Abstract
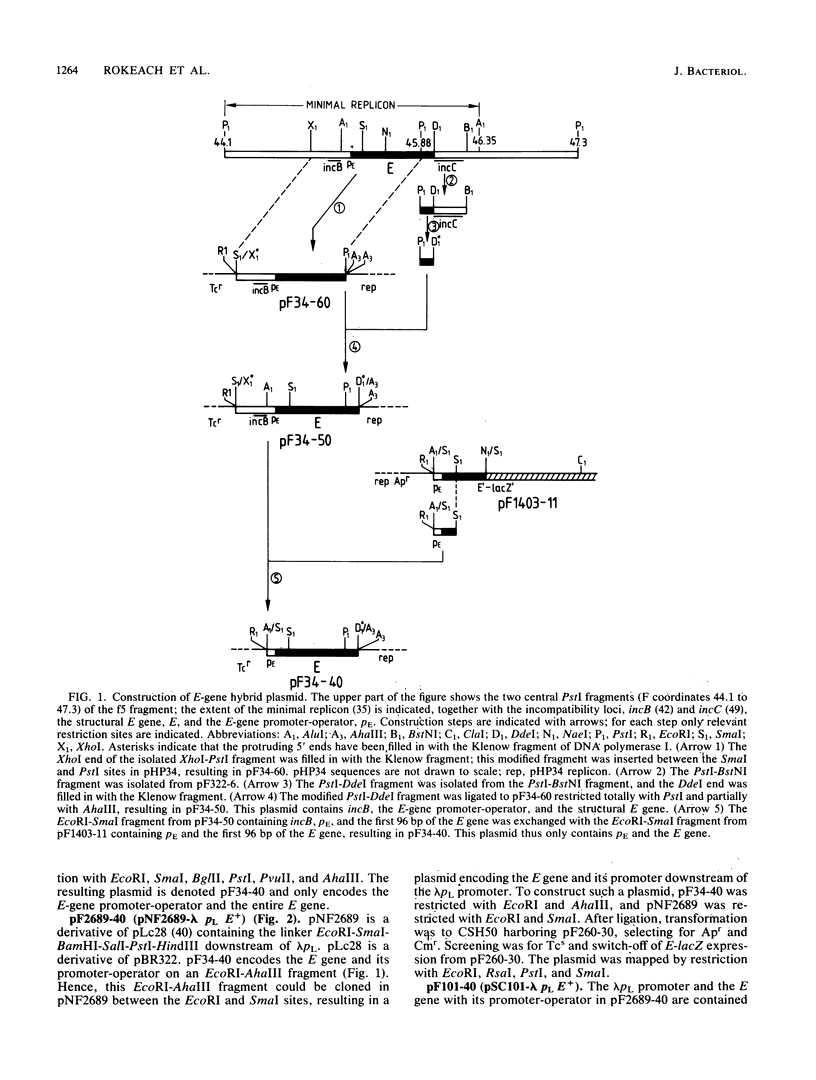
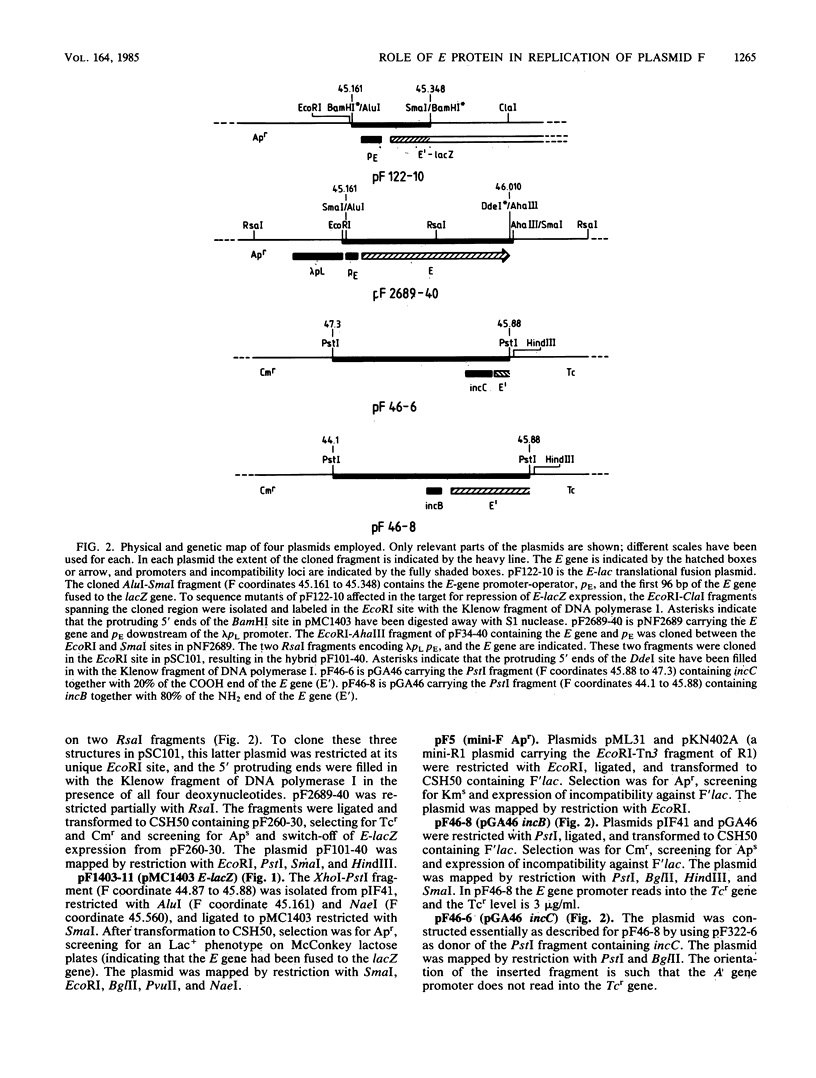
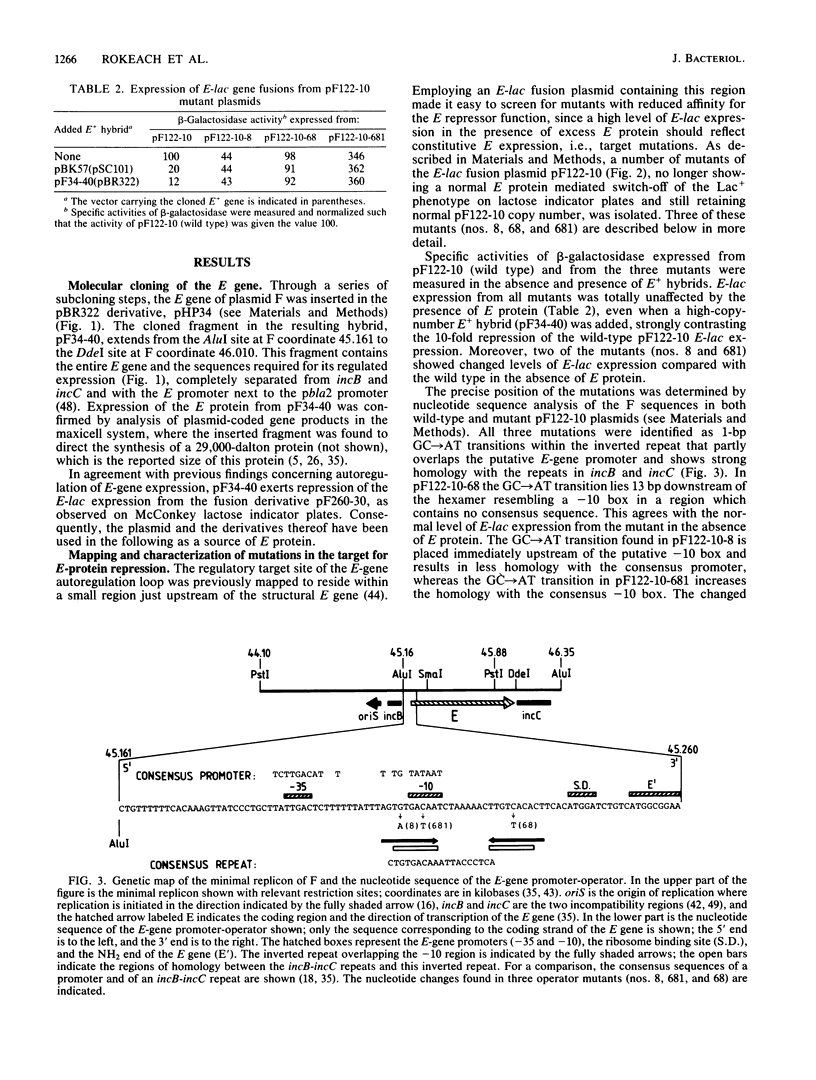
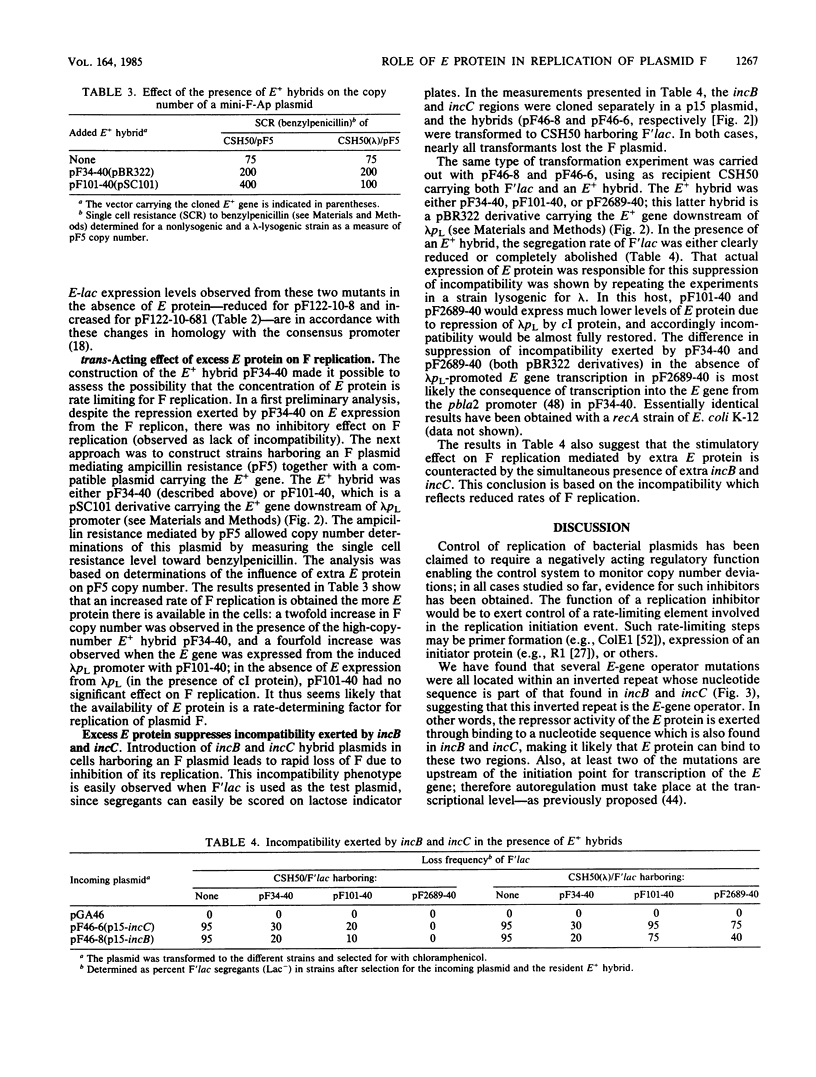
By using a plasmid carrying a translational fusion between the E gene of the IncFI plasmid F and the lacZ gene, we located the operator of the autogenously regulated E gene to an inverted repeat overlapping the E-gene promoter and showing perfect homology to part of the sequence found in all the direct repeats of two regions exerting an inhibitory effect on F replication, incB and incC. Excess E protein provided in trans to an F plasmid increased the replication frequency of the F plasmid. This stimulatory effect was counteracted by increased dosages of incB or incC. A model is proposed for the replication control system of F in which the key elements are autoregulation of E-gene expression and titration of E protein by incB and incC.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles A. L., Snyder K. M., Chattoraj D. K. P1 plasmid replication: replicon structure. J Mol Biol. 1984 Mar 5;173(3):307–324. doi: 10.1016/0022-2836(84)90123-2. [DOI] [PubMed] [Google Scholar]
- An G., Friesen J. D. Plasmid vehicles for direct cloning of Escherichia coli promoters. J Bacteriol. 1979 Nov;140(2):400–407. doi: 10.1128/jb.140.2.400-407.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong K. A., Acosta R., Ledner E., Machida Y., Pancotto M., McCormick M., Ohtsubo H., Ohtsubo E. A 37 X 10(3) molecular weight plasmid-encoded protein is required for replication and copy number control in the plasmid pSC101 and its temperature-sensitive derivative pHS1. J Mol Biol. 1984 May 25;175(3):331–348. doi: 10.1016/0022-2836(84)90352-8. [DOI] [PubMed] [Google Scholar]
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchward G., Linder P., Caro L. The nucleotide sequence of replication and maintenance functions encoded by plasmid pSC101. Nucleic Acids Res. 1983 Aug 25;11(16):5645–5659. doi: 10.1093/nar/11.16.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C. Recircularization and autonomous replication of a sheared R-factor DNA segment in Escherichia coli transformants. Proc Natl Acad Sci U S A. 1973 May;70(5):1293–1297. doi: 10.1073/pnas.70.5.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C. Revised interpretation of the origin of the pSC101 plasmid. J Bacteriol. 1977 Nov;132(2):734–737. doi: 10.1128/jb.132.2.734-737.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J., Pritchard R. H. Relationship between chromosome replication and F'lac episome replication in Escherichia coli. J Mol Biol. 1973 Jun 25;78(1):143–155. doi: 10.1016/0022-2836(73)90434-8. [DOI] [PubMed] [Google Scholar]
- Givskov M., Molin S. Copy mutants of plasmid R1: effects of base pair substitutions in the copA gene on the replication control system. Mol Gen Genet. 1984;194(1-2):286–292. doi: 10.1007/BF00383529. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Smith H. R., Anderson E. S. Mutagenesis of plasmid DNA with hydroxylamine: isolation of mutants of multi-copy plasmids. Mol Gen Genet. 1976 Apr 23;145(1):101–108. doi: 10.1007/BF00331564. [DOI] [PubMed] [Google Scholar]
- Inuzuka M., Helinski D. R. Requirement of a plasmid-encoded protein for replication in vitro of plasmid R6K. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5381–5385. doi: 10.1073/pnas.75.11.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M. L., Figurski D., Ito L., Helinski D. R. Essential regions for replication of a stringent and a relaxed plasmid in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):99–103. doi: 10.1101/sqb.1979.043.01.015. [DOI] [PubMed] [Google Scholar]
- Kamio Y., Tabuchi A., Itoh Y., Katagiri H., Terawaki Y. Complete nucleotide sequence of mini-Rts1 and its copy mutant. J Bacteriol. 1984 Apr;158(1):307–312. doi: 10.1128/jb.158.1.307-312.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamio Y., Terawaki Y. Nucleotide sequence of an incompatibility region of mini-Rts1 that contains five direct repeats. J Bacteriol. 1983 Sep;155(3):1185–1191. doi: 10.1128/jb.155.3.1185-1191.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline B. C., Trawick J. Identification and characterization of a second copy number control gene in mini-F plasmids. Mol Gen Genet. 1983;192(3):408–415. doi: 10.1007/BF00392183. [DOI] [PubMed] [Google Scholar]
- Komai N., Nishizawa T., Hayakawa Y., Murotsu T., Matsubara K. Detection and mapping of six miniF-encoded proteins by cloning analysis of dissected miniF segments. Mol Gen Genet. 1982;186(2):193–203. doi: 10.1007/BF00331850. [DOI] [PubMed] [Google Scholar]
- Light J., Molin S. Replication control functions of plasmid R1 act as inhibitors of expression of a gene required for replication. Mol Gen Genet. 1981;184(1):56–61. doi: 10.1007/BF00271195. [DOI] [PubMed] [Google Scholar]
- Lovett M. A., Helinski D. R. Method for the isolation of the replication region of a bacterial replicon: construction of a mini-F'kn plasmid. J Bacteriol. 1976 Aug;127(2):982–987. doi: 10.1128/jb.127.2.982-987.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki S., Kuribayashi M., Miki T., Horiuchi T. An amber replication mutant of F plasmid mapped in the minimal replication region. Mol Gen Genet. 1983;191(2):231–237. doi: 10.1007/BF00334819. [DOI] [PubMed] [Google Scholar]
- Maki S., Miki T., Horiuchi T. DNA sequence of an amber replication mutant indicates that a 29 kd protein is the product of the F plasmid replication gene. Mol Gen Genet. 1984;194(1-2):337–339. doi: 10.1007/BF00383537. [DOI] [PubMed] [Google Scholar]
- Manis J. J., Kline B. C. F plasmid incompatibility and copy number genes: their map locations and interactions. Plasmid. 1978 Sep;1(4):492–507. doi: 10.1016/0147-619x(78)90007-0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Murotsu T., Matsubara K. Role of an autorepression system in the control of lambda dv plasmid copy number and incompatibility. Mol Gen Genet. 1980;179(3):509–519. doi: 10.1007/BF00271740. [DOI] [PubMed] [Google Scholar]
- Murotsu T., Matsubara K., Sugisaki H., Takanami M. Nine unique repeating sequences in a region essential for replication and incompatibility of the mini-F plasmid. Gene. 1981 Nov;15(2-3):257–271. doi: 10.1016/0378-1119(81)90135-9. [DOI] [PubMed] [Google Scholar]
- Murotsu T., Tsutsui H., Matsubara K. Identification of the minimal essential region for the replication origin of miniF plasmid. Mol Gen Genet. 1984;196(2):373–378. doi: 10.1007/BF00328075. [DOI] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Phillips R. D. A method for the rapid, automated filtration of protein hydrolyzates. Anal Biochem. 1981 Nov 15;118(1):91–95. doi: 10.1016/0003-2697(81)90161-5. [DOI] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. A modified pBR322 vector with improved properties for the cloning, recovery, and sequencing of blunt-ended DNA fragments. Gene. 1982 Feb;17(2):189–196. doi: 10.1016/0378-1119(82)90072-5. [DOI] [PubMed] [Google Scholar]
- Scott J. R. Regulation of plasmid replication. Microbiol Rev. 1984 Mar;48(1):1–23. doi: 10.1016/b978-0-12-048850-6.50006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelke R. W., Kline B. C., Trawick J. D., Ritts G. D. Genetic studies of F plasmid maintenance genes involved in copy number control, incompatability, and partitioning. Plasmid. 1982 Mar;7(2):163–179. doi: 10.1016/0147-619x(82)90075-0. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Hsu M. T., Otsubo E., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. I. Structure of F-prime factors. J Mol Biol. 1972 Nov 14;71(2):471–497. doi: 10.1016/0022-2836(72)90363-4. [DOI] [PubMed] [Google Scholar]
- Stougaard P., Molin S., Nordström K. RNAs involved in copy-number control and incompatibility of plasmid R1. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6008–6012. doi: 10.1073/pnas.78.10.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Organization of transcriptional signals in plasmids pBR322 and pACYC184. Proc Natl Acad Sci U S A. 1981 Jan;78(1):167–171. doi: 10.1073/pnas.78.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søgaard-Andersen L., Rokeach L. A., Molin S. Regulated expression of a gene important for replication of plasmid F in E. coli. EMBO J. 1984 Feb;3(2):257–262. doi: 10.1002/j.1460-2075.1984.tb01794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolun A., Helinski D. R. Direct repeats of the F plasmid incC region express F incompatibility. Cell. 1981 Jun;24(3):687–694. doi: 10.1016/0092-8674(81)90095-7. [DOI] [PubMed] [Google Scholar]
- Tolun A., Helinski D. R. Separation of the minimal replication region of the F plasmid into a replication origin segment and a trans-acting segment. Mol Gen Genet. 1982;186(3):372–377. doi: 10.1007/BF00729456. [DOI] [PubMed] [Google Scholar]
- Tomizawa J., Itoh T. Plasmid ColE1 incompatibility determined by interaction of RNA I with primer transcript. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6096–6100. doi: 10.1073/pnas.78.10.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J., Itoh T., Selzer G., Som T. Inhibition of ColE1 RNA primer formation by a plasmid-specified small RNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1421–1425. doi: 10.1073/pnas.78.3.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trawick J. D., Kline B. C. A two-stage molecular model for control of mini-F replication. Plasmid. 1985 Jan;13(1):59–69. doi: 10.1016/0147-619x(85)90056-3. [DOI] [PubMed] [Google Scholar]
- Tsutsui H., Fujiyama A., Murotsu T., Matsubara K. Role of nine repeating sequences of the mini-F genome for expression of F-specific incompatibility phenotype and copy number control. J Bacteriol. 1983 Jul;155(1):337–344. doi: 10.1128/jb.155.1.337-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui H., Matsubara K. Replication control and switch-off function as observed with a mini-F factor plasmid. J Bacteriol. 1981 Aug;147(2):509–516. doi: 10.1128/jb.147.2.509-516.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlin B. E., Molin S., Gustafsson P., Nordström K. Plasmids with temperature-dependent copy number for amplification of cloned genes and their products. Gene. 1979 Jun;6(2):91–106. doi: 10.1016/0378-1119(79)90065-9. [DOI] [PubMed] [Google Scholar]
- Uhlin B. E., Nordström K. Plasmid incompatibility and control of replication: copy mutants of the R-factor R1 in Escherichia coli K-12. J Bacteriol. 1975 Nov;124(2):641–649. doi: 10.1128/jb.124.2.641-649.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson L. A., Phua S. H., Bergquist P. L., Lane H. E. An Mr 29000 protein is essential for mini-F maintenance in E. coli. Gene. 1982 Sep;19(2):173–178. doi: 10.1016/0378-1119(82)90003-8. [DOI] [PubMed] [Google Scholar]
- Yang R., Lis J., Wu R. Elution of DNA from agarose gels after electrophoresis. Methods Enzymol. 1979;68:176–182. doi: 10.1016/0076-6879(79)68012-6. [DOI] [PubMed] [Google Scholar]


