukocyte emigrationthe multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Butcher E.C., Picker L.J. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- O'Garra A., McEvoy L.M., Zlotnik A. T-cell subsetschemokine receptors guide the way. Curr. Biol. 1998;8:R646–R649. doi: 10.1016/s0960-9822(07)00413-7. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A., Mackay C.R. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol. Today. 1998;19:568–574. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- Sivike J.T., Hamann A. T helper 1 and T helper 2 cells respond differentially to chemokines. J. Immunol. 1998;160:550–554. [PubMed] [Google Scholar]
- Plakhov I.V., Aoki C., Arlund E.G., Reiss C.S. The earliest events in VSV infection of the murine olfactory epithelium and entry of the central nervous system. Virology. 1995;209:257–262. doi: 10.1006/viro.1995.1252. [DOI] [PubMed] [Google Scholar]
- Christian A.Y., Barna M., Bi Z., Reiss C.S. Host immune response to vesicular stomatitis virus infection of the central nervous system in C57BL/6 mice. Viral Immunol. 1996;9:195–205. doi: 10.1089/vim.1996.9.195. [DOI] [PubMed] [Google Scholar]
- Bachmann M.F., Kalinke U., Althage A., Freer G., Burkhart C., Roost H., Aguet M., Hengartner H., Zinkernagel R.M. The role of antibody concentration and avidity in antiviral protection. Science. 1997;276:2024–2027. doi: 10.1126/science.276.5321.2024. [DOI] [PubMed] [Google Scholar]
- Coutelier J.P., Van der Logt J.T.M., Heessen F.W.A., Warnier G., Van Snick J.V. IgG2a restriction of murine antibodies elicited by viral infections. J. Exp. Med. 1987;165:64–69. doi: 10.1084/jem.165.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman F.D., Holmes J., Katona I.M., Urban J., Beckmann M.P., Park L.S., Scholley K.A., Coffman R.L., Mosmann T.R., Paul W.E. Lymphokine control of in vivo immunoglobulin isotype secretion. Annu. Rev. Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- Lee W.T., Vitetta E.S. The differential expression of homing and adhesion molecules on virgin and memory T cells in the mouse. Cell. Immunol. 1991;132:215–222. doi: 10.1016/0008-8749(91)90020-c. [DOI] [PubMed] [Google Scholar]
- Mackay C.R. Homing of naive, memory and effector lymphocytes. Curr. Opin. Immunol. 1993;5:423–427. doi: 10.1016/0952-7915(93)90063-x. [DOI] [PubMed] [Google Scholar]
- Austrup F., Westweber D., Borges E., Löhning M., Bräuer R., Herz U., Renz H., Hallman R., Scheffold A., Radbruch A., Hamann A. P- and E-selectin mediate recruitment of T helper 1 but not T helper 2 cells into inflamed tissues. Nature. 1997;385:81–84. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- Asensio V.C., Campbell I.L. Chemokine gene expression in the brains of mice with lymphocytic choriomeningitis. J. Virol. 1997;71:7832–7840. doi: 10.1128/jvi.71.10.7832-7840.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kündig T.M., Bachmann M.F., Oehen S., Hoffmann U.W., Simard J.J.L., Kalberer C.P., Pircher H., Ohashi P.S., Hengartner H., Zinkernagel R.M. On the role of antigen in maintaining cytotoxic T-cell memory. Proc. Natl. Acad. Sci. USA. 1996;93:9716–9723. doi: 10.1073/pnas.93.18.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M.F., Kündig T.M., Hengartner H., Zinkernagel R.M. Protection against immunopathological consequences of a viral infection by activated but not resting cytotoxic T cellsT cell memory without “memory T cells”? Proc. Natl. Acad. Sci. USA. 1997;94:640–645. doi: 10.1073/pnas.94.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerwenka A., Morgan T.M., Harmsen A.G., Dutton R.W. Migration kinetics and final destination of type 1 and type 2 CD8 effector cells predict protection against pulmonary virus infection. J. Exp. Med. 1999;189:423–434. doi: 10.1084/jem.189.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Z., Quandt P., Komatsu T., Barna M., Reiss C.S. IL-12 promotes enhanced recovery from vesicular stomatitis virus infection of the central nervous system. J. Immunol. 1995;155:5684–5689. [PubMed] [Google Scholar]
- Kündig T.M., Hengartner H., Zinkernagel R.M. T cell dependent interferon gamma exerts an antiviral effect in the central nervous system but not in peripheral solid organs. J. Immunol. 1993;150:2316–2323. [PubMed] [Google Scholar]
- Jonjic S., Pavic I., Lucin P., Rukavina D., Koszinowski U.H. Efficacious control of cytomegalovirus infection after long-term depletion of CD8+ T lymphocytes. J. Virol. 1990;64:5457–5464. doi: 10.1128/jvi.64.11.5457-5464.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manickan E., Francotte M., Kuklin N., Dewerchin M., Molitor C., Gheysen D., Slaoui M., Rouse B.T. Vaccination with recombinant vaccinia virus expressing ICP27 induces protective immunity against herpes simplex virus through CD4+ Th1 T cells. J. Virol. 1995;69:4711–4716. doi: 10.1128/jvi.69.8.4711-4716.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich A., Erlwein O., Niewiesk S., Ter Meulen V., Liebert U.G. CD4+ T cells control measles virus infection in the central nervous system. Immunology. 1992;76:185–191. [PMC free article] [PubMed] [Google Scholar]
- Orange J.S., Wolf S.F., Biron C.A. Effects of IL-12 on the response and susceptibility to experimental viral infections. J. Immunol. 1994;152:1253–1264. [PubMed] [Google Scholar]
- Carr J.A., Rogerson J., Mulqueen M.J., Roberts N.A., Booth R.F.G. Interleukin-12 exhibits potent antiviral activity in experimental herpesvirus infections. J. Virol. 1997;71:7799–7803. doi: 10.1128/jvi.71.10.7799-7803.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutelier J.P., Van Broeck J., Wolf S.F. Interleukin-12 gene expression after viral infection in the mouse. J. Virol. 1995;69:1955–1958. doi: 10.1128/jvi.69.3.1955-1958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenius A., Karrer U., Zinkernagel R.M., Hengartner H. IL-12 is not required for induction of type 1 cytokine responses in viral infections. J. Immunol. 1999;162:965–973. [PubMed] [Google Scholar]
- Kägi D., Ledermann B., Bürki K., Seiler P., Odermatt B., Olsen K.J., Podack E., Zinkernagel R.M., Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Oxenius A., Zinkernagel R.M., Hengartner H. Comparison of activation versus induction of unresponsiveness of virus-specific CD4+ and CD8+ T cells upon acute versus persistent viral infection. Immunity. 1998;9:449–457. doi: 10.1016/s1074-7613(00)80628-7. [DOI] [PubMed] [Google Scholar]