Abstract
Cross-linking of FcεRI induces the activation of three protein tyrosine kinases, Lyn, Syk, and Bruton's tyrosine kinase (Btk), leading to the secretion of a panel of proinflammatory mediators from mast cells. This study showed phosphorylation at Ser-473 and enzymatic activation of Akt/protein kinase B, the crucial survival kinase, upon FcεRI stimulation in mouse mast cells. Phosphorylation of Akt is regulated positively by Btk and Syk and negatively by Lyn. Akt in turn can regulate positively the transcriptional activity of interleukin (IL)-2 and tumor necrosis factor (TNF)-α promoters. Transcription from the nuclear factor κB (NF-κB), nuclear factor of activated T cells (NF-AT), and activator protein 1 (AP-1) sites within these promoters is under the control of Akt activity. Accordingly, the signaling pathway involving IκB-α, a cytoplasmic protein that binds NF-κB and inhibits its nuclear translocation, appears to be regulated by Akt in mast cells. Catalytic activity of glycogen synthase kinase (GSK)-3β, a serine/threonine kinase that phosphorylates NF-AT and promotes its nuclear export, seems to be inhibited by Akt. Importantly, Akt regulates the production and secretion of IL-2 and TNF-α in FcεRI-stimulated mast cells. Altogether, these results revealed a novel function of Akt in transcriptional activation of cytokine genes via NF-κB, NF-AT, and AP-1 that contributes to the production of cytokines.
Keywords: FcεRI, Lyn, Btk, NF-κB, signal transduction
Introduction
Akt/protein kinase B (referred to as Akt hereafter), originally identified by its similarity to protein kinases A and C 1 2 and also found in a rodent oncogenic retroviral genome 3, is a pleiotropic protein serine/threonine kinase composed of an NH2-terminal pleckstrin homology (PH) domain and a COOH-terminal catalytic domain. Akt is activated by numerous stimuli and is implicated in a variety of cellular functions, such as survival, metabolism, transcription, and translation (for reviews, see references 4 5 6 7 8 9). In response to growth factors that activate phosphatidylinositol 3-kinase (PI3K), the membrane-bound lipid phosphatidylinositol 3,4,5-trisphosphate (PIP3) is synthesized and recruits 3-phosphoinositide–dependent kinase (PDK)1 10 11 12 by binding to its PH domain. PIP3 and phosphatidylinositol 3,4-bisphosphate recruit Akt to the plasma membrane by interacting with the PH domain of Akt 13 14. PDK1 phosphorylates Thr-308 of Akt and PDK2, a putative kinase yet to be cloned 10, phosphorylates Ser-473 of Akt. Akt phosphorylated at both sites becomes active and phosphorylates target proteins. Other stimuli such as heat shock, hyperosmolarity, okadaic acid, and cAMP activate Akt in a PI3K-independent manner 15 16 17 18.
Mast cells are crucial effector cells for IgE-dependent immediate hypersensitivity 19. Contact with multivalent antigen activates IgE-bound mast cells, culminating in degranulation releasing vasoactive amines and secretion of lipid mediators, various proteases, and various cytokines. This high-affinity IgE receptor (FcεRI)-dependent activation has been a focus of intense study. FcεRI consists of four subunits, i.e., one IgE-binding α subunit, one β, and two S-S–bonded γ subunits 20. According to the broadly accepted hypothesis 21 22 23, FcεRI cross-linking elicits the activation of β subunit–bound Lyn, a Src family protein tyrosine kinase (PTK). Active Lyn phosphorylates tyrosine residues in the immunoreceptor tyrosine-based activation motifs (ITAMs 24) within both β and γ subunits. Phosphorylated β-ITAM and phosphorylated γ-ITAM recruit Lyn and Syk, respectively, via Src homology (SH)2 domain–phosphotyrosine interactions. Lyn has an SH2 domain, and Syk has two tandem SH2 domains. Activation of these PTKs ensues by phosphorylation of tyrosine residues in the activation loop and conformational changes induced by binding to phosphorylated ITAMs 25 26 27. Active Lyn and Syk phosphorylate numerous target proteins, including phospholipase C (PLC)-γ. Phosphorylation and activation of PLC-γ requires the concerted action of Syk and another PTK, Bruton's tyrosine kinase (Btk [28, 29]). Hydrolysis of phosphatidylinositol 4,5-bisphosphate by PLC generates two second messengers, inositol 1,4,5-trisphosphate (IP3) and diacylglycerol. IP3 recruits Ca2+ from cellular storage sites, and diacylglycerol activates protein kinase C isoforms 30 31. Optimal degranulation requires Ca2+ and protein kinase C 32.
Downstream of the above early activation events, three major subfamilies of mitogen-activated protein kinases (MAPKs), i.e., extracellular signal–regulated kinases (ERKs 33 34 35 36), c-Jun NH2-terminal kinases (JNKs [37, 38]), and p38 39, are activated upon FcεRI cross-linking. In mast cells, ERK1 and ERK2 are under the control of Syk and Ras 39, whereas JNK1 and JNK2 are regulated in a Lyn/Syk/Btk-dependent manner 38 40. p38 is also regulated by Btk 38. MAPKs regulate various transcription factors. For example, JNK phosphorylates and activates c-Jun, which dimerizes with Fos and binds to activator protein 1 (AP-1) sites in various gene promoters, including those for cytokine genes (for a review, see reference 41). Promoters in cytokine genes such as those coding for IL-2 and TNF-α contain other cis-elements that are bound by important transcription factors such as nuclear factor of activated T cells (NF-AT) and nuclear factor κB (NF-κB 42 43 44 45 46). Nuclear location and activity of NF-AT are controlled by Ca2+-dependent phosphatase calcineurin and Ras (for a review, see reference 47). NF-κB is activated by a variety of inflammatory stimuli via the recently defined pathways that involve NF-κB–inducing kinase (NIK [48, 49]), Cot/Tpl-2 50, and MEK kinase (MEKK)1, -2, and -3 51 52 53 54. These MAPK kinase kinases can activate the IκB kinase (IKK) complex of serine/threonine kinases and a cofactor 55 56 57 58 59. IKK in turn phosphorylates and induces the degradation of IκB (an NF-κB–sequestering protein family; for a review, see reference 60). However, signaling pathways leading to the activation of NF-κB in mast cells are little known.
In this study, we found that Akt is activated upon cross-linking of FcεRI in mast cells. Using primary cultured mast cells and mast cell lines deficient in PTK critical for mast cell activation, we investigated the requirements for Akt activation. Downstream of Akt activation, the signaling pathways to transcriptional activation of cytokine genes were analyzed. Importantly, we revealed that Akt is critically involved in cytokine production induced by FcεRI stimulation.
Materials and Methods
Reagents.
Culture media and FCS were purchased from Life Technologies. Sources of commercial antibodies are as follows: anti-Btk (M-138), anti-Lyn 44, anti-Syk (C-20), anti–PLC-γ2 (Q-20), anti-JNK1 (C-17), and anti-ERK1 (C-16) were from Santa Cruz Biotechnology, Inc.; antiphosphotyrosine mAb 4G10, anti–PLC-γ1 mAbs, and anti-Akt antibody were from Upstate Biotechnology; antiphospho-Akt (Ser473), antiphospho-Akt (Thr308), antiphospho–IκB-α (Ser32), and antiphospho-MAPK were from New England Biolabs, Inc.; and antiphospho–glycogen synthase kinase (GSK)-3β (Ser9) and anti–GSK-3α/β were from Biosource International. A PTK inhibitor (genistein), PI3K inhibitors (wortmannin and LY294002), and Pansorbin were purchased from Calbiochem. Other chemicals of the highest grade available were obtained from Sigma-Aldrich or Fisher Scientific, unless otherwise mentioned.
Cells.
btk − /− and lyn − /− mice, each on a mixed C57BL/6 × 129/Sv genetic background, were mated to generate btk +/−lyn+/− F1 progeny. These F1 mice were mated to obtain wild-type (wt), btk − /−, lyn − /−, and btk − /−lyn − /− mice 61. Genotyping was done by Southern blotting or PCR analysis of mouse tail–derived DNAs. Mast cells were cultured as described previously 62. In brief, bone marrow cells derived from femur of the 6–10-wk-old mice were cultured in RPMI 1640 medium supplemented with 10% FCS, 100 μM nonessential amino acids, 50 μM 2-ME, and 8% conditioned medium of IL-3 gene-transfected cells (bone marrow–derived mast cell [BMMC] medium). More than 95% of the Trypan blue–excluding viable cells were mast cells after 4 wk of culture. No discernible differences in morphology and expression of early signaling proteins, including FcεRIβ, FcεRIγ, Syk, Grb2, PLC-γ2, c-Cbl, and Shc were detected among these four types of BMMCs (data not shown). Surface expression of FcεRI at similar levels was confirmed by flow cytometry using a FACSCalibur™ apparatus and CELLQuest™ software (Becton Dickinson). In acute (≤60 min) FcεRI stimulation experiments, BMMCs were sensitized by an overnight incubation with 0.5–1 μg/ml antidinitrophenyl (DNP) IgE mAb, washed once in Tyrode buffer (112 mM NaCl, 2.7 mM KCl, 0.4 mM NaH2PO4, 1.6 mM CaCl2, 1 mM MgCl2, 10 mM Hepes [pH 7.5], 0.05% gelatin, 0.1% glucose), resuspended in Tyrode buffer to 2 × 107 cells/ml, and stimulated by polyvalent antigen, 100 ng/ml DNP conjugates of human serum albumin (DNP-HSA) for the indicated time intervals.
Rat basophilic leukemia (RBL)-2H3 is a rat mast cell line used extensively for studies on FcεRI signal transduction. A Syk-deficient RBL-2H3 variant and its syk cDNA-transfected cell line 63 were provided by Dr. Reuben P. Siraganian (National Institutes of Health, Bethesda, MD).
Retroviral Transfection.
Hemagglutinin (HA)-tagged Akt cDNAs were recloned into the EcoRI and XhoI sites of the retroviral vector pMX-puro 38. These plasmids were transfected into BOSC-23 packaging cells with Lipofectamine (Life Technologies) to generate recombinant retroviruses. BMMCs were infected with the retroviruses in the presence of 10 μg/ml polybrene, and drug selection was started 48 h after infection. Mass populations of puromycin-resistant cells were grown and then cultured in the absence of selection drug for 48 h before FcεRI stimulation.
Measurements of Secreted Cytokines.
For cytokine measurements, mast cells were stimulated in BMMC medium instead of Tyrode buffer. TNF-α and IL-2 secreted into the culture medium for 20 h were measured by ELISA kits (Endogen).
Immunoblotting and Immunoprecipitation.
Cells were lysed in ice-cold 1% NP-40–containing lysis buffer (20 mM Tris-HCl [pH 8.0], 0.15 M NaCl, 1 mM EDTA, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 25 μM p-nitrophenyl p′-guanidinobenzoate, 1 μM pepstatin, and 0.1% sodium azide) immediately after stimulation. Lysates were centrifuged in an Eppendorf microcentrifuge at 4°C for 10 min. Protein concentrations were measured using DC protein assay reagents (Bio-Rad Laboratories). Cleared lysates were either directly analyzed by SDS-PAGE or immunoprecipitated before SDS-PAGE analysis. For immunoprecipitation, lysates were incubated on ice with an appropriate antibody for 2–4 h, and immune complexes were recovered by brief centrifugation after another 30-min incubation with Pansorbin for rabbit polyclonal antibodies or anti–mouse immunoglobulin-conjugated agarose (Sigma-Aldrich) for mouse mAbs. Immune complexes were washed in lysis buffer four times before SDS-PAGE analysis. Proteins separated by SDS-PAGE were electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes (NEN Life Science Products). Membranes were blocked, then incubated consecutively with primary antibody and horseradish peroxidase–conjugated secondary antibody, and immunoreactive proteins were visualized by enhanced chemiluminescence reagents (NEN Life Science Products).
Immune Complex Kinase Assays.
Akt kinase assays were performed as described previously 64. In brief, cells were lysed on ice for 15 min in ice-cold 1% NP-40–containing lysis buffer. Cleared lysates were incubated with anti-Akt (Upstate Biotechnology) and protein G agarose (Santa Cruz Biotechnology, Inc.). Akt immunoprecipitates were washed four times in the lysis buffer and once with kinase buffer (20 mM 3-[N-morpholine] propanesulfonic acid [MOPS], pH 7.2, 25 mM sodium β-glycerophosphate, 1 mM dithiothreitol [DTT], 15 mM MgCl2, 5 mM EGTA, and 1 mM Na orthovanadate). Washed immunoprecipitates were incubated at 30°C for 15 min with kinase buffer supplemented with 1 μg/ml microcystin (Sigma-Aldrich) and 2.5 μg histone H2B in the presence of [γ-32P]ATP. Reactions were terminated with SDS sample buffer and analyzed by SDS-PAGE, followed by electroblotting onto PVDF membranes and autoradiography. ERK and JNK kinase assays were done as described previously 38. Cells were lysed in 1% NP-40–containing lysis buffer. Cleared lysates were immunoprecipiated with anti-ERK1 (Zymed Laboratories) or JNK1 with an aid of Pansorbin. Immunoprecipitates were washed three times with 1% NP-40–containing lysis buffer and once with kinase buffer (20 mM Hepes [pH 7.4], 10 mM MgCl2, 22 mM DTT, 20 mM β-glycerophosphate, and 50 μM Na3VO4). Immune complexes were incubated with 3 μg myelin basic protein (for ERK assays) or 3 μg of glutathione S-transferase (GST)-c-Jun(1–79) (for JNK assays) in the kinase buffer in the presence of [γ-32P]ATP. Reactions were analyzed as above.
Transcriptional Activity Assay with Luciferase Reporter Constructs.
Luciferase reporter constructs, the mouse IL-2 (−321)/luc, the human TNF-α (−200)/luc, NF-κB/luc, AP-1/luc, and NF-AT/luc were described previously 65. 1–1.5 × 107 mast cells were transfected with 5–10 μg reporter plasmids singly or together with 2 or 20 μg each of empty vector, wt Akt 66, E40K Akt, K179M Akt (provided by Dr. P. Blume-Jensen, Salk Institute, La Jolla, CA; reference 67), AAA Akt vectors (provided by Dr. J.R. Woodgett, Ontario Cancer Institute, Toronto, Canada; reference 68), dominant negative (DN) IκB-α (IκBαM; provided by Dr. Van Antwerp, Salk Institute, La Jolla, CA; reference 69), DN IKKα (provided by A. Altman, La Jolla Institute for Allergy and Immunology, San Diego, CA; reference 70), or DN GSK-3β (K85M/K86I mutant; provided by Dr. J.R. Woodgett and Dr. G.R. Crabtree [Stanford University, Palo Alto, CA]; reference 71) by electroporation at 400 V, 950 μF using a Gene Pulser II apparatus (Bio-Rad Laboratories). Both K179M Akt and AAA Akt with K179A/T308A/S473A substitutions are DN mutants. Transfected cells were sensitized overnight with anti-DNP IgE and left unstimulated or stimulated with 30 ng/ml DNP-HSA for 8 h before cell harvest. Cells were lysed in 0.2% Triton X-100 in 100 mM potassium phosphate buffer (pH 7.8)/1 mM DTT. Luminescence of cleared lysates was measured after addition of luciferin solution using a model Monolight 2010 luminometer (Analytical Luminescence Laboratory).
Results
Akt Activation by FcεRI Cross-Linking in Mast Cells.
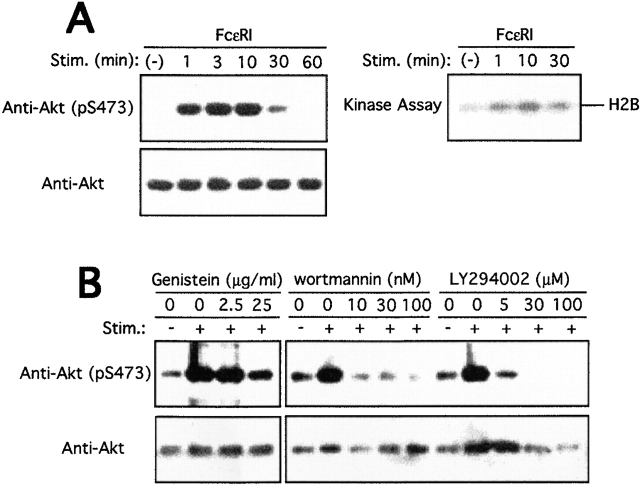
Because Akt is activated by numerous stimuli, we investigated whether FcεRI cross-linking induces Akt activation. In situ Akt activity was monitored before and after cell stimulation by immunoblotting with a phosphospecific antibody to the phosphorylated Ser-473 of Akt. Ser-473 phosphorylation is crucial for Akt activation. Antigen treatment of IgE-primed BMMCs caused a remarkable phosphorylation of Ser-473 at its peak ∼3–10 min after antigen stimulation (Fig. 1 A, left). Enzymatic activation of Akt in a time course similar to that of Ser-473 phosphorylation was shown in in vitro kinase assays on anti-Akt immunoprecipitates using histone H2B as an exogenous substrate (Fig. 1 A, right). Dependence of FcεRI-induced Akt activation on PTK was revealed by pretreatment of BMMCs with genistein, similar to BCR-induced Akt activation 64 72 73. PI3K inhibitors wortmannin and LY294002 also blocked Akt activation very efficiently, confirming the PI3K dependence of Akt activation (Fig. 1 B). These results are consistent with PI3K activation induced by the engagement of FcεRI and other related receptors that induces activation of several PTKs as well 74 75 76 77.
Figure 1.
Activation of Akt in mast cells by growth factor or FcεRI stimulation. (A) BMMCs sensitized overnight with anti-DNP IgE were stimulated with 100 ng/ml DNP-HSA for the indicated amounts of time. Cells were lysed, and lysates were analyzed by SDS-PAGE followed by immunoblotting with antiphospho-Akt antibody that specifically recognizes the phosphorylated Ser-473 residue and its flanking sequence. The same blot was reprobed with anti-Akt antibody (left). Shown is the result representative of at least five independent experiments. Cell lysates were immunoprecipitated with anti-Akt, and immune complexes were subjected to kinase assays using histone H2B as an exogenous substrate (right). The kinase result is representative of three similar experiments. Stim., stimulation. (B) IgE-sensitized BMMCs were pretreated with the indicated concentrations of genistein, wortmannin, or LY294002 for 15 min before antigen (100 ng/ml DNP-HSA) stimulation (Stim.) for 10 min. Cell lysates were analyzed for Ser-473 phosphorylation as above.
Hyperphosphorylation of Akt in Lyn-deficient Cells and Reduced Akt Phosphorylation in Btk- and Syk-deficient Cells.
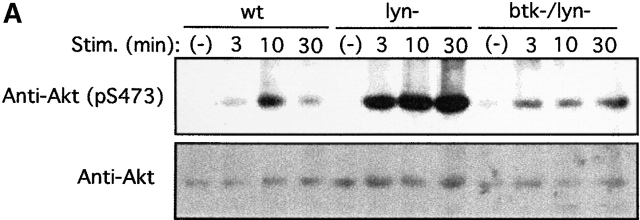
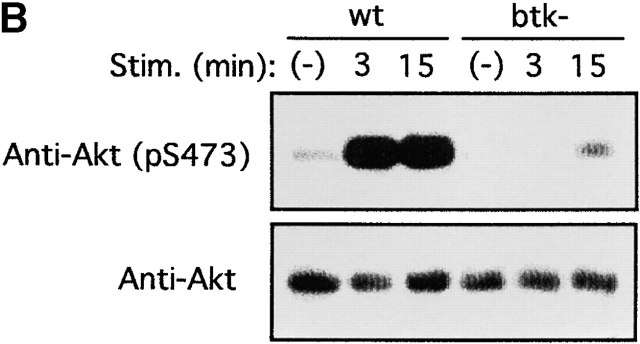
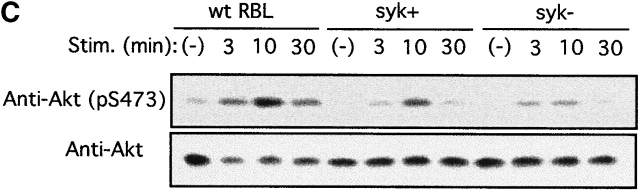
PTK dependence of Akt phosphorylation induced by FcεRI cross-linking was further studied using BMMCs derived from lyn − /− and btk − /− mice and a Syk-deficient variant of RBL-2H3 rat mast cells. Strikingly, lyn − /− cells showed a severalfold more robust and prolonged Akt Ser-473 phosphorylation than wt cells (Fig. 2 A). In contrast, btk − /− mast cells exhibited a reduced phosphorylation of Akt compared to wt cells (Fig. 2 B). btk − /−lyn − /− double-mutant cells showed a prolonged, but not stronger, phosphorylation compared with wt cells (Fig. 2 A), indicating that the enhanced Akt phosphorylation in lyn − /− cells is dependent on Btk. On the other hand, wt RBL-2H3 cells exhibited high basal levels of Akt Ser-473 phosphorylation and further induction upon FcεRI stimulation (Fig. 2 C), consistent with the presence of active mutations of c-Kit, the receptor for stem cell factor (SCF), in this and other mast cell lines 78. But basal and induced levels of Akt phosphorylation were significantly reduced in Syk-deficient RBL-2H3 cells and enhanced in Syk-deficient cells transfected with wt syk cDNA (Fig. 2 C). These results demonstrate that Akt activity is regulated positively by Syk and Btk and negatively by Lyn in mast cells.
Figure 2.
PTK dependence on Akt activation induced by FcεRI cross-linking. (A) BMMCs derived from wt, lyn − /−, and btk − /−lyn − /− mice were sensitized by IgE and stimulated with antigen for the indicated amounts of time. Cells were analyzed for Akt Ser-473 phosphorylation and Akt amounts. Stim., stimulation. (B) wt and btk − /− BMMCs and (C) Syk-deficient (syk−) and Syk-reconstituted (syk+) Syk-deficient RBL-2H3 cells were similarly analyzed. Similar results shown in A–C were reproduced in two more independent experiments. Stim., stimulation.
Regulation of IL-2 and TNF-α Promoters by Akt.
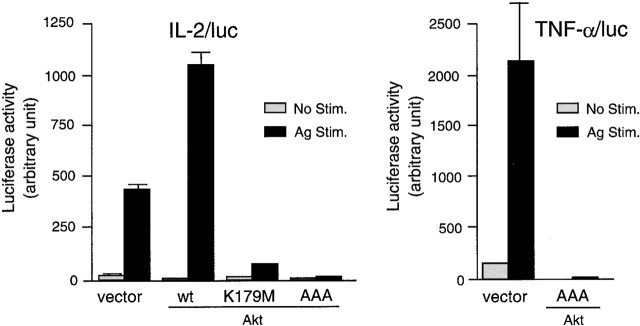
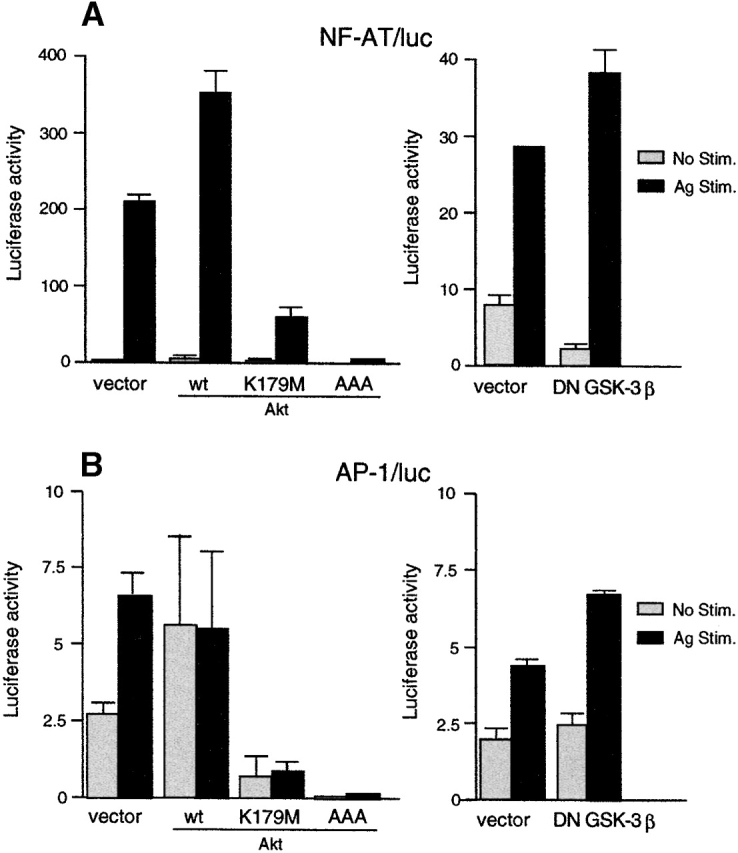
One of the cardinal features of FcεRI-induced mast cell activation is the production and secretion of various cytokines including IL-2 and TNF-α, at least partly through the transcriptional activation of these cytokine genes. Our recent studies indicate that this aspect of mast cell activation is exaggerated in lyn − /− cells 79, and Btk is required for optimal production of these cytokines 80. Given the Akt hyperphosphorylation in lyn − /− cells and the Akt hypophosphorylation in btk − /− cells, we examined whether Akt activity is involved in cytokine gene expression. To this end, we cotransfected BMMCs with wt or DN Akt cDNA expression vectors together with IL-2/luc or TNF-α/luc reporter plasmids (Fig. 3). Overexpression of wt Akt enhanced twofold induction of IL-2 promoter–driven luciferase expression over the vector-transfected cells upon FcεRI cross-linking. FcεRI-induced transcriptional activation was almost abrogated by two different DN Akt mutants. Similar results were obtained with TNF-α/luc. Given these results, together with higher transcriptional activity of IL-2 and TNF-α promoters (Figure 8 in reference 79) and higher production of these cytokines (Figure 7 in reference 79) in lyn − /− mast cells compared with wt cells, higher cytokine production in FcεRI-stimulated lyn − /− cells may be accounted for at least partly by the higher activation levels of Akt via the transcriptional regulation. Indeed, overexpression of wt Akt in lyn − /− cells enhanced the transcriptional activity of IL-2 and TNF-α promoters more than that in wt cells, and DN Akt inhibited it (data not shown).
Figure 3.
Akt regulation of IL-2 and TNF-α promoter activities. wt BMMCs were transfected with IL-2/luc or TNF-α/luc reporter plasmids together with an empty vector or a vector coding for wt, K179M, or AAA Akt. 24 h later, cells were sensitized with IgE overnight. Cells were then stimulated with antigen for the last 8 h before luciferase assays. The IL-2/luc results are representative of three transfection experiments, and the TNF-α/luc results are representative of two experiments. Stim., stimulation.
Transcription Factor NF-κB Is under the Control of Akt.
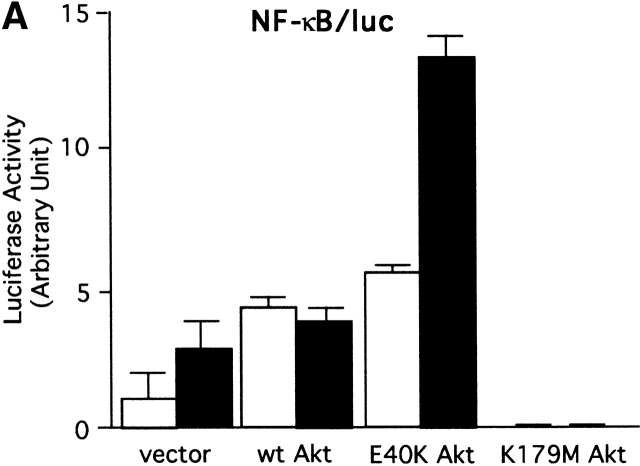
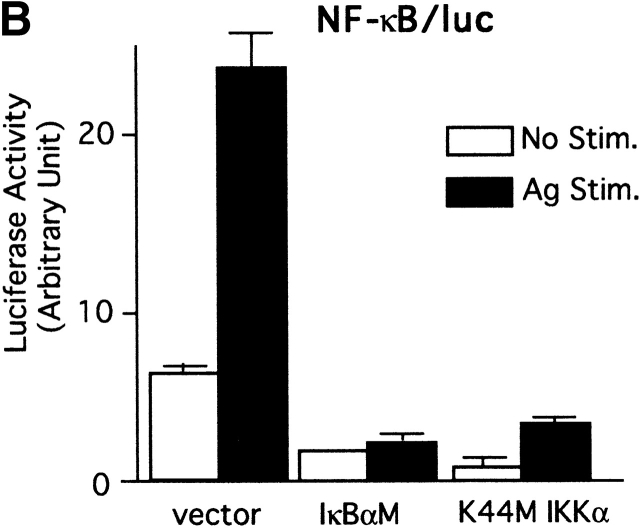
Expression of IL-2 and TNF-α genes requires several transcription factors, including NF-κB, AP-1, and NF-AT 65. Therefore, we examined whether the activity of these transcription factors is regulated by Akt in mast cells. First, the luciferase gene under the control of multiple copies of the NF-κB site (−206 to −202, eight times) of the murine IL-2 promoter was cotransfected into BMMCs with wt, E40K, or DN Akt (Fig. 4 A). wt Akt transfection doubled NF-κB–dependent transcription before stimulation, whereas FcεRI stimulation did not enhance it any further. Constitutively active E40K mutant transfectants exhibited a further enhancement in basal and induced levels of NF-κB activity. Importantly, these activities were strongly inhibited by DN Akt. Because of the possible general negative effect of DN Akt expression, we evaluated whether DN Akt induces apoptosis in BMMCs. Because the efficiency of transient transfection used in the above experiments was too low (<20%), to address this issue, we made stable transfectants using wt or DN Akt retroviral constructs. Puromycin-resistant transfectants were stained with annexin V (to detect early apoptotic cells) and propidium iodide (to detect late apoptotic or dead cells), followed by FACS® analysis. The results indicated no enhanced apoptosis in DN Akt–transfected cells (data not shown; see Fig. 6 A for Akt expression). Therefore, these results indicate that Akt positively regulates the NF-κB activity.
Figure 4.
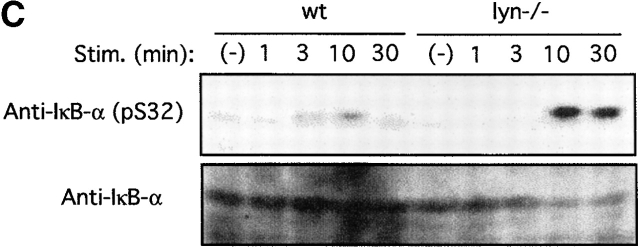
Akt regulation of the NF-κB pathway. (A) wt BMMCs were transfected with an NF-κB/luc reporter plasmid together with an empty vector or a vector encoding wt, E40K, or K179M Akt. 24 h later, cells were sensitized with IgE overnight. Cells were then stimulated with antigen for the last 8 h before luciferase assays. Representative results out of three transfection experiments are shown. (B) wt BMMCs were cotransfected with NF-κB/luc and either an empty vector, DN IκB-α (IκBαM), or DN IKKα (K44M). Results are representative of two experiments. Stim., stimulation. (C) wt and lyn − /− BMMCs were sensitized by IgE and stimulated with antigen for the indicated amounts of time. Cell lysates were analyzed by immunoblotting with antiphospho–IκB-α (phosphorylated Ser-32) antibody followed by reprobing with anti–IκB-α. Results are representative of two similar experiments. Stim., stimulation.
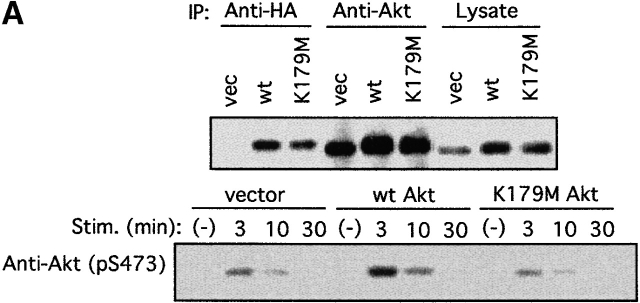
Figure 6.
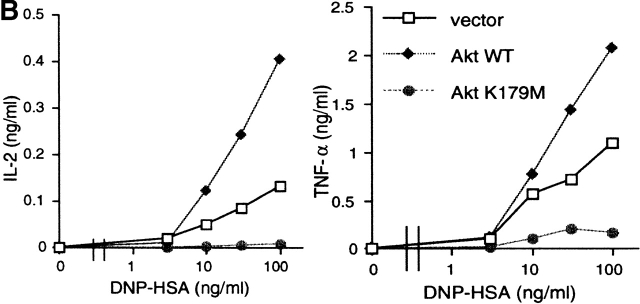
Akt regulates cytokine secretion in FcεRI-stimulated mast cells. Retroviruses coding for HA-tagged wt or K179M Akt were used to overexpress Akt proteins in stably transfected wt BMMCs. (A) Expression of HA-tagged Akt and endogenous Akt proteins was confirmed by immunoblot analysis of lysates or immune complexes precipitated with anti-HA or anti-Akt. Stable transfectants were sensitized with anti-DNP IgE and stimulated with DNP-HSA for the indicated periods of time. Cell lysates were also analyzed for Akt Ser-473 phosphorylation. Stim., stimulation; vec, vector. (B) Transfectants were sensitized with anti-DNP IgE and stimulated with the indicated concentrations of DNP-HSA for 20 h. IL-2 and TNF-α secreted into culture media were measured by ELISA. Values of secreted cytokines varied <10% from the averages among triplicate samples, and therefore SDs were not shown. Similar results were observed in another set of transfectants.
IκB regulates NF-κB by sequestering NF-κB in the cytoplasm, and phosphorylation by IKK and subsequent degradation of IκB releases this inhibition 60. Therefore, we examined whether DN IκB-α (IκBαM) and DN IKKα (K44M) affect NF-κB/luc reporter activity. As shown in Fig. 4 B, transcriptional activation of NF-κB was strongly inhibited by DN IκB-α or DN IKKα. In keeping with these results and the enhanced Akt phosphorylation in lyn − /− cells, phosphorylation of Ser-32 in IκB-α (by IKK) was severalfold higher in FcεRI-stimulated lyn − /− mast cells than that in wt cells (Fig. 4 C). These data are also consistent with a recent study that demonstrated that Akt can directly phosphorylate IKKα at Ser-32 81.
Transcription Factors NF-AT and AP-1 Are also Regulated by Akt.
Luciferase constructs driven by multiple copies of other cis-elements of the IL-2 promoter, i.e., NF-AT (−290 to −261, seven times) and AP-1 65, were also tested by cotransfection with wt or DN Akt (Fig. 5A and Fig. B). NF-AT/luc activity was significantly enhanced in FcεRI-stimulated, wt Akt–transfected cells compared with FcεRI-stimulated, vector-transfected cells. Importantly, these activities were inhibited by two DN Akt (K179M and AAA) mutants, indicating a role for Akt in signal transduction leading to activation of this transcription factor in mast cells. AP-1/luc activity was also affected significantly, albeit to a lesser extent, by wt or DN Akt expression.
Figure 5.
Akt regulation of NF-AT transcription factor. wt BMMCs were transfected with (A) NF-AT/luc or (B) AP-1/luc reporter plasmids together with an empty vector or a vector encoding wt Akt, K179M Akt, AAA Akt, or DN GSK-3β. 24 h later, cells were sensitized with IgE overnight. Cells were then stimulated with antigen for the last 8 h before luciferase assays. Shown are the representative results of two transfection experiments. Stim., stimulation. (C) wt BMMCs stably transfected with an empty vector, wt Akt or K179M Akt, were IgE sensitized and stimulated with antigen. Cell lysates were either directly analyzed by SDS-PAGE followed by immunoblotting with anti-phospho-ERK (for ERK assays) or immunoprecipitated with anti-JNK1 antibody before being subjected to kinase assays using GST-c-Jun as an exogenous substrate (for JNK1 assays). Equal expression of ERK1, ERK2, JNK1, and JNK2 was confirmed by probing immunoblots of total cell lysates with appropriate antibodies. Stim., stimulation. (D) wt and lyn − /− BMMCs were stimulated with IgE and antigen. Cell lysates were analyzed by immunoblotting with phosphospecific anti–GSK-3β (pSer9) antibody. The same blot was then reprobed with anti–GSK-3α/β antibody. Stim., stimulation.
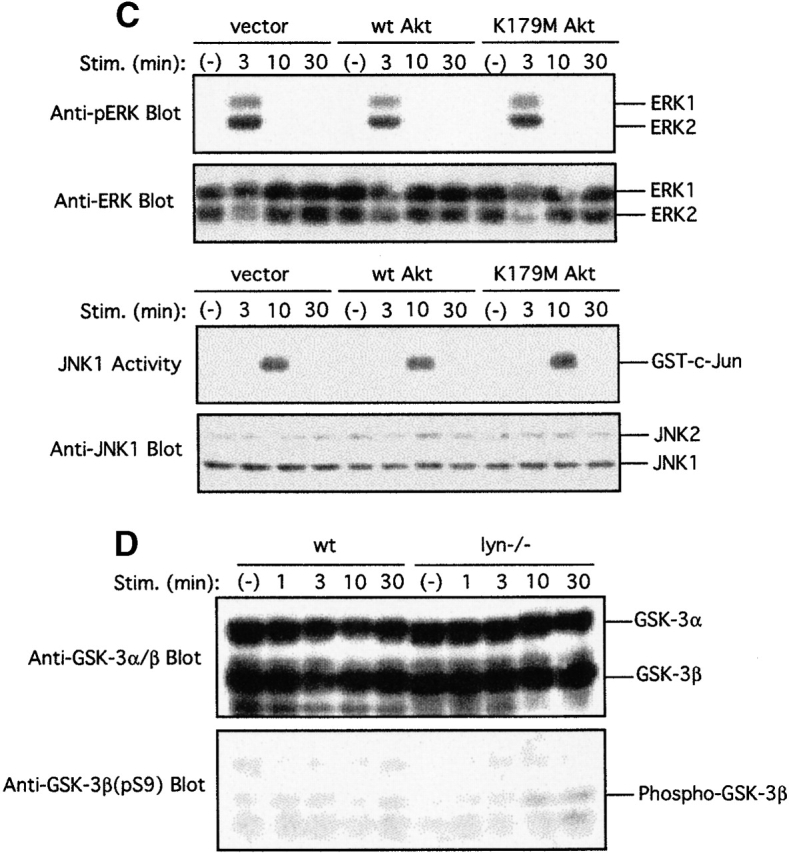
JNK is involved in the regulation of both NF-AT and AP-1 activities 41 65. Therefore, effects of Akt on JNK activity were examined with wt BMMCs stably transfected with wt or DN Akt (Fig. 5 C). FcεRI-induced JNK1 activation was not significantly affected by wt or DN Akt. Similar to JNK activity, FcεRI-induced ERK activation, as measured by phosphorylation at the activation-loop Thr-202 and Tyr-204 residues of ERK1 and ERK2, was barely affected by wt and DN Akt.
These data suggest that the effect of Akt on NF-AT and AP-1 activities is not through the regulation of JNK. GSK-3 controls the nuclear export of NF-AT 71. Phosphorylation at Ser-9 of GSK-3β by Akt inhibits its catalytic activity and blocks the nuclear export of NF-AT. Therefore, we compared the phosphorylation status of Ser-9 of GSK-3β between wt and lyn − /− BMMCs. As shown in Fig. 5 D, Ser-9 phosphorylation in GSK-3β was higher at ∼10–30 min after FcεRI stimulation in lyn − /− cells than in wt counterparts. A slower mobility of GSK-3α on SDS gels was observed at ∼10–30 min in wt cells, and this mobility shift was more pronounced in lyn − /− cells than in wt cells. These data are consistent with the notion that Akt-mediated NF-AT activation in FcεRI-stimulated mast cells is through the phosphorylation and inhibition of GSK-3 by Akt. To further examine whether GSK-3 is involved in the regulation of NF-AT activity, wt BMMC was transiently cotransfected with DN GSK-3β and NF-AT/luc plasmids. DN GSK-3β expression enhanced the FcεRI-induced activation of NF-AT–driven transcription (Fig. 5 A). Expression of DN GSK-3β also enhanced the FcεRI-induced activation of AP-1–driven transcription (Fig. 5 B). Given the lack of the effect of Akt on the activity of JNK and ERK, Akt-mediated AP-1 activation may also be through GSK-3, as GSK-3 is known to inhibit AP-1 activity by phosphorylating Jun 82.
Akt Regulates Cytokine Production in Mast Cells.
All the above data suggest that cytokine production may be regulated by Akt through the regulation of multiple transcription factors including NF-κB, NF-AT, and AP-1. To directly test this possibility, we mused puromycin-resistant, stable transfectants of wt BMMCs by retroviral infection with empty vector, HA-tagged wt, or K179M Akt viruses. Transfected Akt was expressed at approximately two- to threefold more than the endogenous Akt level (Fig. 6 A). Akt Ser-473 phosphorylation induced by FcεRI stimulation in wt Akt–transfected cells was higher than that in control vector–transfected cells, whereas it was less pronounced in K179M Akt–transfected cells (Fig. 6 A). More than 98% of these trasnfected cells were viable in culture medium containing IL-3 and SCF. Upon FcεRI stimulation, wt Akt transfectants secreted more IL-2 and TNF-α than vector-transfected cells, whereas K179M Akt transfectants secreted less than vector-transfectants (Fig. 6 B). lyn − /− BMMCs transfected with wt or K179M Akt viruses exhibited similar patterns of IL-2 and TNF-α production (data not shown). Taken together, these data indicate that Akt positively regulates IL-2 and TNF-α production and secretion in mast cells.
Discussion
This study indicates that Akt is activated by FcεRI stimulation in mast cells. Extracellular stimuli that induce Akt activation include growth factors, cytokines, and antigen receptors. Most, if not all, of these stimuli promote cell survival and proliferation. Indeed, FcεRI cross-linking and stimulation with SCF and IL-3, two major mast cell growth factors, exhibited these properties, i.e., survival/proliferation and Akt activation (Fig. 1; reference 83; data on Akt phosphorylation by IL-3 and SCF not shown). Although mechanisms by which Akt activation contributes to cell proliferation are not fully understood, some of the known Akt targets are involved in cell survival. A proapoptotic member of the Bcl-2 family BAD is phosphorylated at Ser-136 by Akt 67 84 85. The phosphorylated BAD becomes sequestered by 14-3-3 proteins and cannot heterodimerize with and inhibit the survival activity of the proteins Bcl-2 or Bcl-XL. However, some cytokine-mediated cell survival and Akt activation are not correlated with BAD phosphorylation 86 87 88. Caspase-9, an initiator caspase of apoptosis, is another target of Akt, and phosphorylation of caspase-9 at Ser-196 by Akt inhibits its protease activity 89. Another antiapoptotic mechanism by Akt is phosphorylation of forkhead family transcription factors. Phosphorylation of multiple sites in FKHRL1, AFX, and FKHR1 transcription factors results in inhibiting their transcriptional activity 90 91 92. FKHRL1 phosphorylated by Akt is bound to 14-3-3 proteins and retained in the cytoplasm, and is prevented from activating the gene(s) involved in apoptosis 90. It remains to be explored whether any of these known antiapoptotic mechanisms contribute to the mast cell survival/proliferation induced by FcεRI stimulation.
A hallmark of FcεRI-induced mast cell activation is the production and secretion of various cytokines including IL-1, IL-2, IL-3, IL-4, IL-6, IL-9, IL-13, GM-CSF, TNF-α, etc. Among this expanding list of cytokines, TNF-α is known to play a critical role in late phase reactions of hypersensitivity 19. Potential mechanisms for allergic inflammation may include the antiapoptotic effect of TNF-α on monocytes 93. Production of cytokines such as IL-2 and TNF-α is regulated at several steps: gene transcription, mRNA stability, translation, and posttranslational modification. One of the critical regulatory steps is transcription. As shown for other cell types, numerous cis-transcriptional elements that are binding sites for transcription factors are involved in transcriptional activation of the IL-2 gene in FcεRI-stimulated mast cells 65. This study provides evidence that Akt regulates transcriptional activity of NF-κB, NF-AT, and AP-1 that is critical for the expression of the IL-2 and TNF-α genes. Phosphorylation of the inhibitor IκB-α seems to be under the control of Akt as a signaling intermediary from Akt to NF-κB, because phosphorylation of IκB-α at Ser-32 (the phosphorylation site by IKK) was enhanced in lyn − /− mast cells in which Akt is hyperactivated. Because DN IκB-α and DN IKKα inhibit FcεRI-induced NF-κB activity, Akt may phosphorylate and activate IKK, and IKK in turn phosphorylates and promotes the degradation of IκB. Then free from IκB, NF-κB can translocate to the nucleus. During the revision of this manuscript, two groups showed that Btk regulates NF-κB in B cells 94 95, in apparent agreement with our results in mast cells. As several other kinases such as Cot, MEKK1-3, and NF-κB–inducing kinase were shown to be capable of phosphorylating IKK, it will be interesting to determine whether Akt directly phosphorylates IKKα in mast cells, as shown for TNF- and platelet-derived growth factor (PDGF)-stimulated cells 81 96.
GSK-3 phosphorylation by Akt may be involved in NF-AT and AP-1 activation in mast cells. Akt can phosphorylate and inhibit GSK-3 71. Consistent with this established fact, phosphorylation of GSK-3β at Ser-9 was enhanced in lyn − /− mast cells, which exhibit hyperactive Akt upon FcεRI stimulation. GSK-3 in turn is known to phosphorylate NF-AT and regulate the nuclear exit of this transcription factor. GSK-3 is also known to phosphorylate Jun proteins to inhibit AP-1 activity 82. Although Akt overexpression exhibited little effect on ERK and JNK activity, it significantly affected the activities of NF-AT and AP-1 (Fig. 5). Therefore, Akt-dependent phosphorylation of GSK-3 may regulate NF-AT and AP-1 without substantially affecting the canonical MAPK activation pathways, i.e., the Raf-1/MEK/ERK and MEKK/MAPK kinase 4 (or MAPK kinase 7)/JNK pathways.
In summary, we provide the first evidence for the involvement of Akt in FcεRI-induced production of IL-2 and TNF-α. Production of these cytokines is critical to late phase reactions of IgE-dependent hypersensitivity. Although Akt itself may not be a target for pharmaceutical interference to control allergic reactions and immunological diseases, our findings of Akt as a novel component of the signaling pathways leading to cytokine production have provided new insight into the exquisite networks of signaling molecules for mast cell activation.
Acknowledgments
We thank Drs. Amnon Altman, Gerald R. Crabtree, Peter Blume-Jensen, and James R. Woodgett for providing plasmids. We also thank Drs. Masayuki Fujimoto, Masami Narita, and Min Yang for providing some BMMC preparations.
This work was supported in part by National Institutes of Health grants AI-33617 and AI-38348 (to T. Kawakami). This article is publication no. 362 from the La Jolla Institute for Allergy and Immunology.
Footnotes
Abbreviations used in this paper: AP-1, activator protein 1; BMMC, bone marrow–derived mast cell; Btk, Bruton's tyrosine kinase; DN, dominant negative; ERK, extracellular signal–regulated kinase; GSK, glycogen synthase kinase; HA, hemagglutinin; HSA, human serum albumin; IKK, IκB kinase; ITAM, immunoreceptor tyrosine-based activation motif; JNK, c-Jun NH2-terminal kinase; MAPK, mitogen-activated protein kinase; MEKK, MEK kinase; NF-κB, nuclear factor κB; PDK, 3-phosphoinositide–dependent kinase; PH, pleckstrin homology; PI3K, phosphatidylinositol 3-kinase; PLC, phospholipase C; PTK, protein tyrosine kinase; RBL, rat basophilic leukemia; SCF, stem cell factor; SH, Src homology; wt, wild-type.
References
- Jones P.F., Jakubowicz T., Pitossi F.J., Maurer F., Hemmings B.A. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc. Natl. Acad. Sci. USA. 1991;88:4171–4175. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffer P.J., Woodgett J.R. Molecular cloning and characterization of a novel putative protein-serine kinase related to the cAMP-dependent and protein kinase C families. Eur. J. Biochem. 1991;201:475–481. doi: 10.1111/j.1432-1033.1991.tb16305.x. [DOI] [PubMed] [Google Scholar]
- Bellacosa A., Testa J.R., Staal S.P., Tsichlis P.N. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- Franke T.F., Kaplan D.R., Cantley L.C. PI3Kdownstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- Marte B.M., Downward J. PKB/Aktconnecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem. Sci. 1997;22:355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr. Opin. Cell. Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- Hemmings B.A. Akt signalinglinking membrane events to life and death decisions. Science. 1997;275:628–630. doi: 10.1126/science.275.5300.628. [DOI] [PubMed] [Google Scholar]
- Coffer P.J., Jin J., Woodgett J.R. Protein kinase B (c-Akt)a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem. J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T.O., Rittenhouse S.E., Tsichlis P.E. AKT/PKB and other D3 phosphoinositide-regulated kinaseskinase activation by phosphoinositide-dependent phosphorylation. Annu. Rev. Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- Alessi D.R., James S.R., Downes C.P., Holmes A.B., Gaffney P.R.J., Reese C.B., Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Stokoe D., Stephens L.R., Copeland T., Gaffney P.R.J., Reese C.B., Painter G.F., Holmes A.B., McCormick F., Hawkins P.T. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- Stephens L., Anderson K., Stokoe D., Erdjument-Bromage H., Painter G.F., Holmes A.B., Gaffney P.R.J., Reese C.B., McCormick F., Tempst P. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279:710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- Franke T.F., Kaplan D.R., Cantley L.C., Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- Klippel A., Kavanaugh W.M., Pot D., Williams L.T. A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain. Mol. Cell. Biol. 1997;17:338–344. doi: 10.1128/mcb.17.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andjelkovic M., Jakubowicz T., Cron P., Ming X.F., Han J.W., Hemmings B.A. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc. Natl. Acad. Sci. USA. 1996;93:5699–5704. doi: 10.1073/pnas.93.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi H., Matsuzaki H., Tanaka M., Ono Y., Tokunaga C., Kuroda S., Kikkawa U. Activation of RAC-protein kinase by heat shock and hyperosmolarity stress through a pathway independent of phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. USA. 1996;93:7639–7643. doi: 10.1073/pnas.93.15.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippa N., Sable C.L., Filloux C., Hemmings B., Van Obberghen E. Mechanism of protein kinase B activation by cyclic AMP-dependent protein kinase. Mol. Cell. Biol. 1999;19:4989–5000. doi: 10.1128/mcb.19.7.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi H., Fujiyoshi T., Fukui Y., Matsuzaki H., Yamamoto T., Ono Y., Andjelkovic M., Hemmings B.A., Kikkawa U. Activation of protein kinase B induced by H2O2 and heat shock through distinct mechanisms dependent and independent of phosphatidylinositol 3-kinase. J. Biochem. (Tokyo). 1999;126:1136–1143. doi: 10.1093/oxfordjournals.jbchem.a022559. [DOI] [PubMed] [Google Scholar]
- Galli S.J., Lantz C.S. Allergy. In: Paul W., editor. Fundamental Immunology. 4th ed. Lippincott-Raven Publishers; Philadelphia: 1998. pp. 1127–1174. [Google Scholar]
- Ravetch J.V., Kinet J.-P. Fc receptors. Annu. Rev. Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- Jouvin M.-H., Adamczewski M., Numerof R., Letourneur O., Valle A., Kinet J.-P. Differential control of the tyrosine kinases Lyn and Syk by the two signaling chains of the high affinity immunoglobulin E receptor. J. Biol. Chem. 1994;269:5918–5925. [PubMed] [Google Scholar]
- Kihara H., Siraganian R.P. Src homlogy 2 domains of Syk and Lyn bind to tyrosine-phosphorylated subunits of the high affinity IgE receptor. J. Biol. Chem. 1994;269:22427–22432. [PubMed] [Google Scholar]
- Rowley R.B., Burkhardt A.L., Chao H.-G., Matsueda G.R., Bolen J.B. Syk protein-tyrosine kinase is regulated by tyrosine-phosphorylated Igα/Igβ immunoreceptor tyrosine activation motif binding and autophosphorylation. J. Biol. Chem. 1995;270:11590–11594. doi: 10.1074/jbc.270.19.11590. [DOI] [PubMed] [Google Scholar]
- Cambier J.C. New nomenclature for the Reth motif (or ARH1/TAM/ARAM/YXXL) Immunol. Today. 1995;16:110. doi: 10.1016/0167-5699(95)80105-7. [DOI] [PubMed] [Google Scholar]
- Pribluda V., Pribluda C., Metzger H. Transphosphorylation as the mechanism by which the high-affinity receptor for IgE is phosphorylated upon aggregation. Proc. Natl. Acad. Sci. USA. 1994;91:11246–11250. doi: 10.1073/pnas.91.23.11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T., Sakamoto H., Appella E., Siraganian R.P. Conformational changes induced in the protein tyrosine kinase p72syk by tyrosine phosphorylation or by binding of phosphorylated immunoreceptor tyrosine-based activation motif peptides. Mol. Cell. Biol. 1996;16:1471–1478. doi: 10.1128/mcb.16.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hillal O., Kurosaki T., Yamamura H., Kinet J.-P., Scharenberg A.M. syk kinase activation by a src kinase-initiated activation loop phosphorylation chain reaction. Proc. Natl. Acad. Sci. USA. 1997;94:1919–1924. doi: 10.1073/pnas.94.5.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata M., Kurosaki T. A role for Bruton's tyrosine kinase in B cell antigen receptor–mediated activation of phospholipase C-γ2. J. Exp. Med. 1996;184:31–40. doi: 10.1084/jem.184.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluckiger A.-C., Li Z., Kato R.M., Wahl M.I., Ochs H.D., Longnecker R., Kinet J.-P., Witte O.N., Scharenberg A.M., Rawlings D.J. Btk/Tec kinases regulate sustained increases in intracellular Ca2+ following B-cell receptor activation. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:1973–1985. doi: 10.1093/emboj/17.7.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984;308:693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- Ozawa K., Szallasi Z., Kazanietz M.G., Blumberg P.M., Mischak H., Mushinski J.F., Beaven M.A. Ca2+-dependent and Ca2+-independent isozymes of protein kinase C mediate exocytosis in antigen-stimulated rat basophilic RBL-2H3 cells. Reconstitution of secretory responses with Ca2+ and purified isozymes in washed permeabilized cells. J. Biol. Chem. 1993;268:1749–1756. [PubMed] [Google Scholar]
- Fukamachi H., Takei M., Kawakami T. Activation of multiple protein kinases including a MAP kinase upon FcεRI cross-linking. Int. Arch. Allergy Immunol. 1993;102:15–25. doi: 10.1159/000236546. [DOI] [PubMed] [Google Scholar]
- Hirasawa N., Santini F., Beaven M.A. Activation of the mitogen-activated protein kinase/cytosolic phospholipase A2 pathway in a rat mast cell line. J. Immunol. 1995;154:5391–5402. [PubMed] [Google Scholar]
- Tsai M., Chen R.-H., Tam S.-Y., Blenis J., Galli S.J. Activation of MAP kinases, pp90rsk and pp70-S6 kinases in mouse mast cells by signaling through the c-kit receptor tyrosine kinase or FcεRIrapamycin inhibits activation of pp70-S6 kinase and proliferation in mouse mast cells. Eur. J. Immunol. 1993;23:3286–3291. doi: 10.1002/eji.1830231234. [DOI] [PubMed] [Google Scholar]
- Offermanns S., Jones S.V.P., Bombien E., Schultz G. Stimulation of mitogen-activated protein kinase activity by different secretory stimuli in rat basophilic leukemia cells. J. Immunol. 1994;152:250–261. [PubMed] [Google Scholar]
- Ishizuka T., Oshiba A., Sakata N., Terada N., Johnson G.L., Gelfand E.W. Aggregation of the FcεRI on mast cells stimulates c-Jun amino-terminal kinase activity. A response inhibited by wortmannin. J. Biol. Chem. 1996;271:12762–12766. doi: 10.1074/jbc.271.22.12762. [DOI] [PubMed] [Google Scholar]
- Kawakami Y., Miura T., Bissonnette R., Hata D., Khan W.N., Kitamura T., Maeda-Yamamoto M., Hartman S.E., Yao L., Alt F.W., Kawakami T. Bruton's tyrosine kinase regulates apoptosis and JNK/SAPK kinase activity. Proc. Natl. Acad. Sci. USA. 1997;94:3938–3942. doi: 10.1073/pnas.94.8.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa N., Scharenberg A.M., Yamamura H., Beaven M.A., Kinet J.-P. A requirement for Syk in the activation of the microtubule-associated protein kinase/phopholipase A2 pathway by FcεRI is not shared by a G protein-coupled receptor. J. Biol. Chem. 1995;270:10960–10967. doi: 10.1074/jbc.270.18.10960. [DOI] [PubMed] [Google Scholar]
- Kawakami Y., Kitaura J., Hartman S.E., Lowell C.A., Siraganian R.P., Kawakami T. Regulation of protein kinase CβI by two protein-tyrosine kinases, btk and Syk. Proc. Natl. Acad. Sci. USA. 2000;97:7423–7428. doi: 10.1073/pnas.120175097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- Shaw J.-P., Utz P.J., Durand D.B., Toole J.J., Emmel E.A., Crabtree G.R. Identification of a putative regulator of early T cell activation genes. Science. 1988;241:202–205. doi: 10.1126/science.3260404. [DOI] [PubMed] [Google Scholar]
- Rooney J.W., Sun Y.-L., Glimcher L.H., Hoey T. Novel NFAT sites that mediate activation of the interleukin-2 promoter in response to T-cell receptor stimulation. Mol. Cell. Biol. 1995;15:6299–6310. doi: 10.1128/mcb.15.11.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfeld A.E., McCaffrey P.G., Strominger J.L., Rao A. Identification of a novel cyclosporin-sensitive element in the human tumor necrosis factor α gene promoter. J. Exp. Med. 1993;178:1365–1379. doi: 10.1084/jem.178.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai E.Y., Jain J., Pesavento P.A., Rao A., Goldfeld A.E. Tumor necrosis factor alpha gene regulation in activated T cells involves ATF-2/Jun and NFATp. Mol. Cell. Biol. 1996;16:459–467. doi: 10.1128/mcb.16.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai E.Y., Yie J., Thanos D., Goldfeld A.E. Cell-type-specific regulation of the human tumor necrosis factor alpha gene in B cells and T cells by NFATp and ATF-2/JUN. Mol. Cell. Biol. 1996;16:5232–5244. doi: 10.1128/mcb.16.10.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A., Luo C., Hogan P.G. Transcription factors of the NFAT familyregulation and function. Annu. Rev. Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- Song H.Y., Regnier C.H., Kirschning C.J., Goeddel D.V., Rothe M. Tumor necrosis factor (TNF)-mediated kinase cascadesbifurcation of nuclear factor-κB and c-jun N-terminal kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2. Proc. Natl. Acad. Sci. USA. 1997;94:9792–9796. doi: 10.1073/pnas.94.18.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinin N.L., Boldin M.P., Kovalenko A.V., Wallach D. MAP3K-related kinase invoved in NF-κB induction by TNF, CD95 and IL-1. Nature. 1995;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- Lin X., Cunningham E.T., Jr., Mu Y., Geleziunas R., Greene W.C. The proto-oncogene Cot kinase participates in CD3/CD28 induction of NF-κB acting through the NF-κB-inducing kinase and IκB kinases. Immunity. 1999;10:271–280. doi: 10.1016/s1074-7613(00)80027-8. [DOI] [PubMed] [Google Scholar]
- Nakano H., Shindo M., Sakon S., Nishinaka S., Mihara M., Yagita H., Okumura K. Differential regulation of IκB kinase α and β by two upstream kinases, NF-κB-inducing kinase and mitogen-activated protein kinase/ERK kinase kinase-1. Proc. Natl. Acad. Sci. USA. 1998;95:3537–3542. doi: 10.1073/pnas.95.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F.S., Peters R.T., Dang L.C., Maniatis T. MEKK1 activates both IκB kinase α and IκB kinase β. Proc. Natl. Acad. Sci. USA. 1998;95:9319–9324. doi: 10.1073/pnas.95.16.9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto S., DiDonato J.A., Lin A. Coordinate regulation of IκB kinases by mitogen-activated protein kinase kinase kinase 1 and NF-κB-inducing kinase. Mol. Cell. Biol. 1998;18:7336–7343. doi: 10.1128/mcb.18.12.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Lee F.S. Mitogen-activated protein kinase/ERK kinase kinases 2 and 3 activate nuclear factor-κB through IκB kinase-α and IκB kinase-β. J. Biol. Chem. 1999;274:8355–8358. doi: 10.1074/jbc.274.13.8355. [DOI] [PubMed] [Google Scholar]
- DiDonato J.A., Hayakawa M., Rothwarf D.M., Zandi E., Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- Regnier C.H., Song H.Y., Gao X., Goeddel D., Cao Z., Rothe M. Identification and characterization of an IκB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- Zandi E., Rothwarf D.M., Delhase M., Hayakawa M., Karin M. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- Woronicz J.D., Gao X., Cao Z., Rothe M., Goeddel D.V. IκB kinase-βNF-κB activation and complex formation with IκB kinase-α and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- Mercurio F., Zhu H., Murray B.W., Shevchenko A., Bennett B.L., Li J., Young D.B., Barbosa M., Mann M., Manning A., Rao A. IKK-1 and IKK-2cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- Ghosh S., May M.J., Kopp E.B. NF-κB and Rel proteinsevolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Satterthwaite A.B., Lowell C.A., Khan W.N., Sideras P., Alt F.W., Witte O.N. Independent and opposing roles for Btk and Lyn in B and myeloid signaling pathways. J. Exp. Med. 1998;188:833–844. doi: 10.1084/jem.188.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami T., Inagaki N., Takei M., Fukamachi H., Coggeshall K.M., Ishizaka K., Ishizaka T. Tyrosine phosphorylation is required for mast cell activation by FcεRI cross-linking. J. Immunol. 1992;148:3513–3519. [PubMed] [Google Scholar]
- Zhang J., Berenstein E.H., Evans R.L., Siraganian R.P. Transfection of Syk protein tyrosine kinase reconstitutes high affinity IgE receptor-mediated degranulation in a Syk-negative variant of rat basophilic leukemia RBL-2H3 cells. J. Exp. Med. 1996;184:71–79. doi: 10.1084/jem.184.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craxton A., Jiang A., Kurosaki T., Clark E.A. Syk and Bruton's tyrosine kinase are required for B cell antigen receptor-mediated activation of the kinase Akt. J. Biol. Chem. 1999;274:30644–30650. doi: 10.1074/jbc.274.43.30644. [DOI] [PubMed] [Google Scholar]
- Hata D., Kitaura J., Hartman S.E., Kawakami Y., Yokota T., Kawakami T. Bruton's tyrosine kinase-mediated interleukin-2 gene activation in mast cells Dependence on the c- JunN-terminal kinase activation pathway. J. Biol. Chem. 2731998. 10979 10987 [DOI] [PubMed] [Google Scholar]
- Konishi H., Shinomiya T., Kuroda S., Ono Y., Kikkawa U. Molecular cloning of rat RAC protein kinase α and β and their association with protein kinase C ζ. Biochem. Biophys. Res. Commun. 1994;205:817–825. doi: 10.1006/bbrc.1994.2738. [DOI] [PubMed] [Google Scholar]
- Blume-Jensen P., Janknecht R., Hunter T. The Kit receptor promotes cell survival via activation of PI3-kinase and subsequent Akt-mediated phosphorylation of Bad on Ser136. Curr. Biol. 1998;8:779–782. doi: 10.1016/s0960-9822(98)70302-1. [DOI] [PubMed] [Google Scholar]
- Wang Q., Somwar R., Bilan P.J., Liu Z., Jin J., Woodgett J.R., Klip A. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol. Cell. Biol. 1999;19:4008–4018. doi: 10.1128/mcb.19.6.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Antwerp D.J., Martin S.J., Kafri T., Green D.R., Verma I.M. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- Lin X., Mu Y., Cunningham E.T., Jr., Marcu K.B., Geleziunas R., Greene W.C. Molecular determinants of NF-κB-inducing kinase action. Mol. Cell. Biol. 1998;18:5899–5907. doi: 10.1128/mcb.18.10.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beals C.R., Sheridan C.M., Turck C.W., Gardner P., Crabtree G.R. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275:1930–1934. doi: 10.1126/science.275.5308.1930. [DOI] [PubMed] [Google Scholar]
- Li H.-L., Davis W.W., Whiteman E.L., Birnbaum M.J., Pure E. The tyrosine kinases Syk and Lyn exert opposing effects on the activation of protein kinase Akt/PKB in B lymphocytes. Proc. Natl. Acad. Sci. USA. 1999;96:6890–6895. doi: 10.1073/pnas.96.12.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M.R., Scheid M.P., Santos L., Dang-Lawson M., Roth R.A., Matsuuchi L., Duronio V., Krebs D.L. The B cell antigen receptor activates the Akt (protein kinase B)/glycogen synthase kinase-3 signaling pathway via phosphatidylinositol 3-kinase. J. Immunol. 1999;163:1894–1905. [PubMed] [Google Scholar]
- Yano H., Nakanishi S., Kimura K., Hanai N., Saitoh Y., Fukui Y., Nonomura Y., Matsuda Y. Inhibition of histamine secretion by wortmannin through the blockade of phosphatidylinositol 3-kinase in RBL-2H3 cells. J. Biol. Chem. 1993;268:25846–25856. [PubMed] [Google Scholar]
- Eiseman E., Bolen J.B. Engagement of the high-affinity IgE receptor activates src protein-related tyrosine kinases. Nature. 1992;355:78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- Hutchcroft J.E., Geahlen R.L., Deanin G.G., Oliver J.M. FcεRI-mediated tyrosine phosphorylation and activation of the 72-kDa protein-tyrosine kinase, PTK72, in RBL-2H3 rat tumor mast cells. Proc. Natl. Acad. Sci. USA. 1992;89:9107–9111. doi: 10.1073/pnas.89.19.9107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y., Yao L., Miura T., Tsukada S., Witte O.N., Kawakami T. Tyrosine phosphorylation and activation of Bruton tyrosine kinase upon FcεRI cross-linking. Mol. Cell. Biol. 1994;14:5108–5113. doi: 10.1128/mcb.14.8.5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimura T., Furitsu T., Morimoto M., Isozaki K., Nomura S., Matsuzawa Y., Kitamura Y., Kanakura Y. Ligand-independent activation of c-kit receptor tyrosine kinase in a murine mastocytoma cell line P-815 generated by a point mutation. Blood. 1994;83:2619–2626. [PubMed] [Google Scholar]
- Kawakami Y., Kitaura J., Satterthwaite A.B., Kato R.M., Asai K., Hartman S.E., Maeda-Yamamoto M., Lowell C.A., Rawlings D.J., Witte O.N., Kawakami T. Redundant and opposing functions of two tyrosine kinases, btk and lyn, in mast cell activation. J. Immunol. 2000;165:1210–1219. doi: 10.4049/jimmunol.165.3.1210. [DOI] [PubMed] [Google Scholar]
- Hata D., Kawakami Y., Inagaki N., Lantz C.S., Kitamura T., Khan W.N., Maeda-Yamamoto M., Miura T., Han W., Hartman S.E. Involvement of Bruton's tyrosine kinase in FcεRI-dependent mast cell degranulation and cytokine production. J. Exp. Med. 1998;187:1235–1247. doi: 10.1084/jem.187.8.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozes O.N., Mayo L.D., Gustin J.A., Pfeffer S.R., Pfeffer L.M., Donner D.B. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- Nikolakaki E., Coffer P.J., Hemelsoet R., Woodgett J.R., Defize L.H.K. Glycogen synthase kinase 3 phosphorylates Jun family members in vitro and negatively regulates their transactivating potential in intact cells. Oncogene. 1993;8:833–840. [PubMed] [Google Scholar]
- Nechushtan H., Razin E. Regulation of mast cell growth and proliferation. Crit. Rev. Oncol. Hematol. 1996;23:131–150. doi: 10.1016/1040-8428(96)00200-4. [DOI] [PubMed] [Google Scholar]
- Datta S.R., Dudek H., Tao X., Masters S., Fu H., Gotoh Y., Greenberg M.E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- del Peso L., Gonzalez-Garcia M., Page C., Herrera R., Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- Scheid M.P., Duronio V. Dissociation of cytokine-induced phosphorylation of Bad and activation of PKB/aktinvolvement of MEK upstream of Bad phosphorylation. Proc. Natl. Acad. Sci. USA. 1998;95:7439–7444. doi: 10.1073/pnas.95.13.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton H.J., Welham M.J. Cytokine-induced protein kinase B activation and Bad phosphorylation do not correlate with cell survival of hemopoietic cells. J. Immunol. 1999;162:7002–7009. [PubMed] [Google Scholar]
- Dijkers P.F., van Dijk T.B., de Groot R.P., Raaijmakers J.A.M., Lammers J.-W.J., Koenderman L., Coffer P.J. Regulation and function of protein kinase B and MAP kinase activation by the IL-5/GM-CSF/IL-3 receptor. Oncogene. 1999;18:3334–3342. doi: 10.1038/sj.onc.1202678. [DOI] [PubMed] [Google Scholar]
- Cardone M.H., Roy N., Stennicke H.R., Salvesen G.S., Franke T.F., Stanbridge E., Frisch S., Reed J.C. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- Brunet A., Bonni A., Zigmund M.J., Lin M.Z., Juo P., Hu L.S., Anderson M.J., Arden K.C., Blenis J., Greenberg M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Kops G.J.L.P., de Ruiter N.D., De Vries-Smits A.M.M., Powell D.R., Bos J.L., Burgering B.M.Th. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- Biggs W.H., III, Meisenhelder J., Hunter T., Cavenee W.K., Arden K.C. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl. Acad. Sci. USA. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh N., Kraft S., Wessendorf J.H.M., Bieber T. The high-affinity IgE receptor (FcεRI) blocks apoptosis in normal human monocytes. J. Clin. Invest. 2000;105:183–190. doi: 10.1172/JCI6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpai U.D., Zhang K., Teutsch M., Sen R., Wortis H.H. Bruton's tyrosine kinase links the B cell receptor to nuclear factor κB activation. J. Exp. Med. 2000;191:1735–1744. doi: 10.1084/jem.191.10.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petro J.B., Rahman S.M.J., Ballard D.W., Khan W.N. Bruton's tyrosine kinase is required for activation of IκB kinase and nuclear factor κB in response to B cell receptor engagement. J. Exp. Med. 2000;191:1745–1753. doi: 10.1084/jem.191.10.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romashkova J.A., Makarov S.S. NF-κB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]