Abstract
Cytotoxic T lymphocytes (CTLs) play a vital part in controlling viral replication during human viral infections. Most studies in human infections have focused on CTL specificities in chronic infection and few data exist regarding the specificity of the initial CTL response induced in acute infection. In this study, HIV-1 infection in persons expressing human histocompatibility leukocyte antigen (HLA)-A*0201 was used as a means of addressing this issue. In chronic infection, the dominant HLA-A*0201–restricted CTL response is directed towards the epitope SLYNTVATL (“SL9”) in p17 Gag (residues 77–85). This epitope is targeted by 75% of HLA-A*0201–positive adults, and the magnitude of this A*0201-SL9 response shows a strong negative association with viral load in progressive infection. Despite using the highly sensitive peptide–major histocompatibility complex tetramer and intracellular cytokine assays, responses to the SL9 epitope were not detectable in any of 11 HLA-A*0201–positive subjects with acute HIV-1 infection (P = 2 × 10−6), even when assays were repeated using the SL9 peptide variant that was encoded by their autologous virus. In contrast, multiple responses (median 3) to other epitopes were evident in 7 of the 11 A*0201–positive subjects. Longitudinal study of two subjects confirmed that the A*0201-SL9 response emerged later than other CTL responses, and after viral set point had been reached. Together, these data show that the CTL responses that are present and that even may dominate in chronic infection may differ substantially from those that constitute the initial antiviral CTL response. This finding is an important consideration in vaccine design and in the evaluation of vaccine candidates.
Keywords: acute, chronic, HIV infection, immunodominance, epitope targeting
Introduction
Virus-specific CTLs play a critical role in the control of viral infections 1 2 3 4 5 6 7 8 9. Studies in the lymphocytic choriomeningitis virus (LCMV) mouse model have most clearly shown that the early antiviral cellular immunity that is generated strongly influences the subsequent course and outcome from the infection 10. Similar conclusions may be drawn from investigations of human viral infections 3 4 5 11 12 13 14. This implies that the immune responses that are observed in the chronic phase of persistent virus infections may largely be a consequence of what has occurred initially, and that perhaps the most important antiviral immune responses to understand are those responsible for early control of viremia in acute infection. Recent studies in the simian immunodeficiency virus (SIV) macaque model strongly support this hypothesis 15. In the design and testing of vaccines, it is vital to know whether the CTL specificities differ significantly between acute and chronic infection. If differences do exist, it is necessary to determine which responses should optimally be generated by a candidate vaccine.
HIV-1 infection offers an excellent system in which to compare immune responses in acute and chronic infection. In this infection, CTLs are centrally involved both in the initial containment of viremia in acute infection and in limiting viral replication in chronic infection 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30. However, evidence accumulating both from animal models and human viral infections indicate that significant differences exist between the effectiveness of CTLs of different specificities in controlling viremia 28 29 30 31 32 33 34 35 36 37 38 39. A recent study in the SIV macaque model of AIDS virus infection showed that initial immune pressure was directed mainly through an epitope in Tat, whereas one in Gag did not appear to be under strong immune selection pressure 15. Thus, from these data, it is likely that CTLs of different specificities may show dramatic differences in the contribution that each may make to the successful control of viremia.
The best studied HIV-specific CTL response is the dominant HLA-A*0201–restricted specificity that is directed towards an epitope in p17 Gag, SLYNTVATL (residues 77–85; references 22 and 40 41 42 43 44 45 46), referred to as “SL9”. In four independent studies of A*0201-positive adults with chronic HIV infection, 75% of persons studied had a detectable response towards this epitope 22 44 45 46. Using SL9-A*0201 peptide–MHC tetrameric complexes, a strong negative association was shown between levels of SL9-A*0201 CTLs and viral load in A*0201-positive adults with chronic HIV infection 22. This widely quoted landmark study has provided strong indirect evidence that A*0201-SL9–specific CTLs are effective in controlling HIV replication in chronic infection. This has led to the suggestion that an effective vaccine should induce this response.
To determine whether A*0201-SL9–specific CTLs play an important role in the initial control of viremia in acute infection, the HIV-specific CTL responses were characterized in 11 subjects with A*0201 who were enrolled early in the course of HIV infection, in 8 cases before seroconversion. Although CTL responses were observed in the majority of these subjects, none made a detectable response to the A*0201-SL9 epitope.
Materials and Methods
Subjects Studied.
The 11 A*0201-positive subjects studied in early HIV infection were recruited from persons presenting to the Massachusetts General Hospital between 1997 and 1999 who were diagnosed with acute HIV infection. The relevant clinical data for these subjects are described in Table . All but three of the subjects were enrolled before seroconversion: three subjects (AC14, AC23, and AC32) were enrolled within 180 d of seroconversion, as defined by an adapted ELISA assay for HIV Ab 47, and one subject (AC29) was included using clinical criteria that supported a diagnosis of HIV infection within the previous 3 mo. These included a known high-risk exposure to HIV infection, by history a clinical syndrome consistent with acute HIV infection, and a viral load at the time of presentation of >750,000 RNA copies/ml plasma that would be highly unusual as a steady-state viral load in chronic infection 14 48. All 11 subjects were initially studied before initiation of highly active antiretroviral therapy (HAART) as detailed. An additional A*0201-positive subject PI004 whose date of acquiring HIV infection was less certain, and who was never treated with HAART, was also studied as described below. The first sample available for study on PI004 was 7 wk after the first positive HIV Ab test on this subject. An HIV Ab test 21 wk previous to this had been negative.
Table 1.
Subjects with Early/Acute HIV Infection Studied
| Patient ID | Age | Sex | Race | HIV1/2 ELISA | Western blot | Mode of exposure | Viral load | CD4+ cell count | Therapy |
|---|---|---|---|---|---|---|---|---|---|
| y | Copies/ml at diagnosis | Cells/mm3 | |||||||
| AC01 | 31 | M | Caucasian | Negative | ND | Sexual | 0.25 × 106 | 1,023 | ZDV, 3TC, IDV |
| AC07 | 22 | F | Caucasian | Negative | ND | Sexual | 10.6 × 106 | 131 | D4T, 3TC, NFV |
| AC03 | 30 | M | Caucasian | Negative | ND | Sexual | >0.75 × 106 | 463 | ZDV, 3TC, NFV |
| AC04 | 35 | M | Caucasian | Negative | ND | Sexual | 9.62 × 106 | N/A | ZDV, 3TC, NFV |
| AC32 | 34 | M | Caucasian | Positive | IND | Sexual | 0.2 × 106 | 554 | D4T, 3TC, EFV |
| AC13 | 32 | M | Caucasian | Negative | ND | Sexual | 0.73 × 106 | 667 | ZDV, 3TC, IDV |
| AC22 | 29 | M | Caucasian | Negative | ND | Sexual | 95.5 × 106 | 281 | D4T, DDI, HU, NFV |
| AC26 | 45 | M | Caucasian | Positive | IND (3 band) | Sexual | 2.15 × 106 | 390 | ZDV, 3TC, IDV |
| AC23 | 36 | M | Caucasian | Positive | Positive | Sexual | 0.12 × 106 | 396 | ZDV, 3TC, EFZ |
| AC14 | 46 | M | Caucasian | Positive | Positive | Sexual | 0.095 × 106 | 981 | D4T, 3TC, IND |
| AC29 | 54 | M | Caucasian | Positive | Positive | Occupational | >0.75 × 106 | 785 | ZDV, 3TC, EFV |
| PI004 | M | Positive | Positive | 50 | 432 | No treatment |
ID, identification; ND, not done; IND, indeterminate; ZDV, zidovudine; 3TC, lamivudine; IDV, indinivir; d4T, stavudine; NFV, nelfinavir; ddI, didanosine; HU, hydroxyurea; EFV, efavirenz.
HLA Class I Tissue Typing and HLA-A2 Subtyping.
HLA class I typing and A2 subtyping were performed by sequence-specific primer (SSP)-PCR 49. Only subjects with A*0201 were included in the study.
Peptides.
PBMCs in each of the 11 subjects with early HIV infection were screened for recognition in enzyme-linked immunospot (Elispot) assays of epitopes within p17 Gag, p24 Gag, Nef, RT, gp41, gp120, Tat, and Rev using overlapping peptides 12–20 amino acids in length that overlapped by 10 amino acids. Overall, 290 overlapping peptides were used to span these 8 proteins. In addition, from a total of 130 published optimal epitope peptides 50, those that were presented by HLA class I molecules expressed by each subject studied (median 24 peptides) were tested for recognition in Elispot assays. The sequences for the peptides corresponded to the B clade SF2 sequence. Gag, Nef, RT, and gp120 peptides were provided by the National Institute for Biological Standards and Control Centralized Facility for AIDS Reagents, supported by European Union Program EVA and the UK Medical Research Council; 12 additional overlapping p17 Gag peptides, gp41, Tat, and Rev peptides were synthesized commercially (Research Genetics) or at the Massachusetts General Hospital Peptide Synthesis Core Facility.
Elispot Assays.
Fresh PBMCs were plated in 96-well polyvinylidene plates (Millipore) that had been precoated with 0.5 μg/ml anti–IFN-γ mAb, 1-DIK (Mabtech). The peptides were added in a volume of 20 μl and PBMCs were added at 100,000 cells/well in a volume of 180 μl R10 medium (RPMI 1640 [Sigma-Aldrich], 10% FCS [Sigma-Aldrich], and 10 mM Hepes buffer [Sigma-Aldrich] with antibiotics [2 mM l-glutamine, 50 U/ml penicillin-streptomycin]). The end concentration of the peptides was 10 μM. The plates were incubated overnight at 37°C, 5% CO2, and developed as described previously 51 52. The number of specific T cells was calculated by subtracting the negative control values. The background was <20/106 PBMCs (2 spots/well at 100,000 PBMCs/well) in all cases. Responses of >60 IFN-γ spot-forming cells/106 PBMCs were therefore significant positive responses, and these were reconfirmed in intracellular IFN-γ staining assays (see below). Wells that contained >30 spots were not used for accurate quantification. Assays were repeated using lower input numbers of cells as necessary and in duplicate in order to quantitate responses to individual peptides more accurately.
Intracellular IFN-γ Staining.
Intracellular cytokine staining (ICS) assays were performed as described elsewhere 53 54 55. In brief, 0.2–1.0 × 106 PBMCs were incubated with 4 μM peptide and 1 μg/ml each of the mAbs anti-CD28 and anti-CD49d (Becton Dickinson) at 37°C, 5% CO2 for 1 h, before the addition of 10 μg/ml of Brefeldin A (Sigma-Aldrich). After a further 6 h incubation at 37°C, 5% CO2, the cells were placed at 4°C overnight. PBMCs were then washed and stained with surface Abs anti-CD8 and anti-CD3 (Becton Dickinson) at 4°C for 20 min. PBMCs that were also stained with tetramers were incubated with the tetramer at 4°C for 30 min before the addition of the surface Abs. After washing, the PBMCs were then fixed and permeabilized (Caltag) and anti–IFN-γ mAb was added (Becton Dickinson). Cells were then washed and analyzed. Quadrant boundaries for IFN-γ staining were established by exclusion of >99.97% of control CD8+ T cells.
Peptide–MHC Tetramer Assays.
Peptide–MHC tetramers were synthesized as described previously 42 56. The tetramer used in these studies was the HLA-A*0201–SLYNTVATL complex. HLA heavy chain was expressed in Escherichia coli with an engineered COOH-terminal signal sequence containing a biotinylation site for the enzyme BirA. After refolding of heavy chain, β2m, and peptide, the complex was biotinylated by BirA (Avidity) in the presence of ATP-Mg2+ (Sigma-Aldrich). After purification by gel filtration and anion exchange chromatography, tetramer formation was induced by the addition of streptavidin. Use of PE-labeled streptavidin enabled antigen-specific cells to be visualized by flow cytometry.
Staining of lymphocytes was performed by incubating 500,000 PBMCs for 30 min at 4°C with the appropriate tetramer at 0.5 mg/ml of tetramer, then for a further 20 min with saturating amounts of peridinine chlorophyll protein (PerCP)-conjugated anti-CD8 mAb and allophycocyanin (APC)-conjugated anti-CD4 mAb (Becton Dickinson). Stained samples were analyzed on a FACSCalibur™ flow cytometer using CELLQuest™ software (Becton Dickinson). Control samples for the tetramer staining were PBMCs from HLA-mismatched HIV-infected persons. Quadrant boundaries for tetramer staining were established by exclusion of >99.97% of control CD8+ T cells.
Generation of CTL Clones, Precursor Frequency Assays.
CTL clones were generated using methods described previously 57. In brief, PBMCs were plated out in 96-well plates at limiting dilution (30 cells/well down to 1 cell/well) and cultured with irradiated allogeneic feeder PBMCs at 50,000 cells/well in a final volume per well of 200 μl of R10. The anti-CD3 mAb, 12F6, was added at 10 μg/ml. On day 5 and once weekly thereafter, the medium was changed with R10 medium containing 50 U/ml of rIL-2 (provided by Dr. M. Gately, Hoffmann-La Roche, Nutley, NJ). Wells were screened for specific recognition of HLA-matched, peptide-pulsed, 51Cr (New England Nuclear)-labeled EBV-transformed B lymphoblastoid cell line (BCL) target cells after 21–28 d in culture. Wells showing high specific recognition of the relevant peptide were then transferred to 24-well plates and restimulated as above, except 106 feeders were added to each well and rIL-2 was added on day 0. Expanded wells were then retested for lytic activity from 14 d of culture onwards, and maintained in culture by monthly restimulations as described 57.
Cr Release Assays.
BCL target cells were labeled with 51Cr by incubation of pelleted BCL with 50 μCi of Na2CrO4 (New England Nuclear) for 1 h at 37°C, 5% CO2. Targets were washed three times and then incubated with peptide dilutions in the peptide titration assays for a further 90 min, before addition of effectors. The supernatants were harvested after a further 4–6 h of incubation at 37°C, 5% CO2 58.
Sequencing of Viral DNA and Sequence Analyses.
Genomic DNA was extracted from frozen PBMC pellets (3 × 106 cells) using the Puregene™ DNA isolation kit (Gentra). HIV Gag sequences were amplified by nested PCR using inner and outer primer sets and PCR conditions as described previously 44. For sequencing cloned viral sequences, 2 μl of the gel-purified PCR product was used for ligation of the Gag sequences into the Topo2 cloning plasmid according to the manufacturer's recommendation (Invitrogen) and plasmid DNA was obtained after transfection of E. coli cells and DNA purification using the QIAGEN Turbo DNA purification kit. Inserted Gag sequences were determined from both directions on an ABI 377 sequencer using primer sequences located in the plasmid sequence on both sides of the insert (Topo2 cloning kit; Invitrogen). Sequence analysis was performed using the sequencer software version 3.1.1. BLAST was used to compare sequences from each of the study subjects with each other and sequences in the viral subsection of GenBank to screen for potential cross contamination. Signature analysis was done to verify viral sequence identity and phylogenetic tree analyses using the Neighbor TreeMaker program, available at the Los Alamos HIV Database web site (http://hiv-web.lanl.gov/CONTAM/TreeMaker/TreeMaker.html), revealed unique signature sequences for the individuals. These sequence data are available from Genbank/EMBL/DDBJ under accession nos. AF281678 and AF281801.
Results
Recognition of the HLA-A*0201–restricted p17 Gag Epitope in Acute Infection.
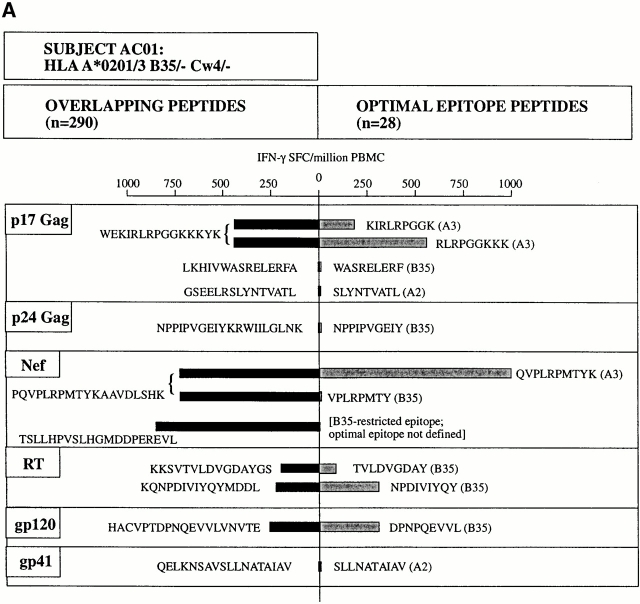
To characterize the HIV-specific CTL response in acute infection for each subject enrolled, IFN-γ responses to epitopes within p17 Gag, p24 Gag, Nef, RT, gp41, gp120, Tat, and Rev were screened in Elispot assays using panels of overlapping 15–20-mer peptides that overlapped by 10 amino acids to span each protein. The approach that was used is illustrated for one subject in Fig. 1 A. In addition, individual peptides previously defined as optimal epitopes corresponding to the HLA class I alleles expressed by each subject were tested for recognition. Thus, for each of the 11 A*0201-positive subjects studied with early HIV infection, 290 overlapping 15–20-mer peptides and between 11 and 29 (median of 24) optimal epitope peptides were used to characterize the CTL response. Overall, 78 different optimal epitope peptides were used in the studies of 11 A*0201-positive subjects with early infection.
Figure 1.
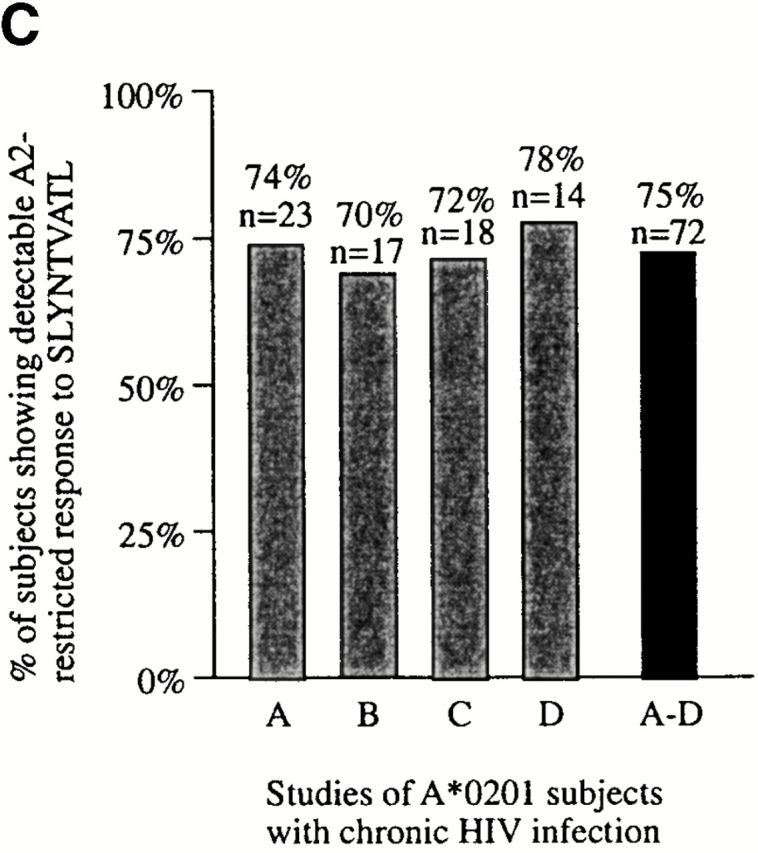
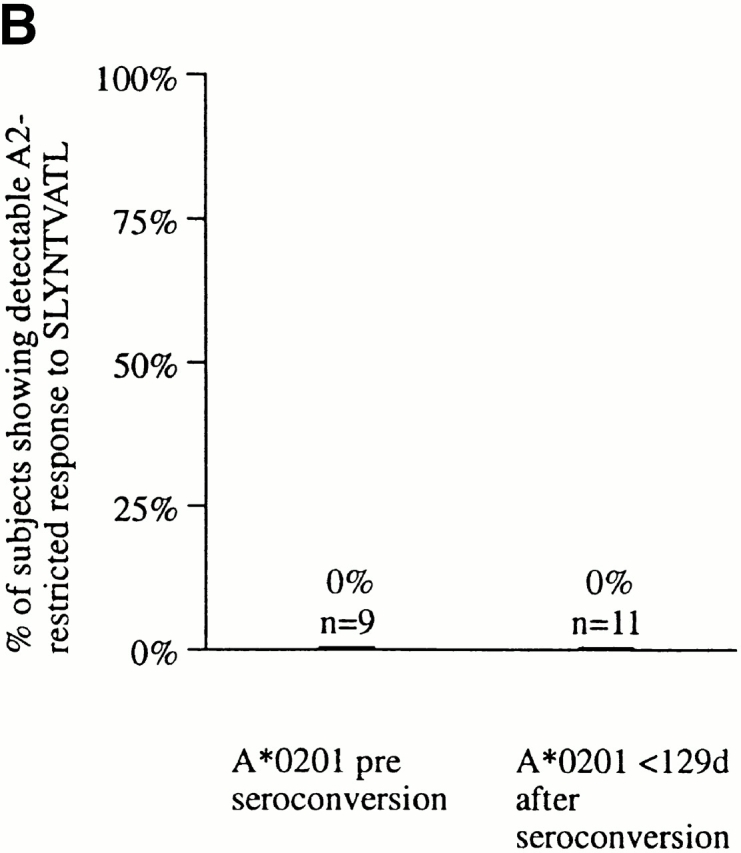
(A) Characterization of the HIV-specific CTL responses made in acute HIV infection. The method used is illustrated for subject AC01. Overlapping peptides spanning p17 Gag, p24 Gag, Nef, RT, gp120, gp41, Rev, and Tat were used in Elispot assays, as well as published optimal peptides presented by HLA-A*0201, A3, B35, or Cw4. Examples of positive and negative responses are shown. No responses to Tat or Rev overlapping peptides were observed in this subject (not shown). SFC, spot-forming cell. (B) Proportion of A*0201-positive subjects in acute infection showing a detectable response to the A*0201-SLYNTVATL epitope. (C) Proportion of A*0201-positive subjects in chronic infection showing a detectable response to the A*0201-SLYNTVATL epitope, using data from four published studies. Criteria for inclusion of a published study were: demonstration of specificity of A*0201-SL9 response by cytotoxicity assays or peptide–MHC tetramers, and more than 1 subject studied.
None of the 11 subjects studied in this way had detectable CTL activity towards the A*0201-SL9 epitope (Fig. 1 B), even when responses were evaluated at several time points in the first 12 mo after presentation. In comparison, responses towards multiple epitopes other than SL9 (range 1–7, median 3) were observed in 7 of these A*0201-positive 11 subjects during this first year after the presumed time of infection 59. These data clearly contrast with the frequent detection of A*0201-SL9–specific responses (in 75% of A*0201-positive adults with chronic infection; n = 72) that has been described in four independent studies 22 44 45 46 (χ2 = 20.9, P = 2 × 10−6). Even restricting the comparison to the seven A*0201-positive subjects who had no detectable SL9 response but who showed evidence of other HIV-specific CTL activity, the absence of an A*0201-SL9 response remains strongly significant (P < 2 × 10−4, Fisher's exact test).
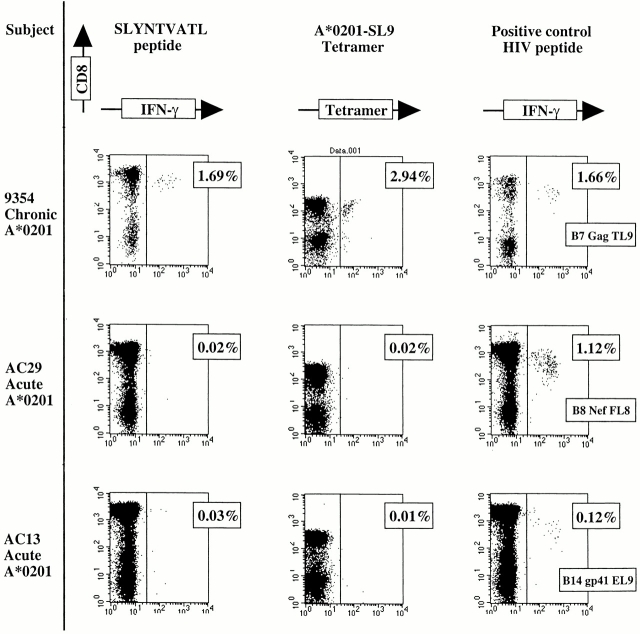
The Elispot assay has a high sensitivity but this does not quite reach the sensitivity of the flow cytometric assays to detect antigen-specific CD8+ T cells either by intracellular IFN-γ staining after peptide stimulation, or by peptide–MHC class I tetrameric complexes 53 60. In addition, tetramer assays have revealed the presence of phenotypically silent antigen-specific T cells in both murine and human viral infections 3 38 39. These assays were therefore used to determine whether any low-frequency or phenotypically silent A*0201-SL9–specific CD8+ T cells could be detected using these highly sensitive assays. All A*0201-positive subjects either showed responses to an A*0201–EBV peptide, GLCTLVAML 61 and/or an A*0201 CMV peptide, NLVPMVATV 62, and/or a positive control HIV peptide in the ICS assay, but none recognized the A*0201-SL9 epitope (Fig. 2, and data not shown). The A*0201-SL9 tetramer and the SL9 peptide in the ICS assay demonstrated responses to this epitope in an A*0201-positive subject 9354 with chronic infection. Thus, no phenotypically silent A*0201-SL9 tetramer-binding cells were detectable in the subjects studied.
Figure 2.
Recognition of SLYNTVATL only in A*0201-positive subjects in chronic infection. Intracellular staining using SL9 and a positive control HIV peptide epitope and A*0201-SL9 tetramer staining of PBMCs from chronically infected subject 9354 (HLA-A*0201/3 B7/35 Cw4/7) and 2 of the 11 A*0201-positive subjects studied in acute infection (expressed as percentage of CD8+ T cells). Controls: percentage of CD8+ T cells showing intracellular IFN-γ staining after incubation with no peptide was, respectively, 0.00% (subject 9354), 0.03% (AC29), and 0.02% (AC13; data not shown). The HIV peptides used as positive controls had previously been established as recognized by these subjects: B7 Gag TL9, TPQDLNTML (p24 Gag); B8 Nef FL8, FLKEKGGL; and B14-gp41-EL9, ERYLKDQQL (reference 50).
To reconfirm that no low-frequency A*0201-SL9–specific CTLs were detectable by any of the most sensitive assays, in two subjects a further method involving stimulation of PBMCs with SL9 peptide before culture in IL-7–containing medium 63 that has been successfully used to detect low-frequency CTL responses 64 was employed. However, this method was only successful in generated SL9-specific responses in A*0201-positive subjects with chronic infection, and not in those with acute infection (data not shown).
Variant Epitope Sequence Encoded by Transmitted Virus Does Not Explain the Absence of an SL9-specific CTL Response in Acute Infection.
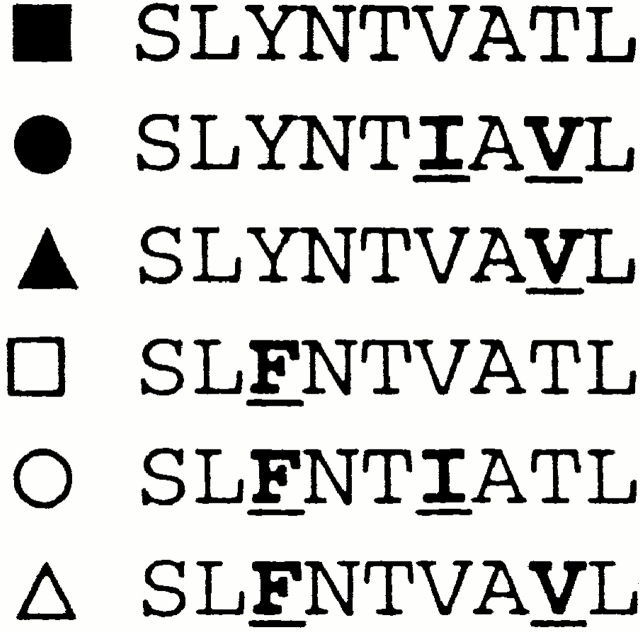
To investigate why no responses were generated towards the A*0201-SL9 epitope in acutely infected subjects with A*0201, autologous virus was sequenced to determine whether mutated epitope sequences had been transmitted. It was hypothesized that this could explain the absence of an SL9-specific response if the autologous SL9-epitope sequence differed significantly from the consensus B clade sequence. In 10 of the 11 A*0201-positive subjects enrolled, the autologous gag sequences encoding the p17 Gag SL9 epitope were determined (Table ). Only sequences encoding full-length p17 and p24 Gag were included in the analysis. In all subjects, the predominant autologous sequence encoded either the B clade consensus sequence SLYNTVATL or variants that occur very frequently in Los Alamos database sequences 50. Even when these A*0201-positive acutely infected subjects were tested for recognition of autologous SL9 variants, still none recognized his autologous epitope. (Fig. 3A and Fig. B).
Table 2.
SLYNTVATL Epitope Variants Encoded in 10 of the 11 Subjects with Early/Acute HIV Infection
| Deduced SLYNTVATL variant sequences | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Date | Clones analyzed | 77 S | 78 L | 79 Y | 80 N | 81 T | 82 V | 83 A | 84 T | 85 L |
| AC23 | 11/10/89 | 8/8 | − | − | F | − | − | − | − | − | − |
| AC29 | 03/09/99 | 11/11 | − | − | − | − | − | − | − | − | − |
| AC04 | 10/17/97 | 13/13 | − | − | F | − | − | − | − | − | − |
| AC01 | 01/31/97 | 11/12 | − | − | − | − | − | I | − | V | − |
| 1/12 | − | − | − | − | P | I | − | V | − | ||
| AC03 | 09/01/97 | 13/15 | − | − | − | − | − | − | − | − | − |
| 1/15 | − | − | − | − | − | I | − | − | − | ||
| 1/15 | − | − | − | − | − | − | − | A | − | ||
| AC14 | 07/27/99 | 13/13 | − | − | − | − | − | I | − | V | − |
| AC13 | 06/25/98 | 11/11 | − | − | − | − | − | − | − | − | − |
| AC22 | 10/30/99 | 12/14 | − | − | − | − | − | − | − | V | − |
| 2/14 | − | − | − | − | − | − | T | V | − | ||
| AC26 | 01/21/99 | 14/14 | − | − | F | − | − | − | − | − | − |
| AC32 | 05/26/99 | 6/6 | − | − | − | − | − | I | − | V | − |
Figure 3.
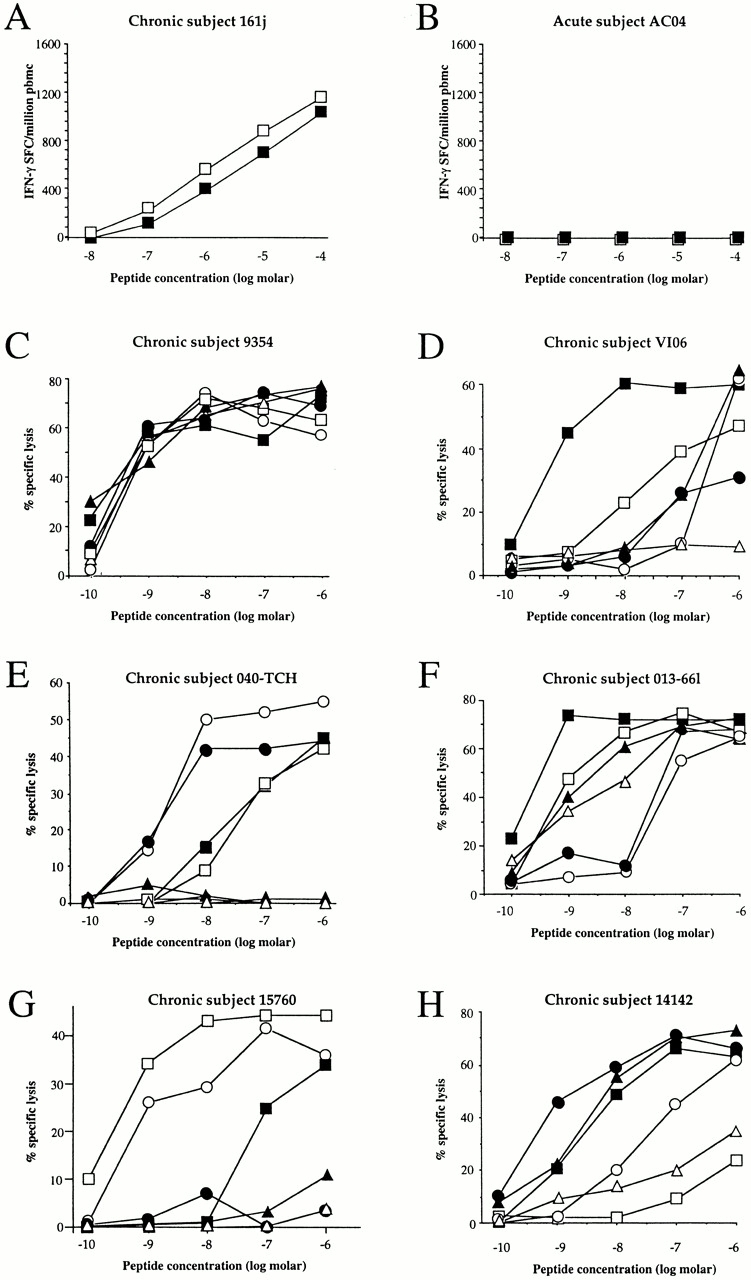
(A and B) Recognition of SLYNTVATL and autologous SL9 variant in Elispot assays. (A) Positive control chronic subject 161j, recognition of SL9, and autologous variant SLFNTVATL (reference 45). (B) Acute subject AC04: no recognition either of SL9 or autologous SL9 variant. Data from the other 10 A*0201 subjects in acute infection are not shown. SFC, spot-forming cell. (C–H) Recognition of SL9 and SL9 variants that arise most often in HIV infection (>90% of published B clade United States originating sequences; reference 50) using CTL clones derived from PBMCs from chronically infected A*0201-positive subjects.
To demonstrate further that variants of SL9 should be able to induce CTL responses, CTL clones specific for this response were generated in chronically infected subjects with A*0201 and the SL9 variants occurring most frequently in published sequences were tested for recognition. The five SL9 variants that were tested, together with the clade B consensus sequence SLYNTVATL, account for 89% of the 272 B clade Los Alamos database sequences originating in the USA 50. Many distinct patterns of variant recognition were observed, in several cases showing that SL9 variants can be recognized as well or better than the consensus sequence (Fig. 3C–H). Although a formal comparison of the ability of SL9 and the different SL9 variants to induce CTL responses was not undertaken, these data together imply that transmission of virus that encodes the commonly occurring SL9 variants (illustrated in Fig. 3) is not a barrier to the generation of CTL responses towards this epitope.
It should be noted that although the SL9 (SLYNTVATL) sequence is considered the consensus clade B sequence, as many sequences in the database contain SL9 variants as contain the consensus sequence within this highly variable region. Thus, it is highly unlikely that the 72 A*0201 subjects studied in chronic infection were all infected with virus encoding the SL9 consensus sequence. Indeed, in one of these studies 45, only 3/11 A*0201-negative chronically infected control subjects had virus encoding this consensus SL9 sequence, a virtually identical proportion to that observed in the acutely infected A*0201-positive subjects described here. However, even if we restrict our analysis to the subjects with A*0201 whose autologous virus encoded the so-called consensus SL9 epitope, the absence of an SL9 response in acute infection still differs significantly from the chronically infected A*0201-positive subjects, 75% of whom made a response to SL9 (P < 0.02, Fisher's exact test).
Late Emergence of the A2-SL9–specific CTL Response.
As the A*0201 Gag (SL9) response is clearly the dominant A*0201-restricted HIV-specific CTL response in chronic infection, but is rarely detectable in acute infection, acutely infected subjects with A*0201 were followed longitudinally in order to characterize further the timing of the development of this response. In subject AC13, in whom five separate HIV-specific CTL specificities were targeted from the first preseroconversion time point onwards, this response first became detectable 20 mo after presentation (Fig. 4 A). The presence of this response was confirmed by the appearance of staining with the A*0201 tetramer for the first time at 20 mo as well. The frequency of A*0201-SL9–specific CTLs by tetramer assay was 0.07% of PBMCs, compared with 0.03% of PBMCs in the Elispot assay (data not shown). The magnitude of this response increased once it had developed, and in the absence of any substantial reduction in the magnitude of the other five responses.
Figure 4.
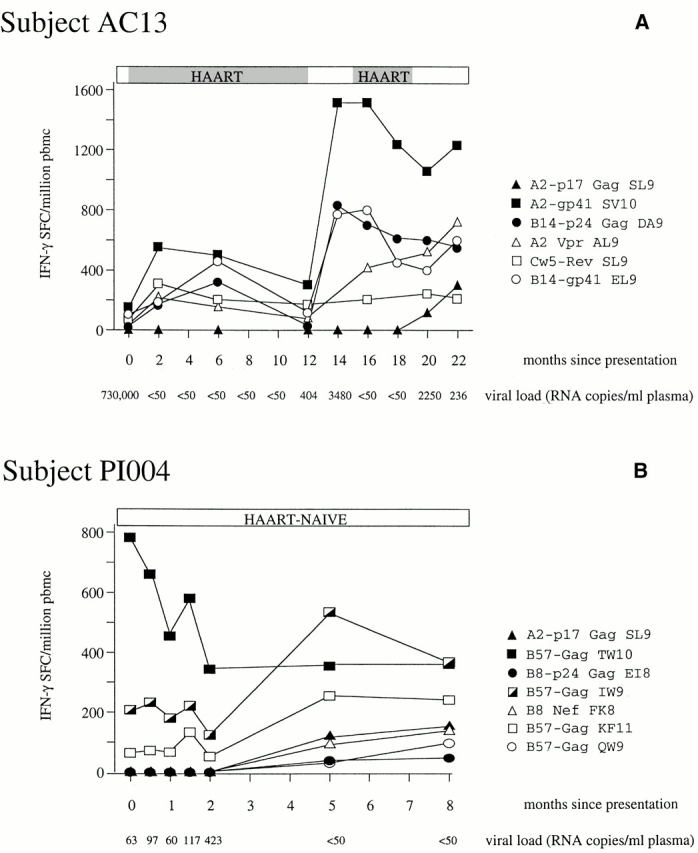
(A and B) Longitudinal study of HIV-specific epitope recognition in subject AC13 (presenting preseroconversion) and subject PI004 (presenting 7–28 wk after seroconversion). Before the onset of HAART in subject 5192d, the viral load had declined from 730,000 HIV-1 RNA copies/ml plasma to 5,000 copies/ml plasma. Subject PI004 did not receive antiretroviral therapy at any time. Peptides that were recognized (references 50 and 76): A2-p17 Gag SL9, SLYNTVATL; A2-gp41 SV10, SLLNATAIAV; B14-p24 Gag DA9, DRFYKTLRA; A2 Vpr, AIIRILQQL; Cw5-Rev SL9 SAEPVPLQL, B14-gp41 ERYLKDQQL; B57-Gag TW10, TSTLQEQIGW; B57-Gag IW9, ISPRTLNAW; B57-Gag KF11, KAFSPEVIPMF; B57-Gag QW9, QASQEVKNW; B8-p24 Gag EI8, EIYKRWII; and B8 Nef FL8, FLKEKGGL. SFC, spot-forming cell.
Although the pattern of responses in subject AC13 over time is of interest, these data are complicated by the fact that AC13 had previously received HAART from 2 wk after presentation and had also undergone two treatment interruptions. At the time the A*0201 Gag response emerged, AC13 was not on antiretroviral therapy and had detectable viremia. In an additional A*0201-positive subject PI004, recruited from a separate small cohort of acutely infected persons from Montreal, who was untreated throughout the time of study, a similar phenomenon of the late appearance of SL9-specific CTLs in the HIV-specific response was observed. This response was detectable 5 mo later than three responses that were present from the first time point available for study onwards (Fig. 4 B). Of note, in both AC13 and PI004, initial control of viremia in acute infection had been achieved in the absence of an A*0201-SL9–specific response. In AC13, the viral load had fallen from 730,000 to 5,000 HIV-1 RNA copies/ml plasma before the initiation of HAART. In PI004, the viral load for the first four time points shown, before the appearance of the A2-Gag–specific CTL response, was between 50 and 400 RNA copies/ml plasma. Thus, the A*0201-SL9–specific response is not required for the initial control of viremia in A*0201-positive subjects.
Discussion
These studies show that the HIV-specific CTL epitopes that may dominate in chronic infection may play no part in the critical antiviral CTL response associated with initial clearance of virus in acute infection. Not one of 11 A*0201-positive subjects with early HIV infection had detectable CTL responses towards the p17 Gag SL9 epitope that is recognized in 75% of A*0201-positive adults with chronic infection (P = 2 × 10−6; references 22 and 44–46). This absence of a response to SL9 in acute infection was especially striking in the seven A*0201 subjects who showed readily detectable responses to multiple other HIV-specific epitopes (P < 0.0002). Finally, the delayed appearance of an A2-restricted CTL response towards the SL9 epitope well after responses were detectable to other epitopes was confirmed in longitudinal studies of two subjects.
In addition to the consensus SL9 sequence, autologous variants were also tested, and there was also no recognition of either autologous or consensus SL9 sequence. This was important because previous studies in EBV have suggested viral evolution over time to mutate immunodominant epitopes 65. We have shown previously that mutations within and adjacent to the SL9 epitope do not alter processing to affect recognition of the SL9 variant epitope 66. Thus, there is no clear explanation from the viral sequence data presented for the absence of the A*0201-SL9 response in acute infection.
To determine whether A*0201-SL9–specific CTLs were present in early infection that were functionally inert 3 38 39, tetramer assays showed conclusively that no phenotypically silent antigen-specific CD8+ T cells could be detectable in the A*0201 subjects studied in early HIV infection. In longitudinal studies of subject AC13, A*0201-SL9 tetramer-binding cells were detectable only when SL9-specific responses in the Elispot and intracellular cytokine assays became evident.
These data are critical in relation to vaccine design, as they raise the question of whether the responses that dominate in chronic infection are in fact important in control of HIV, as was believed previously 22. It is clear from subjects AC13 and PI004 that initial control of viremia in these two subjects was achieved without any contribution from the A*0201-SL9 response. Thus, an A*0201-SL9 response is evidently not required for effective control of acute viremia. It is possible that the A*0201-SL9 CTL response in chronic infection may only be associated with control of viremia indirectly, through the action of CTLs of different HIV specificities, or via HIV-specific T helper responses. Resolving this important question will require further detailed work to understand the HIV-specific T response in A*0201-positive subjects in its entirety, as opposed to the response to a single epitope in chronic infection. Greater focus needs to be placed additionally on characterizing the CTL specificities that make up the acute response in HIV infection.
These data are of importance also to the interpretation of vaccine trials that aim to generate CTL responses. Recent analysis of HIV-uninfected persons immunized with a canarypox vector–based vaccine revealed no responses that were detectable in vaccinees towards the A*0201-SL9 epitope using tetramers. However, responses towards other epitopes in Gag were detectable using overlapping peptides spanning Gag in Elispot assays 67. These initially somewhat puzzling data would be consistent with this study. Thus, demonstration of the late appearance of the A*0201-SL9 response in natural HIV infection indicate that the entire HIV-specific CTL response cannot be estimated adequately by focusing on this single specificity.
It remains unresolved why the A*0201-SL9 responses arise late. The factors likely to contribute towards immunodominance in CTL responses 68 in this case include the binding affinity of SL9 to HLA-A*0201 69 70, the efficiency of processing of the SL9 epitope 41 66 71, and the presence of CTL escape 44 72 in responses that were previously immunodominant.
It is clear from binding studies already performed that SLYNTVATL is not a strong binder to A*0201 45 73 74 75 76, although all the SL9 variants tested do indeed bind. The gp41–A*0201 epitope, SLLNATAIAV (SV10), that dominates the early CTL response in subject AC13 (Fig. 4 A) in fact in recent studies proved to be the strongest binder to A*0201 of all HIV-1 peptides tested that had been selected on the basis of motif 76. Thus, the relatively weak binding of SL9 to A*0201 may contribute to the relatively poor immunogenicity of this epitope.
A second factor likely to contribute to the dominance of the early CTL response would be the efficiency with which individual epitopes are processed. It might be anticipated that epitopes derived from the regulatory and accessory proteins such as Tat, Rev, and Nef, expressed in abundance on the surface of infected cells early in the viral life cycle 77, might dominate the acute antiviral CTL response 15. However, in the cohort of 19 subjects with acute HIV infection being studied in Boston, Gag-specific epitopes were in fact targeted more frequently than Nef-specific at preseroconversion time points (33 vs. 19%; reference 59). Furthermore, no Nef-, Tat-, or Rev-specific A*0201-restricted responses were detectable in any of the 11 A*0201 subjects studied here in early HIV-1 infection (data not shown). Thus, low expression of Gag (relative to Tat, Rev, or Nef) in acute infection does not appear to be an explanation for the absence of A*0201-SL9 responses at this time.
A further possible explanation for the late appearance of the A*0201-SL9 response in the course of HIV-1 infection would be that CTL escape occurring in the dominant epitopes would enable previously subdominant epitopes to become immunodominant over time 72. The limited data available from subject PI004 (Fig. 4 B) do appear to indicate that a substantial reduction in the dominant HLA-B57–restricted responses towards p24 Gag epitope TSTLQEQIGW 34 50 has occurred immediately before appearance of the A*0201-SL9 and several other responses that previously were undetectable. In AC13, the data are more difficult to interpret, as this subject was twice treated with HAART, but the appearance is more suggestive in this case that the A*0201-SL9 response emerges at a time when the CTL response as a whole is increasing in magnitude. These and other subjects who show the late appearance of particular epitopes clearly warrant further study in this regard.
It is important to note that, although SL9 is the dominant A*0201-restricted response in chronic infection, it is not commonly the dominant HIV-specific CTL response overall 34 78 79 80. Thus, it is not unexpected to see that SL9 is subdominant to B14- and B57-restricted responses in subjects AC13 and PI004, and that the SL9 response does not increase to become dominant to these other responses.
The closest precedent for this phenomenon may be in human EBV infection, in which lytic as opposed to latent antigen-specific responses dominate in acute infection 2 81. However, this difference corresponds to the different biological phases of EBV infection. In HIV infection, there is no indication that Gag is not expressed in acute infection; in fact, in 5/7 HLA-A3–positive subjects studied at preseroconversion, p17 Gag-specific HLA-A3–restricted activity was a major component part of the initial anti-HIV immune response 59. There are indications, both in studies of SIV 15 and HIV 59 82, and also in hepatitis C virus infection 3 83, that many clear-cut differences in the specificities of the CTL response in acute and chronic viral infections may exist. However, these data presented with respect to the A*0201-SL9 specificity are apparently the first unequivocal demonstration of this phenomenon.
In conclusion, these data show that the HIV-specific CTL responses that are present in acute infection may differ substantially from those that are frequently detectable in chronic infection. The best-studied response in chronic HIV infection is seen to play little or no part in the antiviral immune response in acute infection that is so critical in determining the ultimate outcome from infection. Further studies are needed to assess the importance of the CTL responses that are frequently detectable in subjects with chronic infection, to determine whether these, or the responses present in acute infection, or both, are effective responses that should be incorporated into HIV vaccine design.
Acknowledgments
We thank Paul Klenerman and David Watkins for helpful comments and discussion of the manuscript.
This work was supported by grants to P.J.R. Goulder from the Elizabeth Glaser Pediatric AIDS Foundation, the Medical Research Foundation (UK; grant G108/274), and the National Institutes of Health (grant AI46995); to M.A. Altfeld through the German Academic Exchange Foundation; to E.S. Rosenberg through the Doris Duke Charitable Foundation and the National Institutes of Health (grant AI01541); to M.M. Addo through the German Research Foundation; to S.A. Kalams through the National Institutes of Health (grant AI39966); and to B.D. Walker through the National Institutes of Health (grants AI28568 and AI30914) and the Doris Duke Charitable Foundation. P.J.R. Goulder is an Elizabeth Glaser Scientist of the Elizabeth Glaser Pediatric AIDS Foundation. B.D. Walker is a Doris Duke Distinguished Clinical Science Professor.
Footnotes
Abbreviations used in this paper: BCL, B lymphoblastoid cell line; Elispot, enzyme-linked immunospot; HAART, highly active antiretroviral therapy; ICS, intracellular cytokine staining; SIV, simian immunodeficiency virus.
P.J.R. Goulder and M.A. Altfeld contributed equally to this work.
References
- Riddell S.R., Watanabe K.S., Goodrich J.M., Agha M.E., Greenberg P.D. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of CTL clones. Science. 1992;257:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- Rickinson A.B., Moss D. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu. Rev. Immunol. 1997;15:405–431. doi: 10.1146/annurev.immunol.15.1.405. [DOI] [PubMed] [Google Scholar]
- Lechner F., Wong D. K.H., Dunbar P.R., Chapman R., Chung R.T., Dohrenwend P., Robbins G., Phillips R., Klenerman P., Walker B.D. Analysis of successful immune responses in patients with hepatitis C virus. J. Exp. Med. 2000;199:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maini M.K., Boni C., Ogg G., King A.S., Reignat S., Lee C.K., Larrubia J.R., Webster G.J., McMichael A.J., Ferrari C. Direct ex vivo analysis of hepatitis B virus-specific CD8+ T cells associated with the control of infection. Gastroenterology. 1999;117:1386–1396. doi: 10.1016/s0016-5085(99)70289-1. [DOI] [PubMed] [Google Scholar]
- Goulder P.J.R, Rowland-Jones S., McMichael A.J., Walker B.D. Anti-human immunodeficiency virus cellular immunityprogress towards vaccine design AIDS. 13Suppl. A1999. S121 S136 [PubMed] [Google Scholar]
- Zinkernagel R.M. Immunology taught by viruses. Science. 1996;271:173–178. doi: 10.1126/science.271.5246.173. [DOI] [PubMed] [Google Scholar]
- Yap K.L., Ada G.L., McKenzie I.F. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature. 1978;273:238–239. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]
- Cooper S.A., Erickson L., Adams E., Kansopon J., Weiner A., Chien D.Y., Houghton M., Parham P., Walker C.M. Analysis of a successful immune response against hepatitis C virus. Immunity. 1999;10:439–449. doi: 10.1016/s1074-7613(00)80044-8. [DOI] [PubMed] [Google Scholar]
- Schmitz J.E., Kuroda M., Santra S., Sasseville V., Simon M., Lifton M., Racz P., Tenner-Racz K., Dalesandro M., Scallon B. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- Klenerman P., Zinkernagel R.M. What can we learn about human immunodeficiency virus infection from a study of lymphocytic choriomeningitis virus? Immunol. Rev. 1997;159:5–16. doi: 10.1111/j.1600-065x.1997.tb01003.x. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Demarest J.F., Schacker T., Vaccarezza M., Cohen O.J., Daucher M., Graziosi C., Schnittman S.S., Quinn T.C., Shaw G.M., Perrin L., Tambussi G., Lazzarin A., Sekaly R.P., Soudeyns H., Corey L., Fauci A.S. The qualitative nature of the primary immune response to HIV infection is a prognosticator of disease progression independent of the initial level of plasma viremia. Proc. Natl. Acad. Sci. USA. 1997;94:254–258. doi: 10.1073/pnas.94.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E.S., Billingsley J.M., Caliendo A., Boswell S.L., Sax P.E., Kalams S.A., Walker B.D. Vigorous HIV-1-specific CD4+ T-cell responses associated with control of viraemia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- Rosenberg E.S., Altfeld M., Eldridge B., Mukerjee J., Phillips M., Brander C., Goulder P.J.R., Walker B.D. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- Mellors J.W., Rinaldo C.R., Gupta P., White R.M., Todd J.A., Kingsley L.A. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- Allen T., O'Connor D., Jing P., Dzuri J.L., Mothe B.R., Vogel T.U., Dunphy E., Liebl M.E., Emerson C., Wilson N. Tat-specific T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature. 2000;407:388–390. doi: 10.1038/35030124. [DOI] [PubMed] [Google Scholar]
- Jin X., Demoitie M.A., Donahoe S.M., Ogg G.S., Bonhoeffer S., Kakimoto W.M., Gillespie G., Moss P.A., Dyer W., Kurilla M.G. High frequency of cytomegalovirus-specific cytotoxic T-effector cells in HLA-A*0201-positive subjects during multiple viral coinfections. J. Infect. Dis. 2000;181:165–175. doi: 10.1086/315201. [DOI] [PubMed] [Google Scholar]
- Borrow P., Lewicki H., Hahn B.H., Shaw G.M., Oldstone M.B.A. Virus-specific CD8+ CTL activity associated with control of viremia in primary HIV infection. J. Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koup R.A., Safrit J.T., Cao Y., Andrew C.A., McLeod G., Borkowsky W., Farthing C., Ho D.D. Temporal association of cellular immune responses with the initial control of viremia in primary HIV infection. J. Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B.D., Chakrabati S., Moss B., Paradis T.J., Flynn T., Durno A.G., Blumberg R.S., Kaplan J.C., Hirsch M.S., and Schoole R.T. HIV-specific T lymphocytes in seropositive individuals. Nature. 1987;328:345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- Klein M.R., van Baalen C.A., Holwerda A.M., Kerkhof Garde S.R., Bende R.J., Keet I.P., Eeftinck-Schattenkerk J.K., Osterhaus A.D., Schuitemaker H., Miedema F. Kinetics of Gag-specific CTL responses during the clinical course of HIV-1 infectiona longitudinal analysis of rapid progressors and long-term, asymptomatics. J. Exp. Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang O.O., Kalams S.A., Rosenzweig M., Trocha A., Jones N., Koziel M., Walker B.D., Johnson R.P. Efficient lysis of human immunodeficiency virus type 1-infected cells by cytotoxic T lymphocytes. J. Virol. 1996;70:5799–5806. doi: 10.1128/jvi.70.9.5799-5806.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg G.S., Jin X., Bonhoeffer S., Dunbar P.R., Nowak M.A., Monard S., Segal J.P., Cao Y., Rowland Jones S.L., Cerundolo V. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- Kalams S.A., Buchbinder S.P., Rosenberg E.S., Billingsley J.M., Colbert D.S., Jones N.G., Shea A.K., Trocha A.K., Walker B.D. Association between virus-specific CTL and helper responses in HIV-1 infection. J. Virol. 1999;73:6715–6720. doi: 10.1128/jvi.73.8.6715-6720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasutomi Y., Reimann K.A., Lord C.I., Miller M.D., Letvin N.L. Simian immunodeficiency virus-specific CD8+ lymphocyte response in acutely infected rhesus monkeys. J. Virol. 1993;67:1707–1711. doi: 10.1128/jvi.67.3.1707-1711.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Bauer D.E., Tuttleton S.E., Gettie A., Blanchard J., Irwin C.E., Safrit J.T., Lewin S., Mittler J., Weinberger L. Dramatic rise in plasma viremia after CD8+ T cell depletion in SIV-infected macaques. J. Exp. Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D.T., O'Connor D.H., Jing P., Dzuris J.L., Sidney J., da Silva J., Allen T.M., Horton H., Venham J.E., Rudersdorf R.A. Virus-specific cytotoxic T-lymphocyte responses select for amino acid variation in simian immunodeficiency virus Env and Nef. Nat. Med. 2000;5:1270–1276. doi: 10.1038/15224. [DOI] [PubMed] [Google Scholar]
- Borrow P., Lewicki H., Wei X., Horwitz M.S., Peffer N., Meyers H., Nelson J.A., Gairin J.E., Hahn B.H., Oldstone M.B., Shaw G.M. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- Price D.A., Goulder P.J.R., Klenerman P., Sewell A.K., Easterbrook P.J., Bangham C.R.M., Phillips R.E. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc. Natl. Acad. Sci. USA. 1997;94:1890–1895. doi: 10.1073/pnas.94.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S., Conley A.J., Brewah Y.A., Jones G.M., Leath S., Boots L.J., Davey V., Pantaleo G., Demarest J.F., Carter C. Transfer of HIV-1-specific cytotoxic T lymphocytes to an AIDS patient leads to selection for mutant HIV variants and subsequent disease progression. Nat. Med. 1995;1:330–336. doi: 10.1038/nm0495-330. [DOI] [PubMed] [Google Scholar]
- Goulder P.J.R., Phillips R.E., Colbert R.A., McAdam S., Ogg G., Nowak M.A., Giangrande P., Luzzi G., Morgan B., Edwards A. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat. Med. 1997;3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- Kaslow R.A., Carrington M., Apple R., Park L., Munoz A., Saah A.J., Goedert J.J., Winkler C., O'Brien S.J., Rinaldo C.R. Influence of human MHC genes on the course of HIV infection. Nat. Med. 1996;2:405–411. doi: 10.1038/nm0496-405. [DOI] [PubMed] [Google Scholar]
- Keet I.P., Tang J., Klein M.R., LeBlanc S., Enger C., Rivers C., Apple R., Mann D., Goedert J.J., Miedema F., Kaslow R.A. Consistent associations of HLA class I and II and transporter gene products with progression of human immunodeficiency virus type 1 infection in homosexual men. J. Infect. Dis. 1999;180:299–309. doi: 10.1086/314862. [DOI] [PubMed] [Google Scholar]
- Carrington M., Nelson G.W., Martin M.P., Kissner T., Vlahov D., Goedert J.J., Kaslow R.A., Buchbinder S., Hoots K., O'Brien S.J. HLA and HIV-1heterozygote advantage and B*35-Cw*04 disadvantage. Science. 1999;283:1748–1752. doi: 10.1126/science.283.5408.1748. [DOI] [PubMed] [Google Scholar]
- Goulder P.J.R., Crowley S., Krausa P., Morgan B., Edwards A., Giangrande P., McIntyre K., McMichael A.J. Novel, cross-restricted, conserved and immunodominant CTL epitopes in long-term slow progressors in HIV-1 infection. AIDS Res. Hum. Retrovir. 1996;12:1691–1698. doi: 10.1089/aid.1996.12.1691. [DOI] [PubMed] [Google Scholar]
- Migueles S.A., Sabbaghian M.S., Shupert W.L., Bettinotti M.P., Marincola F.M., Martino L., Hallahan C.W., Selig S.M., Schwartz D., Sullivan J., Connors M. HLA-B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long-term nonprogressors. Proc. Natl. Acad. Sci. USA. 2000;97:2709–2714. doi: 10.1073/pnas.050567397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A.V.S. The immunogenetics of human infectious diseases. Annu. Rev. Immunol. 1998;16:593–617. doi: 10.1146/annurev.immunol.16.1.593. [DOI] [PubMed] [Google Scholar]
- Gallimore A., Dumrese T., Hengartner H., Zinkernagel R.M., Rammensee H.-G. Protective immunity does not correlate with the hierarchy of virus-specific CTL responses to naturally processed peptides. J. Exp. Med. 1998;187:1647–1657. doi: 10.1084/jem.187.10.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac A.J., Blattman J.N., Murali-Krishna K., Sourdive D.J.D., Suresh M., Altman J.D., Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallimore A., Glithero A., Godkin A., Tissot A.C., Pluckthun A., Elliott T., Hengartner H., Zinkernagel R.M. Induction and exhaustion of lymphocytic choriomeningitis virus–specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I–peptide complexes. J. Exp. Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R.P., Trocha A., Yang L., Mazzara G.P., Panicali D.L., Buchanan T.M., Walker B.D. HIV-1 gag-specific cytotoxic T lymphocytes recognize multiple highly conserved epitopes. Fine specificity of the gag-specific response defined by using unstimulated peripheral blood mononuclear cells and cloned effector cells. J. Immunol. 1991;147:1512–1521. [PubMed] [Google Scholar]
- Tsomides T.J., Aldovini A., Johnson R. P., Walker B.D., Young R.A., Eisen H.N. Naturally processed viral peptides recognized by cytotoxic T lymphocytes on cells chronically infected by human immunodeficiency virus type 1. J. Exp. Med. 1994;180:1283–1293. doi: 10.1084/jem.180.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Moss P.A.H., Goulder P.J.R., Barouch D.H., McHeyzer-Williams M.G., Bell J.I., McMichael A.J., Davis M.M. Direct visualization and phenotypic analysis of virus-specific T lymphocytes in HIV-infected individuals. Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- Harrer T., Harrer E., Barbosa P., Kaufmann F., Wagner R., Bruggemann S., Kalden J.R., Feinberg M., Johnson R.P., Buchbinder S., Walker B.D. Recognition of two overlapping CTL epitopes in HIV-1 p17 by CTL from a long-term non-progressing HIV-1 infected individual. J. Immunol. 1998;161:4875–4881. [PubMed] [Google Scholar]
- Goulder P.J.R., Sewell A.K., Lalloo D.G., Price D.A., Whelan J.A., Evans J., Taylor G.P., Luzzi G., Giangrande P., Phillips R.E., McMichael A.J. Patterns of immunodominance in HIV-1–specific cytotoxic T lymphocyte responses in two HLA-identical siblings with HLA-A*0201 are influenced by epitope mutation. J. Exp. Med. 1997;185:1423–1433. doi: 10.1084/jem.185.8.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brander C., Hartman K.E., Trocha A.K., Jones N.G., Johnson R.P., Korber B., Wentworth P., Buchbinder S.P., Wolinsky S., Walker B.D., Kalams S.A. Lack of strong immune selection pressure by the immunodominant HLA-A*0201 restricted CTL response in chronic HIV-1 infection. J. Clin. Invest. 1998;101:2559–2566. doi: 10.1172/JCI2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C., Lawrence J., Schapiro J.M., Altman J.D., Winters M.A., Crompton M., Loi M., Kundu S.K., David M.M., Merigan T.C. Frequency of class I restricted anti-HIV CD8+ T cells in individuals receiving highly active antiretroviral therapy. J. Immunol. 1999;162:1780–1788. [PubMed] [Google Scholar]
- Janssen R.S., Satten G.A., Stramer S.L., Rawal B.D., O'Brien T.R., Weiblen B.J., Hecht F.M., Jack N., Cleghorn F.R., Kahn J.O. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA. 1998;280:42–48. doi: 10.1001/jama.280.1.42. [DOI] [PubMed] [Google Scholar]
- Lyles R., Munoz H.A., Yamashita T.E., Bazmi H., Detels R., Rinaldo C.R., Margolick J.B., Phair J.P., Mellors J.W. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. Multicenter AIDS Cohort Study. J. Infect. Dis. 2000;181:872–880. doi: 10.1086/315339. [DOI] [PubMed] [Google Scholar]
- Bunce M., O'Neill C.M., Barnardo M.C., Krausa P., Browning M., Morris P., Welsh K. Phototypingcomprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 and DQB1 by PCR with 144 primer mixes utilizing sequence-specific primers (PCR-SSP) Tissue Antigens. 1995;46:355–367. doi: 10.1111/j.1399-0039.1995.tb03127.x. [DOI] [PubMed] [Google Scholar]
- Brander, C., and P.J.R. Goulder. Recent advances in the optimization of HIV-specific CTL epitopes. In HIV Molecular Immunology Database. 1999. B.T.M. Korber, C. Brander, B.D. Walker, R.A. Koup, J. Moore, B. Haynes, and G. Meyers., editors. Los Alamos National Laboratory: Theoretical Biology and Biophysics, Los Alamos, NM (http://www.hiv-lanl.gov).
- Lalvani A., Brookes R., Hambleton S., Britton W.J., Hill A.V.S., McMichael A.J. Rapid effector function in CD8+ memory T cells. J. Exp. Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulder P.J.R., Brander C., Annamalai K., Mngqundaniso N., Govender U., Tang Y., He S., Hartman K.E., O'Callaghan C.A., Ogg G.S. Differential narrow focusing of immunodominant HIV Gag-specific CTL responses in infected African and Caucasoid adults and children. J. Virol. 2000;74:5679–5690. doi: 10.1128/jvi.74.12.5679-5690.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher C.J., Quittner C., Peterson D.M., Connors M., Koup R.A., Maino V.C., Picker L.J. HIV-1 specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat. Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- Goulder P.J.R., Tang Y., Brander C., Trocha A., He S., Rosenberg E.S., Ogg G., O'Callaghan C.A., Kalams S.A., Pelton S.I. Functionally inert HIV-specific cytotoxic T lymphocyte do not play a major role in chronically infected adults and children. J. Exp. Med. 2000;192:1819–1831. doi: 10.1084/jem.192.12.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts M.R., Cassaza J.P., Patterson B.A., Waldrop S., Kern F., Trigona W., Fu T.-M., Picker L.J., Koup R.A. Putative immunodominant human immunodeficiency virus-specific CD8+ T-cell responses cannot be predicted by major histocompatibility complex class I haplotype. J. Virol. 2000;74:9144–9151. doi: 10.1128/jvi.74.19.9144-9151.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C.A., Byford M.F., Jakobsen B.K., McMichael A.J., Bell J.I. BirA enzymeproduction and application in the study of membrane receptor-ligand interactions by site specific biotinylation. Anal. Biochem. 1999;266:9–15. doi: 10.1006/abio.1998.2930. [DOI] [PubMed] [Google Scholar]
- Walker B.D., Flexner C., Birch-Limberger K., Fisher L., Paradis T.J., Aldovini A., Young R., Moss B., Schooley R.T. Long-term culture and fine specificity of human cytotoxic T-lymphocyte clones reactive with human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA. 1989;86:9514–9518. doi: 10.1073/pnas.86.23.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B.D. HIV-1-specific cytotoxic T lymphocytes. In: Aldovini A., Walker B.D., editors. Techniques in HIV Research. Stockton Press; New York: 1990. pp. 201–233. [Google Scholar]
- Altfeld M.A., Rosenberg E.S., Shankarappa R., Hecht R., Eldridge R.L., Addo M.M., Poon S.L., Phillips M.N., Robbins G.K., Mukherjee J.S., Brander C., Goulder P.J.R., Levy J., Mullins J., Walker B.D. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J. Exp. Med. 2001;193:169–180. doi: 10.1084/jem.193.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L.C., Gudgeon N., Annels N.E., Hansasuta P., O'Callaghan C.A., Rowland-Jones S., McMichael A.J., Rickinson A.J., Callan M.F. A reevaluation of the frequency of CD8+ T cells specific for EBV in healthy virus carriers. J. Immunol. 1999;162:1827–1835. [PubMed] [Google Scholar]
- Steven N., Annels N.E., Kumar A., Leese A.M., Kurilla M.G., Rickinson A.B. Immediate early and early lytic cycle proteins are frequent targets of the Epstein-Barr virus–induced cytotoxic T cell response. J. Exp. Med. 1997;185:1605–1617. doi: 10.1084/jem.185.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills M.R., Carmichael A.J., Mynard K., Jin X., Weekes M.P., Plachter B., Sissons J.G. The human cytotoxic T-lymphocyte response to cytomegalovirusis dominated by structural protein pp65frequency, specificity and T-cell receptor usage of pp65-specific CTL. J. Virol. 1996;70:7569–7579. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalvani A.J., Dong T., Ogg G., Patham A.A., Newell H., Hill A., McMichael A.J., Rowland-Jones S. Optimization of a peptide-based protocol employing IL-7 for in vitro restimulation of human cytotoxic T lymphocyte precursors. J. Immunol. Methods. 1997;210:65–77. doi: 10.1016/s0022-1759(97)00177-4. [DOI] [PubMed] [Google Scholar]
- Rowland-Jones S., Sutton J., Ariyoshi K., Dong T., Gotch F.M., McAdam S., Whitby D., Sabally S., Gallimore A., Corrah T. HIV-specific cytotoxic T cells in HIV-exposed but uninfected Gambian women. Nat. Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- De Campos Lima P., Ghavioli R., Zhgang Q.J., Wallace L.E., Dolcetti R., Rowe M., Rickinson A.B., Masucci M.G. A-11 epitope loss in isolates of Epstein-Barr virus from a highly A11+ population. Science. 1993;260:98–100. doi: 10.1126/science.7682013. [DOI] [PubMed] [Google Scholar]
- Brander C., Yang O.O., Jones N.G., Goulder P.J.R., Johnson R.P., Trocha A., Colbert D., Buchbinder S., Bergmann C.C., Zweerink H.J. Efficient processing of the immunodominant HLA-A*0201-restricted HIV-1 CTL epitope despite multiple variations in the epitope flanking sequences. J. Virol. 1999;73:10191–10198. doi: 10.1128/jvi.73.12.10191-10198.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhold, K, G. Ogg, G. Ferrari, T. Evans. M. Keefer, D. Panicali, A.-M. Duliege, M. Klein, L. Corey, and J. McElrath. 2000. Frequency and epitope specificity of CTL elicited in uninfected recipients of candidate AIDS vaccines in AVEG Phase I/II studies: comparison with CTL responses to HIV infection. 7th Conf. on Retroviruses and Opportunistic Infections, San Francisco. 657 (Abstr.).
- Yewdell J.W., Bennink J.R. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu. Rev. Immunol. 1999;17:51–88. doi: 10.1146/annurev.immunol.17.1.51. [DOI] [PubMed] [Google Scholar]
- Van der Most R.G., Sette A., Osiros C., Alexander J., Murali Krishna K., Lau L.L., Southwood S., Sidney J., Chesnut R.W., Matlobian M., Ahmed R. Analysis of cytotoxic T cell responses to dominant and subdominant epitopes during acute and chronic LCMV infection. J. Immunol. 1996;157:5543–5554. [PubMed] [Google Scholar]
- Sette A., Vitellio A., Reherman B., Fowler P., Nayersina R., Kast W.A., Melief C.J.M., Oseroff C., Yuan L., Ruppert J. The relationship between class I binding affinity and immunogenicity of potential cytotoxic T cell epitopes. J. Immunol. 1994;153:5586–5596. [PubMed] [Google Scholar]
- Restifo N.P., Bacik I., Irvine K.R., Yewdell J.W., McCabe B., Anderson R.W., Eisenlohr L.C., Rosenberg S.A., Bennink J.R. Antigen processing in vivo and the elicitation of primary CTL responses. J. Immunol. 1995;154:4414–4422. [PMC free article] [PubMed] [Google Scholar]
- Nowak M.A., May R.M., Phillips R.E., Rowland-Jones S., Lalloo S., McAdam S., Klenerman P., Koppe B., Sigmund K., Bangham C.R.M., McMichael A.J. Antigenic oscillations and shifting immunodominance in HIV-1 infections. Nature. 1995;375:606–611. doi: 10.1038/375606a0. [DOI] [PubMed] [Google Scholar]
- Sewell A.K., Harcourt G.C., Goulder P.J.R., Price D.A., Phillips R.E. Antagonism of cytotoxic T lymphocyte-mediated lysis by natural HIV-1 altered peptide ligands requires simultaenous presentation of agonist and antagonist peptides. Eur. J. Immunol. 1997;27:2323–2329. doi: 10.1002/eji.1830270929. [DOI] [PubMed] [Google Scholar]
- Hunt D.F., Henderson R.A., Shabanowitz J., Sakaguchi K., Michael H., Sevilir N., Cox A.L., Appella E., Engelhard V.H. Characterization of peptides bound to rhew class I molecule HLA-A2.1 by mass spectrometry. Science. 1992;255:1261–1263. doi: 10.1126/science.1546328. [DOI] [PubMed] [Google Scholar]
- Ruppert J, Sidney J., Celis E., Kubo R.T., Grey H.M., Sette A. Prominent role of secondary anchor residues in peptide binding to HLA-A2.1 molecules. Cell. 1993;74:929–937. doi: 10.1016/0092-8674(93)90472-3. [DOI] [PubMed] [Google Scholar]
- Altfeld M., Livingston B., Reshamwala N., Nguyen T., Addo M., Shea M., Newman M., Fikes J., Sidney J., Wentworth P. Identification of novel HLA-A2-restricted HIV-1–specific CTL epitopes predicted by the HLA-A2 supertype peptide-binding motif. J. Virol. 2001;In press doi: 10.1128/JVI.75.3.1301-1311.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotman M.E., Kim S., Buchbinder A., DeRossi A., Baltimore D., Wong-Staal F. Kinetics of expression of multiply spliced RNA in early human immunodeficiency virus type 1 infection of lymphocytes and monocytes. Proc. Natl. Acad. Sci. USA. 1991;88:5011–5015. doi: 10.1073/pnas.88.11.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulder P.J.R., Bunce M., Luzzi G., Edwards A., Phillips R., McMichael A. Underestimated role of HLA-C-restricted CTL epitopes in the anti-HIV immune response. AIDS. 1997;11:1884–1886. doi: 10.1097/00002030-199715000-00016. [DOI] [PubMed] [Google Scholar]
- Goulder P.J.R., Reid S., Price D.A., O'Callaghan C., McMichael A.J., Phillips R.E., Jones E.Y. Combined structural and immunological refinement of CTL epitopes. Eur. J. Immunol. 1997;27:1515–1521. doi: 10.1002/eji.1830270630. [DOI] [PubMed] [Google Scholar]
- Kalams S.A., Goulder P.J.R., Shea A.K., Jones N.G., Trocha A.K., Ogg G.S., Walker B.D. Levels of HIV-1-specific CTL effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J. Virol. 1999;73:6715–6720. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan M.F., Tan L., Annels N., Ogg G.S., Wilson J.D., O'Callaghan C.A., Steven N., McMichael A.J., Rickinson A.B. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J. Exp. Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalod M., Dupuis M., Deschemin J.C., Goujard C., Deveau C., Meyer L., Ngo N., Rouzioux C., Guillet J.G., Delfraissy J.F., Sinet M., Venet A. Weak anti-HIV CD8+ T-cell effector activity in HIV primary infection. J. Clin. Invest. 1999;104:1431–1439. doi: 10.1172/JCI7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner F., Gruener N.H., Urbani S., Uggeri J., Santantonio T., Kammer A.R., Cerny A., Phillips R.E., Ferrari C., Pape G.R., Klenerman P. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur. J. Immunol. 2000;30:2479–2487. doi: 10.1002/1521-4141(200009)30:9<2479::AID-IMMU2479>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]