Abstract
Glucocorticoids (GCs) affect peripheral immune responses by inhibiting T cell immunity at several stages of the activation cascade, causing impaired cytokine production and effector function. The recent demonstration that the thymic epithelium and possibly thymocytes themselves produce steroids suggests that endogenous GCs also play a role in the control of T cell development. As both peripheral responsiveness and thymic differentiation appear to be regulated by the quantity and quality of intracellular signals issued by antigen–major histocompatibility complex-engaged T cell receptor (TCR) complexes, we investigated the effects of GCs on the signaling properties of T cells stimulated by anti-CD3 monoclonal antibodies or agonist peptides. We demonstrate in this work that dexamethasone, a synthetic GC, inhibits the early signaling events initiated upon TCR ligation, such as tyrosine phosphorylation of several TCR-associated substrates including the ζ chain, the ZAP70 kinase, and the transmembrane adapter molecule linker for activation of T cells. Hypophosphorylation was not a consequence of reduced kinase activity of src protein tyrosine kinases, but was correlated with an altered- membrane compartmentalization of these molecules. These observations indicate that in addition to their well-described ability to interfere with the transcription of molecules involved in peripheral responses, GCs inhibit T cell activation by affecting the early phosphorylating events induced after TCR ligation.
Keywords: T lymphocyte, signal transduction, tyrosine kinases, membrane rafts, glycosphingolipid-enriched microdomains
Introduction
T cells undergo a complex and ordered program of phenotypic changes during both differentiation in the thymus (positive versus negative selection) and after Ag encounter in the periphery (helper subset differentiation, choice between activation and unresponsiveness, or apoptosis). Each of these developmental transitions appears to be regulated by the quantity and the quality of intracellular signals issued by Ag–MHC-engaged TCR complexes 1.
Multiple models have been proposed to explain how a single receptor can regulate opposing developmental decisions. A currently favored model postulates that the “strength” of TCR signaling plays a crucial role in T cell development and differentiation 2. Indeed, numerous studies have suggested that the TCR does not act as a simple on/off switch, but rather is able to translate subtle changes in its ligand into unique signaling events leading to different phenotypic outcomes 3. This signaling flexibility was first evidenced from the structural and functional analysis of immunogenic peptide analogues lacking the ability to stimulate some or all T cell effector activities (partial agonists) 4 5 6. Compared with their immunogenic counterparts (which induce activation of peripheral cells and cause negative selection in the thymus), these variant TCR ligands induce distinct biological responses such as T cell anergy in the periphery or positive selection of developing thymocytes 7 8 9 10. Similarly, several studies have shown that altering the strength of TCR stimulation can influence the Th1–Th2 differentiation program of naive T cells 11 12 13 14.
The precise mechanism by which the strength of TCR signaling is translated into distinct developmental responses is presently unknown. Recently, studies performed on mature T cells stimulated by variant TCR ligands have related unique functional responses to a novel pattern of early TCR-associated tyrosine phosphorylations. Stimulation of naive T cells with a strong agonist peptide induces a full spectrum of signaling events, including saturated phosphorylation of the TCR-associated ζ chain, the adaptor molecule linker for activation of T cells (LAT), and the syk kinase ZAP70, leading to sustained mobilization of intracellular calcium and cytokine gene transcription. Stimulation of cells with a low-avidity ligand is followed by an incomplete phosphorylation program, characterized by the predominance of the p21 phosphorylated form of the TCR-associated ζ chain and the lack of phosphorylation of ZAP70 15 16 17. However, studies performed with developing thymocytes suggest that weak TCR ligands known to induce positive selection do not necessarily induce a unique phosphorylation pattern in these cells (qualitatively different), but rather a gradient of phosphorylation events (quantitatively different; reference 9). In either event, these studies suggest that the level of phosphorylation of the receptor-associated immunoreceptor tyrosine-based activation motifs (ITAMs) reflects the intensity of the early signals derived by the TCR and may therefore serve as a first level of regulation for determining signaling thresholds 1.
In most models studied to date, the nature of the signals issued by the TCR and the resulting functional response appears to be determined by the overall avidity of the APC–T cell interaction, which is influenced by the affinity of the Ag–MHC ligand for the TCR 18 19, the extent of receptor–coreceptor aggregation, and the nature of accessory signals provided by the APCs 2 20. However, numerous studies have shown that immune responses in vivo are not simply controlled by Ag recognition and cell–cell interactions between lymphoid cells, but are also sensitive to the endocrine milieu. Glucocorticoids (GCs) are well known immunoregulatory agents widely used for the treatment of autoimmune and allergic diseases and to prevent or delay graft rejection 21. GCs are thought to exert their antiinflammatory effect by inhibiting (directly or via interaction with other transcription factors) the transcription of molecules (including cytokines) involved in peripheral immune responses 22. Recently GCs have been shown to regulate cell fate decisions both in the thymus and in the periphery 23 24, suggesting that they may affect TCR signaling in a more complex manner than previously anticipated. The observation that GCs regulate developmental steps known to be sensitive to the strength of signals delivered to the TCR prompted us to study the mechanisms by which dexamethasone (DEX), a GC analogue, inhibits T cell activation. We demonstrate in this report that GCs affect the signaling properties of the TCR by inhibiting the early phosphorylating events induced after TCR ligation, possibly through altered membrane compartmentalization of several key enzymes and substrates. These observations indicate that the nature of membrane-proximal signals delivered by the TCR is not only determined by the ligand structure and avidity, but is also sensitive to the hormonal environment, and suggest therefore a novel mechanism by which steroids may regulate T cell differentiation and responsiveness.
Materials and Methods
Mice.
Balb/c mice, 6–8 wk old, were purchased from Charles River Laboratories. Mice transgenic for the αβ TCR, from the F5 cytotoxic T cell clone, were generated as described previously 25. These mice were crossed onto β2-microglobulin (β2m)-negative and recombination activating gene (Rag)-1–negative backgrounds and are referred to as F5 β2m−/−Rag1−/−.
Cell Culture, Reagents, and Abs.
The pigeon cytochrome c/IEk-specific 3B4.15 murine hybridoma cell line was described elsewhere 26. The thymic epithelial cell line YO1 (H-2b) was generated as described previously 27. DEX (soluble in ethanol), cycloheximide, saponin, and methyl-β-cyclodextrin were purchased from Sigma-Aldrich. RU486 was obtained from Roussel UCLAF. Cells were cultured in RPMI 1640 (Life Technologies) supplemented with 5% FCS, nonessentials amino acids, 2 mM l-glutamine, penicillin-streptomycin, and 5 × 10−5 M β-mercaptoethanol. Abs used in this study were specific for the following markers: CD3ε (hamster mAb 145-2C11, American Type Culture Collection; and mouse mAb 7D6, reference 28); CD3ζ (hamster mAb H146-968; provided by Dr. R. Kubo, National Jewish Medical Center, Denver, CO); CD4 (rat mAb GK1.5; American Type Culture Collection); TCR-β (hamster mAb H57-95; provided by Dr. R. Kubo); ZAP70 (rabbit serum 1222; provided by Dr. A. Weiss, University of California at San Francisco, San Francisco, CA); LAT (rabbit serum 3023; provided by Dr. L. Samelson, National Institutes of Health, Bethesda, MD); Lck (mouse mAb; Santa Cruz Biotechnology, Inc.); Fyn (rabbit serum; Santa Cruz Biotechnology, Inc.); CD90.1 (mouse mAb OX7; provided by D.P. Draper, Academy of Sciences of the Czech Republic, Prague, Czech Republic); phospholipase C (PLC)γ1 (rabbit serum; Santa Cruz Biotechnology, Inc.); phosphotyrosine (mouse mAb 4G10; Upstate Biotechnology); CD45 (rat mAb M1/9.3.4.HL.2; American Type Culture Collection); and CD90 (mouse mAb HO-13.4; American Type Culture Collection). When indicated, Abs from ascitic fluids grown in nude mice were purified by anion exchange chromatography (mono Q; Amersham Pharmacia Biotech). F5 β2m−/−Rag1−/− thymocytes were stimulated with the following peptides: NP68 (from the nucleoprotein of influenza virus A/NT/60/68 [NP366–374]) and the control peptide glycosaminoglycan (GAG) (from the GAG protein of the SF2 strain of HIV [390–398]). They were both synthesized on a peptide synthesizer (model 430A; Applied Biosystems).
Cell Stimulation, Immunoprecipitations, and Western Blot Analyses.
Thymocytes and T cell hybridomas were cultured for 16 h at 37°C in DEX or solvent (ethanol)-supplemented media, pelleted, and resuspended at 4 × 107–108 cells/ml in complete media. Cells were incubated for 5 min with anti-CD3 mAbs (clone 7D6, 10 μg/ml) and then cross-linked with rabbit anti–mouse Ig (20 μl serum/ml) for 90 s at 37°C. F5 β2m−/−Rag1−/− (5 × 107) thymocytes were centrifuged briefly onto a monolayer of YO1 cells that were preincubated with the peptides for 2 h at 37°C. After 5 min, thymocytes were removed and rapidly sedimented. Cells were lysed in ice-cold 1% Brij97 lysis buffer (200 mM boric acid, 150 mM NaCl, pH 8.0) containing 5 mM EDTA, 2 mM sodium orthovanadate, 1 mM PMSF, 5 mM sodium fluoride, and 1 U/ml aprotinin). After clarification (13,000 rpm for 15 min), cell lysates were subjected to immunoprecipitation with the indicated Abs. Immunoprecipitations were performed using mAbs directly coupled to CNBr-activated Sepharose beads (Amersham Pharmacia Biotech) or using polyclonal rabbit Abs and protein A/G–Sepharose beads (Santa Cruz Biotechnology, Inc.). Note that TCR complex–associated structures were immunoprecipitated using the 145-2C11 anti-CD3ε mAb. The 7D6 anti-CD3 mAb used for TCR cross-linking does not compete with the 145-2C11 mAb for binding to the TCR complex 28, enabling 145-2C11–coupled beads to immunoprecipitate all TCR structures. Immunoprecipitates were resolved on SDS-PAGE (14% gels; Novex) under reducing conditions. Secondary reagents included horseradish peroxidase (HRP)-coupled protein A (Sigma-Aldrich), HRP-sheep anti–mouse IgG (SAM; Abcam), and HRP-goat anti–rat IgG (GAR; Southern Biotechnology Associates, Inc.). Western blotting and visualization of proteins by enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech) were performed as described previously 26. Densitometric analysis was performed with the Molecular Analyst® software (Bio-Rad Laboratories).
Subcellular Fractionation.
Membrane and cytosol fractions were obtained as follows. Cells (4 × 107) were pelleted and suspended at 108 cells/ml in ice-cold lysis buffer containing 50 mM Tris, 5 mM EDTA, and a complete, EDTA-free protease inhibitor cocktail (Roche Biochemicals). The lysate was homogenized and centrifuged at 1,600 rpm for 2 min at 4°C to remove nonlysed cells and nuclei. The supernatant was ultracentrifuged at 22,000 rpm for 15 min at 4°C in an AH650 Sorvall rotor. The supernatant was collected as the cytosol preparation (108 cell equivalent/ml). The pellet was washed, resuspended at 2 × 108 cell equivalent/ml in lysis buffer, and used as the membrane preparation. Proteins from the samples were then separated by SDS-PAGE as described in the above section.
Kinase Assays.
Kinase immune complexes from 2 × 107 hybridomas were incubated with 5 μCi of adenosine triphosphate ([γ-32P]ATP; Amersham Pharmacia Biotech) and the exogenous kinase substrate enolase (Roche Biochemicals) in kinase buffer (Hepes 20 mM, pH 7.4, 100 mM NaCl, 10 mM MgCl2, 10 mM MnCl2, 50 mM NaF, and 1 μM ATP) for 2 or 10 min at 30°C. Reactions were stopped by adding ice-cold reducing buffer. Immune complexes were then separated by SDS-PAGE and exposed to x-ray film (Eastman Kodak Co.). One fourth of the immunoprecipitates were subjected to Western blotting with the indicated Abs.
TCR Downregulation.
Control and DEX-treated T cell hybridomas were incubated for 1 h on flat-bottomed 96-well tissue culture plates precoated with 10 μg/ml purified anti–TCR-β in PBS solution. CD3ε expression was assayed by flow cytometry (FACSort™; Becton Dickinson) using FITC-coupled 145-2C11 (produced in our laboratory using standard techniques).
Glycolipid-enriched Membrane Microdomain Isolation.
Purification of glycolipid-enriched membrane microdomains (GEMs) was performed as described 29 30. 108/ml T cell hybridomas were lysed on ice in 1 ml MNE buffer (morpholinoethane sulfonic acid [MES] 50 mM, pH 6.5, 150 mM NaCl, 5 mM EDTA) containing 1% Triton X-100 (wt/vol), 1 mM Na3VO4, 1 mM PMSF, and 1 mM NaF. Lysates were gently sonicated (five bursts of 5 s at 5 W) and cleared at 5,000 rpm for 10 min at 4°C. 1 ml supernatant was mixed with an equivalent volume of 80% sucrose made with MNE buffer and transferred to a 5-ml ultracentrifuge tube. This solution was carefully overlaid with 2 ml of 35% sucrose followed by 1 ml of 5% sucrose (both prepared in MNE buffer) and the tubes were placed in a cooled AH650 Sorvall rotor. The gradients were then ultracentrifuged at 39,000 rpm for 16 h at 4°C. The GEM-containing fraction was obtained by collecting the 5–35% sucrose interface, and the non-GEMs and cytosolic fractions were harvested at the bottom of the gradient. Fractions were then subjected to SDS-PAGE under reducing conditions except for CD90 and CD45, which required nonreducing conditions for adequate detection. Blots were revealed using the appropriate primary and HRP secondary reagents and developed by chemiluminescence. GM1 gangliosides were visualized using HRP-coupled cholera toxin B subunit (Calbiochem). GEM fractions were concentrated as described 31 and phosphoproteins were detected by Western blot analysis.
Results
DEX Inhibits TCR-proximal Tyrosine Phosphorylations.
GCs inhibit T cell–dependent immunity by blocking both cytokine production and cell proliferation 22. The 3B4.15 murine T cell hybridoma 26 was used in this study as a model cell line to gain insight into the mechanisms by which GCs antagonize signals delivered via the TCR, as DEX inhibits TCR-induced IL-2 synthesis in this cell line (Fig. 1 A), without affecting cell viability (as assessed by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide [MTT] colorimetric assay; data not shown). 3B4.15 cells were incubated overnight in DEX or solvent (ethanol)-supplemented media and an equivalent number of cells were stimulated with an anti-CD3ε mAb (clone 7D6) at 37°C for 90 s. Whole cell lysates from control and Ab-stimulated cells were submitted to immunoprecipitation using anti-CD3ε–coupled agarose beads (clone 145-2C11) and analyzed for protein tyrosine phosphorylation by immunoblotting (Fig. 1 B). Stimulation of control cells led to the tyrosine phosphorylation of several CD3ε-associated substrates, including both a p23 and a p21 form of phospho-ζ, a TCR-associated phosphoprotein of 28–29 kD probably related to the phosphorylated form of the CD3ε subunit, and a molecule of 70 kD that was identified as ZAP70 by immunoblotting (Fig. 1 B). The intensity of some of these bands was reduced in extracts from mAb-stimulated DEX-treated cells, suggesting that DEX affected the early steps of TCR signaling in this cell line. TCR stimulation appeared to be qualitatively affected by GCs, as ZAP70, phospho-CD3ε, and the high molecular weight form of phospho-ζ (p23) appeared to be selectively hypophosphorylated in DEX-treated cells compared with the p21 form of phospho-ζ (Fig. 1 B). The preferential inhibition of the p23 high molecular weight form of phospho-ζ over the p21 isoform by DEX was further confirmed by scan densitometry analysis of multiple independent experiments (Fig. 1 C). Although DEX inhibited the tyrosine phosphorylation of ZAP70 in T cell hybrids, it did not affect its ability to associate with the TCR complex upon CD3 triggering (Fig. 1 B). Accordingly, immunoprecipitation studies using anti-ZAP70 Abs revealed that only unphosphorylated forms of ZAP70 were found to associate with the TCR complex (Fig. 1 D). Of note, the integral membrane adaptor molecule LAT is phosphorylated at multiple tyrosine residues after TCR activation, creating docking sites for numerous signaling molecules, including PLCγ1, Gads, and Grb2 32 33 34. As LAT represents a likely substrate for ZAP70, we investigated the effect of GCs on its phosphorylation status. As shown in Fig. 1 E, DEX was found to downregulate LAT tyrosine phosphorylation induced upon TCR triggering, without affecting LAT protein expression.
Figure 1.
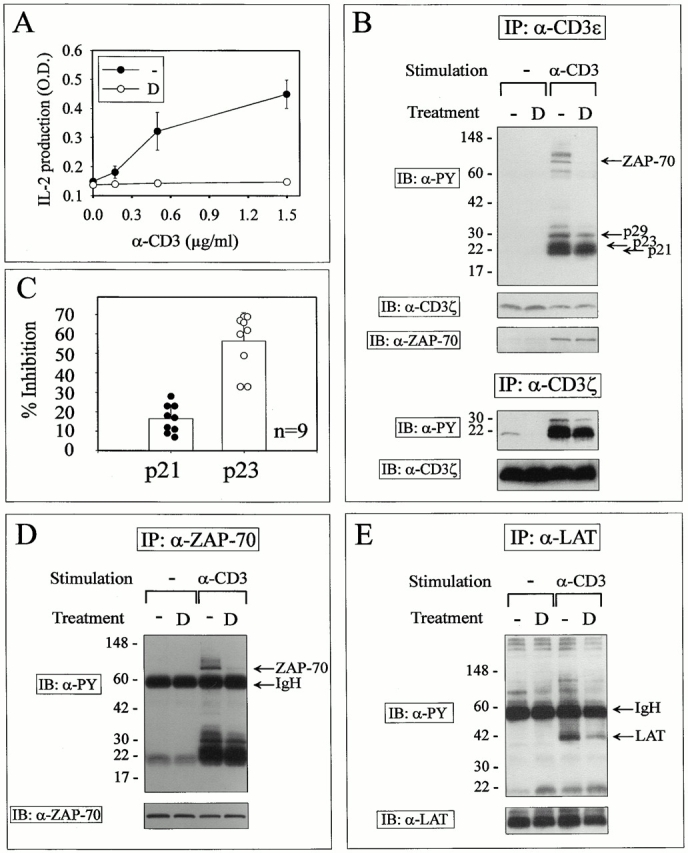
DEX causes partial signaling in anti-CD3–stimulated T cell hybridoma cells. (A) T cell hybrids (3 × 104 cells/well) were stimulated by graded doses of plastic-adsorbed anti-CD3ε mAbs in control or 1 μM DEX-supplemented media. Supernatants were collected after overnight culture and IL-2 content was determined using a standard ELISA. Results are expressed as the mean ± SD of triplicate determinations. TCR signaling studies were performed as follows: T cell hybridomas (2 × 107 cells/lane) were cultured for 16 h with 1 μM DEX or solvent (ethanol). An equivalent number of cells were left untreated or stimulated by TCR cross-linking for 2 min and lysed in Brij97 lysis buffer. (B) Lysates were immunoprecipitated (IP) with Sepharose beads coupled to anti-CD3ε or anti-CD3ζ mAbs as indicated, resolved by SDS-PAGE, and immunoblotted (IB) with antiphosphotyrosine mAbs. The blot was subsequently stripped and reprobed with an mAb against CD3ζ and a ZAP70-specific rabbit antiserum. (C) The intensity of phospho-ζ isoforms resolved by SDS-PAGE from nine independent experiments was quantitated by densitometric analysis. Results are expressed as percent inhibition of the relative intensity of bands in DEX- versus control-treated cells (mean ± SD). (D and E) Lysates were immunoprecipitated with rabbit antisera raised against ZAP70 and LAT. Immunoprecipitates were resolved by SDS-PAGE and immunoblotted with antiphosphotyrosine mAbs. Stripped membranes were reprobed with antisera raised against ZAP70 and LAT (bottom). Similar results were obtained in three independent experiments. D, DEX.
Steroid hormones may affect cell function through both classical “genomic” effects (requiring binding to intracellular receptors and novel protein synthesis) and “nongenomic” rapid (within minutes) effects mediated through binding to putative membrane receptors 35. To investigate the mechanism by which DEX affects the early wave of intracellular tyrosine phosphorylation, T cell hybrids were incubated in DEX-supplemented media and the kinetics and the sensitivity to an intracellular GC receptor antagonist and to a protein synthesis inhibitor were analyzed. As shown in Fig. 2 (and keeping with our own previous observations, reference 26), the inhibitory effects of DEX on LAT phosphorylation were characterized by delayed onset (>6 h) and required both binding to the GCs receptor and de novo protein synthesis. Accordingly, DEX did not inhibit TCR signal transduction in the Jurkat GC receptor–negative cell line (data not shown).
Figure 2.
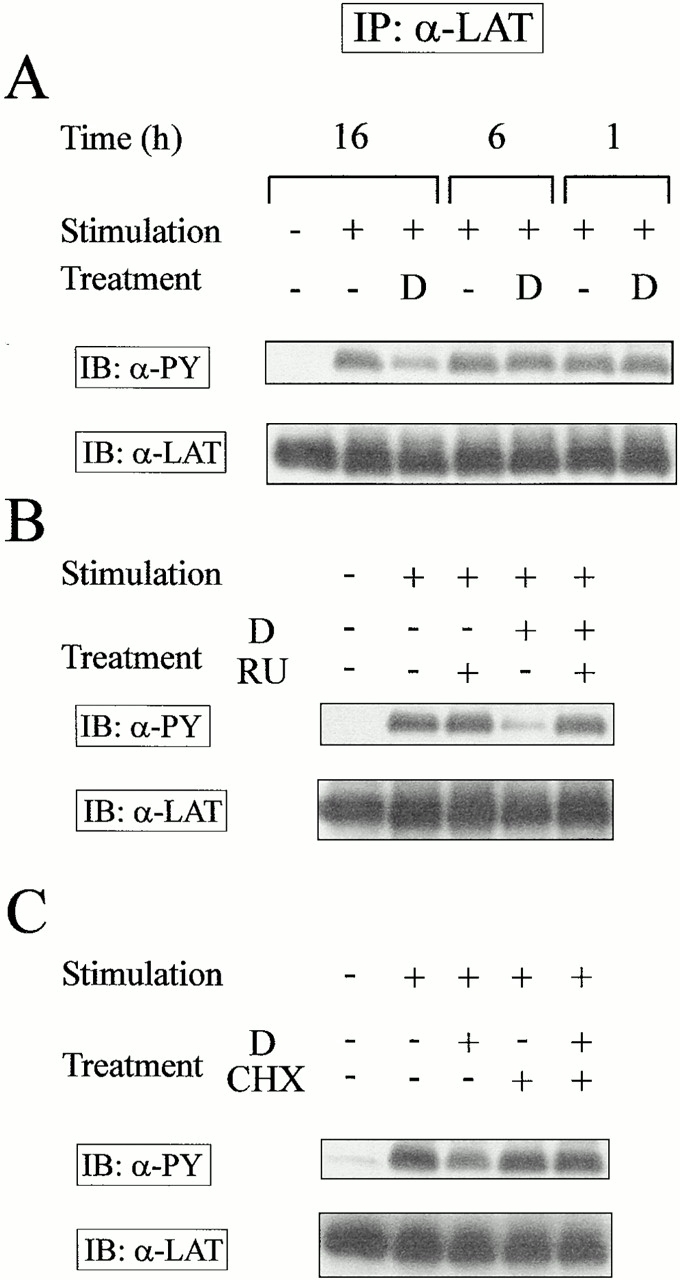
Inhibition of TCR signaling by DEX requires binding to the GC receptor and de novo protein synthesis. (A) T cell hybridomas were incubated with media or 1 μM DEX for the indicated times before stimulation by anti-CD3 mAbs. T cell hybridomas were preincubated for 2 h in the presence of (B) the GC receptor antagonist RU486 (1 μM RU) or (C) the protein synthesis inhibitor cycloheximide (0.25 μg/ml CHX) before addition of 0.1 μM DEX. The signaling properties of these cells were analyzed after a 16-h incubation period as described in the legend for Fig. 1. The results are representative of three independent experiments. D, DEX. IP, immunoprecipitation.
To establish whether DEX could inhibit signal transduction in murine thymic cells, thymocytes from adult Balb/c mice were treated in vitro with doses of DEX that did not significantly affect cell viability or cell surface TCR expression (data not shown) and were stimulated by anti-CD3 mAbs as described previously. Whole cell extracts were submitted to immunoprecipitation using anti-CD3ε–coupled agarose beads and the phosphorylation status of CD3-associated chains was determined by immunoblotting. In agreement with published reports 36 37, the CD3ζ chain was constitutively phosphorylated in thymocytes, migrating as a single p21 molecular species (Fig. 3 A). TCR stimulation led to the appearance of the p23 phospho-ζ isoform, the p29 phospho-CD3ε chain, and the p70 ZAP70 phosphoband (see also Fig. 3 B). In keeping with studies performed on a monoclonal cell line, treatment with low doses (in the nanomolar range) of DEX inhibited the early phosphorylating events secondary to TCR engagement, as shown by reduced intensity of multiple phosphotyrosine-containing bands, including p70 (ZAP70), p29 (CD3ε), and the p21 and p23 isoforms of CD3ζ. Reduction in the intensity of phospho-ζ was not related to a diminished expression of ζ, as revealed by immunoblotting with an anti-ζ chain mAb.
Figure 3.
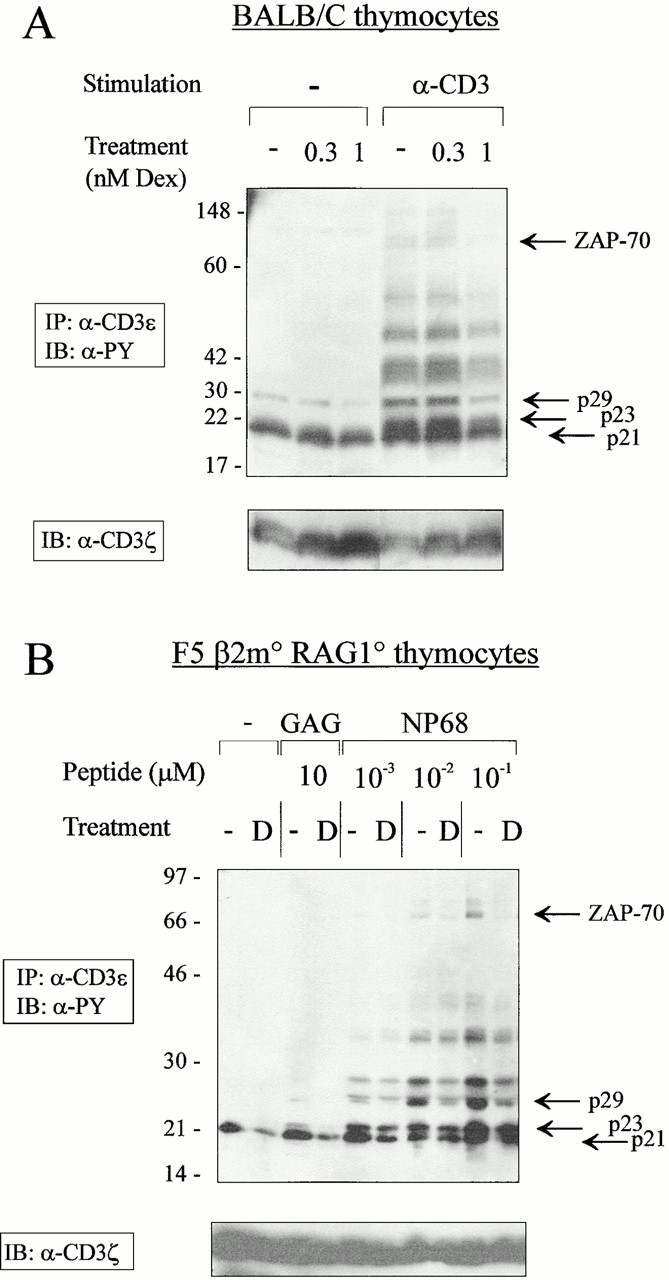
DEX inhibits the early steps of TCR signaling in thymocytes. (A) Balb/c thymocytes were incubated for 16 h in the indicated concentrations of DEX or solvent (ethanol). Cultured thymocytes were left untreated or stimulated by anti-CD3 mAbs at 37°C for 2 min and lysed in Brij97 lysis buffer. Cell lysates (2 × 107 cells/lane) were immunoprecipitated (IP) with an anti-CD3ε mAb directly coupled to beads. The immunoprecipitates were resolved by SDS-PAGE and immunoblotted (IB) with antiphosphotyrosine mAbs. The amount of protein was compared by reblotting the membrane with anti-CD3ζ mAbs (bottom). (B) F5 β2m−/−Rag1−/− thymocytes were cultured overnight in 1 nM DEX or solvent (ethanol) and left untreated or stimulated with the indicated doses of agonist (NP68) or irrelevant (GAG) peptides presented by YO1 thymic epithelial cells. Cell lysates (4 × 107 cells/lane) were immunoprecipitated with anti-CD3ε mAbs and the immunoprecipitates were resolved by SDS-PAGE and immunoblotted with antiphosphotyrosine mAbs. Membranes were then stripped and blotted with anti-CD3ζ mAbs (bottom). Similar results were obtained in three independent experiments. D, DEX.
To further investigate the effect of DEX on developing thymocytes, we used an in vitro model in which all T lineage cells expressed a single defined TCR. Because of the lack of MHC class I expression, thymocytes from F5 β2m−/− Rag1−/− mice represent a homogeneous population of immature CD4+CD8+ cells 9. These cells were incubated overnight in the presence of control or DEX-supplemented media, washed, and exposed to graded doses of agonist (NP68) or irrelevant (GAG) peptides presented by a thymic epithelial cell line in the absence of GCs. The phosphorylation state of TCR-associated substrates was assessed by phosphotyrosine-specific immunoblotting of anti-CD3ε immunoprecipitates. As shown in Fig. 3 B, ligation of the F5 TCR on double positive thymocytes with NP68 resulted in the phosphorylation of several substrates, including ZAP70, CD3ε, and the p23 isoform of the ζ chain. The unrelated GAG peptide inefficiently stimulated the thymocytes in this assay (reference 9, and data not shown), inducing detectable p23 only when added at high concentrations (10 mM, Fig. 3 B; data not shown). Consistent with previous experiments (Fig. 1 B and 3 A), DEX attenuated TCR signaling, as judged by decreased phosphorylation of several CD3-associated substrates, including CD3ε itself, ZAP70, and ζ. DEX also inhibited the constitutive phosphorylation of the ζ chain, as shown by reduced intensity of the p21 band. A similar decrease in the ζ chain p21 and p23 isoforms was also observed in anti-ZAP70 immunoprecipitates from DEX-treated, NP68-stimulated thymocytes (data not shown).
Collectively, these data demonstrate that DEX affects an early step of the TCR signaling cascade, resulting in decreased phosphorylation of several substrates known to play a key role in the recruitment and/or activation of downstream effectors regulating gene transcription.
DEX Inhibits TCR Downregulation.
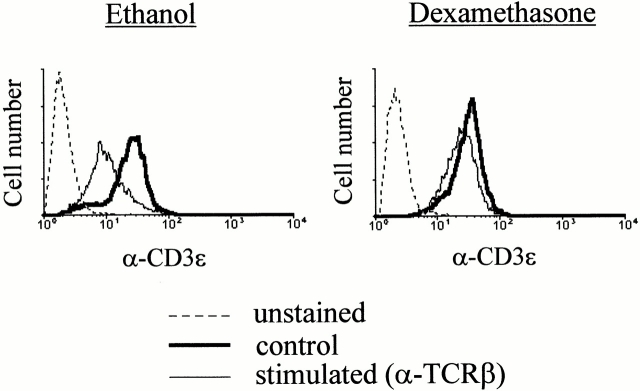
TCR-mediated activation of T lymphocytes results in rapid and long-lasting downregulation of cell surface–exposed TCR structures 38. This phenomenon has been shown to be positively controlled by the src kinase p56lck 39. To further characterize the effect of DEX on the early steps of the TCR signaling cascade, we analyzed the TCR cell surface expression of T cell hybrids stimulated by a short exposure to plastic-adsorbed anti-TCR mAbs. In keeping with the idea that GCs inhibit an early step of TCR signaling, DEX treatment inhibited the early downregulation of the TCR complex induced upon Ab-mediated activation (Fig. 4).
Figure 4.
DEX inhibits activation-induced TCR downregulation. Control and DEX-treated (1 μM) T cell hybridomas were stimulated by plastic-coated anti–TCR-β mAbs (H57-957) for 1 h at 37°C in 96-well culture plates. Cells were then recovered and analyzed for CD3ε expression by flow cytometry.
DEX Does Not Inhibit the Expression or the Enzymatic Activity of the Tyrosine Kinases p56lck and p59fyn.
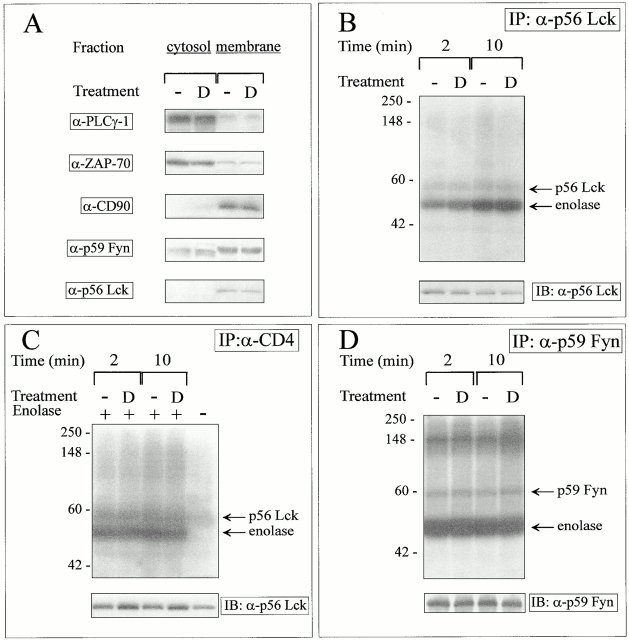
Genetic and mutational analyses have demonstrated that tyrosine kinases of the src family are responsible for the early wave of intracellular phosphorylations initiated upon TCR triggering and for the subsequent TCR downmodulation 39 40 41. Therefore, we wished to assess whether GC treatment affected the protein level and/or tyrosine kinase activity of the p56lck and p59fyn protein tyrosine kinases (PTKs). Immunoblotting experiments performed on whole cell extracts from the 3B4 cell line indicated that the expression levels of these kinases were not affected by DEX exposure (data not shown). To verify whether GCs affected protein intracellular partition, cytosolic and membrane extracts were prepared after overnight incubation of T cell hybrids in ethanol or DEX-supplemented media. The purification procedure was assessed by probing the blots with Abs to membrane-bound (CD90) and cytoplasmic proteins (PLCγ1, ZAP70). As shown in Fig. 5 A, DEX did not affect the intracellular localization of the p56lck and p59fyn PTKs. The in vitro enzymatic activity of the p56lck and the p59fyn PTKs was not significantly affected by overnight incubation in DEX-supplemented media, as assayed on the exogenous substrate enolase (Fig. 5B and Fig. D). The faint bands migrating in the 56–60-kD range comigrate with bands revealed by antikinase Abs in Western blots performed in parallel gels and probably represent the autophosphorylated forms of these kinases (data not shown). DEX did not affect the protein levels and kinase activity of the CD4-associated forms of p56lck, as shown by immunoprecipitation studies performed using Abs to CD4 (Fig. 5 C). Collectively, these data indicated that DEX exposure did not affect the expression level or the in vitro enzymatic activity of the p56lck and p59fyn kinases.
Figure 5.
DEX does not affect the cellular distribution or the kinase activity of p56Lck and p59Fyn. (A) Cytosolic and membrane proteins from unstimulated control and DEX-treated hybridomas (4 × 107 cells/condition) were separated as described in Materials and Methods. Equivalent amounts of proteins from both fractions were separated by SDS-PAGE and blotted with the indicated Abs. Lysates from DEX-treated T cell hybridomas (2 × 107 cells/lane) were immunoprecipitated (IP) with (B) anti-p56Lck mAbs, (C) anti-CD4 mAbs, or (D) a rabbit serum raised against p59Fyn, and the resulting immune complexes were incubated for the indicated times at 30°C with [γ-32P]ATP and the exogenous substrate enolase, separated by SDS-PAGE, and exposed to x-ray film for 24 h. One fourth of the immune complexes were analyzed by immunoblotting with the relevant anti-kinase Ab (bottom). The results are representative of three independent experiments. D, DEX.
DEX Affects the Membrane Compartmentalization of Key Transducing Molecules.
Recently, the important role of GEMs or detergent-insoluble lipid rafts in cell signaling has been recognized 42 43. These GEMs are enriched in glycosyl phosphatidylinositol (GPI)-linked glycoproteins and in other lipid-modified proteins such as tyrosine kinases of the src family, monomeric and heterotrimeric G proteins, G-coupled protein receptors, and the adaptor protein LAT 30 42 43 44 45. The confinement of signaling molecules to membrane subdomains suggests that these compartments function as platforms for the formation of multicomponent transduction complexes 46 47. Accordingly, studies performed on T lymphocytes have shown that raft integrity is required for effective TCR signal transduction 44 48 49 50. To determine the effects of GCs on GEM composition, T cell hybrids were lysed in a cold buffer containing a nonionic detergent, and detergent-resistant membrane constituents were fractionated by ultracentrifugation in a sucrose gradient 51. The GEMs and the detergent-soluble fraction were analyzed by Western blot analysis using Abs and reagents to membrane components and signaling molecules. As expected, CD45, a transmembrane protein known to be excluded from the GEMs 52, and PLCγ1, a cytoplasmic protein, were only detected in the soluble fraction (Fig. 6 A). DEX did not overtly affect the plasma membrane microdomains, as GEMs enriched for both the GPI-linked CD90/Thy-1 protein and the ganglioside GM1 could be isolated from both control and DEX-treated cells. However, GCs were found to selectively affect the submembrane localization of several key signaling molecules, since GEMs purified from DEX-treated cells displayed a marked decrease in the amount of p56lck, p59fyn, and LAT, as determined by Western blotting. DEX treatment did not affect the cellular expression level of these proteins, as judged by Western blots performed on whole cell extracts (data not shown), membrane fractions (Fig. 5 A), or specific immunoprecipitates (Fig. 1 E and Fig. 5B and Fig. D). Noteworthy, since proteins in GEMs only represent a minor fraction of the total cellular protein content (∼1–2%), loss of a given protein from the GEMs does not always lead to a detectable increase in the detergent-soluble fraction. Consistent with previous publications 44 45, TCR activation led to the tyrosine phosphorylation of GEM-associated proteins such as LAT (Fig. 6 B). In keeping with observations performed on immunoprecipitates, DEX inhibited the amount of phospho-LAT in the GEM fraction (Fig. 6 B), possibly as a consequence of altered membrane compartmentalization and/or reduced access to the appropriate kinase.
Figure 6.
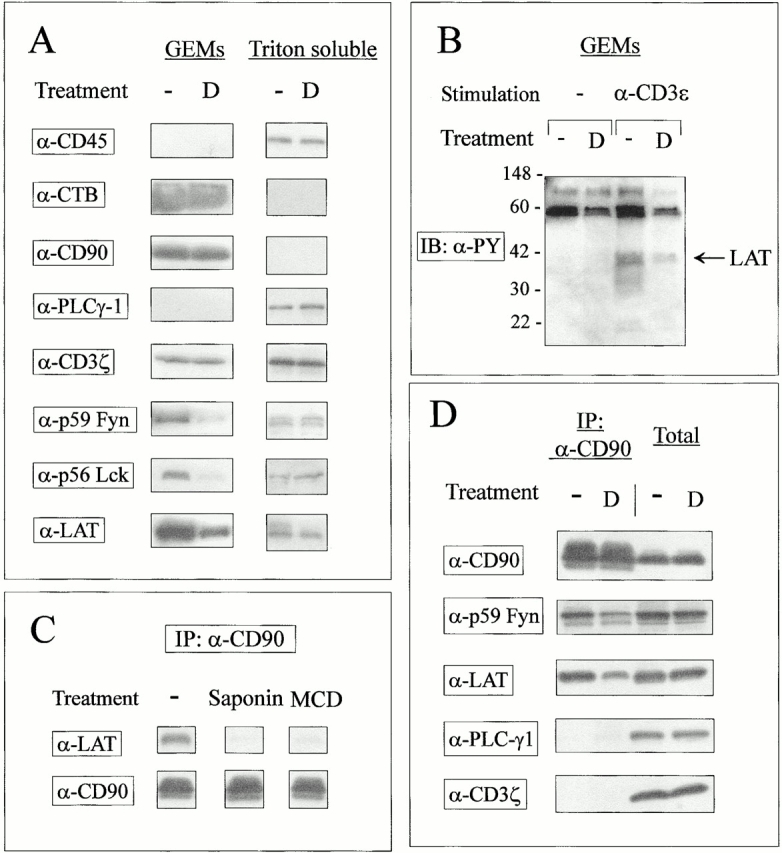
DEX affects membrane signaling complexes. (A) GCs affect protein raft composition. Control and DEX-treated T cell hybridomas (108 cells/condition) were solubilized in 1% Triton X-100 MES lysis buffer, gently sonicated, and the subsequent lysates were ultracentrifuged in a sucrose gradient as described in Materials and Methods. Undiluted GEMs and cytoplasmic fractions (1:4 dilution to avoid abnormal migration) from control and DEX-treated unstimulated cells were separated by SDS-PAGE and immunoblotted with Abs to membrane-associated (CD90, CD45, CD3ζ, p59Fyn, p56Lck, LAT) and cytoplasmic (PLCγ1) proteins. The ganglioside GM1 was detected using HRP-coupled cholera toxin B subunit. (B) Reduced phospho-LAT in the GEM fraction of DEX-treated cells. Control and DEX-treated hybridomas were left untreated or stimulated by CD3 cross-linking for 2 min and lysed in 1% Triton X-100 MES buffer. Lysates were ultracentrifuged as described previously and GEM fractions were concentrated as described in Materials and Methods, separated by SDS-PAGE, and immunoblotted (IB) with antiphosphotyrosine mAbs. Similar results were obtained in five independent experiments. (C) Coimmunoprecipitation of LAT with CD90 requires membrane cholesterol. T cell hybridomas (107 cells/condition) were pretreated with 10 mM methyl-β-cyclodextrin (MCD) for 30 min at 37°C or with 0.2% saponin for 10 min at 4°C in PBS before lysis in 1% Triton X-100 MES buffer. After sonication, lysates were subjected to immunoprecipitation (IP) with anti-CD90 (clone HO-13.4)–coupled Sepharose beads. Immunoprecipitates were resolved by SDS-PAGE and immunoblotted with the indicated Abs. (D) DEX affects signaling complexes. T cell hybridomas were incubated in control and 1 μM DEX-supplemented media before lysis and immunoprecipitation with anti-CD90 mAbs. Proteins in total extracts (2 × 105 cells/lane) or anti-CD90 immunoprecipitates (from 107 cells/lane) were revealed by immunoblotting with the indicated Abs. D, DEX.
The existence of signaling complexes at the cell membrane can also be demonstrated by coimmunoprecipitation studies. In particular, the GPI-anchored Thy-1 molecule is known to associate with elements of the intracellular signaling machinery 53 54. Noteworthy, these molecular associations require membrane cholesterol, suggesting that such linkages may occur by virtue of colocalization to membrane subdomains 54. To further document the effects of GCs on these signaling complexes, postnuclear supernatants obtained after extraction of 3B4 cells in 1% Triton X-100 were immunoprecipitated using an anti–Thy-1 mAb and analyzed by immunoprecipitation. In keeping with previous observations, signaling proteins were found to coimmunoprecipitate with Thy-1 under these experimental conditions, whereas transmembrane (CD3ζ) and cytoplasmic (PLCγ1) proteins were excluded from these complexes (Fig. 6 D). Noteworthy, cholesterol depletion by methyl-β-cyclodextrin or saponin prevented these molecular associations (including Thy-1/LAT coimmunoprecipitation, shown as an example in Fig. 6 C), suggesting that coimmunoprecipitation required membrane subdomain integrity. In agreement with our previous observations, anti–Thy-1 immunoprecipitates from DEX-treated cells displayed reduced amounts of p59fyn and LAT proteins, suggesting that GCs affected the association of several signaling molecules with membrane microdomains enriched in GPI-anchored proteins and cholesterol. Collectively, the observations reported in Fig. 6 strongly suggest that DEX inhibits the early steps of TCR signaling by affecting the submembrane localization of important signaling molecules.
Discussion
GCs are potent immunosuppressive agents able to inhibit the expression of several proteins involved in inflammatory immune responses. In particular, their ability to inhibit T cell responses is thought to be related to the modulation of gene expression induced upon TCR triggering. Negative regulation of gene transcription in activated T lymphocytes can occur directly by competition for DNA binding sites in the promoter region of several cytokine-encoding genes, or indirectly, by sequestration/binding to transcription factors such as activator protein-1, nuclear factor κB, cAMP response element–binding protein (CREB), octamer binding factor 1, and signal transducer and activator of transcription 5 (55, and references therein). Previously, we have shown that in addition to their effect on gene transcription, GCs can inhibit an early step of TCR-initiated signal transduction, causing impaired calcium flux in activated murine T lymphocytes and T cell hybrids 26. Recently, numerous reports have underlined the importance of the early signaling events in determining cell fate decision in the immune system, leading us to further explore the mechanisms by which GCs antagonize TCR-issued signals.
In this study we provide evidence that DEX, a synthetic GC analogue, affects the response of T cells to a given TCR ligand by interfering with a membrane-proximal phosphorylative event, thus identifying a novel mechanism by which GCs exert their antiinflammatory properties. TCR stimulation of DEX-treated cells led to a defective phosphorylation of CD3-associated substrates, including the ζ chain. In keeping with numerous observations suggesting a key role for ζ in transducing activation signals 3 56, cells expressing reduced amounts of phospho-ζ also displayed reduced phosphorylation of other molecules known to play a major role in relaying activation signals to downstream effectors, including the ZAP70 kinase and the adaptor molecule LAT. The pattern of phosphorylative events evoked in DEX-treated cells suggests that GCs may affect T responses in a partial agonist fashion, as TCR occupancy in a DEX-treated T cell hybridoma only evoked a subset of the signaling events observed in control-stimulated cells. In particular, the recruitment of ZAP70 to the TCR complex and the accumulation of p21 phospho-ζ were not or were only weakly affected by GCs in activated cells, whereas other intracellular events (phosphorylation of CD3ζ p23 form, ZAP70, LAT, PLCγ1, and sustained calcium influx; this study and reference 26) were inhibited. Similarly, the profile of phosphorylative events observed in DEX-treated thymocytes was reminiscent of the pattern of phosphoproteins induced by altered peptide ligands in developing thymocytes. Indeed, in the F5 TCR transgenic model, peptides known to favor positive selection in vitro were shown to reduce ζ chain phosphorylations in a more quantitative that qualitative fashion 9. Previous studies have suggested that the ordered phosphorylation of CD3ζ establishes the threshold for T cell activation 56. These studies identified the CD3-associated ITAM modules as critical signaling thresholds detectors, able to integrate graded signals such as TCR signal strength 15 16 17, costimulation 57 58 59, and CTLA4 engagement 60 into all-or-none developmental decisions. This study demonstrates that CD3ζ is also sensitive to the hormonal environment, suggesting an important and complex role for CD3-associated ITAMs in relaying external cues to the interior of the cell.
The mechanism by which DEX affects the early steps of TCR signaling was further studied using a T cell hybrid known to resist the proapoptotic effects of GCs 26. In this cell line, DEX did not affect the expression or the in vitro PTK activity of p56lck and p59fyn, which have been shown to positively regulate the early steps of signaling events (such as phosphorylation of CD3ζ and TCR downmodulation) inhibited by GCs. This observation suggests that PTK activity is not the primary target of DEX, arguing against a role for Csk (a tyrosine kinase known to inhibit the enzymatic activity of p56lck; reference 61), CD45, or src homology 2 domain–bearing protein tyrosine phosphatase 1 (SHP-1) (hematopoietic tyrosine phosphatases able to downregulate Lck kinase activity; references 62, 63).
Analysis of membrane subdomains revealed a possible mechanism by which DEX may modulate TCR signaling. Recently it has been suggested that src PTKs and other important signaling molecules including LAT are confined into specific detergent-resistant plasma membrane domains (GEMs) enriched in sphingolipids and GPI-anchored proteins 30 43 44. The fact that components of the TCR–CD3 complex are recruited into the GEMs upon stimulation and phosphorylated on tyrosine residues within this compartment argues for an important role of these membrane domains in signal transduction 46. Accordingly, numerous experiments indicate that disruption of GEMs (and the consequent dispersion of their content into the detergent-soluble membrane fraction) inhibits TCR signal transduction 43 44 47 48. The present report suggests that DEX affects the submembrane compartmentalization of several molecules associated with the inner (cytoplasmic) lipid layer of the GEMs, including the src PTKs, p56lck and p59fyn. Based on the current literature, displacement of Src family kinases and LAT from the GEMs might be because of modification of the GEM lipid content (such as lower cholesterol content and/or the phospholipid composition; references 47–49) or by altered protein acylation. Indeed, studies using Lck and LAT mutant proteins lacking acylation sites have shown that these proteins are excluded from the GEMs in transfected T cells and are unable to transduce signals issued from the TCR 30 64 65. However, note that an Lck mutant that cannot be palmitoylated is unable to associate with membranes 65, whereas DEX failed to affect stable membrane interaction of Lck (Fig. 5 A). Moreover, no effect of DEX on membrane-associated cholesterol was evidenced by filipin staining (unpublished observations). In any event, the results reported in this study are consistent with the view that disruption of GEMs may be the primary cause of partial signaling in DEX-treated T cells. Dispersion of signaling molecules such as Lck or Fyn in the soluble membrane fraction may affect their enzymatic activity, as it has been recently recognized that these kinases exhibit optimal activity only when associated with cholesterol-enriched domains 66. Moreover, altered membrane localization may impede an adequate juxtaposition of PTKs with their substrate upon TCR stimulation or cause their sequestration in ineffective signaling complexes, resulting in a novel pattern of phosphorylating events and altered biological response. Of interest, it has been recently demonstrated that inhibition of CD4 association with the Ag–MHC-engaged TCR leads to partial signaling (predominant accumulation of the p21 isoform of phospho-ζ) in response to an agonist 67. Collectively, these data strongly suggest that partial signaling may be a consequence of ineffective clustering of TCR subunits with the relevant membrane-associated src PTKs. The analysis of phospho-ζ p21 isoform levels in unstimulated cells is in agreement with this hypothesis. Reduced levels of p21 in these cells (Fig. 1 B) appear to be the consequence of altered membrane localization of PTKs (Fig. 6 A), as both CD3ζ submembrane compartmentalization (Fig. 6 A) and PTK enzymatic activity (Fig. 5) were unaffected by DEX treatment.
The interest in the phenomenon reported here lies in its possible in vivo relevance. Thymic selection, a process whereby autoreactive thymocytes are induced to die (negative selection) while thymocytes displaying self-MHC–restricted potential are allowed to differentiate into mature T cells (positive selection), is thought to depend on subtle differences in the signals issued by the TCR in response to self-peptide–MHC complexes expressed in the thymus 1 68 69. Recently, it has been demonstrated that the thymic epithelium and possibly thymocytes themselves produce steroids, suggesting a potential role of GCs in the control of T cell development and repertoire development 23 70. Accordingly, downregulation of GC receptors on thymocytes and/or pharmacological inhibition of GC synthesis have been shown to affect thymic differentiation in vivo 71 72. These authors have proposed that GCs produced in the thymus antagonize TCR signals, thus shifting the positive selection window towards higher TCR avidities for self-Ag–MHC complexes. The work presented here provides a sound basis for explaining these observations. We propose that GCs may affect thymocyte development by modifying the early signals issued by the TCR, converting an agonist signal (causing negative selection) into a positively selecting, partial agonist–like signal. Similarly, GCs may cause cell death by the neglect of cells expressing TCRs with very low self-reactivity. Therefore, it is tempting to speculate that by lowering TCR sensitivity, GCs may shift the range of positively selected TCRs towards a higher self-reactivity, possibly ensuring that T cells surviving positive selection display a sufficient reactivity to self-MHC in the periphery. Noteworthy, this hypothesis postulates that lack of thymic GC receptor expression would lead to subtle changes in repertoire selection, with no major alteration in overall thymic cellularity. In keeping with this hypothesis, lack of GC responsiveness led to the specific loss of T cells bearing a TCR specific for a given Ag 73, with no major impact on thymic cell numbers and phenotype 74.
Signal strength has also been proposed to regulate the development of helper subsets in the periphery. Numerous studies have suggested that “weak” and/or partial signaling in mature lymphocytes favors Th2 cell development. Priming of naive T cells with optimal doses of an agonist peptide will induce Th1 development, whereas very low doses of an agonist peptide or stimulation by a low-avidity altered peptide ligand will favor Th2 differentiation 11 75. GCs in vivo and in vitro have been shown to favor Th2 cell differentiation in response to Ag 24 76 77, suggesting again that by reducing TCR signal strength, GCs may affect cell fate decisions in the periphery. Noteworthy, we and others have proposed previously that GCs may favor Th2 development by affecting both survival of dendritic cells and IL-12 production 78 79 80. These observations suggest that by affecting both APC function and TCR signaling, endogenous GCs produced during acute infection may favor the development of Th2-like cells with antiinflammatory properties.
In conclusion, our study illustrates a novel aspect of the signaling flexibility of the TCR, revealing that in addition to avidity for Ag–MHC and costimulation, environmental factors such as GC hormones determine the nature of intracellular signals induced by the TCR. Further studies are required to better understand how all these influences are translated in vivo into distinct functional responses.
Acknowledgments
We would like to thank Drs. L. Samelson, R. Kubo, P. Draper, and A. Weiss for providing reagents; Drs. H.T. He, O. Williams, and D. Nolan for interesting discussions and comments on the manuscript; Dr. M. Moser for careful review of the manuscript; and G. Dewasme, F. Thielemans, M. Swaenepoel, and P. Veirman for technical assistance.
This work was funded by the Belgian Program in Interuniversity Poles of Attraction initiated by the Belgian State, Prime Minister's Office, Science Policy Programming, and by a Research Concerted Action of the Communauté Française de Belgique. The scientific responsibility is assumed by its authors. F. Van Laethem is supported by the Fonds pour la Formation à la Recherche dans l'Industrie et dans l'Agriculture; and E. Baus is supported by the Fonds National de la Recherche Scientifique.
Footnotes
Abbreviations used in this paper: β2m, β2-microglobulin; DEX, dexamethasone; GAG, glycosaminoglycan; GC, glucocorticoid; GEM, glycolipid-enriched membrane microdomain; GPI, glycosyl phosphatidylinositol; HRP, horseradish peroxidase; ITAM, immunoreceptor tyrosine-based activation motif; LAT, linker for activation of T cells; MES, morpholinoethane sulfonic acid; PLC, phospholipase C; PTK, protein tyrosine kinase; Rag, recombination activating gene.
References
- Germain R.N., Stefanova I. The dynamics of T cell receptor signalingcomplex orchestration and the key roles of tempo and cooperation. Annu. Rev. Immunol. 1999;17:467–522. doi: 10.1146/annurev.immunol.17.1.467. [DOI] [PubMed] [Google Scholar]
- Constant S.L., Bottomly K. Induction of Th1 and Th2 CD4+ T cell responsesthe alternative approaches. Annu. Rev. Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- Kersh E.N., Shaw A.S., Allen P.M. Fidelity of T cell activation through multistep T cell receptor ζ phosphorylation. Science. 1998;281:572–575. doi: 10.1126/science.281.5376.572. [DOI] [PubMed] [Google Scholar]
- Evavold B.D., Sloan-Lancaster J., Allen P.M. Tickling the TCRselective T-cell functions stimulated by altered peptide ligands. Immunol. Today. 1993;14:602–609. doi: 10.1016/0167-5699(93)90200-5. [DOI] [PubMed] [Google Scholar]
- Sloan-Lancaster J., Allen P.M. Altered peptide ligand-induced partial T cell activationmolecular mechanisms and role in T cell biology. Annu. Rev. Immunol. 1996;14:1–27. doi: 10.1146/annurev.immunol.14.1.1. [DOI] [PubMed] [Google Scholar]
- Jameson S.C., Bevan M.J. T cell receptor antagonists and partial agonists. Immunity. 1995;2:1–11. doi: 10.1016/1074-7613(95)90074-8. [DOI] [PubMed] [Google Scholar]
- Sloan-Lancaster J., Evavold B.D., Allen P.M. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature. 1993;363:156–159. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- Sebzda E., Kundig T.M., Thomson C.T., Aoki K., Mak S.Y., Mayer J.P., Zamborelli T., Nathenson S.G., Ohashi P.S. Mature T cell reactivity altered by peptide agonist that induces positive selection. J. Exp. Med. 1996;183:1093–1104. doi: 10.1084/jem.183.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth L.A., Williams O., Huby R.D., Norton T., Acuto O., Ley S.C., Kioussis D. Altered peptide ligands induce quantitatively but not qualitatively different intracellular signals in primary thymocytes. Proc. Natl. Acad. Sci. USA. 1998;95:8193–8198. doi: 10.1073/pnas.95.14.8193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist K.A., Jameson S.C., Heath W.R., Howard J.L., Bevan M.J., Carbone F.R. T cell receptor antagonist peptides induce positive selection. Cell. 1994;14:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Constant S., Pfeiffer C., Woodard A., Pasqualini T., Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J. Exp. Med. 1995;182:1591–1596. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosken N.A., Shibuya K., Heath A.W., Murphy K.M., O'Garra A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-αβ–transgenic model. J. Exp. Med. 1995;182:1579–1584. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X., Grant C., Constant S., Bottomly K. Induction of IL-4-producing CD4+ T cells by antigenic peptides altered for TCR binding. J. Immunol. 1994;158:4237–4244. [PubMed] [Google Scholar]
- Smith J.A., Tang Q., Bluestone J.A. Partial TCR signals delivered by FcR-nonbinding anti-CD3 monoclonal antibodies differentially regulate individual Th subsets. J. Immunol. 1998;160:4841–4849. [PubMed] [Google Scholar]
- Sloan-Lancaster J., Shaw A.S., Rothbard J.B., Allen P.M. Partial T cell signalingaltered phospho-ζ and lack of zap70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Madrenas J., Wange R.L., Wang J.L., Isakov N., Samelson L.E., Germain R.N. ζ phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science. 1995;267:515–518. doi: 10.1126/science.7824949. [DOI] [PubMed] [Google Scholar]
- Reis é Sousa, C., E.H. Levine, and R.N. Germain Partial signaling by CD8+ T cells in response to antagonist ligands. J. Exp. Med. 1996;184:149–157. doi: 10.1084/jem.184.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam S.M., Travers P.J., Wung J.L., Nasholds W., Redpath S., Jameson S.C., Gascoigne N.R. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996;381:616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- Allen P.M. Peptides in positive and negative selectiona delicate balance. Cell. 1994;76:593–596. doi: 10.1016/0092-8674(94)90497-9. [DOI] [PubMed] [Google Scholar]
- Madrenas J., Chau L.A., Smith J., Bluestone J.A., Germain R.N. The efficiency of CD4 recruitment to ligand-engaged TCR controls the agonist/partial agonist properties of peptide-MHC molecule ligands. J. Exp. Med. 1997;185:219–229. doi: 10.1084/jem.185.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato A.C., Wade E. Molecular mechanisms of anti-inflammatory action of glucocorticoids. Bioessays. 1996;18:371–378. doi: 10.1002/bies.950180507. [DOI] [PubMed] [Google Scholar]
- Ashwell J.D., Lu F.W., Vacchio M.S. Glucocorticoids in T cell development and function. Annu. Rev. Immunol. 2000;18:309–345. doi: 10.1146/annurev.immunol.18.1.309. [DOI] [PubMed] [Google Scholar]
- Vacchio M.S., Papadopoulos V., Ashwell J.D. Steroid production in the thymusimplications for thymocyte selection. J. Exp. Med. 1994;179:1835–1846. doi: 10.1084/jem.179.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daynes R.A., Araneo B. Contrasting effects of glucocorticoids on the capacity of T cells to produce the growth factors interleukin 2 and interleukin 4. Eur. J. Immunol. 1989;19:2319–2325. doi: 10.1002/eji.1830191221. [DOI] [PubMed] [Google Scholar]
- Mamalaki C., Norton T., Tanaka Y., Townsend A.R., Chandler P., Simpson E., Kioussis D. Thymic depletion and peripheral activation of class I major histocompatibility complex-restricted T cells by soluble peptide in T-cell receptor transgenic mice. Proc. Natl. Acad. Sci. USA. 1992;89:11342–11346. doi: 10.1073/pnas.89.23.11342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baus E., Andris F., Dubois P.M., Urbain J., Leo O. Dexamethasone inhibits the early steps of antigen receptor signaling in activated T lymphocytes. J. Immunol. 1996;156:4555–4561. [PubMed] [Google Scholar]
- Tanaka Y., Williams O., Tarazona R., Wack A., Norton T., Kioussis D. In vitro positive selection of αβ TCR transgenic thymocytes by a conditionally immortalized cortical epithelial clone. Int. Immunol. 1997;9:381–393. doi: 10.1093/intimm/9.3.381. [DOI] [PubMed] [Google Scholar]
- Coulie P.G., Uyttenhove C., Wauters P., Manolios N., Klausner R.D., Samelson L.E., Van Snick J. Identification of a murine monoclonal antibody specific for an allotypic determinant on mouse CD3. Eur. J. Immunol. 1991;21:1703–1709. doi: 10.1002/eji.1830210718. [DOI] [PubMed] [Google Scholar]
- Nolan D.P., Jackson D.G., Biggs M.J., Brabazon E.D., Pays A., Van Laethem F., Paturiaux-Hanocq F., Elliot J.F., Voorheis H.P., Pays E. Characterization of a novel alanine-rich protein located in surface microdomains in Trypanosoma brucei . J. Biol. Chem. 2000;275:4072–4080. doi: 10.1074/jbc.275.6.4072. [DOI] [PubMed] [Google Scholar]
- Zhang W., Trible R.P., Samelson L.E. LAT palmitoylationits essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- Wessel D., Flugge U.I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- Zhang W., Sloan-Lancaster J., Kitchen J., Trible R.P., Samelson L.E. LATthe ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- Weber J.R., Orstavik S., Torgersen K.M., Danbolt N.C., Berg S.F., Ryan J.C., Tasken K., Imboden J.B., Vaage J.T. Molecular cloning of the cDNA encoding pp36, a tyrosine-phosphorylated adaptor protein selectively expressed by T cells and natural killer cells. J. Exp. Med. 1998;187:1157–1161. doi: 10.1084/jem.187.7.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finco T.S., Kadlecek T., Zhang W., Samelson L.E., Weiss A. LAT is required for TCR-mediated activation of PLCγ1 and the Ras pathway. Immunity. 1998;9:617–625. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- Wehling M. Specific, nongenomic actions of steroid hormones. Annu. Rev. Physiol. 1997;59:365–393. doi: 10.1146/annurev.physiol.59.1.365. [DOI] [PubMed] [Google Scholar]
- van Oers N.S., Killeen N., Weiss A. ZAP-70 is constitutively associated with tyrosine-phosphorylated TCR ζ in murine thymocytes and lymph node T cells. Immunity. 1994;1:675–685. doi: 10.1016/1074-7613(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Wiest D.L., Ashe J.M., Abe R., Bolen J.B., Singer A. TCR activation of ZAP70 is impaired in CD4+CD8+ thymocytes as a consequence of intrathymic interactions that diminish available p56lck. Immunity. 1996;4:495–504. doi: 10.1016/s1074-7613(00)80415-x. [DOI] [PubMed] [Google Scholar]
- Valitutti S., Muller S., Cella M., Padovan E., Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- D'Oro U., Vacchio M.S., Weissman A.S., Ashwell J.D. Activation of Lck tyrosine kinase targets cell surface T cell antigen receptors for lysosomal degradation. Immunity. 1997;7:619–628. doi: 10.1016/s1074-7613(00)80383-0. [DOI] [PubMed] [Google Scholar]
- van Oers N.S., Killeen N., Weiss A. Lck targets the tyrosine phosphorylation of the T cell receptor subunits and ZAP-70 in murine thymocytes. J. Exp. Med. 1996;183:1053–1062. doi: 10.1084/jem.183.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillette A., Bookman M.A., Hork E.M., Samelson L.E., Bolen J.B. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine kinase p56Lck. Nature. 1989;338:257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]
- Brown D.A., London E. Function of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- Simons K., Toomre D. Lipid rafts and signal transduction. Nat. Rev. (Mol. Cell. Biol.). 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Xavier R., Brennan T., Li Q., McCormack C., Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- Montixi C., Langlet C., Bernard A.M., Thimonier J., Dubois C., Wurbel M.A., Chauvin J.P., Pierres M., He H.T. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:5334–5348. doi: 10.1093/emboj/17.18.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola A., Lanzavecchia A. T-cell activation and the dynamic world of rafts. APMIS. 1999;107:615–623. doi: 10.1111/j.1699-0463.1999.tb01450.x. [DOI] [PubMed] [Google Scholar]
- Ilangumaran S., He H.T., Hoessli D.C. Microdomains in lymphocyte signalingbeyond GPI-anchored proteins. Immunol. Today. 2000;21:2–7. doi: 10.1016/s0167-5699(99)01494-2. [DOI] [PubMed] [Google Scholar]
- Stulnig T.M., Berger M., Sigmund T., Stockinger H., Horesji V., Waldhausl W. Signal transduction via glycosyl phosphatidyl-anchored proteins in T cells is inhibited by lowering cellular cholesterol. J. Biol. Chem. 1997;272:19242–19247. doi: 10.1074/jbc.272.31.19242. [DOI] [PubMed] [Google Scholar]
- Stulnig T.M., Berger M., Sigmund T., Stockinger H., Horesji V., Waldhausl W. Polyunsaturated fatty acids inhibit T cell signaling transduction by modification of detergent-insoluble membrane domains. J. Cell Biol. 1998;143:637–644. doi: 10.1083/jcb.143.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb Y., Hermida-Matsumoto L., Resh M.D. Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J. Biol. Chem. 2000;275:261–270. doi: 10.1074/jbc.275.1.261. [DOI] [PubMed] [Google Scholar]
- Brown D.A., Rose J.K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Rodgers W., Rose J.K. Exclusion of CD45 inhibits activity of p56lck associated with glycolipid-enriched membrane domains. J. Cell Biol. 1996;135:1515–1523. doi: 10.1083/jcb.135.6.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P.M., Samelson L.E. The glycophosphatidylinositol-anchored Thy-1 molecule interacts with the p60fyn protein tyrosine kinase in T cells. J. Biol. Chem. 1992;267:12317–12322. [PubMed] [Google Scholar]
- Draberova L., Amoui M., Draber P. Thy-1-mediated activation of rat mast cellsthe role of Thy-1 membrane microdomains. Immunology. 1996;87:141–148. [PMC free article] [PubMed] [Google Scholar]
- De Bosscher K., Schmitz M.L., Vanden Berghe W., Plaisance S., Fiers W., Haegeman G. Glucocorticoid-mediated repression of nuclear factor-κB-dependent transcription involves direct interference with transactivation. Proc. Natl. Acad. Sci. USA. 1997;94:13504–13509. doi: 10.1073/pnas.94.25.13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving B.A., Chan A.N., Weiss A. Functional characterization of a signal transducing motif present in the T cell antigen receptor ζ chain. J. Exp. Med. 1993;177:1093–1103. doi: 10.1084/jem.177.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuosto L., Acuto O. CD28 affects the earliest signaling events generated by TCR engagement. Eur. J. Immunol. 1998;28:2131–2142. doi: 10.1002/(SICI)1521-4141(199807)28:07<2131::AID-IMMU2131>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Boussiotis V.A., Barber D.L., Lee B.J., Gribben J.G., Freeman G.J., Nadler N.M. Differential association of protein tyrosine kinases with the T cell receptor is linked to the induction of anergy and its prevention by B7 family-mediated costimulation. J. Exp. Med. 1996;184:365–376. doi: 10.1084/jem.184.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran M., Miceli M.C. Engagement of GPI-linked CD48 contributes to TCR signals and cytoskeletal reorganizationa role for lipid rafts in T cell activation. Immunity. 1998;9:787–796. doi: 10.1016/s1074-7613(00)80644-5. [DOI] [PubMed] [Google Scholar]
- Lee K.M., Chuang E., Griffin M., Khattri R., Hong D.K., Zhang W., Straus D., Samelson L.E., Thompson C.B., Bluestone J.A. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282:2256–2263. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- Chow L.M., Fournel M., Davidson D., Veillette A. Negative regulation of T-cell receptor signaling by tyrosine protein kinase p50csk. Nature. 1993;365:156–160. doi: 10.1038/365156a0. [DOI] [PubMed] [Google Scholar]
- D'Oro U., Ashwell J.D. The CD45 tyrosine phosphatase is an inhibitor of Lck activity in thymocytes. J. Immunol. 1999;162:1879–1883. [PubMed] [Google Scholar]
- Dittel B.N., Stefanova I., Germain R.N., Janeway C.A., Jr. Cross-antagonism of a T cell clone expressing two distinct T cell receptors. Immunity. 1999;11:289–298. doi: 10.1016/s1074-7613(00)80104-1. [DOI] [PubMed] [Google Scholar]
- Melkonian K.A., Ostermeyer A.G., Chen J.Z., Roth M.G., Brown D. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J. Biol. Chem. 1999;274:3910–3917. doi: 10.1074/jbc.274.6.3910. [DOI] [PubMed] [Google Scholar]
- Kabouridis P.S., Magee A.L., Ley S.C. S-acylation of LCK protein tyrosine kinase is essential for its signaling function in T lymphocytes. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:4983–4998. doi: 10.1093/emboj/16.16.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilangumaran S., Arni S., van Echten-Deckert G., Borisch B., Hoessli D.C. Microdomain-dependent regulation of Lck and Fyn protein-tyrosine kinases in T lymphocyte plasma membranes. Mol. Biol. Cell. 1999;10:891–905. doi: 10.1091/mbc.10.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrenas J., Chau L.A., Smith J., Bluestone J.A., Germain R.N. The efficiency of CD4 recruitment to ligand-engaged TCR controls the agonist/partial agonist properties of peptide-MHC molecule ligands. J. Exp. Med. 1997;185:219–229. doi: 10.1084/jem.185.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldrath A.W., Bevan M.J. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402:255–262. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- Mariathasan S., Jones R.G., Ohashi P.S. Signals involved in thymocyte positive and negative selection. Semin. Immunol. 1999;11:263–272. doi: 10.1006/smim.1999.0182. [DOI] [PubMed] [Google Scholar]
- Lechner O., Wiegers G.J., Oliveira-Dos-Santos A.J., Dietrich H., Recheis H., Waterman M., Boyd R., Wick G. Glucocorticoid production in the murine thymus. Eur. J. Immunol. 2000;30:337–346. doi: 10.1002/1521-4141(200002)30:2<337::AID-IMMU337>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Vacchio M.S., Ashwell J.D. Thymus-derived glucocorticoids regulate antigen-specific positive selection. J. Exp. Med. 1997;185:2033–2038. doi: 10.1084/jem.185.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacchio M.S., Lee J.Y., Ashwell J.D. Thymus-derived glucocorticoids set the thresholds for thymocyte selection by inhibiting TCR-mediated thymocyte activation. J. Immunol. 1999;163:1327–1333. [PubMed] [Google Scholar]
- Lu F.W., Yasutomo K., Goodman G.B., McHeyzer-Williams L.J., McHeyzer-Williams M.G., Germain R.N., Ashwell J.D. Thymocyte resistance to glucocorticoids leads to antigen-specific unresponsiveness due to “holes” in the T cell repertoire. Immunity. 2000;12:183–192. doi: 10.1016/s1074-7613(00)80171-5. [DOI] [PubMed] [Google Scholar]
- Purton J.F., Boyd R.L., Cole T.J., Godfrey D.I. Intrathymic T cell development and selection proceeds normally in the absence of glucocorticoid receptor signaling. Immunity. 2000;13:179–186. doi: 10.1016/s1074-7613(00)00018-2. [DOI] [PubMed] [Google Scholar]
- Leitenberg D., Bottomly K. Regulation of naive T cell differentiation by varying the potency of TCR signal transduction. Semin. Immunol. 1999;11:283–292. doi: 10.1006/smim.1999.0184. [DOI] [PubMed] [Google Scholar]
- Ramirez F., Fowell D.J., Puklavec M., Simmonds S., Mason D. Glucocorticoids promote a Th2 cytokine response by CD4+ T cells in vitro. J. Immunol. 1996;156:2406–2412. [PubMed] [Google Scholar]
- Dozmorov I.M., Miller R.A. Generation of antigen-specific Th2 cells from unprimed mice in vitroeffects of dexamethasone and anti-IL-10 antibody. J. Immunol. 1998;160:2700–2705. [PubMed] [Google Scholar]
- Moser M., De Smedt T., Sornasse T., Tielemans F., Chentoufi A.A., Muraille E., Van Mechelen M., Urbain J., Leo O. Glucocorticoids down-regulate dendritic cell function in vitro and in vivo. Eur. J. Immunol. 1995;25:2818–2824. doi: 10.1002/eji.1830251016. [DOI] [PubMed] [Google Scholar]
- Vieira P.L., Kalinski P., Wierenga E.A., Kapsenberg M.L., de Jong E.C. Glucocorticoids inhibit bioactive IL-12p70 production by in vitro-generated human dendritic cells without affecting their T cell stimulatory potential. J. Immunol. 1998;161:5245–5251. [PubMed] [Google Scholar]
- DeKruyff R.H., Fang Y., Umetsu D.T. Corticosteroids enhance the capacity of macrophages to induce Th2 cytokine synthesis in CD4+ lymphocytes by inhibiting IL-12 production. J. Immunol. 1998;160:2231–2237. [PubMed] [Google Scholar]