Abstract
Little is known of age-associated functional changes in hematopoietic stem cells (HSCs). We studied aging HSCs at the clonal level by isolating CD34−/lowc-Kit+Sca-1+ lineage marker–negative (CD34−KSL) cells from the bone marrow of C57BL/6 mice. A population of CD34−KSL cells gradually expanded as age increased. Regardless of age, these cells formed in vitro colonies with stem cell factor and interleukin (IL)-3 but not with IL-3 alone. They did not form day 12 colony-forming unit (CFU)-S, indicating that they are primitive cells with myeloid differentiation potential. An in vivo limiting dilution assay revealed that numbers of multilineage repopulating cells increased twofold from 2 to 18 mo of age within a population of CD34−KSL cells as well as among unseparated bone marrow cells. In addition, we detected another compartment of repopulating cells, which differed from HSCs, among CD34−KSL cells of 18-mo-old mice. These repopulating cells showed less differentiation potential toward lymphoid cells but retained self-renewal potential, as suggested by secondary transplantation. We propose that HSCs gradually accumulate with age, accompanied by cells with less lymphoid differentiation potential, as a result of repeated self-renewal of HSCs.
Keywords: mouse, bone marrow transplantation, hematopoiesis, self-renewal, differentiation
Introduction
Aging of individuals is defined by two criteria: increasing probability of death with age and characteristic changes in phenotype over time 1. As hematopoietic stem cells (HSCs) are able to self-renew and to differentiate along all hematopoietic lineages throughout the entire lifetime of an organism 2, it has been questioned whether HSCs age. Evaluation of HSC life-span has been challenged by serial transplantation. Aged bone marrow cells were able to reconstitute hematopoiesis of the recipients after multiple transfers as wells as young bone marrow cells 3 4 5. This observation was supported by a recent study showing that HSCs from young and old mice exhibited indistinguishable repopulating abilities at the clonal level 6. It has been suggested that transplantation, as a procedure, adversely affects the repopulating activity of HSCs 5 7. Nonetheless, it is evident that HSCs can function far longer than the life-span of their donor even if their capacity for self-renewal is limited 8 9.
Competitive repopulation has revealed another age-associated feature of hematopoiesis. The repopulating activity of bone marrow cells from aged mice is higher than that of cells from young mice 10. This might be caused by qualitative and/or quantitative changes in aged HSCs as compared with young HSCs. Analyses using the binomial formula with covariance suggested that the frequency of HSCs was relatively higher in aged bone marrow cells 11. However, the mechanism by which repopulating activity rises in aged bone marrow cells remains elusive. These findings collectively argue against defective hematopoiesis in aged mice.
Our study focused on the characterization of age-associated functional changes in HSCs in mice. A twofold increase in numbers of multilineage repopulating cells from 2 to 18 mo of age was demonstrated by limiting dilution analysis with unseparated bone marrow cells. We noticed the existence of recipients in which only myeloid lineage was reconstituted at significant level among irradiated mice transplanted with a limited dose of bone marrow cells from aged mice, but not from young mice. To extend these observations, we analyzed repopulating cells at the clonal level.
We have shown that CD34−/lowc-Kit+Sca-1+ lineage marker–negative (CD34−KSL) bone marrow cells are highly enriched for HSCs 12. A population of these cells appeared to expand with age. It was shown that multilineage repopulating cell number also increased twofold among CD34−KSL cells from 2 to 18 mo of age. We thereupon found another compartment of repopulating cells, whose differentiation potential was skewed toward myeloid lineage, among aged CD34−KSL cells. Successful secondary transplantation indicated that self-renewal potential was maintained in these unusual repopulating cells. Cell cycle kinetics was taken into consideration in determining that CD34−KSL cell numbers expanded. We present an age-associated effect on hematopoiesis at the HSC level.
Materials and Methods
Mice and Cells.
C57BL/6 male (B6-Ly5.2) mice were purchased from Charles River Japan, Inc. Aged C57BL/6 mice were provided by Dr. K. Hirokawa (Tokyo Medical and Dental University, Tokyo, Japan). Mice congenic for the Ly5 locus (B6-Ly5.1) were bred and maintained in the University of Tsukuba Animal Research Center. The Animal Experiment Committee of the University of Tsukuba approved animal use and care. B6-Ly5.1/Ly5.2 (B6-F1) mice were obtained from mating pairs of B6-Ly5.1 and B6-Ly5.2 mice. Bone marrow cells were obtained from both right and left femora and tibiae of B6-Ly5.1 and B6-F1 male mice. Test donor cells were prepared from B6-Ly5.1 mice at 2, 6, 12, and 18 mo of age. Competitor cells were prepared from B6-F1 mice at 2 mo of age. Recipients were B6-Ly5.2 male mice aged 2 mo.
Analysis and Purification of CD34−KSL Cells.
CD34−KSL cells were purified from bone marrow cells as previously described 12. In brief, cells with low density (<1.077 g/ml) were stained with an appropriate amount of biotinylated lineage–antibody mixture consisting of anti–Gr-1 (RB6-8C5), Mac-1 (M1/70), B220 (RA3-6B2), CD4 (GK1.5), CD8 (53-6.7), and Ter-119 mAbs. Lineage-positive cells were depleted with streptavidin–magnetic beads (M-280; Dynal). The cells were further stained with PE-conjugated anti–Sca-1, allophycocyanin (APC)-conjugated anti–c-Kit (ACK-2), and fluorescein isothiocyanate (FITC)-conjugated anti-CD34 (49E8) antibodies. Biotinylated antibodies were developed with streptavidin–Texas Red (Life Technologies). All antibodies other than ACK-2 (a gift of Dr. S.I. Nishikawa, Kyoto University, Kyoto, Japan) were purchased from PharMingen. Four-color analysis was performed on a FACS Vantage™ (Becton Dickinson) using CELLQuest™ software (Becton Dickinson). CD34−KSL cells were sorted with the counter mode. Dead cells stained with propidium iodide (PI) were excluded from analysis and sorting.
Competitive Repopulation Assay.
The Ly5 system was adapted to a competitive repopulation assay 13 as described 14. Unfractionated or purified bone marrow cells to be tested were mixed with 2 × 105 bone marrow cells as competitor cells and were transplanted into B6-Ly5.2 mice irradiated at a dose of 9.5 Gy. In vivo limiting dilution was performed as described 15. Three or more different numbers of test donor cells were subjected to competitive repopulation against 2 × 105 bone marrow cells. At indicated time points after transplantation, peripheral blood cells of the recipients were obtained by retroorbital bleeding and were stained with biotinylated anti-Ly5.1 (A20) and FITC-conjugated anti-Ly5.2 antibodies (104). The cells were simultaneously stained with APC-conjugated anti-B220 antibody together with a mixture of PE-conjugated anti–Mac-1 and –Gr-1 antibodies or PE-conjugated anti-CD4 and -CD8 antibodies. The cells were stained last with streptavidin–Texas Red. Four-color analysis was performed on a FACS®. The percent chimerism was calculated, based on FACS® analyses, as follows: (percent test donor–derived cells) × 100/(percent test donor–derived cells + percent competitor-derived cells). In this study, test donor cells were prepared from B6-Ly5.1 mice, and competitor cells were prepared from B6-F1 mice. Thus, percent chimerism = (percent Ly5.1 cells) × 100/(percent Ly5.1 cells + percent F1 cells). The percent chimerism for myeloid and B or T lymphoid cells was calculated using the same formula. For example, the percent chimerism for B lymphoid cells = (percent B220+ Ly5.1 cells) × 100/(percent B220+ Ly5.1 + percent B220+ F1 cells). When the percent chimerism was >1.0 in all lineages (myeloid and B and T lymphoid lineages), recipient mice were considered to be multilineage reconstituted.
Secondary Transplantation.
Bone marrow cells from primary recipient mice were transferred into lethally irradiated B6-Ly5.2 mice (2 × 106 cells per recipient). Competition between test and competitor donor cells in secondary recipients was evaluated as in primary recipients.
In Vivo and In Vitro Colony Assays.
CFU-S assay was performed as described 16. Purified cells were injected into lethally irradiated mice. Spleens were removed from the recipients at day 12 after transplantation. After fixation with Bouin solution, numbers of spleen colonies were counted. For in vitro colony assays, individual cells were directly sorted into a round-bottomed 96-well plate containing 10% FCS, 5 × 10−4 2-β-mercaptoethanol, 10 ng/ml mouse (m)IL-3, and 10 ng/ml mouse stem cell factor (mSCF) in α-medium. Both mIL-3 and mSCF were provided by Kirin Brewery Co., Takasaki, Japan. Colony formation was observed on day 14 of culture.
Analysis of Cell Cycle.
Sorted cells were fixed with 70% ethanol in water at 4°C for one night. The cells were stained with 30 μg/ml PI in staining medium containing 11.25 Kunitz units of RNase A at 37°C for 30 min. Cell cycle analyses were performed on a FACSCalibur™ (Becton Dickinson). Collected data were analyzed by ModFit LT (version 2.0; Verity Software House, Inc.).
To investigate the turnover rate of CD34−KSL cells, bromodeoxyuridine (BrdU; Sigma-Aldrich) was administered continuously to mice via drinking water. A bottle of water containing 0.5 mg/ml BrdU was changed weekly. After BrdU incorporation in vivo, CD34−KSL cells were isolated and stained with FITC-conjugated anti-BrdU (Becton Dickinson) and PI as previously described 17. The cells were analyzed on a FACS Vantage™.
Results
Repopulating Activity in Bone Marrow Cells of Aging Mice.
We compared repopulating activity of unseparated bone marrow cells from B6 mice of different ages. Table shows the result of reconstitution analyses 24 wk after transplantation. The percentages of chimerism obtained after transplantation by bone marrow cells from mice aged 6, 12, and 18 mo were higher than those obtained by bone marrow cells from mice aged 2 mo. These data are consistent with previous observations 6 10. We noticed that the degree of myeloid reconstitution was significantly higher than that of B lymphoid reconstitution when bone marrow cells from mice aged 18 mo were transplanted.
Table 1.
Repopulating Activities of Bone Marrow Cells from Mice of Different Ages
| Percent chimerism | ||||
|---|---|---|---|---|
| Age of mice | Total | Myeloid | B lymphoid | T lymphoid |
| mo | ||||
| 2 | 30.9 ± 31.9 (n = 11) | 28.8 ± 35.6 (n = 11) | 30.9 ± 28.9 (n = 11) | 34.3 ± 31.7 (n = 11) |
| 6 | 62.0 ± 17.9 (n = 7) | 65.9 ± 24.4 (n = 7) | 57.6 ± 16.1 (n = 7) | 63.6 ± 22.5 (n = 7) |
| 12 | 49.4 ± 28.6 (n = 8) | 55.5 ± 35.2 (n = 8) | 43.3 ± 24.6 (n = 8) | 47.9 ± 30.0 (n = 8) |
| 18 | 60.1 ± 19.7 (n = 11) | 72.3 ± 35.2 (n = 11) | 52.5 ± 20.6 (n = 11) | 55.5 ± 26.4 (n = 11) |
2 × 105 bone marrow cells from 2-, 6-, 12-, or 18-mo-old B6-Ly5.1 mice were mixed with 2 × 105 bone marrow cells from 2-mo-old B6-F1 mice and transplanted into lethally irradiated B6-Ly5.2 mice. 24 wk after transplantation, peripheral blood cells were analyzed for the percent chimerism for total leukocytes and for myeloid and B and T lymphoid cells. The data are expressed as the mean ± SD. The numbers of recipients are shown in parentheses.
Absolute Numbers of Multilineage Repopulating Cells.
To compare numbers of repopulating cells in mouse bone marrow cells at 2 and 18 mo of age, we performed in vivo limiting dilutions with unseparated bone marrow cells from mice of each age. At 12 wk after transplantation, peripheral blood cells were analyzed for the presence of test donor cell–derived cells for each lineage. Reconstitution in all myeloid and B and T lymphoid lineages was obligatory for multilineage repopulating cells.
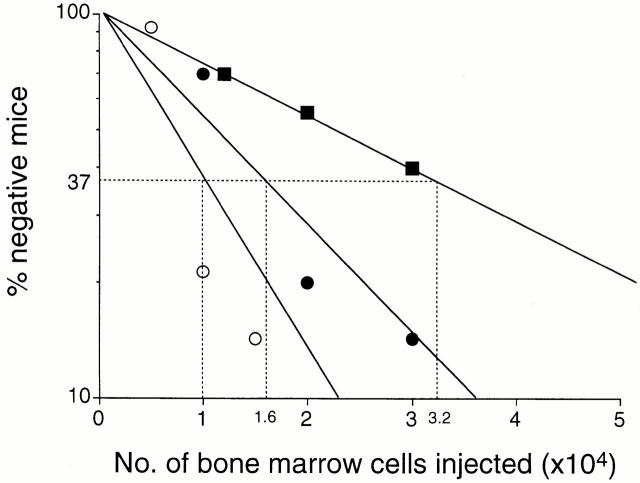
The percentages of negative mice in a logarithmic function were plotted against the number of cells transplanted (Fig. 1). According to the Poisson distribution, the frequency of competitive repopulating units 15 was estimated using the maximum likelihood 18 19. 1 in 1.6 × 104 bone marrow cells was a multilineage repopulating cell in aging mice, whereas in young mice, 1 in 3.2 × 104 cells was such as a cell. As there was no significant difference in total numbers of nucleated bone marrow cells between 2 and 18 mo of age (Table ), it was concluded that the number of HSCs increased twofold from 2 to 18 mo of age.
Figure 1.
Limiting dilution analysis based on competitive repopulation. Varying numbers of bone marrow cells from either 2- or 18-mo-old mice were mixed with 2 × 105 bone marrow cells from 2-mo-old mice and injected into lethally irradiated mice. Reconstitution in myeloid and B and T lymphoid lineages was evaluated 12 wk after transplantation. Mice were considered to be negative when percent chimerism for myeloid or B or T lymphoid lineage was <1.0. 1 in 3.2 × 104 cells and 1 in 1.6 × 104 cells was a multilineage repopulating cell for 2-mo-old (▪) and 18-mo-old (•) mice. When uni- and bilineage repopulating cells were included in estimations of frequencies, 1 in 1.0 × 104 cells was a repopulating cell in 18-mo-old mice (○).
Table 2.
Bone Marrow Cellularity and Populations of CD34−KSL Cells at Different Ages
| Age of mice | No. mice analyzed | No. bone marrow cells | Lin− cells | CD34−KSL cells |
|---|---|---|---|---|
| mo | ×107/hindlimbs | % | % | |
| 2 | 8 | 6.4 ± 1.2 | 1.57 ± 0.23 | 0.005 ± 0.002 |
| 6 | 6 | 6.5 ± 0.6 | 1.49 ± 1.20 | 0.011 ± 0.005 |
| 12 | 8 | 5.4 ± 0.9 | 1.58 ± 0.25 | 0.042 ± 0.015 |
| 18 | 6 | 6.4 ± 0.5 | 2.03 ± 0.30 | 0.087 ± 0.039 |
Bone marrow cells were collected from both right and left femora and tibiae. The number of bone marrow cells is presented as the mean ± SD per mouse. Percent lineage marker–negative (Lin−) cells and percent CD34−KSL cells in bone marrow cells is shown.
We observed recipient mice in which the myeloid lineage appeared to be reconstituted, without significant repopulation of B and/or T lymphoid cells, when 1.5 × 104 or fewer test donor cells from 18-mo-old mice were transplanted. When these reconstituted mice were taken into account, the frequency of repopulating cells was 1 in 1.0 × 104 bone marrow cells. Thus, by calculation, at 18 mo of age, there were 6.3 multilineage repopulating cells, and 3.7 repopulating cells that were less capable of giving rise to lymphoid cells, per 105 bone marrow cells. As we did not detect the latter type of repopulating cells in 2-mo-old mice, we postulated that unusual repopulating cells were present in aging mice in addition to multilineage repopulating cells.
CD34−KSL Cells in the Bone Marrow.
We have shown that HSCs are highly enriched in a population of CD34−KSL bone marrow cells. Consistent with the previous data 12, CD34−KSL cells accounted for 0.005% of bone marrow cells at 2 mo of age. The percentage of these cells increased about 2-, 4-, and 17-fold on average by 6, 12, and 18 mo of age, respectively, as shown in Table . As the total number of bone marrow cells remained constant from 2 to 18 mo of age, the absolute number of CD34−KSL cells increased proportionally with age.
In Vivo and In Vitro Colony-forming Ability of Aged CD34−KSL Cells.
To clarify whether CD34−KSL cells that increased in number with age were functionally similar to those of young mice, colony-forming abilities of CD34−KSL cells at different ages were compared (Table ). Regardless of age, most CD34−KSL cells did not give rise to day 12 spleen colonies (CFU-Sd12), nor did these cells form in vitro colonies in the presence of IL-3 alone. However, a large proportion (70–80%) of the CD34−KSL cells formed colonies in the presence of SCF and IL-3, irrespective of age. These data indicate that expanded CD34− KSL cells in aging mice have myeloid differentiation potential similar to that of such cells in young mice.
Table 3.
In Vivo and In Vitro Colony Formation by CD34−KSL Cells of Different Ages
| Percent colony-forming cells | |||
|---|---|---|---|
| Age of mice | CFU-Sd12 | CFU-CIL-3 | CFU-CIL-3+SCF |
| mo | |||
| 2 | 0.8 ± 0.7 | 0.3 ± 0.5 | 79.8 ± 5.5 |
| 6 | 1.2 ± 0.7 | 2.1 ± 0.5 | 70.5 ± 8.5 |
| 12 | 0.7 ± 0.2 | 1.3 ± 0.9 | 76.7 ± 3.9 |
| 18 | 1.1 ± 0.2 | 1.5 ± 1.0 | 76.7 ± 3.9 |
The frequency of colony-forming cells among CD34−KSL cells is presented as the mean ± SD (n = 4). 200 CD34−KSL cells were injected into irradiated mice for day 12 CFU (CFU-Sd12) assay. A single cell culture of CD34−KSL cells was performed in the presence of IL-3 alone (CFU-CIL-3) or of IL-3 plus SCF (CFU-CIL-3+SCF). In vitro colony formation was evaluated on day 14 of culture.
Long-Term Reconstitution with Limited Numbers of CD34− KSL Cells from Old Mice.
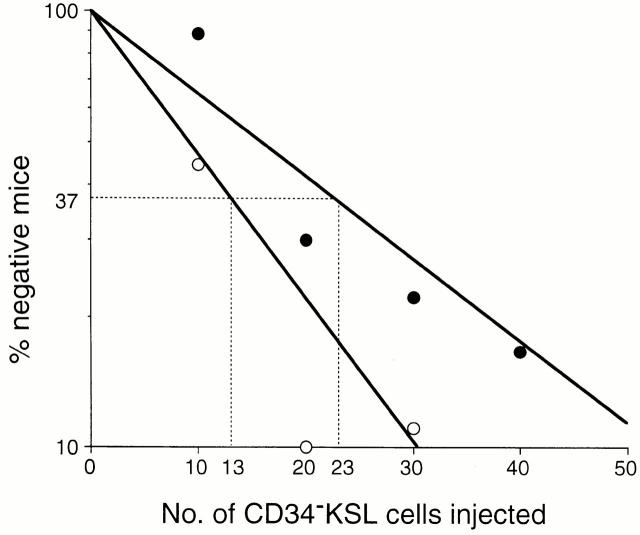
We evaluated the in vivo function of CD34−KSL cells from 18-mo-old mice and determined the frequency of multilineage repopulating cells among these cells by limiting dilution analysis. Graded numbers of CD34−KSL cells were transplanted into irradiated mice. The recipient mice were analyzed at 16 wk after transplantation. As shown in Fig. 2, multilineage repopulating cells were detected at a frequency of 1 in 23 cells. Multilineage reconstitution was detected in a recipient mouse, as demonstrated in Fig. 3 A. Both CD34−KSL cells at 18 mo of age and competitor cells at 2 mo of age contributed to reconstitution in myeloid and B and T lymphoid lineages. Based on the percentage of CD34−KSL cells among bone marrow cells (Table ) and the estimated frequency of multilineage repopulating cells among CD34−KSL cells (Fig. 2), it was calculated that at 18 mo of age, 3.8 cells per 105 bone marrow cells were multilineage repopulating with the CD34−KSL phenotype. This frequency was nearly twice that in young mice 12.
Figure 2.
Limiting dilution analysis on CD34−KSL cells from 18-mo-old mice. Graded numbers of CD34−KSL cells were mixed with 2 × 105 bone marrow competitor cells and transplanted into irradiated mice. Peripheral blood cells of the recipients were analyzed for the presence of CD34−KSL-derived cells 16 wk after transplantation. The frequencies of multilineage repopulating cells (•) and a total of multilineage repopulating cells and myeloid-dominant repopulating cells (○) were estimated as 1 in 23 and 1 in 13 CD34−KSL cells, respectively.
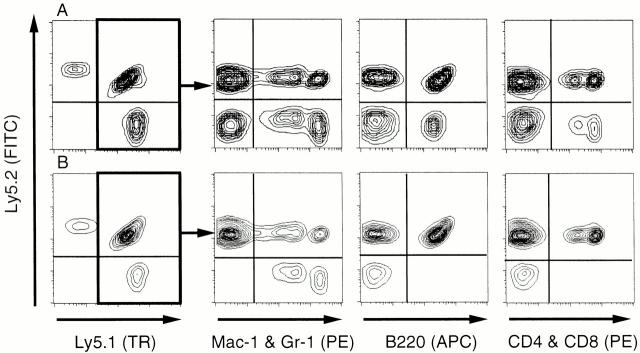
Figure 3.
FACS® profiles for reconstitution analysis. Data are from recipients transplanted with a limited number of CD34−KSL cells from 18-mo-old mice. At 16 wk after transplantation, peripheral blood cells were analyzed for contributions of test donor cells, which were distinguished as Ly5.1 cells. Multilineage reconstitution was detected in a mouse transplanted with 20 CD34−KSL cells, as shown in A. Myeloid lineage was predominately reconstituted in a mouse transplanted with 10 CD34−KSL cells (Table , mouse No. 2), as shown in B. Note good repopulation in all lineages by F1 competitor cells in both cases.
Of note is that among mice transplanted with 10 CD34−KSL cells, a dose less than the threshold number for the presence of a multilineage repopulating cell, we again observed mice in which reconstitution was detected only for myeloid lineage, as demonstrated in Fig. 3 B. In four out of nine cases, total chimerism and myeloid chimerism were both >1%, but B and T lymphoid chimerism was <1% (Table ). We designated the repopulating cells with undetectable lymphoid differentiation potential as defective repopulating cells. The percentages of chimerism for total leukocytes were relatively low in the cases reconstituted with defective repopulating cells, reflecting low chimerism in lymphoid lineage. It was estimated that multilineage and defective repopulating cells were present, taken together, at a frequency of 1 per 13 CD34−KSL cells (Fig. 2). Thus, the total of both types of repopulating cells was calculated to be 6.7 cells per 105 bone marrow cells. As the frequency of multilineage repopulating cells was estimated to be 3.8 cells per 105 bone marrow cells, defective repopulating cells were inferred to be present at a frequency of 2.9 cells per 105 bone marrow cells from 18-mo-old mice. An accumulation of defective repopulating cells might contribute to high myeloid reconstitution, with low lymphoid reconstitution, after transplantation with aging bone marrow cells. Expansion in numbers of these two types of repopulating cells accounted for high repopulating activity of aging bone marrow cells.
Table 4.
Transplantation of 10 CD34−KSL Bone Marrow Cells from 18-mo-old Mice
| Percent chimerism | ||||
|---|---|---|---|---|
| Mouse no. | Total | Myeloid | B lymphoid | T lymphoid |
| 1 | 49.4 | 93.7 | 1.5 | 3.7 |
| 2 | 11.7 | 47.9 | 0.1 | 0.2 |
| 3 | 7.3 | 15.7 | 0.7 | 0.2 |
| 4 | 2.1 | 7.5 | 0.3 | 0.2 |
| 5 | 1.1 | 2.8 | 0.8 | 0.1 |
| 6 | 0.8 | 3.2 | 0.0 | 0.3 |
| 7 | 0.4 | 0.3 | 0.2 | 0.1 |
| 8 | 0.2 | 0.1 | 0.2 | 0.7 |
| 9 | 0.1 | 0.2 | 0.1 | 0.0 |
Irradiated B6-Ly5.2 mice were injected with a mixture of 10 CD34−KSL cells from 18-mo-old B6-Ly5.1 mice and 2 × 105 bone marrow cells from 2-mo-old B6-F1 mice. Peripheral blood cells of the recipients were evaluated for engraftment 16 wk after transplantation. Percent chimerism is shown for all Ly5.1 leukocytes and Ly5.1 cells in myeloid and B and T lymphoid cells.
In addition, we concluded that most CD34−KSL cells in aging mice were myeloid lineage–committed precursor cells. Over 70 percent of these cells formed colonies on stimulation by SCF and IL-3 but lacked CFU-S activity. Most cells did not show repopulating activity. We inferred that such cells lie in the developmental stages between repopulating cells and CFU-S.
Secondary Transplantation of Bone Marrow Cells Repopulated with 10 CD34−KSL Cells.
We addressed whether or not defective repopulating cells still have self-renewal potential. One case with multilineage reconstitution and two cases with myeloid dominant reconstitution (Table ; mice Nos. 1, 2, and 3) were subjected to secondary transplantation. Bone marrow cells of the primary recipients were transferred into irradiated mice 16 wk after primary transplantation. The secondary recipient mice were analyzed between 12 and 16 wk after transplantation.
Table summarizes the percentages of chimerism in the secondary recipients. With mouse No. 1, high levels of reconstitution occurred in myeloid and B and T lymphoid lineages in all secondary recipients. Multilineage repopulating cells appeared to be forced to self-renew in secondary recipients.
Table 5.
Secondary Transplantation of Defective Repopulating Cells
| Percent chimerism | ||||
|---|---|---|---|---|
| Primary recipient | Total | Myeloid | B lymphoid | T lymphoid |
| No. 1 | 88.8 ± 10.8 (n = 5) | 77.5 ± 18.8 (n = 5) | 94.9 ± 8.4 (n = 5) | 90.0 ± 9.0 (n = 5) |
| No. 2 | 51.0 ± 25.8 (n = 10) | 73.4 ± 34.6 (n = 10) | 4.0 ± 5.5 (n = 10) | 21.0 ± 21.3 (n = 10) |
| 80.7 | 97.4 | 11.9 | 55.8 | |
| 68.0 | 91.9 | 14.3 | 10.9 | |
| 58.3 | 80.0 | 1.6 | 2.0 | |
| 55.9 | 71.6 | 1.4 | 61.3 | |
| 50.7 | 93.7 | 8.7 | 20.6 | |
| 12.3 | 12.2 | 1.2 | 19.6 | |
| 78.8 | 93.0 | 0.4 | 22.3 | |
| 53.3 | 98.2 | 0.4 | 3.4 | |
| 50.5 | 89.0 | 0.1 | 13.4 | |
| 1.5 | 7.2 | 0.0 | 0.2 | |
| No. 3 | 9.2 ± 7.9 (n = 6) | 26.2 ± 15.5 (n = 6) | 0.1 ± 0.1 (n = 6) | 0.3 ± 0.2 (n = 6) |
The primary recipients listed as mice Nos. 1, 2, and 3 in Table served as donors for secondary transplantation. Lethally irradiated mice were transplanted with 2 × 106 bone marrow cells per mouse 16 wk after primary transplantation. Between 12 and 16 wk after secondary transplantation, the recipients were analyzed for percent chimerism of CD34−KSL-derived cells.
On the other hand, with mouse No. 2, the recipients showed wide variations in reconstitution. Reconstitution levels in individual recipients are shown in Table . Reconstitution in myeloid and both B and T lymphoid lineages occurred in 6 out of 10 recipients. Myeloid and T lymphoid, but not B lymphoid, cells were reconstituted in 3 cases. In one recipient, only myeloid reconstitution was significant. On average, lymphoid lineage chimerism remained significantly lower than myeloid lineage. With mouse No. 3, 3 out of 9 recipients were not reconstituted with CD34−KSL-derived cells. Reconstitution was detectable only in myeloid lineage for the rest. It should be emphasized that predominance of myeloid lineage reconstitution persisted in secondary recipients with both mice Nos. 2 and 3 and that defective repopulating cells exhibited particularly profound reductions in generation of B cells.
These data indicate the existence of repopulating cells whose differentiation potential along lymphoid lineage is reduced but whose self-renewal potential is maintained. Significant levels of lymphoid lineage reconstitution appeared in some of the mice transplanted with defective repopulating cells (mouse No. 2). This indicates that the percentage of chimerism in primary recipients shows only relative repopulating activity but not the presence or absence of repopulating activity in test donor cells. We concluded that the differentiation potential of defective HSCs was amplified into detectability when these cells were forced to self-renew by secondary transplantation.
Cell Cycle Analysis of CD34−KSL Cells.
We analyzed the cell cycle status of an expanded population of CD34−KSL cells. CD34−KSL cells isolated from mice of different ages were stained with PI and analyzed on a FACS®. As summarized in Table , 2.2, 0.8, 1.5, and 1.8% of the CD34−KSL cells were in S/G2/M phases of the cell cycle at 2, 6, 12, and 18 mo of age, respectively. Thus, at any one time, the cell cycle status of these cells was steady from 2 to 18 mo of age.
Table 6.
Cell Cycle Status of CD34−KSL Cells
| Age of mice (mo) | ||||
|---|---|---|---|---|
| Cell cycle phase | 2 | 6 | 12 | 18 |
| G0/G1 | 97.8% | 99.2% | 98.5% | 98.2% |
| G2/M | 0.1% | 0.4% | 1.0% | 0.7% |
| S | 2.1% | 0.4% | 0.5% | 1.1% |
CD34−KSL cells were sorted from bone marrow cells of 2-, 6-, 12-, or 18-mo-old mice and stained with PI. Approximately 5,000 cells were analyzed on a FACS® using ModFit software.
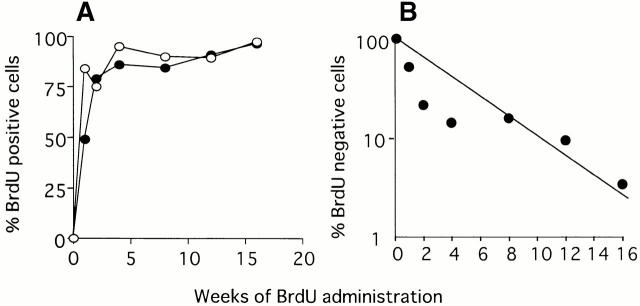
To study the turnover rate of a population of CD34−KSL cells, mice aged 2 mo were fed water containing BrdU over varying periods of time. CD34−KSL cells were then isolated, and BrdU incorporation by these cells was analyzed. As shown in Fig. 4, ∼50% of CD34−KSL cells were labeled with BrdU by day 7 of administration, whereas >80% of the lineage marker–negative cells were labeled with BrdU. However, both cell populations gradually came to be labeled with BrdU after 2 wk of administration. From the transformed data as presented in Fig. 4 B, it was estimated that the average turnover time and 50% turnover time of CD34−KSL cells was 30 and 21 d, respectively. These estimations were consistent with data obtained using rhodamine 123 low, Hoechst 33342 low cells, which have been shown to represent HSCs in the murine bone marrow 17.
Figure 4.
BrdU incorporation by CD34−KSL cells. After administration of BrdU to mice, CD34−KSL cells (•) and lineage marker–negative cells (○) were isolated from the mice and analyzed for BrdU uptake (A). The data are shown with a function of logarithmic percent of BrdU-negative cells (B).
Discussion
We have demonstrated age-associated features of HSCs in B6 mice: (a) an expansion of the HSC pool and (b) an accumulation of defective HSCs and myeloid lineage–committed progenitors. Defective HSCs were defined as repopulating cells capable of both self-renewal and differentiation into myeloid cells but incapable of balanced generation of lymphoid cells.
Table summarizes the kinetics of CD34−KSL cells. The absolute number of CD34−KSL cells increased ∼17-fold from 2 to 18 mo of age. Features of these cells at both ages were calculated based on the data presented above. The frequencies of HSCs and defective HSCs with CD34−KSL phenotype at 2 mo of age, which have been recently estimated using a single cell transplantation assay (Sudo, K., H. Ema, Y. Morita, and H. Nakauchi, manuscript in preparation), are also included in Table . Approximately 20% of the CD34−KSL cells at 2 and 18 mo of age did not show any activity in colony assays and in competitive repopulation. These cells are listed as unidentified cells.
Table 7.
Frequencies of CD34−KSL Cells among Bone Marrow Cells
| Age of mice | |||
|---|---|---|---|
| Cells | 2 mo | 18 mo | Fold increase |
| Total CD34−KSL cells | 5 | 87 | 17.4 |
| HSCs | 1.6 | 3.8 | 2.2 |
| Defective HSCs | <0.5 | 2.9 | >5.8 |
| Progenitor cells | 1.9 | 60.0 | 31.6 |
| Unidentified cells | 1.0 | 20.3 | 20.3 |
The number of cells per 105 bone marrow cells is shown. The fold increase from 2 to 18 mo of age is also shown. The frequency of total CD34−KSL cells in bone marrow cells was obtained by FACS® analysis. Frequencies of HSCs, defective HSCs, and progenitor cells among CD34−KSL cells were estimated using functional assays. Proceeding from the assumption that all HSCs and defective HSCs have colony-forming ability in vitro, the numbers of colony-forming cells without repopulating activity were calculated; these cells were classified as myeloid progenitors. Cells without colony-forming and repopulating ability are shown as unidentified cells.
Numbers of HSCs increased about twofold among CD34−KSL cells. When unseparated bone marrow cells were examined, it was estimated that HSCs were present at rates of 3.2 and 6.3 cells in 105 bone marrow cells at 2 and 18 mo of age, respectively (Fig. 1). Thus, a twofold increase of HSC over 16 mo of aging was consistent. In bone marrow cells at 2 mo of age, defective HSCs were undetectable by limiting dilution analysis of unseparated bone marrow cells, but they were detected at 3.7 cells per 105 bone marrow cells from 18-mo-old mice. According to single cell reconstitution data, defective HSCs were present at rates of <0.5 CD34−KSL cells per 105 bone marrow cells at 2 mo of age. In 18-mo-old mice, defective HSCs were estimated to be present at 2.9 CD34−KSL cells per 105 bone marrow cells. Therefore, defective HSC increased nearly sixfold in a population of CD34−KSL cells.
An important question that should be addressed is how HSCs become defective with time. It has been suggested that the length of telomeres limits a life-span of normal somatic cells 20 21, including HSCs 13. Correlation of telomerase activity and self-renewal potential has been suggested for hematopoietic cells 22. Telomere length can be an indicator of replicative history for HSCs. However, aging in HSCs may be not related solely to shortening of telomeres, since, as shown in this study, aging preferentially affects the lymphoid differentiation potential of HSCs but does not affect their myeloid differentiation potential nor their self-renewal potential.
Apart from repopulating cells, the majority of expanded CD34−KSL cells were interpreted as members of a class of myeloid progenitors with in vitro colony-forming ability but without in vivo repopulating potential. However, it is possible that these cells are repopulating cells with defective homing ability, as previously suggested 6.
Consistent with a pervious report 6, the cell cycle status of CD34−KSL cells was shown to be steady from 2 to 18 mo of age. Fewer than 3% of these cells were in S/G2/M phases of the cell cycle at any point in time. However, the 50% turnover time was estimated to be only 21 d when 2-mo-old mice were studied. Our data support the notion that most HSCs continuously enter the cell cycle in a shorter interval than has been previously considered 17 23. However, it remains uncertain whether CD34−KSL cells from 18-mo-old mice show cell cycle kinetics like those of young mice.
From the viewpoint of self-renewal, the properties described above can be related. We propose an aging model for HSCs. HSCs could have a certain likelihood of self-renewal via symmetric division. Most of the time, HSCs in cell cycle undergo asymmetric divisions, but sometimes symmetric rather than asymmetric division takes place under physiological conditions. Expanded HSCs may have some sort of defect in maintenance of lymphoid lineage repopulating potential and may give rise to defective HSCs, which may finally commit to myeloid progenitor cells. As a result, aged mice exhibit accumulations of HSCs, defective HSCs, and myeloid progenitors, which together form a hierarchy in a primitive hematopoietic compartment. We hope that further studies on the regulation of self-renewal of HSCs will test this hypothesis.
Acknowledgments
The authors thank M. Osawa for his help in initiating this work and H. Kodama, H. Miyoshi, A. Shibuya, and A.S. Knisely for critical reading of the manuscript.
This work was supported by grants from Core Research for Evolutional Science and Technology of Japan Science and Technology Corporation and the Ministry of Education, Science, Sports and Culture of Japan.
Footnotes
Abbreviations used in this paper: HSCs, hematopoietic stem cells; PI, propidium iodide; SCF, stem cell factor.
References
- Johnson F.B., Sinclair D.A., Guarente L. Molecular biology of aging. Cell. 1999;96:291–302. doi: 10.1016/s0092-8674(00)80567-x. [DOI] [PubMed] [Google Scholar]
- Till J.E., McCulloch E.A., Siminovitch L. A stochastic model of stem cell proliferation, based on the growth of spleen colony-forming cells. Proc. Natl. Acad. Sci. USA. 1964;51:29–36. doi: 10.1073/pnas.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D.E. Normal production of erythrocytes by mouse marrow continuous for 73 months. Proc. Natl. Acad. Sci. USA. 1973;70:3184–3188. doi: 10.1073/pnas.70.11.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden D.A., Micklem H.S. The fate of serially transplanted bone marrow cell populations from young and old donors. Transplantation. 1976;22:287–293. doi: 10.1097/00007890-197609000-00010. [DOI] [PubMed] [Google Scholar]
- Harrison D.E., Astle C.M., Delaittre J.A. Loss of proliferative capacity in immunohemopoietic stem cells caused by serial transplantation rather than aging. J. Exp. Med. 1978;147:1526–1531. doi: 10.1084/jem.147.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S.J., Wandycz A.M., Akashi K., Globerson A., Weissman I.L. The aging of hematopoietic stem cells. Nat. Med. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- Ross E.A., Anderson N., Micklem H.S. Serial depletion and regeneration of the murine hematopoietic system. J. Exp. Med. 1982;155:432–444. doi: 10.1084/jem.155.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D.E. Normal function of transplanted mouse erythrocyte precursors for 21 months beyond donor life spans. Nat. New Biol. 1972;237:220–222. doi: 10.1038/newbio237220a0. [DOI] [PubMed] [Google Scholar]
- Harrison D.E., Astle C.M. Loss of stem cell repopulating ability upon transplantation. Effects of donor age, cell number, and transplantation procedure. J. Exp. Med. 1982;156:1767–1779. doi: 10.1084/jem.156.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D.E. Long-term erythropoietic repopulating ability of old, young, and fetal stem cells. J. Exp. Med. 1983;157:1496–1504. doi: 10.1084/jem.157.5.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D.E., Astle C.M., Stone M. Numbers and functions of transplantable primitive immunohematopoietic stem cells. Effect of age. J. Immunol. 1989;142:3833–3840. [PubMed] [Google Scholar]
- Osawa M., Hanada K., Hamada H., Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- Vaziri H., Dragowska W., Allsopp R.C., Thomas T.E., Harley C.B., Lansdorp P.M. Evidence for a mitotic clock in human hematopoietic stem cellsloss of telemeric DNA with age. Proc. Natl. Acad. Sci. USA. 1994;11:9857–9860. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema H., Nakauchi H. Expansion of hematopoietic stem cells in the developing liver of a mouse embryo. Blood. 2000;95:2284–2288. [PubMed] [Google Scholar]
- Szilvassy S.J., Humphries R.K., Landsdorp P.M., Eaves A.C., Eaves C.J. Quantitative assay for totipotent reconstituting hematopoietic stem cells by a competitive repopulation strategy. Proc. Natl. Acad. Sci. USA. 1990;87:8736–8740. doi: 10.1073/pnas.87.22.8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till J.E., McCulloch E.A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat. Res. 1961;14:213–222. [PubMed] [Google Scholar]
- Bradford G.B., Williams B., Rossi R., Bertoncello I. Quiescence, cycling, and turnover in the primitive hematopoietic stem cell compartment. Exp. Hematol. 1997;25:445–453. [PubMed] [Google Scholar]
- St. Groth S.F. The evaluation of limiting dilution assays. J. Immunol. Methods. 1982;49:R11–R23. doi: 10.1016/0022-1759(82)90269-1. [DOI] [PubMed] [Google Scholar]
- Finney D.J. Statistical method in biological assay. 3rd Ed. Charles Griffin & Company Limited; High Wycombe, UK: 1978. [Google Scholar]
- Harley C.B., Futcher A.B., Greider C.W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Hastie N.D., Dempster M., Dunlop M.G., Thompson A.M., Green D.K., Allshire R.C. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990;30:866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- Morrison S.J., Prowse K.R., Ho P., Weissman I.L. Telemerase activity in hematopoietic cells is associated with self-renewal potential. Immunity. 1996;5:207–216. doi: 10.1016/s1074-7613(00)80316-7. [DOI] [PubMed] [Google Scholar]
- Cheshier S.H., Morrison S.J., Liao X., Weissman I.L. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc. Natl. Acad. Sci. USA. 1999;96:3120–3125. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]