Abstract
Several immune-based approaches are being considered for modulation of inflammatory T cells and amelioration of autoimmune diseases. The most recent strategies include simulation of peripheral self-tolerance by injection of adjuvant free antigen, local delivery of cytokines by genetically altered T cells, and interference with the function of costimulatory molecules. Although promising results have been obtained from these studies that define mechanisms of T cell modulation, efficacy, practicality, and toxicity, concerns remain unsolved, thereby justifying further investigations to define alternatives for effective downregulation of aggressive T cells. In prior studies, we demonstrated that an immunoglobulin (Ig) chimera carrying the encephalitogenic proteolipid protein (PLP)1 peptide corresponding to amino acid sequence 139–151 of PLP, Ig-PLP1, is presented to T cells ∼100-fold better than free PLP1. Here, we demonstrate that aggregation endows Ig-PLP1 with an additional feature, namely, induction of interleukin (IL)-10 production by macrophages and dendritic cells, both of which are antigen-presenting cells (APCs). These functions synergize in vivo and drive effective modulation of autoimmunity. Indeed, it is shown that animals with ongoing active experimental allergic encephalomyelitis dramatically reduce the severity of their paralysis when treated with adjuvant free aggregated Ig-PLP1. Moreover, IL-10 displays bystander antagonism on unrelated autoreactive T cells, allowing for reversal of disease involving multiple epitopes. Therefore, aggregated Ig-PLP1 likely brings together a peripheral T cell tolerance mechanism emanating from peptide presentation by APCs expressing suboptimal costimulatory molecules and IL-10 bystander suppression to drive a dual-modal T cell modulation system effective for reversal of autoimmunity involving several epitopes and diverse T cell specificities.
Keywords: autoimmunity, antigen delivery, bystander downregulation, cytokine antagonism, T cell modulation
Introduction
During development, T cells whose antigen receptors are devoid of self-reactivity exit the thymus and participate in immune surveillance, whereas those bearing receptors endowed with self-reactivity are negatively selected and deleted by programmed cell death 1. This process of T cell screening and selection, known as central tolerance, requires the antigen to be available in the thymus in sufficient quantities and in a form presentable by MHC molecules 2 3 4 5. Although central tolerance exerts a tight control on the shaping of the T cell repertoire, some self-reactive T cells still escape the thymus and migrate to the periphery 6 7 8. If the antigen is available in the periphery, a second round of T cell screening, known as peripheral tolerance, will follow to further minimize autoreactivity 3 9 10 11 12 13. Presumably, peripheral tolerance develops as a consequence of presentation of autoantigen by nonactivated APCs expressing minimal or no costimulatory molecules 3 14.
For sequestered autoantigens that are not available for presentation in either the thymus or the periphery, the corresponding T cells will circulate harmlessly. However, events that trigger exposure of those autoantigens, which are usually accompanied by conditions favorable for activation of local APCs, lead to an optimal presentation to and activation of the circulating T cells 15 16 17 18. The results of this T cell activation may be the escalation of inflammatory reactions and injury of specific tissues and organs 19 20 21.
Supply of antigen in an adjuvant free form might not stimulate the expression of costimulatory molecules on APCs and thereby drive an antigen presentation inadequate for T cell activation 3 22 23. Prior studies have in fact indicated that this approach modulates autoreactive T cells and promotes recovery from illness 24 25 26. However, the usefulness of this approach for modulation of autoimmunity is hampered by the unlimited availability of autoantigen at the injury site and the consequent continuous activation of the self-reactive T cells. In addition, as bystander suppression is unlikely to occur, the approach holds little promise for modulation of T cell–mediated autoimmunity involving multiple antigens. To overcome these issues, an in vitro approach that uses plasmid 27 and viral 28 29 vector-driven modulatory cytokines was adopted. Indeed, autoreactive T cell clones or hybridomas expressing the cytokine IL-4 or IL-10, as a consequence of transfection or infection, induced recovery from disease when injected into animals with ongoing experimental autoimmune encephalomyelitis (EAE) 27 28. This is a promising approach and bodes well for the development of practical strategies that could combine both peripheral tolerance and cytokine antagonism to combat autoimmunity.
It has previously been shown that peptide delivery on Igs increases presentation by 100–1,000-fold relative to free peptide 30 31. It is also known that cross-linking of Fc receptors (FcRs) on target cells by antigen–antibody complexes can trigger the production of cytokines 32 33 34. Moreover, aggregation of Igs confers the effector functions associated with the Fc fragment without the need for complex formation 35 36. Here, the encephalitogenic proteolipid protein (PLP)1 was genetically engineered into an Ig molecule 30, and the resulting Ig-PLP1 chimera was aggregated and assayed for modulation of autoreactive T cells and amelioration of active EAE. The results show that aggregated (agg) Ig-PLP1 induced IL-10 secretion by both macrophages and dendritic cells (DCs) but not B cells. In vitro, APCs incubated with agg Ig-PLP1 presented PLP1 to specific T cells. However, because of the IL-10 secreted by the presenting APCs, IFN-γ production by the T cells was impaired. In vivo, when soluble (sol) Ig-PLP1 was injected into mice with ongoing EAE, the severity of disease was slightly reduced. However, when mice were given agg Ig-PLP1, full recovery was achieved. Moreover, agg Ig-PLP1 was able to modulate disease induced by either an encephalitogenic peptide other than PLP1 or by a central nervous system (CNS) homogenate. Neutralization of endogenous IL-10 by injection of anti–IL-10 antibody during administration of agg Ig-PLP1 restored disease severity. Therefore, agg Ig-PLP1 triggers IL-10 production by APCs, drives inadequate peripheral presentation of PLP1, and couples both events to modulate autoimmunity involving diverse T cell specificities.
Materials and Methods
Animals.
SJL/J (H-2S) mice were purchased from Harlan, bred, and maintained in our animal care facility for the duration of the experiments.
Antigens
Peptides.
The peptides used in this study were purchased from Research Genetics, and were HPLC purified to >90% purity. PLP1 peptide (HSLGKWLGHPDKF) encompasses amino acid (aa) residues 139–151 of PLP and is encephalitogenic in SJL/J mice 37. PLP2 peptide (NTWTTCQSIAFPSK), encompassing aa 178–191 of PLP, is likewise encephalitogenic in SJL/J 38. Myelin basic protein (MBP)-3 peptide (VHFFKNIVTPRTP) corresponding to aa residues 87–99 of MBP is also I-AS restricted, and induces EAE in SJL/J mice 39. Hemagglutinin (HA) peptide, an I-Ed–restricted epitope 31, corresponding to aa residues 110–120 of HA, was used for negative control purposes.
CNS Homogenate.
50 frozen unstripped rat brains (Pelfreez Biologicals) were homogenized in PBS using a Waring blender and adjusted to 300 mg/ml with PBS. CNS homogenate was stored at −20°C.
Ig-PLP Chimeras.
The Ig-PLP1 and Ig-PLP2 chimeras harbor, within the heavy chain CDR3 region, PLP1 and PLP2, respectively, and have been described previously 30 40 41. Ig-W is the parental IgG2b antiarsonate antibody, 91A3, not encompassing any PLP peptide, and has been described elsewhere 30. Large-scale cultures of Ig-W, Ig-PLP1, and Ig-PLP2 transfectants were performed in DMEM containing 10% serum supreme (BioWhittaker) and purified on separate rat anti–mouse κ chain sepharose columns to avoid cross-contamination. Subsequently, the Ig chimeras were dialyzed against PBS and concentrated on collodion membranes (Schleicher & Schuell). The chimeras were aggregated by precipitation with 50% saturated (NH4)2SO4 as described 42. In brief, filtered 100% saturated (NH4)2SO4 was added at an equal volume to the sol Ig chimera preparation. The mixture was incubated at 24°C for 1 h with gentle agitation every 20 min. Subsequently, the samples were spun down at 10,000 rpm, and the pellet was resuspended at 1 mg/ml in PBS. Electrophoresis on a 10% acrylamide gel indicated that the sol Ig chimera entered the gel and migrated ∼160 kD. However, the agg Ig chimera did not enter the gel. Knowing that we applied the equivalent of 2 μg of agg Ig chimera, and that the sensitivity of the technology is 0.1 μg, we concluded that at least 95% of the agg Ig chimera preparation is in an aggregate form.
Induction of EAE
6–8-wk-old mice were induced for EAE by subcutaneous injection in the footpads, and at the base of the limbs and tail with a 200 μl IFA/PBS (vol/vol) solution containing the autoantigen and 200 μg Mycobacterium tuberculosis H37Ra. 6 h later, the mice were given intravenous 5 × 109 inactivated Bordetella pertussis (Bioport). A second injection of B. pertussis was given after 48 h. Subsequently, the mice were scored daily for clinical signs of EAE as follows: 0, no clinical score; 1, loss of tail tone; 2, hindlimb weakness; 3, hindlimb paralysis; 4, forelimb paralysis; and 5, moribund or death. In some experiments, purified pertussis toxin (List Biological Laboratories, Inc.) was used instead of whole B. pertussis organism.
Treatment of EAE with Ig-PLP1
Mice induced for EAE with PLP1, PLP2, a mixture of PLP1 plus PLP2, or CNS homogenate began receiving treatment with Ig-PLP1 after loss of tail tone, which occurs regularly between days 6 and 8 after disease induction. Treatment injections were given intraperitoneally in PBS on days 9, 13, and 17.
Histopathology
Mice treated with agg Ig-PLP1 or agg Ig-W were killed at the peak of the initial phase of disease (day 28 after disease induction), and the brain and spinal cord were removed, fixed with formalin, and embedded in paraffin. Serial cross-sections (6 μm) from the cerebellum, cerebrum, and lumbar cord were cut and stained with hematoxylin and eosin. Perivascular clusters containing at least 20 mononuclear cells were counted as inflammatory foci.
T Cells
TCC-PLP1-1B10.
Adult SJL mice were immunized subcutaneously with 100 μg PLP1 peptide in CFA, and 10 d later the draining LNs were removed and the cells (5 × 106 cells/ml) were stimulated with PLP1 (15 μg/ml). After 5 d, the blasts were separated on a Histopaque gradient (Sigma-Aldrich), and then restimulated with peptide and fresh irradiated (3,000 rads) syngeneic APCs. 10 d later, the cells were washed, resuspended in media containing 10% T-Stim (Collaborative Research), and rested for 7 d. After three cycles of stimulation/resting, the cells were cloned by limiting dilution (1 cell/3 wells) and positives were subjected to a second round of limiting dilution cloning. Subsequently, one clone, designated TCC-PLP1-1B10, was selected for these studies.
Isolation of Macrophages, DCs, and B Cells
Macrophages.
Macrophages were obtained from the peritoneal cells of mice injected with thioglycollate broth as described previously 43. In brief, 2 ml of thioglycollate broth was injected intraperitoneally, and after 5 d the macrophages were removed by washing the peritoneal cavity with 8 ml of HBSS, 4 μM EDTA. Macrophage purity was ≥93% as determined by FACS® analysis using antibody to F4/80 marker.
DCs.
DCs were purified from SJL/J spleen according to the standard collagenase/differential adherence method 44. Cell purity was ≥94% as determined by FACS® analysis using antibody to the 33D1 marker.
B Cells.
SJL/J splenocytes were panned on plates coated with rat anti–mouse κ (1 mg/ml) for 15 min at 25°C. Nonadherent cells were washed out with PBS. B cells were then dissociated from the plate by incubation with lidocaine HCl (0.8 mg/ml), followed by vigorous pipetting. Cell purity was ≥90% as determined by FACS® analysis for expression of B220 marker.
Proliferation Assays.
SJL/J splenocytes (10 × 105 cells/well/100 μl) were pulsed with graded amounts of antigen on round-bottomed 96-well plates for 4 h, pelleted, fixed with 1% paraformaldehyde for 15 min, washed, and transferred to a fresh 96-well plate. TCC-PLP1-1B10 cells (0.5 × 105 cells/well/100 μl) were then added and incubated for 3 d. Subsequently, 1 μCi [3H]thymidine was added per well, and the incubation continued for an additional 14.5 h. The cells were then harvested on glass fiber filters, and incorporated [3H]thymidine was counted using an Innotech β counter (Wohlen).
Cytokine Detection
ELISA.
ELISA was done according to BD PharMingen's standard protocol. The capture antibodies were rat anti–mouse IL-2, JES6-1A12; rat anti–mouse IL-4, 11B11; rat anti–mouse IFN-γ, R4-6A2; rat anti–mouse IL-10, JES5-2A5; and rat anti–mouse IL-5, TRFK5. The biotinylated anticytokine antibodies were rat anti–mouse IL-2, JES6-5H4; rat anti–mouse IL-4, BVD6-24G2; rat anti–mouse IFN-γ, XMG1.2; rat anti–mouse IL-10, JES5-16E3; and rat anti–mouse IL-5, TRFK4. ELISA for the detection of active TGF-β was preformed using the human TGF-β1 DuoSet kit (Genzyme) according to the manufacturer's instructions. Bound ligand was revealed using the TMB microwell peroxidase substrate system (Kirkegaard & Perry Laboratories). Assays were read on a SpectraMAX 340 counter (Molecular Devices). Graded amounts of recombinant mouse IL-2, IL-4, IFN-γ, IL-10, IL-5, and TGF-β were included in all experiments for construction of standard curves. The cytokine concentration in culture supernatants was estimated by extrapolation from the linear portion of the standard curve.
Enzyme-linked Immunospot.
Enzyme-linked immunospot (ELISPOT) assays were used to measure the cytokines produced by LN T cells upon stimulation with antigen as described 41. In brief, LN cells (5 × 105 cells/100 μl/well) and the antigen (100 μl/well) were incubated in HA-multiscreen plates (Millipore) coated with capture antibody for 24 h. Bound cytokines were revealed with peroxidase and anticytokine antibodies. The anticytokine antibody pairs used here were those described for the ELISA technique. Spots were counted under a dissecting microscope.
Stimulation of Cytokine Production by TCC-PLP1-1B10
Stimulation was performed with both irradiated and fixed APCs. In one case, SJL/J splenocytes were irradiated (3,000 rads) and plated (at 5 × 105 cells/well/50 μl) with graded concentrations of antigens (100 μl/well). After 1 h, TCC-PLP1-1B10 cells (0.5 × 105 cells/well/50 μl) were added and the culture was incubated for 24 h. For fixed APCs, SJL/J splenocytes (10 × 105 cells/well/100 μl) were pulsed with graded amounts of antigen, fixed with 1% paraformaldehyde, and incubated with TCC-PLP1-1B10 cells (0.5 × 105 cells/well/100 μl) for 24 h. Detection and quantification of cytokines were then assessed by ELISA from 100 μl of culture supernatant.
Results
Soluble Ig-PLP1 Reduces Paralytic Severity and Suppresses Clinical Relapses in Mice with Ongoing EAE.
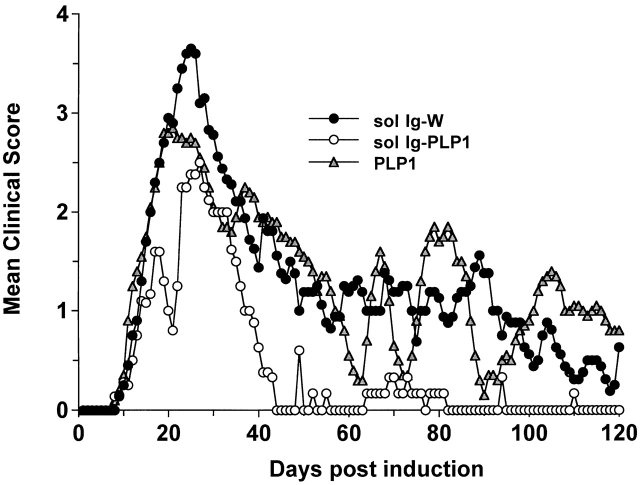
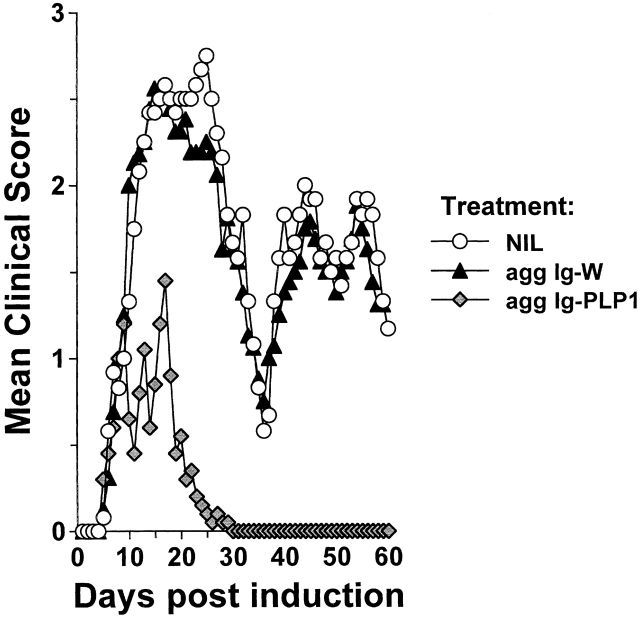
Prevention and treatment of active autoimmune disease have been achieved by injection of adjuvant-free autoantigens or peptides 24 25 26 45 46 47 48 49. However, repetitive injections of the autoantigens are required, and the disease rebounds when the supply of antigen is discontinued 48. One approach that may overcome these setbacks and modulate active disease is the delivery of the self-peptide on Igs. Igs have long half-lives and grant the peptides access to newly synthesized MHC molecules 31 50, which could lead to efficient peptide loading onto MHC molecules 50 over an extended period of time. To test the Ig delivery system for treatment of active autoimmunity, SJL/J mice were induced for EAE with free PLP1 peptide, and when the clinical signs of disease became apparent the animals were given three injections of sol Ig-PLP1 in saline at 4-d intervals and assessed for reduction in disease severity. Control mice were given sol Ig-W, the parental Ig without any PLP1 peptide. The results illustrated in Fig. 1 show that mice treated with the sol Ig-W had an initial severe phase of paralysis with a mean maximal score of 3.7 ± 0.5 and displayed relapses throughout the 120-d period of examination. The mice treated with sol Ig-PLP1, however, had a reduced severity of paralysis at the initial phase of disease with a mean maximal score of 2.5 ± 0.3 (P < 0.005) and fully recovered by day 42. Mice treated with 10-fold excess of free PLP1 peptide had a slight reduction in the severity of paralysis at the initial phase of disease (mean maximal clinical score 3.0 ± 0.2), but never recovered and underwent relapses throughout the entire 120-d observation period (Fig. 1).
Figure 1.
Inhibition of paralytic relapses by treatment with sol Ig-PLP1. Groups of 6–8-wk-old SJL/J mice were induced for EAE with 100 μg PLP1 peptide, and then treated intraperitoneally with 500 μg sol Ig-PLP1, 500 μg sol Ig-W, or 100 μg free PLP1 peptide in PBS on days 9, 13, and 17 after disease induction. Each point represents the mean clinical score of eight mice. These results are representative of two independent experiments. On the basis of 150-kD molecular mass for Ig-PLP1 and 1.5-kD molecular mass for free PLP1, we estimated that the 300 μg free PLP1 given during the three injections correspond to ∼200 nmol of PLP1 peptide, and the 1,500 μg sol Ig-PLP1 encompasses ∼20 nmol PLP1 peptide. Therefore, when free PLP1 is used for treatment the mice were given ∼10-fold more peptide than for treatment with sol Ig-PLP1.
Aggregated Ig-PLP1 Displays Higher Efficacy Than Soluble Ig-PLP1 in Reversing Active EAE.
Binding of antigen–antibody complexes to FcRs on target cells induces the production of cytokines 34 51 52. IL-10, produced by macrophages upon exposure to antigen–antibody complexes, exerts antagonist effects on IL-12 production and reverses proinflammatory responses 34 52. Similar to complexation of antibodies with antigen, aggregation confers to Igs the effector functions associated with the Fc fragment 35 36. These include the binding of complement and cross-linking of FcRs. We then reasoned that aggregation of Ig-PLP1 should be able to cross-link FcRs on APCs and drive both cytokine production and efficient loading of PLP1 peptide onto MHC molecules. If this hypothesis proved accurate and IL-10 was among the cytokines produced by APCs, agg Ig-PLP1 should be much more efficient than sol Ig-PLP1 for modulation of active EAE. To investigate this issue, mice were induced for EAE with free PLP1 peptide and when signs of clinical EAE became apparent, they were given three injections of 300 μg agg Ig-PLP1 at 4-d intervals and assessed for signs of paralysis. Control mice were treated with agg Ig-W instead of agg Ig-PLP1. As can be seen in Fig. 2 a, the initial phase of paralytic disease severity was reduced from a mean maximum score of 3.3 ± 0.3 in agg Ig-W–treated animals to 1.1 ± 0.5 (P < 0.001) in the agg Ig-PLP1 recipient mice. In addition, the animals fully recovered within 9 d of completion of the treatment and never relapsed throughout the entire 120-d observation period, whereas agg Ig-W–treated mice never recovered and showed relapses throughout the entire period of clinical assessment. The effectiveness of agg Ig-PLP1 is also apparent when the paralytic clinical signs of agg Ig-PLP1–treated animals were compared with those of animals injected with sol Ig-PLP1 (P < 0.001) (Fig. 2 b). Indeed, the mean maximum clinical score was much lower and the recovery was faster. Histologic examination of the cerebellum at the peak of disease indicated a lower number of foci and a reduced number of infiltrating mononuclear cells per foci in the mice treated with agg Ig-PLP1 versus those given agg Ig-W (Fig. 3). Moreover, when serial histologic cross-sections were prepared from both the brain and spinal cord and the mean foci per cross section were estimated, there was a two- to threefold reduction in the number of foci in agg Ig-PLP1–treated mice versus mice recipient of agg Ig-W (Table ). Furthermore, the foci in agg Ig-PLP1–treated mice had fewer infiltrating mononuclear cells than those of agg Ig-W–treated mice (agg Ig-W, 73 ± 39; agg Ig-PLP1: 32 ± 14, P < 0.005).
Figure 2.
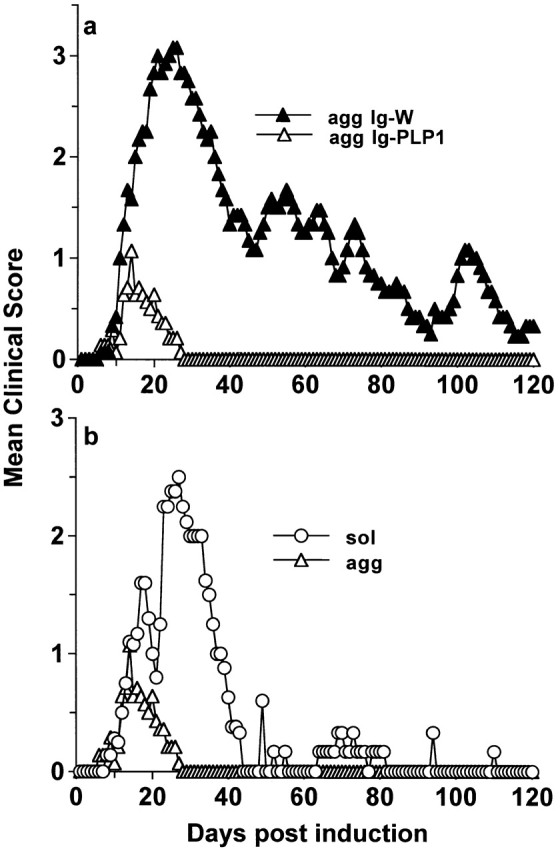
Effective amelioration of EAE by agg Ig-PLP1. (a) Groups of mice (eight per group) were induced for EAE with 100 μg PLP1 and then treated with 300 μg of agg Ig-PLP1 or agg Ig-W in PBS on days 9, 13, and 17 after disease induction. (b) Direct comparison of the disease course of PLP1 peptide induced EAE after treatment with sol Ig-PLP1 (from Fig. 1) versus agg Ig-PLP1 (from 2 a). Each point represents the mean clinical score of eight mice. These results are representative of three independent experiments. The 900 μg agg Ig-PLP1 given to mice contains ∼12 nmol PLP1. This amount is ∼17-fold lower than the 200 nmol given as free PLP1, yet disease modulation was much more effective.
Figure 3.
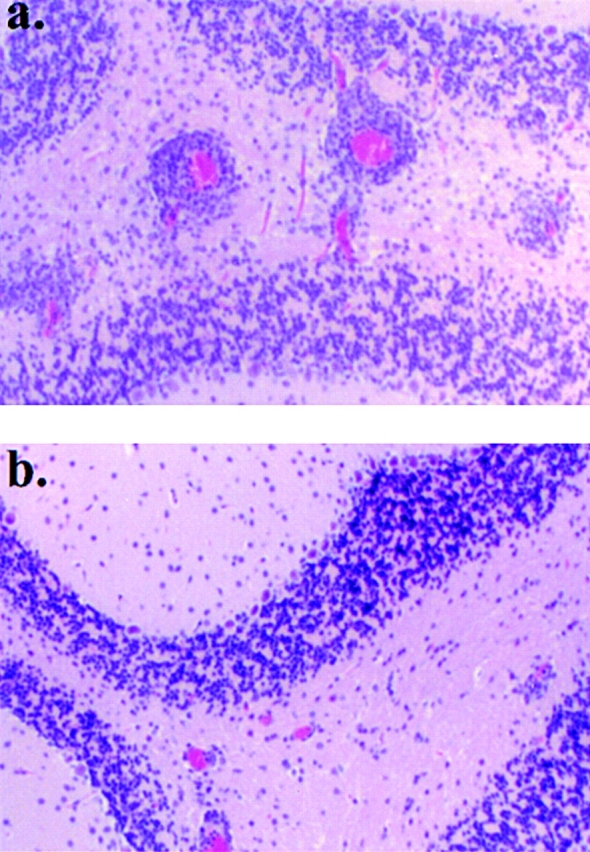
Agg Ig-PLP1 significantly reduces the number of inflammatory foci within the CNS. Serial cross-sections were prepared at the peak of disease (day 28) from the cerebellum of mice treated with agg Ig-W (a) and agg Ig-PLP1 (b) and described in the legend to Fig. 2. The brains were fixed with formalin, embedded in paraffin, and stained with hematoxylin and eosin as described in Materials and Methods. Magnifications: ×40.
Table 1.
Treatment with Agg Ig-PLP1 Ablates Clinical and Histologic EAE
| Histologic EAE: foci/cross-section | ||||||
|---|---|---|---|---|---|---|
| Treatment | Clinical EAE: mean maximum severity | Cerebrum | Lumbar spinal cord | |||
| Agg Ig-W | 3.3 ± 0.3 | 11.7 ± 2.1 | 18.5 ± 3.3 | |||
| (P < 0.001) | (P < 0.001) | (P < 0.001) | ||||
| Agg Ig-PLP1 | 1.1 ± 0.5 | 6.5 ± 0.5 | 6.9 ± 1.1 | |||
6–8-wk-old mice were induced for EAE with PLP1 as described in Materials and Methods, and then treated with 300 μg agg Ig-PLP1 or agg Ig-W on days 9, 13, and 17 after disease induction and scored daily for clinical disease. The mean maximum severity was determined by averaging the maximal clinical score obtained from each mouse within a group. To determine histological disease, brains and spinal cords were removed from mice on day 28 after disease induction (peak of disease), fixed in formalin, paraffin embedded, serially cross-sectioned at 6 μm, and then stained with hemotoxylin and eosin. Inflammatory foci represent a minimum of 20 mononuclear cells per perivascular cluster.
Aggregated Ig-PLP1 Induces the Production of IL-10 by APCs and Downregulates IFN-γ Secretion by Specific T Cells In Vitro.
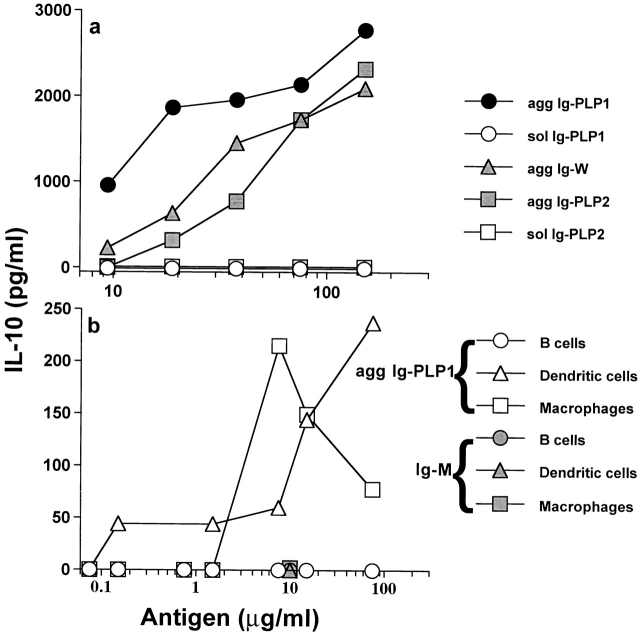
To delineate the mechanism underlying the effective modulation of EAE by agg Ig-PLP1, we sought to investigate whether agg Ig-PLP1 stimulates the production of IL-10 by APCs, and whether such IL-10 would display an inhibitory function on T cells engaged in the recognition of the PLP1 peptide presented by IL-10–producing APCs. To this end, naive splenocytes were incubated with sol or agg Ig chimeras, and the supernatants were used for IL-10 detection. As indicated in Fig. 4 a, agg Ig-PLP1, Ig-PLP2, and Ig-W chimeras stimulated the production of IL-10 by splenic cells in a dose-dependent manner. The soluble forms of the chimeras did not induce detectable levels of IL-10. Furthermore, we decided to investigate whether cells known to function as professional APCs are able to produce IL-10 upon incubation with agg Ig chimeras. To address this issue, thioglycollate-induced peritoneal macrophages and splenic B and DCs were isolated and tested for IL-10 production upon incubation with agg Ig-PLP1. Fig. 4 b indicates that macrophages and DCs, but not B cells, produce IL-10 upon incubation with agg Ig-PLP1. Mouse IgM was unable to stimulate IL-10 production by any of the APCs tested. These results indicate that agg Ig-PLP1 cross-links FcγR and induces the production of IL-10 by APCs. Furthermore, preincubation of APCs with soluble mouse IgG inhibited agg Ig-PLP1–induced IL-10 production (data not shown). These results indicate that the IL-10 produced by the APCs was due to cross-linking of FcγR rather than to contamination with endotoxin.
Figure 4.
Agg Ig-PLP chimeras induce the production of IL-10 by APCs. (a) Irradiated (3,000 rads) SJL/J splenocytes (5 × 105 cells/well) were incubated with graded amounts of sol Ig-PLP1, agg Ig-PLP1, agg Ig-W, sol Ig-PLP2, or agg Ig-PLP2 for 24 h, and the supernatant was used to quantitate IL-10 production by ELISA. (b) Irradiated (3,000 rads) B cells (2 × 105 cells/well), macrophages (0.2 × 105 cells/well), and DCs (0.2 × 105 cells/well) were incubated with graded amounts of agg Ig-PLP1 (open symbols) or mouse IgM (filled symbols) for 24 h, and cell culture supernatant was used to measure IL-10 production. Each point represents the mean of triplicate wells. These data are representative of four independent experiments.
To investigate the effect that APC-derived IL-10 might have on T cells specifically engaged with the APCs through antigen presentation, a PLP1-specific Th0 clone able to produce both type I and type II cytokines upon peptide stimulation was used. This clone, designated TCC-PLP1-1B10, proliferates upon incubation with paraformaldehyde-fixed splenic APCs that were previously pulsed with free PLP1 peptide or agg Ig-PLP1 (Fig. 5 a). TCC-PLP1-1B10 did not show significant proliferation when the APCs were pulsed with the negative control PLP2 or agg Ig-PLP2. When tested for cytokine production upon incubation with nonfixed splenic APCs and free PLP1 peptide, TCC-PLP1-1B10 produced significant amounts of IL-2, IL-4, and IFN-γ (Fig. 5b–d). All three cytokines were also detected when agg Ig-PLP1 was used for stimulation (Fig. 5b–d). However, IL-10 was detectable at significant levels when the stimulator was agg Ig-PLP1 but not free PLP1 (Fig. 5 e). As agg Ig-PLP1 induces IL-10 production by macrophages and DCs, it is likely that the IL-10 seen in the T cell cytokine assessment assay was the product of splenic APCs rather than TCC-PLP1-1B10. In fact, this statement is confirmed by the observation that IL-10 was undetectable when APCs, pulsed with agg Ig-PLP1, were washed and fixed with paraformaldehyde before incubation with TCC-PLP1-1B10 (Fig. 6). The other striking observation from the T cell cytokine assessment assay was that the production of IFN-γ seemed to be decreased as IL-10 production by APCs increased (Fig. 5c and Fig. e). To investigate this issue further, an extended range of Ig-PLP1 concentrations was used for stimulation of bulk and purified APCs, and IL-10 and IFN-γ production was assessed simultaneously from the same tissue culture well. The results presented in Fig. 7 clearly indicate that the IL-10 secreted by the APCs antagonizes the production of IFN-γ by the T cells. Indeed, when the stimulation assay was performed using splenocytes, purified DCs, or enriched peritoneal macrophages as APCs (all of which produce IL-10 upon incubation with agg Ig-PLP1) (Fig. 4), IFN-γ production by the T cells decreased dramatically and became undetectable as the production of IL-10 by APCs increased (Fig. 7, a–c). However, when B cells were used as APCs, which do not produce IL-10 upon incubation with agg Ig-PLP1 (Fig. 4 b), the secretion of IFN-γ by T cells was not affected (Fig. 7 d). Overall, these results indicate that agg Ig-PLP1 triggers IL-10 production by presenting APCs (DC and macrophage), and that IL-10 antagonizes the production of IFN-γ by the T cells.
Figure 5.
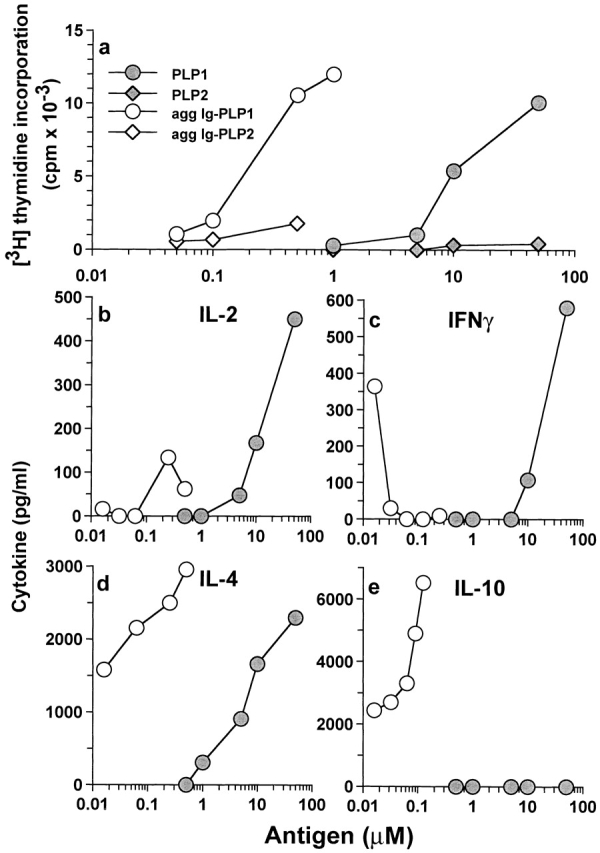
Specificity and cytokine response of a Th0 T cell clone to agg Ig-PLP1. (a) The specificity of the TCC-PLP1-1B10 T cell clone. SJL/J splenocytes (10.0 × 105 cells/well) were pulsed for 4 h with graded amounts of free PLP1, free PLP2, agg Ig-PLP1, or agg Ig-PLP2, and then fixed with paraformaldehyde as described in Materials and Methods. Subsequently, TCC-PLP1-1B10 cells (0.5 × 105 cells/well) were added to the wells, and proliferation was assessed by [3H]thymidine incorporation. (b–e) The pattern of cytokine production by TCC-PLP1-1B10 cells upon stimulation with PLP1 versus agg Ig-PLP1. Irradiated (3,000 rads) SJL/J splenocytes (5 × 105 cells/well) were incubated with graded amounts of PLP1 peptide (•) or agg Ig-PLP1 (○) for 1 h, after which TCC-PLP1-1B10 cells (0.5 × 105 cells/well) were added and the incubation continued for an additional 24 h. Cytokine production was measured by ELISA from 100 μl of culture supernatant. Each point represents the mean of triplicate wells.
Figure 6.
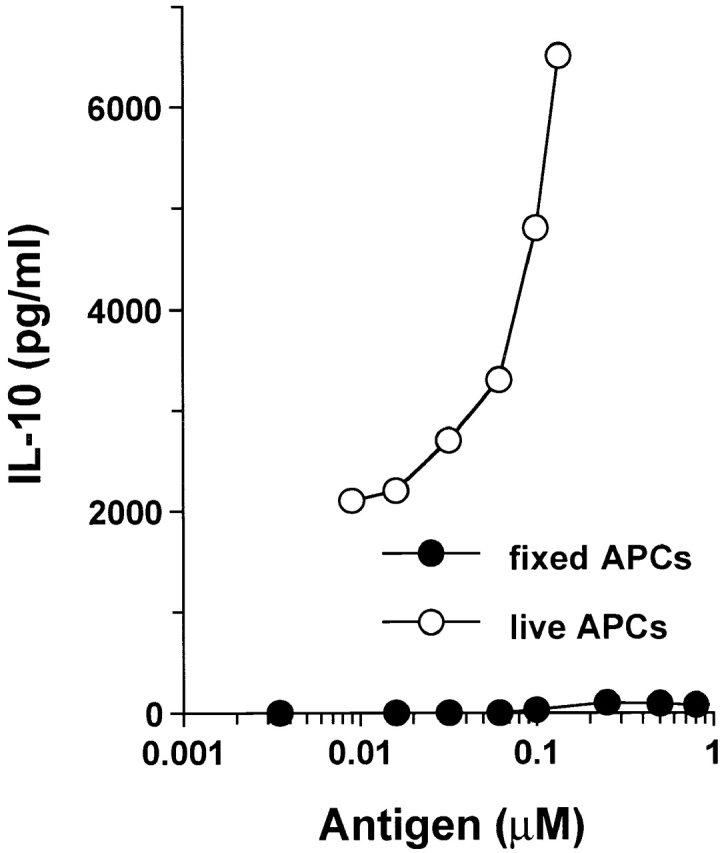
Agg Ig-PLP1 does not induce IL-10 production by the TCC-PLP1-1B10 T cell clone. Fixed and live APCs were used to identify the source of IL-10 in T cell activation by agg Ig-PLP1. In the fixed APC assay, SJL/J splenocytes (10 × 105 cells/well) were pulsed with graded amounts of agg Ig-PLP1 for 4 h, washed extensively, and fixed with paraformaldehyde. In the live APC assay, irradiated (3,000 rads) SJL/J splenocytes (5 × 105 cells/well) were mixed with graded amounts of agg Ig-PLP1 and incubated for 1 h. Subsequently, TCC-PLP1-1B10 cells (0.5 × 105 cells/well) were added to both assays and the incubation continued for an additional 24 h. IL-10 production was measured by ELISA from 100 μl of culture supernatant. Each point represents the mean of triplicate wells.
Figure 7.
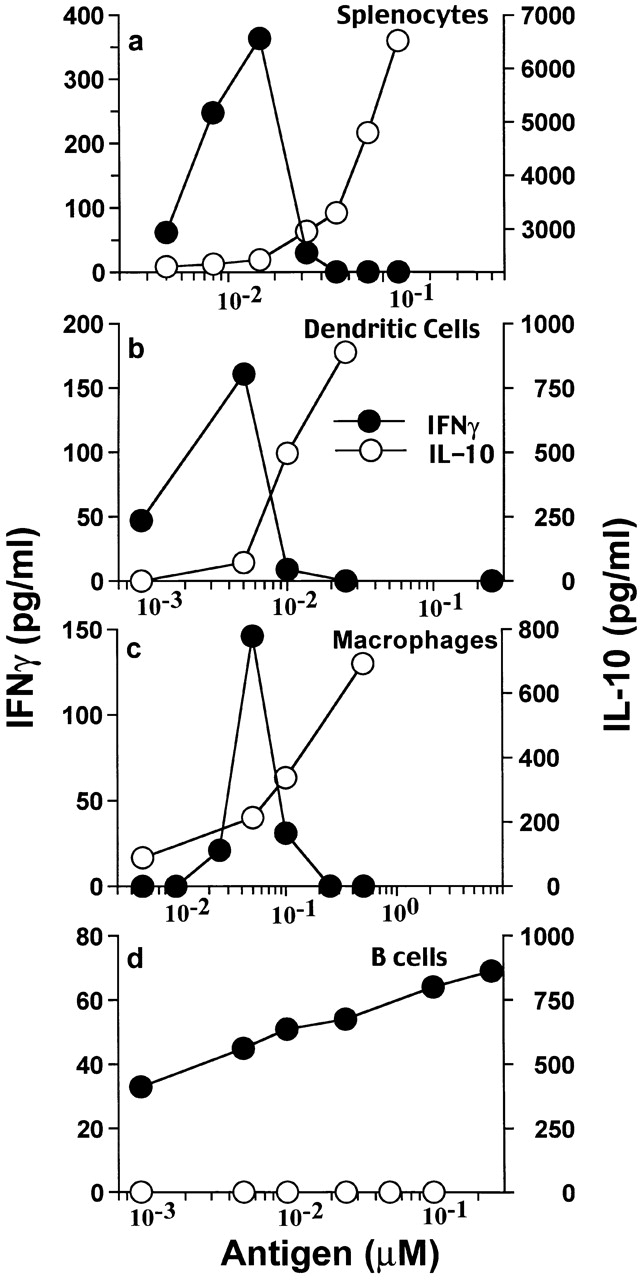
APCs' IL-10 induced by agg Ig-PLP1 antagonizes IFN-γ production by T cells. Irradiated (3,000 rads) SJL/J splenocytes (5 × 105 cells/well), DCs (0.2 × 105 cells/well), macrophages (0.2 × 105 cells/well), or B cells (2 × 105 cells/well) were incubated with graded amounts of agg Ig-PLP1, and after 1 h TCC-PLP1-1B10 cells (0.5 × 105 cells/well) were added and the incubation continued for an additional 24 h. IFN-γ and IL-10 production in the same culture well was measured by ELISA. Each point represents the mean of triplicate wells.
Synergy between Endogenous IL-10 and Peripheral Tolerance for In Vivo Modulation of Aggressive T Cells.
Systemic antigen given to animals without adjuvant usually drives tolerance operating through antigen presentation by peripheral APCs expressing minimal or no costimulatory molecules 3 22 23. Incubation of purified macrophages or DCs with sol or agg Ig-PLP1, which allows for efficient loading of peptide onto MHC class II molecules, does not lead to upregulation of B7-1, B7-2, or CD40 (data not shown). Furthermore, as agg Ig-PLP1 causes the production of IL-10 by APCs (Fig. 4), it is likely that IL-10, as has previously been shown 53 54, inhibits upregulation of costimulatory molecules on APCs.
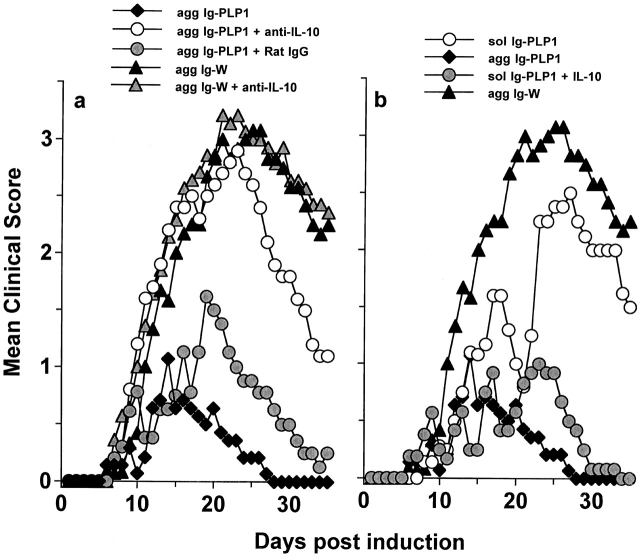
As IL-10 has been defined to antagonize Th1 cytokines 55 and possibly interfere with inflammatory functions, we postulated that the effectiveness of agg Ig-PLP1 in T cell modulation and reversal of disease lies on inadequate peptide presentation by APCs expressing minimal costimulatory molecules and the inhibitory function of IL-10 produced by such APCs. To test this hypothesis, mice were induced for EAE with PLP1 peptide, and when the signs of paralysis became apparent, the mice were given agg Ig-PLP1 together with anti–IL-10 antibody and assessed for reduction in disease severity. The results presented in Fig. 8 a indicate that the severity of paralysis was restored when in vivo IL-10 was neutralized by the anti–IL-10 antibody. In fact, mice treated with agg Ig-PLP1 alone had a mean maximal clinical score of 1.1 ± 0.5, whereas the mice injected with both agg Ig-PLP1 and anti–IL-10 antibody had a score of 3.0 ± 0.3, which is comparable to the 3.3 ± 0.3 (P > 0.23) score seen in mice treated with agg Ig-W. Furthermore, control mice given agg Ig-PLP1 together with rat IgG, instead of anti–IL-10 antibody, did not restore disease severity and had a mean maximal score of 1.6 ± 0.2. Injection of anti–IL-10 antibody together with agg Ig-W neither reduced nor exacerbated the severity of disease. These results indicate that agg Ig-PLP1–induced IL-10 plays a significant role in controlling disease severity, and that for the effects of IL-10 to occur, a specific interaction between APCs and the target T cells is required. In support of this statement is the observation that treatment with sol Ig-PLP1 plus exogenous IL-10 reduces the severity of paralysis to the same extent as agg Ig-PLP1 (Fig. 8 b). Sol Ig-PLP1, which does not induce detectable levels of IL-10, ameliorates the disease slightly with a mean maximal score of 2.5 ± 0.3, whereas sol Ig-PLP1 together with exogenous IL-10 further reduces the disease to a mean maximal clinical score of 1.1 ± 0.3, which is comparable to the 1.1 ± 0.5 score obtained with mice treated with agg Ig-PLP1. Furthermore, for endogenous IL-10 to modulate the disease, a physical bridging of the APCs to the T cells seems to be required. This conclusion is drawn from the observation that treatment of diseased mice with a mixture of agg Ig-W and free PLP1 peptide, instead of agg Ig-PLP1, did not reduce the severity of disease (Fig. 9). Overall, effective T cell downregulation requires physical interaction between IL-10–producing APCs and the target pathogenic T cell. The likely explanation for this requirement is that IL-10 as a paracrine cytokine needs to be in close proximity to T cells in order to achieve antagonism.
Figure 8.
Neutralization of Ig-PLP1–induced endogenous IL-10 restores severity of paralysis. (a) SJL/J mice (eight per group) were induced for EAE with 100 μg PLP1, and on days 9, 13, and 17 were given intraperitoneally in PBS 300 μg agg Ig-PLP1 (agg Ig-PLP1); 300 μg agg Ig-PLP1 plus 500 μg rat anti–mouse IL-10 antibody, 2A5 (agg Ig-PLP1 + anti-IL-10); 300 μg agg Ig-PLP1 plus 500 μg rat IgG (agg Ig-PLP1 + rat IgG); 300 μg agg Ig-W (agg Ig-W); or 300 μg agg Ig-W plus 500 μg rat anti–mouse IL-10 antibody, 2A5 (agg Ig-W + anti-IL-10). All the injections were done intraperitoneally in PBS. (b) Exogenous IL-10 synergizes with sol Ig-PLP1 to reduce the severity of EAE. Groups of mice (eight per group) were induced for EAE with 100 μg PLP1 and on days 9, 13, and 17 were given intraperitoneally in PBS 300 μg sol Ig-PLP1 (sol Ig-PLP1); 300 μg agg Ig-PLP1 (agg Ig-PLP1); 300 μg sol Ig-PLP1 plus 400 U recombinant IL-10 (sol Ig-PLP1 + IL-10); or 300 μg agg Ig-W (agg Ig-W).
Figure 9.
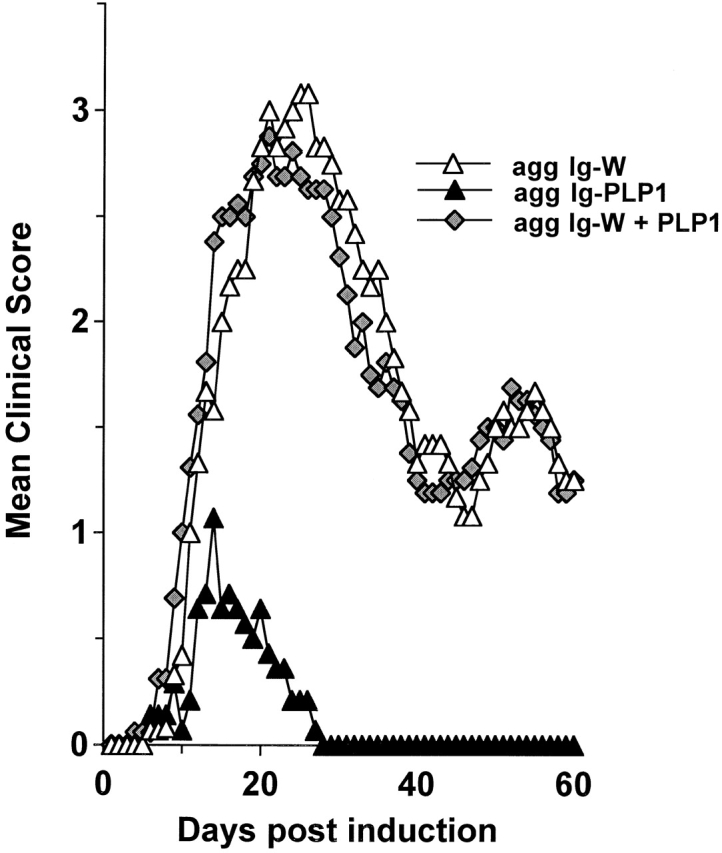
Requirement for a physical linkage of the Ig vehicle to PLP1 peptide for amelioration of EAE. Groups of mice (eight per group) were induced for EAE with 100 μg PLP1, and then treated with 300 μg of agg Ig-PLP1 (agg Ig-PLP1), 300 μg agg Ig-W (agg Ig-W), or 300 μg agg Ig-W plus 100 μg PLP1 (agg Ig-W + PLP1) in PBS on days 9, 13, and 17 after disease induction. The onset of disease was at day 7 in these experimental groups.
Treatment with Aggregated Ig-PLP1 Decreases the Clinical Severity of Active EAE Induced by Multiple Epitopes.
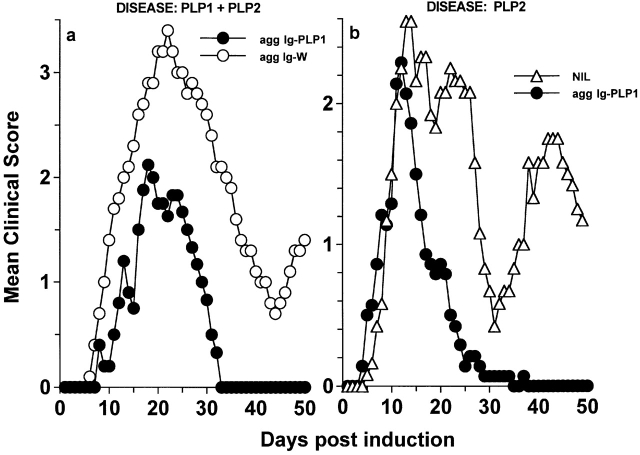
IL-10 produced by APCs as a result of agg Ig-PLP1–mediated FcR cross-linking may antagonize specific T cells engaged to the PLP1–MHC ligand on the APCs as well as neighboring T cells with unrelated specificity. This phenomenon, known as bystander suppression, has proven effective in IL-4 56 and IL-10 57 settings. One way to find out if bystander suppression could be ascribed to the IL-10 in our experimental system is to induce EAE with a mixture of epitopes and test if treatment with agg Ig-PLP1 could modulate unrelated autoreactive T cells and ameliorate the disease. This experiment was carried out, and the results presented in Fig. 10 a show that mice with ongoing EAE induced by a mixture of PLP1 and PLP2 peptides manifested reduced severity of paralysis and fully recovered by day 33 after disease induction upon treatment with agg Ig-PLP1, whereas animals treated with agg Ig-W had severe paralysis and did not recover from the disease during the 50-d period of clinical assessment. Therefore, endogenous IL-10 may have displayed downregulatory effects on PLP2-specific T cells. Induction of disease with PLP2 peptide should expose whole PLP and drive spreading and activation of PLP1-specific T cells 17 58. In this case, injection of agg Ig-PLP1 should bridge IL-10–producing APCs to PLP1-specific T cells and promote bystander suppression of these cells as well as neighboring PLP2-specific T cells. To address this issue, mice were induced for EAE with PLP2 peptide, and when signs of paralysis became apparent, they were treated with agg Ig-PLP1. Fig. 10 b shows that although the initial phase of paralysis in these mice is only slightly milder than untreated mice, the animals quickly recovered by day 26, and unlike the untreated mice, did not relapse for the remaining period of clinical assessment. These results strengthen the notion of bystander suppression and suggest that epitope spreading offers an opportunity to modulate disease at a later stage of paralysis.
Figure 10.
Aggregated Ig-PLP1 modulates disease involving intramolecular epitopes. (a) Groups of SJL/J mice (eight per group) were induced for EAE with a mixture of 100 μg PLP1 and 100 μg PLP2 and on days 9, 13, and 17 treated with 300 μg agg Ig-PLP1 or agg Ig-W per injection. All treatments were intraperitoneally in PBS. The onset of disease was at day 7 in these experimental groups. Each point represents the mean clinical score of eight mice. (b) Groups of SJL/J mice (eight per group) were induced for EAE with 100 μg PLP2 and on days 9, 13, and 17 treated intraperitoneally with 300 μg agg Ig-PLP1 per injection. A group of untreated mice (NIL) was included for comparison purposes.
To further explore the broadness of this approach in T cell downregulation, we tested agg Ig-PLP1 for modulation of disease induced with CNS homogenate, which incorporates a full range of myelin autoantigens. The results presented in Fig. 11 clearly indicate that mice injected with agg Ig-PLP1 had mild signs of paralysis in the initial phase of paralysis and fully recovered by day 24 after disease induction without any relapses for the 60-d period of clinical assessment. Control mice treated with agg Ig-W instead of agg Ig-PLP1 had a disease pattern similar to that of untreated animals (Fig. 11). These results indicate that the downregulatory function of agg Ig-PLP1 extends to both intra- and intermolecular epitopes and suppresses diverse T cell specificities.
Figure 11.
Agg Ig-PLP1 modulates disease involving intra- and intermolecular epitopes. Groups of SJL/J mice (nine per group) were induced for EAE with 6 mg of CNS homogenate and on days 9, 13, and 17 treated intraperitoneally with 300 μg agg Ig-PLP1 or agg Ig-W per injection. A group of untreated mice (NIL) was included for comparison purposes.
Exposure to IL-10 seems to be the likely mechanism underlying downregulation and suppression of pathogenic myelin-specific T cells. The source of IL-10, as demonstrated in Fig. 4 and Fig. 6, is APCs such as DCs and macrophages. However, the broadened effectiveness and the endurance of T cell modulation in this setting raise the question of whether the bystander suppression was due to antagonism of the pathogenic T cells by APCs' IL-10, or to downregulation by regulatory T cells generated under the effect of such IL-10 59. The rationale for this statement derives from previous studies suggesting that IL-10 enables naive T cells to develop into regulatory cells 59 that could produce IL-10, IL-5, or TGF-β and inhibit the function of pathogenic T cells, thereby sustaining suppression 46 60 61 62 63. To address this issue, the LN T cells from mice that were recovering from CNS-induced paralysis subsequent to treatment with agg Ig-PLP1 were stimulated with antigen and tested for proliferation and production of cytokines markers of regulatory T cells. The results presented in Fig. 12 show that 2 d after the final injection of agg Ig chimeras, proliferation to myelin peptides was significant in the mice treated with the control Ig-W but at background levels for those recipient of agg Ig-PLP1. Similarly, while the mice injected with agg Ig-W had significant amounts of IL-2 and IFN-γ, those treated with agg Ig-PLP1 had neither Th1- nor Th2-type cytokines and did not produce IL-10, IL-5, or TGF-β. Similar results were obtained when the mice were tested at day 9 after completion of the treatment regimen (data not shown). Furthermore, splenic T cells and cells harvested from the peritoneum showed a similar pattern of responses (data not shown). Overall, these results suggest that the typical proliferative and cytokine responses trademark of regulatory T cells are undetectable in this particular setting of systemic treatment of active autoimmunity.
Figure 12.
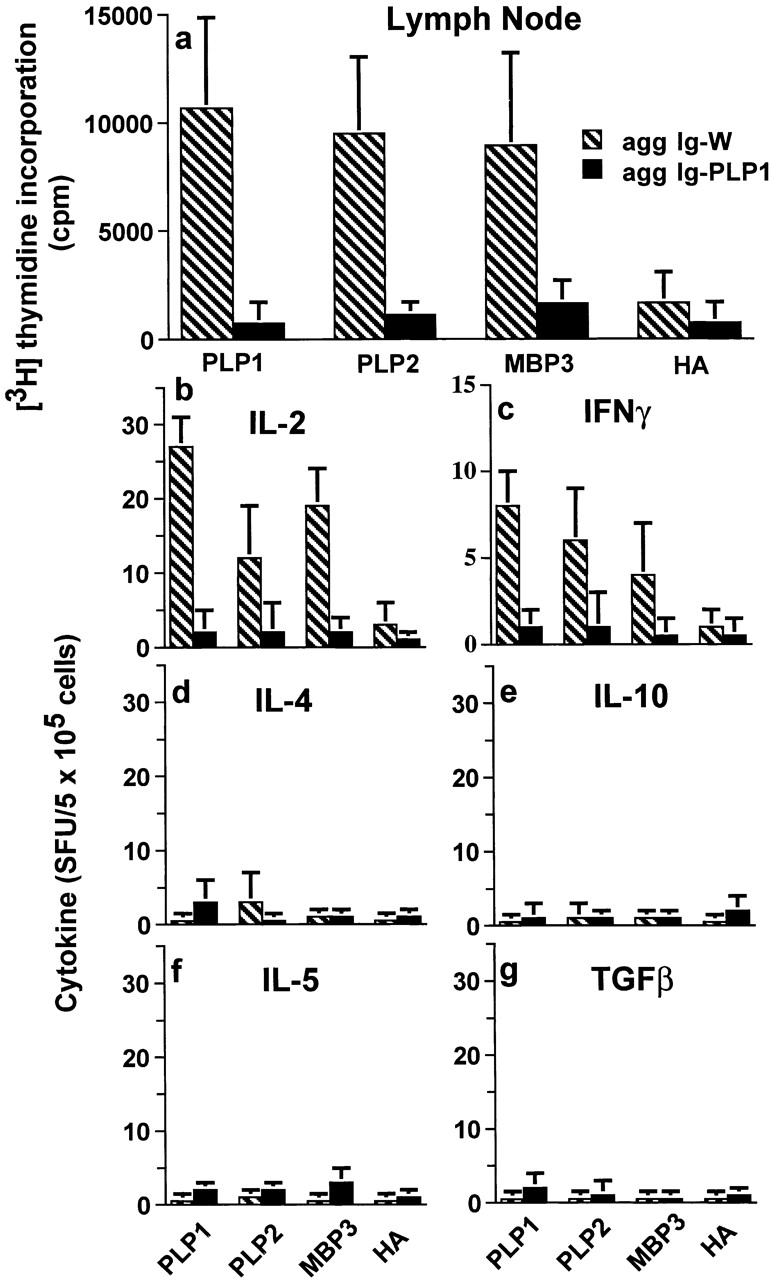
Treatment of CNS homogenate–induced EAE with aggregated Ig-PLP1 suppresses proliferative and cytokine responses to diverse myelin epitopes. Mice (six per group) were induced for EAE with CNS homogenate and then treated with agg Ig-W (hatched bars) or agg Ig-PLP1 (black bars) on days 9, 13, and 17 as described in the legend to Fig. 11. 2 d after completion of the treatment regimen, the LNs (axillary, lateral axillary, and popliteal) were harvested, and the cells (4 × 105 cells/100 μl/well) were stimulated with 100 μl/well of antigen. (a) Cell proliferation was assessed 3 d later using [3H]thymidine incorporation assay. (b–g) Cytokine responses were analyzed after 24 h of incubation with antigen by enzyme-linked immunospot (ELISPOT) assay using 5 × 105 cells per well. The antigens were used at the defined optimal concentrations of 15 μg/ml for PLP1, PLP2, and HA and 30 μg/ml for MBP-3. Control wells of media without addition of antigen were included and used as background. Each bar represents the mean ± SD of six individually tested mice. Similar results were observed when the in vitro stimulators were used with two times higher concentrations. Also, the pattern of proliferation and cytokine production was similar when the splenic and peritoneal cells from these animals were analyzed.
Discussion
Previous studies have demonstrated that delivery of peptides on Igs increases presentation to T cells in vitro by 100–1,000-fold relative to free peptide 30 31 and induces stronger T cell responses in vivo 31 64. Internalization of Ig-peptide chimeras into APCs via FcRs and access of the attached peptide to newly synthesized MHC class II molecules are most likely responsible for such effective presentation 31 50. The efficacy of peptide delivery by Igs seems to extend to peripheral APCs expressing minimal or no costimulatory molecules, as injection of the Ig-PLP1 chimera without adjuvant into diseased mice modulates PLP1-specific pathogenic T cells and ameliorates EAE (Fig. 1). This statement is supported by the finding that 200 nmol of PLP1 in the form of free peptide reduced the severity of disease only slightly and the animals never recovered, but 20 nmol of peptide in the form of sol Ig-PLP1 reduced the severity of the initial phase of disease and most of the animals fully recovered by day 42 (Fig. 1). The effectiveness of Ig-PLP1 in disease reversal was more dramatic when the chimera was given to mice in an aggregated form (Fig. 2 and Fig. 3, and Table ). The explanation we wish to put forth for this observation is that agg Ig-PLP1 displays an antagonistic function against T cells at two levels. On the one hand, as Ig-PLP1 was injected without any adjuvant it is likely that presentation was carried out mostly by peripheral APCs not expressing optimal costimulatory molecules, thereby leading to inactivation of the T cells. In fact, incubation of purified DCs and macrophages with sol or agg Ig-PLP1 did not induce upregulation of B7 or CD40 costimulatory molecules. On the other hand, the presenting APCs produce IL-10, which further antagonizes the T cells engaged to them through PLP1 peptide. The evidence for this dual-modal mechanism for T cell turn off derives from two essential observations. First, soluble adjuvant-free Ig-PLP1, which does not induce detectable levels of IL-10 (Fig. 4 a), modulates the disease with significantly fewer copies of peptide than free PLP1 peptide (Fig. 1). Second, agg Ig-PLP1, which induces IL-10 production by macrophages and DCs (Fig. 4 b), enhances T cell antagonism, as the mice display a significant reduction in the frequency and size of inflammatory foci both in the brain and spinal cord (Fig. 3, and Table ), manifest very mild clinical signs of EAE, and fully recover from paralysis by day 25 after disease induction (Fig. 2). The contribution of IL-10 to T cell modulation and disease amelioration is supported by both in vitro and in vivo data. Indeed, the TCC-PLP1-1B10 T cell clone, which proliferates and produces both type I and II cytokines upon incubation with free PLP1 (Fig. 5), significantly reduced IFN-γ production when agg Ig-PLP1 was used for stimulation (Fig. 5). This is well illustrated in Fig. 7, which shows that the production of IFN-γ by T cells is inversely proportional to the IL-10 production by DCs and macrophages within the same cell culture. In vivo, neutralization of APCs' IL-10 by anti–IL-10 antibodies during treatment with agg Ig-PLP1 restores the severity of disease to scores comparable with Ig-W treatment (Fig. 8 a). Furthermore, the combination of recombinant IL-10 and sol Ig-PLP1 reduces disease severity to the same extent as agg Ig-PLP1 (Fig. 8 b). All together, these results suggest that effective control of EAE requires synergy of antagonistic functions against T cells, and points out the need for a multimodal strategy to contain autoimmunity. In fact, systemic injection of IL-10 has proven inefficient for modulation of autoimmunity 65, and only overexpression of IL-10 by means of transgenic manipulation 66, targeted delivery of the cytokine by means of engineered T cells 27, or viral gene transfer 29 has proven effective for control of T cell–mediated autoimmunity. The agg Ig-peptide approach brings together a peripheral tolerance-like mechanism and the antagonism activity of IL-10 to strengthen the modulatory functions against aggressive T cells. Furthermore, as IL-10 exerts its downregulatory function in a paracrine fashion, it may counteract neighboring Th1 cells regardless of antigen specificity. This behavior, known as bystander suppression 56 57, seems to contribute to disease modulation by agg Ig-PLP1. Indeed, mice induced for EAE with a mixture of PLP1 and PLP2 peptides recovered from paralysis when they were treated with agg Ig-PLP1 (Fig. 10). The likely explanation for these results is that IL-10 produced by the APCs exerts an antagonist effect on neighboring T cells specific for PLP2 peptide, thereby modulating disease involving two epitopes. Moreover, when the disease was initiated with PLP2 peptide, the treatment with agg Ig-PLP1 allowed paralysis to peak to a significant clinical score but the recovery was expeditious and relapses did not occur. This pattern could suggest that IL-10–mediated bystander suppression had to await exposure of PLP and generation of PLP1-specific T cells to focus the presenting APCs and their IL-10 on the pathogenic T cells. This has twofold importance for the usefulness of this approach for treatment of autoimmunity. First, using this strategy for intervention at the initial phase of disease will likely modulate paralysis, as the target T cells should become available to focus the APCs and their IL-10 on neighboring pathogenic T cells regardless of their specificity. Second, as epitope spreading seems to follow a sequential order and sustain specific relapses 58, one could devise a regimen to target T cell modulation at later phases in the relapses. The data presented in Fig. 11, showing that agg Ig-PLP1 modulates disease induced with CNS homogenate, argue for the broad effectiveness of the approach and support the notion that bystander suppression offers a strategy for intervention at various stages of the disease.
At the mechanistic level, the in vitro data demonstrate that while IL-10 downregulates the Th1 type cytokine IFN-γ, produced by the TCC-PLP1-1B10 Th0 clone, production of the Th2 cytokine IL-4 was not affected (Fig. 5). This suggests that IL-10 antagonism partially affects the specific functions of the T cell rather than driving full inactivation or death of the cells. Whether IL-10 exerts a similar activity on differentiated Th1 cells in vivo remains to be investigated. On a speculative basis, if IL-10 affects IL-12 synthesis by the APCs, a complex regulatory mechanism could be triggered around T cell–APC interactions possibly via CD40–CD40L, resulting in downregulation of Th1 function and suppression of IFN-γ production 67 68 69.
On the other hand, one could speculate that APCs' IL-10 may promote the development of regulatory T cells 46 59 60 61 62 63, which in turn would help in downregulation of the pathogenic T cells. In fact, this would provide a good argument for the broad and sustainable bystander suppression, as regulatory T cells could produce suppressive cytokines such as IL-10 and TGF-β 61 63. However, the results presented in Fig. 12 provided no support for this speculation and neither proliferation nor IL-10, IL-5, or TGF-β were obtained subsequent to treatment with agg Ig-PLP1. Although high concentrations of peptide were used for in vitro stimulation and cells from various tissues have been assayed for production of IL-10, IL-5, and TGF-β, this does not provide a definitive exclusion of regulatory cells. A few decades ago, Jerne 70 postulated that the immune system keeps itself in check by sustaining an equilibrium of interaction among its components. Pathogenic T cells could be kept harmless by a tight control from regulatory T cells. A perturbation of these interactions would lead to oscillations in the levels of each component. At the time of recovery, the regulatory T cells may have returned to minimal levels below the sensitivity of our assays.
Overall, agg Ig-PLP1 promotes efficient peptide loading onto MHC molecules and couples a peripheral tolerance-like mechanism and directed IL-10 bystander suppression to counteract aggressive T cells and modulate complex autoimmunity involving multiple epitopes. One should emphasize that the effectiveness of the approach and its bystander suppression likely lies on the physical link of the Ig vehicle to the peptide, which serves to bridge the APCs to the T cell and focus IL-10 on the neighboring pathogenic lymphocytes. This dual-modal approach, which proves to be efficient in this autoimmune setting where a high precursor frequency probably entertains the severe paralysis 71, may prove feasible for treatment of human multiple sclerosis, which likely involves several myelin autoantigens 19 72.
Acknowledgments
This work was supported by grant RG2967A2/1 from the National Multiple Sclerosis Society, grant RO1-NS37406 from the National Institutes of Health, and grant RO1-101564 from Astral, Inc. (a subsidiary of Alliance Pharmaceutical Corp., San Diego, CA).
Footnotes
Abbreviations used in this paper: agg, aggregated; aa, amino acid; CNS, central nervous system; DC, dendritic cell; EAE, experimental autoimmune encephalomyelitis; HA, hemagglutinin; MBP, myelin basic protein; PLP, proteolipid protein; sol, soluble.
References
- Goldrath A.W., Bevan M.J. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402:255–262. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- Cibotti R., Kanellopoulos J.M., Cabaniols J.P., Halle-Panenko O., Kosmatopoulos K., Sercarz E., Kourilsky P. Tolerance to self-protein involves its immunodominant but does not involve its subdominant determinants. Proc. Natl. Acad. Sci. USA. 1992;89:416–420. doi: 10.1073/pnas.89.1.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlkes B.J., Ramsdell F. T-cell tolerance. Curr. Opin. Immunol. 1993;5:873–879. doi: 10.1016/0952-7915(93)90099-e. [DOI] [PubMed] [Google Scholar]
- Herold K.C., Montag A.G., Buckingham F. Induction of tolerance to autoimmune diabetes with islet antigens. J. Exp. Med. 1992;176:1107–1114. doi: 10.1084/jem.176.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamula M.J. The inability to process a self-peptide allows autoreactive T cells to escape tolerance. J. Exp. Med. 1993;177:567–571. doi: 10.1084/jem.177.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J., Rosenzweig A., Zweiman B., Lisak R.P. Isolation of myelin basic protein-reactive T cell lines from normal human blood. Cell. Immunol. 1983;81:435–440. doi: 10.1016/0008-8749(83)90250-2. [DOI] [PubMed] [Google Scholar]
- Liu G.Y., Fairchild P.J., Smith R.M., Prowle J.R., Kioussis D., Wraith D.C. Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity. 1995;3:407–415. doi: 10.1016/1074-7613(95)90170-1. [DOI] [PubMed] [Google Scholar]
- Yan T., Burkhardt H., Ritter T., Broker B., Mann K.H., Bertling W.M., von der Mark K., Emmrich F. Specificity and TCR β chain usage of a human collagen type II-reactive T cell clone derived from a healthy individual. Eur. J. Immunol. 1992;22:51–56. doi: 10.1002/eji.1830220109. [DOI] [PubMed] [Google Scholar]
- Arnold B., Schonrich G., Hammerling G. Multiple levels of peripheral tolerance. Immunol. Today. 1993;14:12–14. doi: 10.1016/0167-5699(93)90317-E. [DOI] [PubMed] [Google Scholar]
- Kosaka H., Sprent J. Tolerance of CD8+ T cells developing in parent F1 chimeras prepared with supralethal irradiationstep-wise induction of tolerance in the intrathymic and extrathymic environments. J. Exp. Med. 1993;177:367–378. doi: 10.1084/jem.177.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J.E., Callahan J.E., Kappler J., Marrack P.C. Profound deletion of mature T cells in vivo by chronic exposure to exogenous superantigen. J. Immunol. 1993;150:3785–3792. [PubMed] [Google Scholar]
- Rocha B., von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- Webb S., Morris C., Sprent J. Extrathymic tolerance of mature T cellsclonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- Jenkins M.K., Johnson J.G. Molecules involved in T-cell costimulation. Curr. Opin. Immunol. 1993;5:361–367. doi: 10.1016/0952-7915(93)90054-v. [DOI] [PubMed] [Google Scholar]
- Brocke S., Gaur A., Piercy C., Gautam A., Gijbels K., Fathman C.G., Steinman L. Induction of relapsing paralysis in experimental autoimmune encephalomyelitis by bacterial superantigen. Nature. 1993;365:642–644. doi: 10.1038/365642a0. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig K.W., Strominger J.L. Molecular mimicry in T cell-mediated autoimmunityviral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae B.L., Vanderlugt C.L., Dal Canto M.C., Miller S.D. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J. Exp. Med. 1995;182:75–85. doi: 10.1084/jem.182.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sercarz E.E., Lehmann P.V., Ametani A., Benichou G., Miller A., Moudgil K. Dominance and crypticity of T cell antigenic determinants. Annu. Rev. Immunol. 1993;11:729–766. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- Steinman L. Multiple sclerosisa coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- Tisch R., McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–297. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Brennan F.M., Maini R.N. Rheumatoid arthritis. Cell. 1996;85:307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- Jacobs M.J., van den Hock A.E., van de Putte L.B., van den Berg W.B. Anergy of antigen-specific T lymphocytes is a potent mechanism of intravenously induced tolerance. Immunology. 1994;82:294–300. [PMC free article] [PubMed] [Google Scholar]
- Mueller D.L., Jenkins M.K. Molecular mechanisms underlying functional T-cell unresponsiveness. Curr. Opin. Immunol. 1995;7:375–381. doi: 10.1016/0952-7915(95)80113-8. [DOI] [PubMed] [Google Scholar]
- Elliott E.A., McFarland H.I., Nye S.H., Cofiell R., Wilson T.M., Wilkins J.A., Squinto S.P., Matis L.A., Mueller J.P. Treatment of experimental encephalomyelitis with a novel chimeric fusion protein of myelin basic protein and proteolipid protein. J. Clin. Invest. 1996;98:1602–1612. doi: 10.1172/JCI118954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur A., Wiers B., Liu A., Rothbard J., Fathman C.G. Amelioration of autoimmune encephalomyelitis by myelin basic protein synthetic peptide-induced anergy. Science. 1992;258:1491–1494. doi: 10.1126/science.1279812. [DOI] [PubMed] [Google Scholar]
- Liblau R., Tisch R., Bercovici N., McDevitt H.O. Systemic antigen in the treatment of T-cell-mediated autoimmune diseases. Immunol. Today. 1997;18:599–604. doi: 10.1016/s0167-5699(97)01171-7. [DOI] [PubMed] [Google Scholar]
- Mathisen P.M., Yu M., Johnson J.M., Drazba J.A., Tuohy V.K. Treatment of experimental autoimmune encephalomyelitis with genetically modified memory T cells. J. Exp. Med. 1997;186:159–164. doi: 10.1084/jem.186.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw M.K., Lorens J.B., Dhawan A., DalCanto R., Tse H.Y., Tran A.B., Bompane C., Eswaran S.L., Brocke S., Sarvetnick N. Local delivery of interleukin 4 by retrovirus-transduced T lymphocytes ameliorates experimental autoimmune encephalomyelitis. J. Exp. Med. 1997;185:1711–1714. doi: 10.1084/jem.185.9.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Thornton S., Duwel L.E., Boivin G.P., Giannini E.H., Leiden J.M., Bluestone J.A., Hirsch R. Inhibition of collagen-induced arthritis in mice by viral IL-10 gene transfer. J. Immunol. 1998;161:1516–1524. [PubMed] [Google Scholar]
- Legge K.L., Min B., Potter N.T., Zaghouani H. Presentation of a T cell receptor antagonist peptide by immunoglobulins ablates activation of T cells by a synthetic peptide or proteins requiring endocytic processing. J. Exp. Med. 1997;185:1043–1053. doi: 10.1084/jem.185.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghouani H., Steinman R., Nonacs R., Shah H., Gerhard W., Bona C. Presentation of a viral T cell epitope expressed in the CDR3 region of a self immunoglobulin molecule. Science. 1993;259:224–227. doi: 10.1126/science.7678469. [DOI] [PubMed] [Google Scholar]
- Deo Y.M., Graziano R.F., Repp R., van de Winkel J.G. Clinical significance of IgG Fc receptors and FcγR-directed immunotherapies. Immunol. Today. 1997;18:127–135. doi: 10.1016/s0167-5699(97)01007-4. [DOI] [PubMed] [Google Scholar]
- Polat G.L., Laufer J., Fabian I., Passwell J.H. Cross-linking of monocyte plasma membrane Fc alpha, Fc gamma or mannose receptors induces TNF production. Immunology. 1993;80:287–292. [PMC free article] [PubMed] [Google Scholar]
- Sutterwala F.S., Noel G.J., Salgame P., Mosser D.M. Reversal of proinflammatory responses by ligating the macrophage Fcγ receptor type I. J. Exp. Med. 1998;188:217–222. doi: 10.1084/jem.188.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian C.L. Studies on aggregated gamma-globulin I & II. J. Immunol. 1960;84:112–121. [PubMed] [Google Scholar]
- Rosenqvist E., Jossang T., Feder J. Thermal properties of human IgG. Mol. Immunol. 1987;24:495–501. doi: 10.1016/0161-5890(87)90024-1. [DOI] [PubMed] [Google Scholar]
- Tuohy V.K., Lu Z., Sobel R.A., Laursen R.A., Lees M.B. Identification of an encephalitogenic determinant of myelin proteolipid protein for SJL mice. J. Immunol. 1989;142:1523–1527. [PubMed] [Google Scholar]
- Greer J.M., Kuchroo V.K., Sobel R.A., Lees M.B. Identification and characterization of a second encephalitogenic determinant of myelin proteolipid protein (residues 178–191) for SJL mice. J. Immunol. 1992;149:783–788. [PubMed] [Google Scholar]
- Brocke S., Gijbels K., Allegretta M., Ferber I., Peircy C., Blankenstein T., Martin R., Utz U., Karin N., Mitchell D. Treatment of experimental encephalomyelitis with a peptide analog of myelin basic protein. Nature. 1996;379:343–346. doi: 10.1038/379343a0. [DOI] [PubMed] [Google Scholar]
- Legge K.L., Min B., Pack C., Caprio J., Zaghouani H. Differential presentation of an altered peptide within fetal central and peripheral organs supports an avidity model for thymic cell development and implies a peripheral readjustment for activation. J. Immunol. 1999;162:5738–5746. [PubMed] [Google Scholar]
- Min B., Legge K.L., Pack C., Zaghouani H. Neonatal exposure to a self-peptide–immunoglobulin chimera circumvents the use of adjuvant and confers resistance to autoimmune disease by a novel mechanism involving interleukin 4 lymph node deviation and interferon γ–mediated splenic anergy. J. Exp. Med. 1998;188:2007–2017. doi: 10.1084/jem.188.11.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase M.W., Gidez L., Levine L., Mach B., Murakami W.T., Williams C.A. Chemical analyses. In: Williams C.A., Chase M.W., editors. Methods in Immunology and Immunochemistry. Vol. 2. Academic Press; New York: 1968. pp. 249–341. [Google Scholar]
- Doyle A.G., Fraser I.P. Murine macrophagesisolation, cultivation, and characterization Herzenberg L.A., Weir D., Herzenberg L.A., Blackwell C. Weir's Handbook of Experimental Immunology 1996. 154 Blackwell Science; Cambridge, MA: 1–154.8. [Google Scholar]
- Romani N., Bhardwaj N., Pope M., Koch F., Swiggard W.J., Doherty U.O., Witmer-Pack M.D., Hoffman L., Schuler G., Inaba K. Dendritic cells Herzenberg L.A., Weir D., Herzenberg L.A., Blackwell C. Weir's Handbook of Experimental Immunology 1996. 156 Blackwell Science; Cambridge, MA: 1–156.14. [Google Scholar]
- Critchfield J.M., Racke M.K., Zuniga-Pflucker J.C., Cannella B., Raine C.S., Goverman J., Lenardo M.J. T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science. 1994;263:1139–1143. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- Chen Y., Inobe J.I., Kuchroo V.K., Baron J.L., Janeway C.A., Jr., Weiner H.L. Oral tolerance in myelin basic protein T-cell receptor transgenic micesuppression of autoimmune encephalomyelitis and dose-dependent induction of regulatory cells. Proc. Natl. Acad. Sci. USA. 1996;93:388–391. doi: 10.1073/pnas.93.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux B., Enderlin F., Wallner B., Smilek D.E. Induction of EAE in mice with recombinant human MOG, and treatment of EAE with a MOG peptide. J. Neuroimmunol. 1997;75:169–173. doi: 10.1016/s0165-5728(97)00019-2. [DOI] [PubMed] [Google Scholar]
- Leadbetter E.A., Bourque C.R., Devaux B., Olson C.D., Sunshine G.H., Hirani S., Wallner B.P., Smilek D.E., Happ M.P. Experimental autoimmune encephalomyelitis induced with a combination of myelin basic protein and myelin oligodendrocyte glycoprotein is ameliorated by administration of a single myelin basic protein peptide. J. Immunol. 1998;161:504–512. [PubMed] [Google Scholar]
- Staykova M.A., Simmons R.D., Willenborg D.O. Infusion of soluble myelin basic protein protects long-term against induction of experimental autoimmune encephalomyelitis. Immunol. Cell Biol. 1997;75:54–64. doi: 10.1038/icb.1997.9. [DOI] [PubMed] [Google Scholar]
- Brumeanu T.D., Swiggard W.J., Steinman R.M., Bona C.A., Zaghouani H. Efficient loading of identical viral peptide onto class II molecules by antigenized immunoglobulin and influenza virus. J. Exp. Med. 1993;178:1795–1799. doi: 10.1084/jem.178.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S., Ballo H., Stutte H.J. Immune complex-induced interleukin-6, interleukin-10 and prostaglandin secretion by human monocytesa network of pro- and anti-inflammatory cytokines dependent on the antigen:antibody ratio. Eur. J. Immunol. 1996;26:1297–1301. doi: 10.1002/eji.1830260618. [DOI] [PubMed] [Google Scholar]
- Berger S., Chandra R., Ballo H., Hildenbrand R., Stutte H.J. Immune complexes are potent inhibitors of interleukin-12 secretion by human monocytes. Eur. J. Immunol. 1997;27:2994–3000. doi: 10.1002/eji.1830271136. [DOI] [PubMed] [Google Scholar]
- Steinbrink K., Wolfl M., Jonuleit H., Knop J., Enk A.H. Induction of tolerance by IL-10-treated dendritic cells. J. Immunol. 1997;159:4772–4780. [PubMed] [Google Scholar]
- Ding L., Linsley P.S., Huang L.-Y., Germain R.N., Shevach E.M. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J. Immunol. 1993;151:1224–1234. [PubMed] [Google Scholar]
- Fiorentino D.F., Zlotnik A., Vieira P., Mosmann T.R., Howard M., Moore K.W., O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- Falcone M., Bloom B.R. A T helper cell 2 (Th2) immune response against non-self antigens modifies the cytokine profile of autoimmune T cells and protects against experimental allergic encephalomyelitis. J. Exp. Med. 1997;185:901–907. doi: 10.1084/jem.185.5.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohlman S.A., Pei L., Cua D.J., Li Z., Hinton D. Activation of regulatory cells suppresses experimental allergic encephalomyelitis via secretion of IL-10. J. Immunol. 1999;163:6338–6344. [PubMed] [Google Scholar]
- Tuohy V.K., Yu M., Kawczak J.A., Johnson J.M., Mathisen P.M., Weinstock-Guttman B., Kinkel R.P. The epitope spreading cascade during progression of experimental autoimmune encephalomyelitis and multiple sclerosis. Immunol. Rev. 1998;164:93–100. doi: 10.1111/j.1600-065x.1998.tb01211.x. [DOI] [PubMed] [Google Scholar]
- Groux H., O'Garra A., Bigler M., Rouleau M., Antonenko S., de Vries J.E., Roncarolo M.G. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Asseman C., Mauze S., Leach M.W., Coffman R.L., Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 1999;190:995–1003. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H., Powrie F. Regulatory T cells and inflammatory bowel disease. Immunol. Today. 1999;20:442–445. doi: 10.1016/s0167-5699(99)01510-8. [DOI] [PubMed] [Google Scholar]
- Seddon B., Mason D. Peripheral autoantigen induces regulatory T cells that prevent autoimmunity. J. Exp. Med. 1999;189:877–881. doi: 10.1084/jem.189.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon B., Mason D. The third function of the thymus. Immunol. Today. 2000;21:95–99. doi: 10.1016/s0167-5699(99)01559-5. [DOI] [PubMed] [Google Scholar]
- Legge K.L., Min B., Cestra A.E., Pack C.D., Zaghouani H. TCR agonist and antagonist exert in vivo cross-regulation when presented on Igs. J. Immunol. 1998;161:106–111. [PubMed] [Google Scholar]
- Cannella B., Gao Y.L., Brosnan C., Raine C.S. IL-10 fails to abrogate experimental autoimmune encephalomyelitis. J. Neurosci. Res. 1996;45:735–746. doi: 10.1002/(SICI)1097-4547(19960915)45:6<735::AID-JNR10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Cua D.J., Groux H., Hinton D.R., Stohlman S.A., Coffman R.L. Transgenic interleukin 10 prevents induction of experimental autoimmune encephalomyelitis. J. Exp. Med. 1999;189:1005–1010. doi: 10.1084/jem.189.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J.T., Shevach E.M., Segal B.M. Regulation of interleukin (IL)-12 receptor β2 subunit expression by endogenous IL-12a critical step in the differentiation of pathogenic autoreactive T cells. J. Exp. Med. 1999;189:969–978. doi: 10.1084/jem.189.6.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal I.S., Flavell R.A. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- Stuber E., Strober W., Neurath M. Blocking the CD40L–CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. J. Exp. Med. 1996;183:693–698. doi: 10.1084/jem.183.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerne N.K. Towards a network theory of the immune system. Ann. Immunol. (Paris). 1974;125C:373–389. [PubMed] [Google Scholar]
- Anderson A.C., Nicholson L.B., Legge K.L., Turchin V., Zaghouani H., Kuchroo V.K. High frequency of autoreactive myelin proteolipid protein–specific T cells in the periphery of naive micemechanisms of selection of the self-reactive repertoire. J. Exp. Med. 2000;191:761–770. doi: 10.1084/jem.191.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinissen P., Raus J., Zhang J. Autoimmune pathogenesis of multiple sclerosisrole of autoreactive T lymphocytes and new immunotherapeutic strategies. Crit. Rev. Immunol. 1997;17:33–75. doi: 10.1615/critrevimmunol.v17.i1.20. [DOI] [PubMed] [Google Scholar]