Abstract
Bruton's tyrosine kinase (Btk) is a nonreceptor tyrosine kinase involved in precursor B (pre-B) cell receptor signaling. Here we demonstrate that Btk-deficient mice have an ∼50% reduction in the frequency of immunoglobulin (Ig) λ light chain expression, already at the immature B cell stage in the bone marrow. Conversely, transgenic mice expressing the activated mutant BtkE41K showed increased λ usage. As the κ/λ ratio is dependent on (a) the level and kinetics of κ and λ locus activation, (b) the life span of pre-B cells, and (c) the extent of receptor editing, we analyzed the role of Btk in these processes. Enforced expression of the Bcl-2 apoptosis inhibitor did not alter the Btk dependence of λ usage. Crossing 3-83μδ autoantibody transgenic mice into Btk-deficient mice showed that Btk is not essential for receptor editing. Also, Btk-deficient surface Ig+ B cells that were generated in vitro in interleukin 7-driven bone marrow cultures manifested reduced λ usage. An intrinsic defect in λ locus recombination was further supported by the finding in Btk-deficient mice of reduced λ usage in the fraction of pre-B cells that express light chains in their cytoplasm. These results implicate Btk in the regulation of the activation of the λ locus for V(D)J recombination in pre-B cells.
Keywords: Btk, B lymphocytes, Ig L chain, receptor editing, V(D)J rearrangements
Introduction
During early B cell development in the bone marrow, Ig H and L chain variable region genes are assembled from component V, (D), and J gene segments (for reviews, see references 1 2 3). V(D)J recombination is a highly ordered process, initiated by DNA rearrangements at the H chain in pro-B cells. The expression of a functional μ H chain is monitored through the formation of a (pre-) B cell receptor (BCR) complex, together with λ5 and Vpre-B proteins. Signaling through the pre-BCR results in feedback inhibition of H chain VDJ recombination to ensure allelic exclusion and IL-7–dependent proliferative expansion. The rapidly proliferating pre-B cells then exit the cell cycle and perform Ig L chain rearrangements, leading to the deposition of complete Ig molecules on the cell surface 2 3. The murine κ L chain locus contains 70–90 functional Vκ gene segments, present in both orientations relative to the four functional Jκ elements. By contrast, the λ locus in the mouse is very small; it consists of only three functional Vλ and three functional Jλ segments. Ig κ and λ L chain rearrangements occur independently and at different kinetics, with sequential activation of the κ and λ loci 4 5 6. The privileged activation of the κ locus is thought to determine the final ratio of κ+ to λ+ mature B cells of 95:5 in wild-type (WT) mice.
PCR analyses of the Ig κ L chain locus in single developing B cells in the bone marrow have demonstrated the presence of multiple in- and out-of-frame rearrangements in small pre-B cells 6. These findings indicated that the BCR does not signal allelic or isotypic exclusion of the Ig κ or λ L chain loci, allowing secondary L chain rearrangements to occur. Cells with a first productive rearrangement on one allele are rapidly selected to enter the immature B cell compartment 6. The rearrangement machinery remains active in immature B cells and is only turned off at the transition to mature B cells 7 8 9. If an immature B cell expresses an Ig that has reactivity with an autoantigen in the bone marrow, continued Ig L chain rearrangement can be induced to rescue autoreactive B cells from tolerance elimination, a phenomenon called receptor editing 10 11 12.
One of the molecules that is involved in pre-BCR signaling and thereby directs B cell development is the Tec family nonreceptor Bruton's tyrosine kinase (Btk; for a review, see reference 13). Upon BCR stimulation, Btk phosphorylation and kinase activity are increased 14 15 16 probably by the activity of Src family kinases 13 17. Concomitantly, Btk is targeted to the plasma membrane by the binding of its pleckstrin homology (PH) domain to the second messenger phospatidylinositol-(3,4,5)triphosphate 18. Binding of Btk to the linker protein BLNK/SLP-65 is crucial for phosphorylation and activation of phospholipase Cγ2 by Btk, implicating Btk as a mediator of BCR-induced Ca2+ mobilization 19.
Mutations in Btk lead to X-linked agammaglobulinemia (XLA) in humans and X-linked immunodeficiency (xid) in mice (for reviews, see references 20 21 22 23). XLA is characterized by an almost complete arrest of B cell development at the pre-B cell level 24 25. As a result, XLA patients have profoundly reduced numbers of B cells in the peripheral blood, and serum Ig levels of all classes are very low 24. Btk-deficient mice display a milder phenotype, mainly reflecting poor survival of peripheral B cells. B cell numbers in the spleen and lymph nodes are reduced by 50%, and IgM and IgG3 levels in the serum are low 20 26. Nevertheless, mouse Btk-deficient cells also showed an impaired transition from pre-B to immature B cells by analysis of competition in vivo between Btk+ and Btk− cells in females heterozygous for a targeted mutation in the Btk gene 27.
Both in XLA patients and in xid mice the absence of Btk appeared to result in a decrease of Ig λ L chain usage in peripheral B cells 24 28 29. The reduced frequency of Ig λ L chain–expressing cells could either reflect an intrinsic feature of the L chain rearrangement process in the absence of Btk signaling or could alternatively be secondary to an altered antigen-dependent peripheral repertoire selection in Btk-deficient mice. To distinguish between these two possibilities, we determined the proportions of Ig λ+ B cells during B cell differentiation in Btk-deficient mice, as well as in transgenic mice that express a constitutively activated form of Btk, the E41K PH domain mutant 27 30. As the κ to λ ratio is dependent on (a) the level and kinetics of the activation of the κ and λ loci for recombination, (b) the life span of pre-B cells that are in the process of L chain rearrangement, and (c) the extent of receptor editing of autoreactive B cells, we analyzed the involvement of Btk in these processes in detail.
Materials and Methods
Mice.
Btk − /lacZ mice 27 were crossed on a C57BL/6 background for over five generations. CD19-hBtk WT and CD19-hBtk E41K transgenic mice that express WT and E41K-mutated human Btk (hBtk), respectively, under the control of the CD19 promoter region have been described previously 30 and were on a mixed background containing 129/Sv, C57BL/6, and FVB. For the generation of λ5-hBtk E41K mice we used a 1.5-kb NotI-BglII fragment containing the murine λ5 promoter and a 6.5-kb ClaI-NotI fragment with the 3′ locus control region of the λ5-Vpre-B1 locus 31. The promoter and locus control region were cloned into the unique SwaI and SmaI sites, respectively, present in the cosmid vector pTL5 containing a BglII-NotI-XhoI-SwaI-PvuI-KpnI-SmaI-NotI-BglII polylinker 32. Next, an ∼27-kb PvuI-NotI fragment containing the E41K-mutated hBtk cDNA/genomic DNA fragment 32 was cloned into the λ5/pTL5 vector, using the PvuI and KpnI sites in the polylinker. The ∼35-kb NotI insert of the λ5-hBtk E41K construct was excised from the vector and purified, using standard methods. DNA (∼2–4 ng/μl) was injected into the pronuclei of FVB fertilized oocytes, which were subsequently implanted into pseudopregnant foster mice. Founder mice were crossed with Btk − /lacZ mice. Eμ -2-22 Bcl-2 transgenic mice 33 and 3-83μδ mice 10 were on a C57BL/6 and a B10.D2 background, respectively. All mice were bred and maintained in the animal care facility at the Erasmus University Rotterdam.
Mouse Genotyping.
To determine the Btk genotype and score the presence of the CD19-hBtk WT or CD19-hBtk E41K transgenes, tail DNA was analyzed by Southern blot analysis of BamHI digests, as described previously 27 30. The presence of the Eμ -2-22 Bcl-2 transgene was evaluated by PCR, using primers that are specific for the SV40 DNA sequences flanking the Bcl-2 transgene: 5′-GGCACTATACATCAAATATTC-3′ and 5′-TGAAGGAACCTTACTTCTGT-3′. The presence of the 3-83μδ transgene was identified by Southern blot analysis of BamHI or EcoRI digests, using a Jκ-specific probe as described previously 34.
Flow Cytometric Analyses.
Preparation of single cell suspensions, flow cytometry, and determination of β-galactosidase activity by loading cells with fluorescein-di-β-galactopyranoside substrate have been described previously 27 32. Events (5 × 104–5 × 105) were scored using a FACSCalibur™ flow cytometer and analyzed by CELLQuest™ software (Becton Dickinson). The following mAbs were obtained from BD PharMingen: FITC-conjugated anti-B220–RA3-6B2, anti-κ–R5-240, and anti-IgM, PE-conjugated anti-CD19, anti-CD43, and anti-H2–Kd; and CyChrome-conjugated anti-B220–RA3-6B2 and biotinylated anti-CD19, anti-λ1 and λ2–R26-46, anti–IgM, and anti-H2–Kb. PE-conjugated anti-IgD was from Southern Biotechnology Associates, Inc. The anti–3-83 clonotype 54.1 antibody has been described previously 35. Secondary antibodies were PE-conjugated goat anti–rat, Tri-color, or allophycocyanin-conjugated Streptavidin, purchased from Caltag. Affinity-purified polyclonal rabbit anti-Btk (BD PharMingen) was used for intracellular flow cytometric detection of cytoplasmic Btk protein using FITC-conjugated goat anti–rabbit total Ig (Nordic) as a secondary antibody 32.
IL-7–driven Bone Marrow Cultures.
Primary pre-B cell cultures were performed as described previously 36. In brief, bone marrow cells were depleted of erythrocytes by standard ammonium-chloride lysis, and subsequently IgM− B cells were purified by negative selection. Cell suspensions were labeled with biotinylated anti-IgM (BD PharMingen) and incubated with Streptavidin-coated microbeads (Miltenyi Biotec). After cell separation using MACS column purification, the IgM− fraction was collected and purity was confirmed by flow cytometry. Cells were cultured in IMDM medium, supplemented with 10% heat-inactivated FCS at 2 × 106 cells/well in 24-well plates at 37°C in the presence of 100 U/ml of recombinant murine IL-7 (R&D Systems). After 5 d of culture, cells were washed and recultured on S17 stromal cells with or without 100 U/ml IL-7 for 48 h.
Results
Reduced Ig λ L Chain Usage throughout B Cell Development in Btk-deficient Mice.
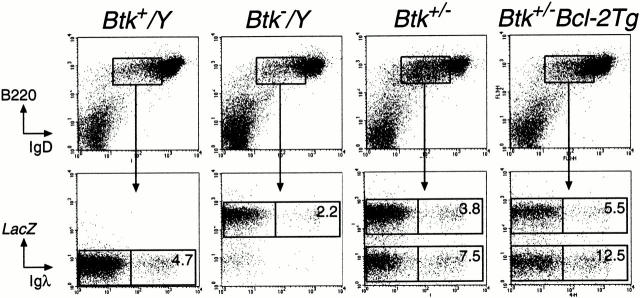
The expression of Ig λ L chain was investigated in Btk-deficient mice, in which the Btk gene is inactivated by a targeted insertion of a lacZ reporter 27. Total splenic cell suspensions were analyzed by three-color flow cytometry, using an antibody specific for the Ig λ1 and λ2 L chain constant regions in conjunction with anti-B220 and anti-CD19. The proportions of B cells that expressed Ig λ L chain on the cell surface were 4.9% ± 0.2 (n = 7) and 5.0 ± 0.2 (n = 6) in the spleen of Btk+ and Btk+/− control mice, respectively. By contrast, we observed a significant reduction of this proportion in Btk− mice (2.3 ± 0.4; n = 10).
Next, λ expression was determined in individual B cell subpopulations in bone marrow and spleen. We performed four-color flow cytometry experiments, using anti-λ L chain antibodies in combination with mAbs specific for the B220, IgM, and IgD surface markers, which define successive stages of B cell development (Fig. 1). In Btk+ mice, 10–16% of cells within the immature B cell subpopulations, including immature IgM+IgD− and transitional IgM+ IgDlow B cells in the bone marrow and immature IgMhigh IgDlow B cells in the spleen, were found to express Ig λ L chain. By contrast, in the Btk− mice only 5–7% of cells within these subpopulations were λ+, i.e., about half the number found in WT mice. In the mature B cell subpopulations, including the IgM+IgDhigh cells in bone marrow and spleen, Ig λ L chain was expressed in 4–5% of cells in Btk+ mice and in 2.5–3.5% of cells in Btk− mice. Finally, we found that in the subpopulation of large lymphoblastoid cells in the spleen of Btk+ and Btk− mice 8–12% and 4–6% were λ+, respectively (Fig. 1). In Btk+/− heterozygous mice, intermediate values of λ+ cells were found in the bone marrow immature cells (data not shown).
Figure 1.
Ig λ L chain expression during B cell development in Btk+ and Btk− mice. Bone marrow and spleen single cell suspensions were stained with mAbs specific for B220, IgM, IgD, and Ig λ. Spleen lymphoblasts were gated based on large forward scatter values. The indicated B cell subpopulations were gated (top) and analyzed for B220 and Ig λ L chain expression (bottom). On the basis of IgM and IgD expression, B cell subpopulations were defined in the bone marrow: E, IgM+IgD− immature B cells; ET, IgM+IgDlow transitional immature B cells; F, IgM+IgD+ mature recirculating B cells (reference 47). In the spleen: I, mature IgMlow IgDhigh; II, IgMhighIgDhigh; III, immature IgMhighIgDlow B cells. Data are displayed as dot plots, and the percentages of λ+ cells of the B220+ B cell population are indicated. Data shown are representative of 10 Btk+ and 8 Btk− mice animals examined.
These findings demonstrate that the absence of Btk leads to a significantly reduced frequency of λ usage, already from the immature B cell stage onwards.
Reduced λ L Chain Usage Is an Intrinsic Feature of Btk-deficient B Cells.
Although we demonstrated that Ig L chain isotype usage is determined in the bone marrow, the possibility remained that the reduced λ usage did not reflect an intrinsic feature of Btk-deficient B cells. Alternatively, this phenomenon could originate from the xid immune status of the Btk-deficient mice, which may, directly or indirectly, affect selection events at the pre-B to B cell progression.
To address this issue, we analyzed heterozygous Btk+/− female mice, which do not manifest the xid phenotype due to the selective advantage of B cells that have the intact Btk + allele on their active X chromosome 27. Because of the process of random X chromosome inactivation, ∼50% of the B cell progenitors have the disrupted Btk − /lacZ + allele on the active X chromosome. When cells reach a differentiation stage at which Btk is required, their further development is hampered. As a consequence of this selective disadvantage, the proportions of Btk − /lacZ + cells decrease below the value of 50%, finally to undetectable levels in the mature peripheral B cells 27.
We analyzed splenic cell samples from Btk+/− heterozygous females for λ usage in four-color flow cytometry experiments, using fluorescein-di-β-galactopyranoside as a fluorogenic substrate in conjunction with surface labeling to define the immature IgDlowB220+ B cell subpopulation in the spleen. In this fraction, ∼30% of the cells expressed lacZ, enabling a separate analysis of the Btk + /lacZ − and Btk − /lacZ + B cell populations. We found that the proportion of Ig λ+ cells within the Btk− immature IgDlowB220+ subpopulation was reduced to 3.6% ± 0.7, when compared with Btk+ immature B cells (6.3% ± 0.9; Fig. 2).
Figure 2.
Separate effects of Btk and Bcl-2 on Ig λ L chain usage. Four-color flow cytometric analyses of spleen cells of the indicated mice. The immature IgDlowB220+ compartment was gated (top) and analyzed for lacZ and λ L chain expression (bottom). The percentages of λ+ cells within the indicated lacZ+ or lacZ− subpopulations are given. Data are shown as dot plots representative of the 4–10 mice examined.
These results show that reduced λ usage is an intrinsic feature of Btk-deficient B cells, which is independent of the xid immune status of the mice.
Bcl-2 Overexpression Does Not Alter the Btk Dependence of Ig L Chain Isotype Usage.
The finding of reduced λ expression in Btk-deficient cells may be explained by a role for Btk signal transduction in extending the life span of pre-B cells that are in the process of L chain rearrangement. Enforced expression of the antiapoptotic Bcl-2 gene results in elevated Ig λ expression in mature B cells 37. As the Ig κ and λ loci are sequentially activated for VL-JL recombination, Bcl-2 gene is assumed to provide an extended time window per cell for Ig L chain rearrangement 37. To investigate a role for Btk in pre-B cell survival, we have crossed Btk − /lacZ mice onto an Eμ-Bcl-2 transgenic background 33. We investigated λ usage in Eμ-Bcl-2 transgenic Btk+/− female mice, in which the Btk+ and Btk− immature splenic IgDlowB220+ B cell population can be analyzed within a single animal. In these mice the Btk+ subpopulation had a significantly higher percentage (12.8% ± 1.3; n = 4) of λ+ B cells, compared with the Btk− subpopulation (7.3% ± 1.5; Fig. 2).
These analyses demonstrate that even if the life span of pre-B cells is extended by the presence of the Bcl-2 transgene, the Btk-deficient B cell population still manifests an ∼50% reduction in λ usage. Therefore, we conclude that protection of pre-B cells from apoptosis does not alter the Btk dependence of the frequency of λ usage.
Expression of a Constitutively Activated Btk Mutant Increases λ Usage.
To further test the involvement of Btk signaling in the mechanism that sets the κ to λ isotype ratio in vivo, we examined mice that express the constitutively activated BtkE41K mutant. This Glu-to-Lys PH domain Btk mutant shows increased membrane localization in quiescent cells, independent of phosphatidylinositol 3-kinase activity 38 39. In CD19-hBtk E41K transgenic mice, which express BtkE41K under the control of the CD19 promoter region, B cell development is almost completely arrested at the immature B cell stage in the bone marrow, probably because the BtkE41K mutant mimics BCR occupancy by autoantigens (30; Fig. 3).
Figure 3.
Increased λ usage in CD19-hBtk E41K mice independent of cell survival. Four-color flow cytometric analyses of bone marrow and spleen of the indicated mice. Single cell suspensions were stained with mAbs specific for B220, IgM, IgD, and Ig λ. On the basis of IgM and IgD expression, the immature IgM+IgD−/low B cell population in the bone marrow (top, fraction E+ET; see legend to Fig. 1) and the total IgM+IgD+ B cell population in the spleen (bottom) were gated and analyzed for λ usage. Data shown are representative of five to nine mice examined per group. At the bottom, the total splenic B cell numbers of the indicated animals are given as mean values ± SD (determined by flow cytometric analysis using mAbs to B220 and CD19).
In four-color flow cytometry experiments using antibodies to B220, IgM, IgD, and Ig λ, the CD19-hBtk E41K transgenic mice manifested a significant increase in the frequency of λ usage (∼11% in the IgM+IgD−/low immature B cell fraction in the bone marrow compared with ∼6% in nontransgenic littermates; Fig. 3). This increase was specifically associated with the expression of the Btk E41K mutation, as the control CD19-hBtk WT transgenic mice in which the CD19 promoter drives expression of WT hBtk contained normal proportions of λ+ B cells (Fig. 3). We conclude that the presence of constitutively activated Btk enhances λ usage.
Ig λ Usage in CD19-hBtkE41K Eμ-Bcl-2 Double Transgenic Mice.
To confirm that Btk-mediated signaling increases λ usage, independent of the life span of pre-B cells, we crossed CD19-hBtk E41K and CD19-hBtk WT transgenic mice onto the Eμ-Bcl-2 background. The enforced expression of Bcl-2 partially prevented central deletion of B cells in CD19-hBtk E41K transgenic mice, as was evident from the presence of substantial numbers of mature B cells in bone marrow and spleen of CD19-hBtk E41K Eμ-Bcl-2 double transgenic mice (Fig. 3). We analyzed Ig λ L chain expression in the B cell populations in bone marrow and spleen from Eμ-Bcl-2 transgenic, CD19-hBtk E41K Eμ-Bcl-2, and CD19-hBtk WT Eμ-Bcl-2 double transgenic mice (Fig. 3). Consistent with previous reports 37, Eμ-Bcl-2 mice or CD19-hBtk WT Eμ-Bcl-2 double transgenic mice had significantly higher percentages of λ+ cells in the immature IgM+IgD−/low B cell population in the bone marrow (∼24%), when compared with nontransgenic or CD19-hBtk WT littermates (∼7%). Most importantly, in CD19-hBtk E41K Eμ-Bcl-2 double transgenic mice we found even higher proportions of Ig λ+ cells (∼35–40%). Similar significant differences were found in the mature recirculating IgM+IgD+ B cell population in the bone marrow and in the spleen (data not shown, and Fig. 3). Therefore, we conclude that the BtkE41K-mediated increase of λ usage cannot be explained by an effect of this mutant on pre-B cell survival.
Btk Signaling Is Not Essential for Receptor Editing.
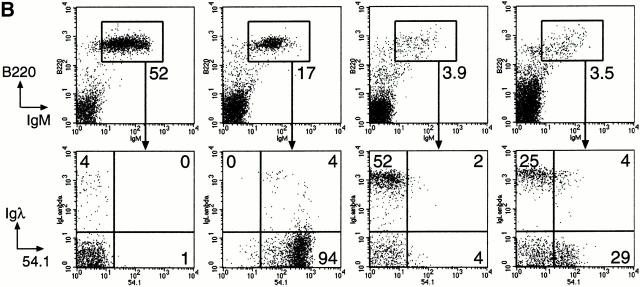
As receptor editing may occur frequently during normal B cell development and is accompanied by increased λ usage 10 12 40, we tested whether the reduced λ expression in Btk-deficient B cells results from the inability of these cells to perform receptor editing. Therefore, Btk-deficient mice were crossed with 3-83μδ transgenic mice bearing rearranged H and L chain genes encoding an antibody that specifically recognizes MHC class I H-2Kk,b 10. On a nondeleting H-2Kd background, the 3-83μδ mice contain a virtually monoclonal B cell population bearing the 3-83μδ BCR, as identified by the antiidiotypic antibody 54.1 (Fig. 4). In contrast, centrally deleting 3-83μδ/H2-Kb mice exhibit the phenotype of central B cell tolerance in which idiotype-positive B cells are present in the bone marrow (Fig. 4 A), but are efficiently deleted from the spleen and lymph nodes 10. Only small numbers of B cells are present in the spleen and lymph nodes and most of these lack the autoreactive specificity (>94% and >99%, respectively; Fig. 4b and Fig. c). These B cells, which have performed receptor editing, express the transgenic H chain together with endogenous L chain, a large fraction (∼50%) of which is λ (10; Fig. 4b and Fig. c). Btk-deficient 3-83μδ/H2-Kb mice manifested similar B cell numbers in the spleen and lymph nodes, when compared with Btk+ littermates. Deletion of autoreactive 3-83μδ–expressing B cells occurred in the absence of Btk, but was less efficient in the spleen than in lymph nodes (∼70 and ∼97% of B cells were 54.1−, respectively; Fig. 4b and Fig. c). In the Btk-deficient 3-83μδ/H2-Kb mice, significant numbers of idiotype-negative B cells were present, 25–40% of which expressed Ig λ L chain. Therefore, we conclude that receptor editing can occur in Btk-deficient autoreactive immature B cells.
Figure 4.
Analysis of receptor editing in 3-83μδ transgenic Btk+ and Btk− mice. Single cell suspensions were stained with mAbs specific for B220, IgM, Ig λ, as well as the clonotype-specific rat mAb 54.1 that detects idiotype-positive B cells in 3-83μδ transgenic mice. The Btk dependence of receptor editing was analyzed in Btk+ and Btk− centrally deleting 3-83μδ transgenic mice on an H-2Kd/H-2Kb F1 background. As controls, nondeleting 3-83μδ transgenic mice (H-2Kd) and nontransgenic (H-2Kb) Btk+ mice are shown. (A) Presence of 54.1+ B cells in the bone marrow of the indicated mice. In the spleen (B) and mesenteric lymph node (C), the B cell population was gated (numbers indicate the percentages of B220+IgM+ cells from total lymphoid cells) and analyzed for the expression of λ and the 54.1 idiotype. The numbers in the quadrants indicate the percentages of B220+IgM+ cells that are λ+54.1−, λ+54.1+, and λ−54.1+. Data shown are representative of the mice examined.
Reduced λ Usage in Btk-deficient B Cells Generated in Bone Marrow Cultures In Vitro.
To further investigate the relation between Btk signaling and λ chain rearrangement, we performed IL-7–driven bone marrow culture experiments as described previously 7 41. When surface IgM− bone marrow cell suspensions from WT or Btk-deficient mice were cultured for 5 d in the presence of IL-7, the majority of cells consisted of B220+IgM− pre-B cells that expressed μ H chain in their cytoplasm. In these cultures, a considerable fraction of the WT B220+ cells (∼15%) matured to surface IgM+ B cells, while <5% of Btk-deficient B220+ cells were surface IgM+, suggesting that the progression from pre-B cell to sIg+ B cell is hampered in Btk-deficient mice. When cells were subsequently cultured without IL-7 on S17 stroma cells for 48 h, allowing the cells to exit from the cell cycle and further differentiate 36, significant fractions of the WT (∼40%) and Btk-deficient (∼30%) B220+ cells were surface IgM+. In these cultures, in vitro–generated Btk-deficient surface IgM+ B cells showed significantly reduced usage of λ L chains, compared with WT B cells (Fig. 5 A).
Figure 5.
Decreased λ L chain usage in in vitro–generated Btk− B cells and in Btk− pre-B cells in vivo. (A) Fraction of B220+ cells that express κ and λ L chain on the cell surface in bone marrow cultures from Btk+ and Btk− mice (n = 10). Cells were grown in IL-7 for 5 d and subsequently recultured on S17 stroma in the absence of IL-7 for 48 h. Cells were stained with mAbs specific for B220, IgM, IgD, and either κ or λ. (B) Fraction of sIgM−sIgD−B220+ cells that express L chains in their cytoplasm in the bone marrow of Btk+ and Btk− mice (n = 8).
Reduced Cytoplasmic L Chain Expression in Btk-deficient Pre-B Cells.
About one fifth of all pre-B cells have been reported to express μ H chain and L chains in their cytoplasm without depositing IgM molecules on their surface 3. In the bone marrow of Btk+ and Btk− mice, we determined the proportions of sIgM− cells that express κ and λ L chain in their cytoplasm. In four-color flow cytometry experiments, intracellular staining for H or L chain expression was combined with surface labeling to define the IgM−IgD−B220+ pre-B cells. In Btk+ and Btk− mice, the proportions of sIgM− B cells that expressed cytoplasmic μ H chain were similar. By contrast, the percentage of sIgM− B cells that were positive for κ or λ L chains in their cytoplasm was significantly reduced in Btk-deficient mice, compared with WT mice (Fig. 5 B). The absence of Btk had a much stronger effect on λ expression, resulting in decreased frequencies of λ usage in Btk-deficient sIgM− cells (1.9 compared with 5.8% in WT mice).
Transient Expression of BtkE41K in Pre-B Cells Increases λ Usage.
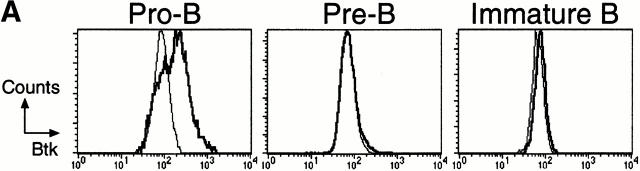
To confirm that the Btk E41K mutant exerts its role on λ L chain usage at the pre-B cell stage, we generated transgenic mice in which Btk E41K expression was driven by the λ5 promoter and the 3′ λ5-Vpre-B1 locus control region. As shown in Fig. 6 A, the transgene was selectively expressed in early CD43+B220+ B cell precursors. In contrast to the CD19-hBtk E41K transgenic mice, the λ5-hBtk E41K mice did not manifest an arrest of B cell development, as the sizes of the B cell subpopulations in bone marrow and peripheral organs were in the normal ranges (Fig. 6, and data not shown). When λ5-hBtk E41K transgenic and nontransgenic mice on a Btk− background were compared, expression of the transgene was found to increase λ usage. The frequencies of λ+ cells in the IgM+IgD−/low immature B cell population were 4.9 ± 0.3 in Btk− mice (n = 4) and 8.9 ± 0.7 in λ5-hBtk E41K transgenic Btk− mice (n = 4; Fig. 6 B). This finding showed that transient expression of BtkE41K in CD43+ pro- and pre-B cells increased the frequency of λ usage.
Figure 6.

Increase of λ usage by transient Btk E41K expression in λ5-hBtk E41K mice. (A) Intracellular Btk expression in B cells from λ5-hBtk E41K transgenic mice on a Btk− background. Btk expression profiles are displayed as histograms for CD43+B220+IgM− pro-B cells, CD43− B220+IgM− pre-B cells, and B220+IgM+IgD− immature B cells of λ5-hBtk E41K transgenic mice (black lines), together with those of Btk− mice, which served as background stainings (thin lines). (B) Four-color flow cytometric analyses of bone marrow of the indicated mice. On the basis of IgM and IgD expression (top), the immature IgM+IgD−/low B cell population (fraction E+ET; see legend to Fig. 1) and the IgM+IgD+ recirculating B cell population (fraction F) were gated and analyzed for B220 and λ usage (bottom). Data shown are representative of the mice examined.
Discussion
In this report we show that the absence of Btk during murine B cell development results in a 50% reduction of the proportion of Ig λ L chain–expressing cells. Conversely, expression of the constitutively activated BtkE41K mutant significantly promotes λ usage. The observed λ expression profiles of Btk-deficient, CD19hBtk E41K, and λ5-hBtk E41K mice during B cell development show that the effect of Btk signaling is at the level of the Ig L chain rearrangement events in pre-B cells, and not a result of antigen-dependent selection processes in the periphery. Moreover, the comparison of Btk+ and Btk− B cells in the spleen of heterozygous females shows that reduced λ usage is an intrinsic feature of Btk-deficient B cells and not associated with the immunodeficient status of Btk-deficient mice. We conclude that the regulation of Vλ-Jλ recombination events in the bone marrow is partially dependent on Btk signal transduction.
It has been shown that the κ to λ ratio of 95:5 in murine cells reflects a developmental hit-and-run program of B cells in which κ gene rearrangements precede λ rearrangements, whereby λ usage is also dependent on the pre-B cell life span and the extent of receptor editing in immature B cells 4 5 6 37.
The finding of increased λ L chain usage in Bcl-2 transgenic animals indicates that the extent of secondary rearrangement events in pre-B cells with a nonfunctional L chain rearrangement is dependent on their life span 37. Although Btk is involved in the survival of mature peripheral B cells 20 42, a role for Btk in the survival of pre-B cells in the mouse is not very likely. The absolute numbers of pre-B cells that are generated in the bone marrow of Btk-deficient mice are normal, and Btk-deficient B cell precursors in the bone marrow have the same kinetics of turnover 20 26 27 43. Moreover, our analyses in Btk-deficient and BtkE41K mice on an Eμ-Bcl-2 transgenic background show that protection of pre-B cells from apoptosis by the expression of the Bcl-2 transgene did not alter the Btk dependence of the frequency of λ usage (Fig. 2 and Fig. 3). Therefore we conclude that the influence of Btk on λ usage is not at the level of the regulation of the time window for Ig L chain rearrangements in pre-B cells.
As our findings argue against a role of Btk in pre-B cell survival, the relationship between Btk activity and λ usage would point at a role for Btk either in the regulation of the initiation of gene rearrangement at the λ L chain locus or in the induction of receptor editing. As comparable numbers of 54.1 idiotype-negative B cells were present in Btk+ and Btk− centrally deleting 3-83μδ autoantibody transgenic mice, we conclude that Btk signaling is not essential for receptor editing. Nevertheless, a 50% reduction of λ usage was found in the spleen and lymph nodes of Btk-deficient 3-83μδ transgenic mice (Fig. 4). This finding may indicate that in Btk-deficient mice the mechanism of receptor editing is intact, but λ usage is decreased due to impaired initiation of λ L chain rearrangement, or alternatively that the overall level of Ig L chain replacement is reduced. Recent experiments indicated that receptor editing represents a major force in shaping the antibody repertoire in the mouse, as it was found that ∼25% of all Ig L chains are produced by gene replacement 12. Whether all of these replacements are induced by self-reactive or nonpairing receptors is currently unknown. Thus, it is possible that the 15–20% of all small pre-B cells that have been reported to express Ig L chains in their cytoplasm 44 may have deposited IgM on the membrane, but sIgM expression has subsequently been downregulated due to binding of an autoantigen. Such cells would only be present in the bone marrow for a very short time, as receptor editing has been estimated to take only 2 h 12. In this model, the reduced cytoplasmic λ L chain expression observed in Btk-deficient pre-B cells may reflect reduced receptor editing in the absence of Btk signaling.
Alternatively, the pre-B cells that express μ H chain and L chains in their cytoplasm, but not on their surface, may produce Ig L chains that are not capable of pairing with the μ H chain in that cell 6. On the basis of these findings, it could be assumed that the κ to λ ratio in cytoplasmic L chain–positive pre-B cells reflects the intrinsic probabilities for productive rearrangements on the κ and λ loci. In this alternative model, the observed significant reduction of the frequencies of cytoplasmic λ L chain expressing pre-B cells would point at an intrinsic defect in λ gene rearrangement in Btk-deficient cells. Such a role for Btk in the initiation of λ gene rearrangement would, together with the observation of hampered in vitro progression of Btk-deficient pre-B cells into surface IgM+ B cells in IL-7–driven bone marrow cultures, implicate Btk in pre-BCR signaling in the mouse. In this context, impaired L chain rearrangement of Btk-deficient cells would be consistent with their selective disadvantage at the transition from small pre-B to immature B cell, as we previously observed in an in vivo competition assay 27. Obviously, in this model Btk signaling could mediate developmental progression to a stage of B cell development in which λ L chain rearrangements are initiated, or alternatively Btk signaling could directly control the rate or efficiency of L chain rearrangement. The observed increase in coding and signal broken DNA ends at multiple gene segments in the Ig κ locus across the pro-B to pre-B cell transition supported the hypothesis that the role of pre-BCR signaling is not limited to expanding the population undergoing L chain gene rearrangement, but actually increases the activity of V(D)J recombination at the Ig κ locus 45. During the rounds of DNA replication that follow pre-BCR expression, pre-BCR signaling could then, e.g., induce alterations of the chromatin structure of the L chain loci to provide accessibility to the V(D)J recombinase system 1 46. As Btk does not appear to influence the size of the pre-B cell population or its kinetics of turnover in the mouse 20 26 27 43, we would propose that Btk is most likely involved in the activation of the L chain loci, in particular the λ locus, for recombination. In this context, the differential effect of Btk signaling on the κ and λ loci would reflect a different regulation of the activation of the two loci for recombination. This is to be expected, as the loci become accessible for recombination at different stages of B cell development, i.e., large cycling pre-B cells for the κ locus and small quiescent pre-B cells for the λ locus 5.
A role for Btk in the initiation of λ L chain rearrangement rather than receptor editing would be supported by the finding of increased Ig λ usage, when the constitutively activated BtkE41K mutant was selectively expressed in early CD43+ pro- and large pre-B cells, but not in CD43− small pre-B cells (Fig. 6). Normally, both the initiation of λ L chain rearrangement and receptor editing take place at the small pre-B cell stage 5 12 where the BtkE41K mutant is no longer expressed in the λ5-hBtk E41K mice. Therefore, the increased Ig λ usage in λ5-hBtk E41K mice may reflect premature initiation of Vλ-Jλ recombination. However, as our findings do not provide direct evidence for the presence of a Btk-mediated signaling pathway controlling the initiation of L chain rearrangements, further experiments are required to directly demonstrate whether Btk signaling activates the λ locus for recombination, e.g., by changing its accessibility for the V(D)J recombinase.
In summary, the observed correlation between Btk activity and the frequency of λ usage indicates a role for Btk-mediated signaling in the activation of Ig λ L chain locus for V(D)J recombination. As a result, Btk signaling contributes significantly to the mechanism that sets the κ/λ isotype ratio in the mouse. Although we have shown that Btk is not essential for receptor editing in the 3-83μδ autoreactive model, it remains possible that the absence of Btk signaling results in a decrease in the extent of L chain replacement events, and thereby in decreased λ usage in Btk-deficient B cells. Alternatively, Btk could be involved in the activation of the λ L chain locus for recombination. A role for Btk in the initiation of L chain rearrangements may also explain the developmental arrest in those XLA patients with normal pre-B cell numbers that nevertheless show impaired maturation to surface Ig+ B cells 23.
Acknowledgments
We thank N. Dillon (MRC Clinical Sciences Centre, London, UK), D. Nemazee (Scripps Research Institute, La Jolla, CA), C. Kanellopoulos-Langevin (Institut Jacques Monod, Paris, France), L. Braam, D. Drabek, W. van Ewijk, J.P. van Hamburg, J. Mahabier, and T. de Wit for assistance at various stages of the project.
This work was supported by Netherlands Organization for Scientific Research grants 901-07-224 to K. Dahlenborg and A. Maas and 901-07-209 to S. Middendorp, as well as by the Royal Academy of Arts and Sciences to R.W. Hendriks.
Footnotes
Abbreviations used in this paper: BCR, B cell receptor; Btk, Bruton's tyrosine kinase; PH, pleckstrin homology; WT, wild-type; Xid, X-linked immunodeficiency; XLA, X-linked agammaglobulinemia.
References
- Alt F.W., Blackwell T.K., Yancopoulos G.D. Development of the primary antibody repertoire. Science. 1987;238:1079–1087. doi: 10.1126/science.3317825. [DOI] [PubMed] [Google Scholar]
- Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- Melchers F., ten Boekel E., Seidl T., Kong X.C., Yamagami T., Onishi K., Shimizu T., Rolink A.G., Andersson J. Repertoire selection by pre-B-cell receptors and B-cell receptors, and genetic control of B-cell development from immature to mature B cells. Immunol. Rev. 2000;175:33–46. [PubMed] [Google Scholar]
- Arakawa H., Shimizu T., Takeda S. Re-evaluation of the probabilities for productive arrangements on the κ and λ loci. Int. Immunol. 1996;8:91–99. doi: 10.1093/intimm/8.1.91. [DOI] [PubMed] [Google Scholar]
- Engel H., Rolink A., Weiss S. B cells are programmed to activate κ and λ for rearrangement at consecutive developmental stages. Eur. J. Immunol. 1999;29:2167–2176. doi: 10.1002/(SICI)1521-4141(199907)29:07<2167::AID-IMMU2167>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Yamagami T., ten Boekel E., Andersson J., Rolink A., Melchers F. Frequencies of multiple IgL chain gene rearrangements in single normal or κ L chain-deficient B lineage cells. Immunity. 1999;11:317–327. doi: 10.1016/s1074-7613(00)80107-7. [DOI] [PubMed] [Google Scholar]
- Rolink A., Grawunder U., Haasner D., Strasser A., Melchers F. Immature surface Ig+ B cells can continue to rearrange κ and λ L chain gene loci. J. Exp. Med. 1993;178:126–127. doi: 10.1084/jem.178.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grawunder U., Leu T.M., Schatz D.G., Werner A., Rolink A.G., Melchers F., Winkler T.H. Down-regulation of RAG1 and RAG2 gene expression in preB cells after functional immunoglobulin heavy chain rearrangement. Immunity. 1995;3:601–608. doi: 10.1016/1074-7613(95)90131-0. [DOI] [PubMed] [Google Scholar]
- Hertz M., Kouskoff V., Nakamura T., Nemazee D. V(D)J recombinase induction in splenic B lymphocytes is inhibited by antigen-receptor signalling. Nature. 1998;394:292–295. doi: 10.1038/28419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiegs S.L., Russell D.M., Nemazee D. Receptor editing in self-reactive bone marrow B cells. J. Exp. Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow C.C. Balancing immunity and tolerancedeleting and tuning lymphocyte repertoires. Proc. Natl. Acad. Sci. USA. 1996;93:2264–2271. doi: 10.1073/pnas.93.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casellas R., Shih T.A., Kleinewietfeld M., Rakonjac J., Nemazee D., Rajewsky K., Nussenzweig M.C. Contribution of receptor editing to the antibody repertoire. Science. 2001;291:1541–1544. doi: 10.1126/science.1056600. [DOI] [PubMed] [Google Scholar]
- Yang W.-C., Colette Y., Nunes J.A., Olive D. Tec kinasesa family with multiple roles in immunity. Immunity. 2000;12:373–382. doi: 10.1016/s1074-7613(00)80189-2. [DOI] [PubMed] [Google Scholar]
- Saouaf S.J., Mahajan S., Rowley R.B., Kut S.A., Fargnoli J., Burkhardt A.L., Tsukada S., Witte O.N., Bolen J.B. Temporal differences in the activation of three classes of non-transmembrane protein tyrosine kinases following B-cell antigen receptor surface engagement. Proc. Natl. Acad. Sci. USA. 1994;91:9524–9528. doi: 10.1073/pnas.91.20.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weers M., Brouns G.S., Hinshelwood S., Kinnon C., Schuurman R.K., Hendriks R.W., Borst J. B-cell antigen receptor stimulation activates the human Bruton's tyrosine kinase, which is deficient in X-linked agammaglobulinemia. J. Biol. Chem. 1994;269:23857–23860. [PubMed] [Google Scholar]
- Aoki Y., Isselbacher K.J., Pillai S. Bruton tyrosine kinase is tyrosine phosphorylated and activated in pre-B lymphocytes and receptor-ligated B cells. Proc. Natl. Acad. Sci. USA. 1994;91:10606–10609. doi: 10.1073/pnas.91.22.10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings D.J., Scharenberg A.M., Park H., Wahl M.I., Lin S., Kato R.M., Fluckiger A.C., Witte O.N., Kinet J.P. Activation of BTK by a phosphorylation mechanism initiated by SRC family kinases. Science. 1996;271:822–825. doi: 10.1126/science.271.5250.822. [DOI] [PubMed] [Google Scholar]
- Scharenberg A.M., El-Hillal O., Fruman D.A., Beitz L.O., Li Z., Lin S., Gout I., Cantley L.C., Rawlings D.J., Kinet J.P. Phosphatidylinositol-3,4,5-trisphosphate (PtdIns-3,4,5-P3)/Tec kinase-dependent calcium signaling pathwaya target for SHIP-mediated inhibitory signals. EMBO J. 1998;17:1961–1972. doi: 10.1093/emboj/17.7.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki T., Tsukada S. BLNKconnecting Syk and Btk to calcium signals. Immunity. 2000;12:1–5. doi: 10.1016/s1074-7613(00)80153-3. [DOI] [PubMed] [Google Scholar]
- Wicker L.S., Scher I. X-linked immune deficiency (xid) of CBA/N mice. Curr. Top. Microbiol. Immunol. 1986;124:87–101. doi: 10.1007/978-3-642-70986-9_6. [DOI] [PubMed] [Google Scholar]
- Vihinen M., Kwan S.P., Lester T., Ochs H.D., Resnick I., Valiaho J., Conley M.E., Smith C.I. Mutations of the human BTK gene coding for Bruton tyrosine kinase in X-linked agammaglobulinemia. Hum. Mutat. 1999;13:280–285. doi: 10.1002/(SICI)1098-1004(1999)13:4<280::AID-HUMU3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Conley M.E., Cooper M.D. Genetic basis of abnormal B cell development. Curr. Opin. Immunol. 1998;10:399–406. doi: 10.1016/s0952-7915(98)80112-x. [DOI] [PubMed] [Google Scholar]
- Smith C.I., Islam K.B., Vorechovsky I., Olerup O., Wallin E., Rabbani H., Baskin B., Hammarstrom L. X-linked agammaglobulinemia and other immunoglobulin deficiencies. Immunol. Rev. 1994;138:159–183. doi: 10.1111/j.1600-065x.1994.tb00851.x. [DOI] [PubMed] [Google Scholar]
- Conley M.E. B cells in patients with X-linked agammaglobulinemia. J. Immunol. 1985;134:3070–3074. [PubMed] [Google Scholar]
- Nomura K., Kanegane H., Karasuyama H., Tsukada S., Agematsu K., Murakami G., Sakazume S., Sako M., Tanaka R., Kuniya Y. Genetic defect in human X-linked agammaglobulinemia impedes a maturational evolution of pro-B cells into a later stage of pre-B cells in the B-cell differentiation pathway. Blood. 2000;96:610–617. [PubMed] [Google Scholar]
- Khan W.N., Alt F.W., Gerstein R.M., Malynn B.A., Larsson I., Rathbun G., Davidson L., Muller S., Kantor A.B., Herzenberg L.A. Defective B cell development and function in Btk-deficient mice. Immunity. 1995;3:283–299. doi: 10.1016/1074-7613(95)90114-0. [DOI] [PubMed] [Google Scholar]
- Hendriks R.W., de Bruijn M.F., Maas A., Dingjan G.M., Karis A., Grosveld F. Inactivation of Btk by insertion of lacZ reveals defects in B cell development only past the pre-B cell stage. EMBO J. 1996;15:4862–4872. [PMC free article] [PubMed] [Google Scholar]
- Timmers E., Hermans M.M., Kraakman M.E., Hendriks R.W., Schuurman R.K. Diversity of immunoglobulin κ light chain gene rearrangements and evidence for somatic mutation in V κ IV family gene segments in X-linked agammaglobulinemia. Eur. J. Immunol. 1993;23:619–624. doi: 10.1002/eji.1830230306. [DOI] [PubMed] [Google Scholar]
- Burkly L.C., Zaugg R., Eisen H.N., Wortis H.H. Influence of the nude and X-linked immune deficiency genes on expression of κ and λ light chains. Eur. J. Immunol. 1982;12:1033–1039. doi: 10.1002/eji.1830121209. [DOI] [PubMed] [Google Scholar]
- Maas A., Dingjan G.M., Grosveld F., Hendriks R.W. Early arrest in B cell development in transgenic mice that express the E41K Bruton's tyrosine kinase mutant under the control of the CD19 promoter region. J. Immunol. 1999;162:6526–6533. [PubMed] [Google Scholar]
- Sabbattini P., Georgiou A., Sinclair C., Dillon N. Analysis of mice with single and multiple copies of transgenes reveals a novel arrangement for the λ5-VpreB1 locus control region. Mol. Cell. Biol. 1999;19:671–679. doi: 10.1128/mcb.19.1.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingjan G.M., Maas A., Nawijn M.C., Smit L., Voerman J.S., Grosveld F., Hendriks R.W. Severe B cell deficiency and disrupted splenic architecture in transgenic mice expressing the E41K mutated form of Bruton's tyrosine kinase. EMBO J. 1998;17:5309–5320. doi: 10.1093/emboj/17.18.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A., Whittingham S., Vaux D.L., Bath M.L., Adams J.M., Cory S., Harris A.W. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc. Natl. Acad. Sci. USA. 1991;88:8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed D., Nemazee D. Self-antigen does not accelerate immature B cell apoptosis, but stimulates receptor editing as a consequence of developmental arrest. Proc. Natl. Acad. Sci. USA. 1997;94:9267–9272. doi: 10.1073/pnas.94.17.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemazee D., Buerki K. Clonal deletion of autoreactive B lymphocytes in bone marrow chimeras. Proc. Natl. Acad. Sci. USA. 1989;86:8039–8043. doi: 10.1073/pnas.86.20.8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolink A., Streb M., Melchers F. The κ/λ ratio in surface immunoglobulin molecules on B lymphocytes differentiating from DHJH-rearranged murine pre-B cell clones in vitro. Eur. J. Immunol. 1991;21:2895–2898. doi: 10.1002/eji.1830211137. [DOI] [PubMed] [Google Scholar]
- Lang J., Arnold B., Hammerling G., Harris A.W., Korsmeyer S., Russell D., Strasser A., Nemazee D. Enforced Bcl-2 expression inhibits antigen-mediated clonal elimination of peripheral B cells in an antigen dose–dependent manner and promotes receptor editing in autoreactive, immature B cells. J. Exp. Med. 1997;186:1513–1522. doi: 10.1084/jem.186.9.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Tsukada S., Satterthwaite A., Havlik M.H., Park H., Takatsu K., Witte O.N. Activation of Bruton's tyrosine kinase (BTK) by a point mutation in its pleckstrin homology (PH) domain. Immunity. 1995;2:451–460. doi: 10.1016/1074-7613(95)90026-8. [DOI] [PubMed] [Google Scholar]
- Varnai P., Rother K.I., Balla T. Phosphatidylinositol 3-kinase-dependent membrane association of the Bruton's tyrosine kinase pleckstrin homology domain visualized in single living cells. J. Biol. Chem. 1999;274:10983–10989. doi: 10.1074/jbc.274.16.10983. [DOI] [PubMed] [Google Scholar]
- Retter M.W., Nemazee D. Receptor editing occurs frequently during normal B cell development. J. Exp. Med. 1998;188:1231–1238. doi: 10.1084/jem.188.7.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed D., Benschop R.J., Cambier J.C., Nemazee D. Developmental regulation of B lymphocyte immune tolerance compartmentalizes clonal selection from receptor selection. Cell. 1998;92:173–182. doi: 10.1016/s0092-8674(00)80912-5. [DOI] [PubMed] [Google Scholar]
- Woodland R.T., Schmidt M.R., Korsmeyer S.J., Gravel K.A. Regulation of B cell survival in xid mice by the proto-oncogene bcl-2. J. Immunol. 1996;156:2143–2154. [PubMed] [Google Scholar]
- Reid G.K., Osmond D.G. B lymphocyte production in the bone marrow of mice with X-linked immunodeficiency (xid) J. Immunol. 1985;135:2299–2302. [PubMed] [Google Scholar]
- Pelanda R., Schaal S., Torres R.M., Rajewsky K. A prematurely expressed Igκ transgene, but not VκJκ gene segment targeted into the Igκ locus, can rescue B cell development in λ5-deficient mice. Immunity. 1996;5:229–239. doi: 10.1016/s1074-7613(00)80318-0. [DOI] [PubMed] [Google Scholar]
- Constantinescu A., Schlissel M.S. Changes in locus-specific V(D)J recombinase activity induced by immunoglobulin gene products during B cell development. J. Exp. Med. 1997;185:609–620. doi: 10.1084/jem.185.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanhope-Baker P., Hudson K.M., Shaffer A.L., Constantinescu A., Schlissel M.S. Cell type-specific chromatin structure determines the targeting of V(D)J recombinase activity in vitro. Cell. 1996;85:887–897. doi: 10.1016/s0092-8674(00)81272-6. [DOI] [PubMed] [Google Scholar]
- Hardy R.R., Carmack C.E., Shinton S.A., Kemp J.D., Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J. Exp. Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]