Abstract
Apoptosis is a key for CD4+ T cell destruction in HIV-1–infected patients. In this study, human peripheral blood lymphocyte (PBL)-transplanted nonobese diabetic (NOD)-severe combined immunodeficient (SCID) (hu-PBL-NOD-SCID) mice were used to examine in vivo apoptosis after HIV-1 infection. As the hu-PBL-NOD-SCID mouse model allowed us to see extensive infection with HIV-1 and to analyze apoptosis in human cells in combination with immunohistological methods, we were able to quantify the number of apoptotic cells with HIV-1 infection. As demonstrated by terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL), massive apoptosis was predominantly observed in virus-uninfected CD4+ T cells in the spleens of HIV-1–infected mice. A combination of TUNEL and immunostaining for death-inducing tumor necrosis factor (TNF) family molecules indicated that the apoptotic cells were frequently found in conjugation with TNF-related apoptosis-inducing ligand (TRAIL)-expressing CD3+CD4+ human T cells. Administration of a neutralizing anti-TRAIL mAb in HIV-1–infected mice markedly inhibited the development of CD4+ T cell apoptosis. These results suggest that a large number of HIV-1–uninfected CD4+ T cells undergo TRAIL-mediated apoptosis in HIV-infected lymphoid organs.
Keywords: HIV-1, apoptosis, TRAIL, hu-PBL-NOD-SCID mouse, TUNEL
Introduction
HIV infection induces gradual loss of CD4+ T cells, leading to AIDS, but the mechanism of this cell loss remains unclear. Severe impairment of T cell development 1 and increased destruction of CD4+ T cells in secondary lymphoid organs such as lymph nodes and spleens have been reported in HIV-infected individuals 2. Histopathological analyses of HIV-1– or simian immunodeficiency virus (SIV)-infected lymph nodes showed that apoptosis occurs mostly in virus-uninfected bystander CD4+ T cells 3. The role of apoptosis in the CD4+ T cell loss has also been suggested by the following in vitro experiments: (a) a greater proportion of peripheral blood T cells from HIV-1–infected individuals became apoptotic in culture after TCR-mediated activation (activation-induced cell death [AICD]) 4 5; and (b) the proportion of Fas-expressing T cells in HIV-1–infected individuals increased with disease progression, and ligation of Fas with specific Abs induced apoptosis of T cells in vitro 6 7. However, possible involvement of the Fas–Fas ligand (FasL) pathway in AICD of CD4+ T cells from HIV-1–infected individuals 8 9 remains controversial. Another report has indicated that AICD was independent of Fas 10, and it has been shown that neither Fas protein nor biologically active FasL were detectable at significant levels in freshly isolated T cells from HIV-1–infected individuals 11. These results strongly suggested the involvement of other mechanisms in the apoptosis of CD4+ T cells in HIV-infected individuals.
TNF-related apoptosis-inducing ligand (TRAIL)/Apo-2L is a new member of death-inducing ligands in the TNF family 12. While it has been reported that TRAIL induced apoptosis in various tumor cells in vivo, pathophysiological roles of TRAIL are largely unknown. It has been shown that TRAIL was expressed in IFN-α–stimulated CD4+ T cells 13, monocytes 14, and dendritic cells (DCs) 15. In addition, it was recently reported that infection with measles virus augmented TRAIL expression in DCs 16. Therefore, it is possible that TRAIL may be involved in HIV pathologies. In fact, it has been reported that TRAIL, but not FasL, was involved in AICD of CD4+ T cells isolated from HIV-1–infected individuals in vitro 17 18.
Lymphoid organs are thought to be the major site of HIV replication and pathogenesis 19 20. To explore the immunopathological processes occurring in these organs in HIV-infected humans, it is helpful to develop an appropriate animal model. We have previously shown that the nonobese diabetic (NOD)-SCID mouse is a useful strain for both successful engraftment of human PBLs and massive HIV-1 replication, probably due to its severe immunodeficiency, including innate immunity 21. In this model, engrafted human cells migrated to various organs, including the spleen, and human CD4+ T cells were efficiently killed by HIV-1 injection. In this study, we found that this model is useful for analysis of apoptotic cells and that TRAIL played a critical role in inducing the apoptosis of HIV-1–uninfected human CD4+ T cells. The possible clinical relevance of this finding is discussed.
Materials and Methods
Mice.
NOD (NOD/Shi) scid/scid mice 21 were maintained in the Central Institute for Experimental Animals (Kawasaki, Japan). The mice were screened for immunodeficiency by immunodiffusion assay (Medical and Biological Laboratory, Nagoya, Japan) for serum IgM and were 6–8 wk old at the time of human PBMC transfer. The experimental protocol was approved by the Ethics Review Committees for Animal Experimentation of the participating institutions.
Reconstitution and HIV-1 Infection of hu-PBL-NOD-SCID Mice.
Reconstitution with human PBLs and infection with HIV-1 were performed as previously described 21 22. 1,000 ID50 of HIV-1 was inoculated intraperitoneally. Cloned HIV-1 isolates, including macrophage-tropic viruses JR-FL or green fluorescent protein (GFP)-carrying HIV-1 (data not shown), were employed. Mice were killed 2–4 wk after infection. 1 mg of anti–human TRAIL mAb (RIK-2) 23, anti–human FasL mAb (NOK-1) 24, or control mouse IgG (Inter-Cell Technologies, Inc.) was injected intraperitoneally 9 d after HIV-1JR-FL infection. These mice were killed 3 d later, and the spleens were collected.
Histological Analyses.
Mouse spleen was fixed in 4% periodate-lysine-paraformaldehyde fixative 25 26, embedded in either paraffin or Tissue-Tek OCT compound and cut into 6- or 10-μm-thick sections. Paraffin sections were dried, dewaxed in xylene, ethanol, and water, and then stained with hematoxylin and eosin. The paraffin sections were initially either treated with 0.0025% trypsin solution for human CD68 detection or microwaved for human CD8 and HIV-1 p24gag detection. Both treatments were required for human CD3 and CD4 detection. Tissue sections were incubated with primary Abs against human CD3 (rabbit polyclonal IgG; Dako), human CD4 (clone 1F6, mouse IgG; NeoMarkers), human CD8 (clone C8/144B, mouse IgG; Dako), human CD68 (clone PGM1, mouse IgG; Dako), human CD20 (clone L26, mouse IgG; Dako), or HIV-1 p24gag (clone Kal-1, mouse IgG; Dako). Subsequently, the avidin-biotinylated peroxidase complex (ABC) method (Vector Laboratories) for human CD4 staining, the enhanced polymer one-step staining (EPOS) method (Dako) for human CD68 and CD20 staining, the EnVision™+ method (Dako) for human CD3 and CD8 staining, or the TSA™-Indirect method (NEN Life Science Products) for HIV-1 p24gag staining was performed as described previously 27 28. Nonimmunized mouse IgG (Inter-Cell Technologies, Inc.) or rabbit IgG (Dako) was used as a negative control.
Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) was carried out using an indirect method according to the instructions provided by the manufacturer. Frozen sections were incubated with TdT reaction solution (0.02 μg/μl digoxigenin-labeled dUTP, 0.012 U/μl TdT; Roche), followed by tetramethylrhodamine isothiocyanate (TRITC)-conjugated antidigoxigenin Ab (Roche). The specificity of the TUNEL staining was confirmed by the following: (a) absence of either digoxigenin-labeled dUTP or TdT served as a negative control; (b) DNase-treated section as a positive control showed positive staining in all nuclei.
Combinations of TUNEL and immunostaining using specific Abs were performed as follows. The first combination was for human CD4, TUNEL, and HIV p24gag. Labeled streptavidin biotin (LSAB) technique with Cy5 (Amersham Pharmacia Biotech) was performed using anti–human CD4 mAb (clone MT310, mouse IgG; Dako). TSA™-Direct method was performed for TUNEL using horseradish peroxidase (HRP)-conjugated antidigoxigenin Ab (sheep IgG Fab fragment; Roche) and TRITC-conjugated tyramide (NEN Life Science Products). Final staining was carried out with FITC-conjugated anti-HIV p24gag mAb (Verostat Inc.) enhanced with anti-FITC Ab (rabbit polyclonal IgG; Molecular Probes, Inc.) and FITC-conjugated goat anti–rabbit IgG Ab (Zymed Laboratories). Biotinylated horse anti–mouse IgG Ab (Vector Laboratories), which was the secondary Ab for human CD4, did not react with rabbit and sheep IgG. To block the unoccupied binding sites of this secondary Ab, sections were incubated with nonimmunized mouse IgG (Inter-Cell Technologies, Inc.) and nonimmunized goat serum (Vector Laboratories) before incubation of anti-HIV p24gag mAb. FITC-conjugated anti–rabbit IgG Ab, which was used as the third-step Ab for p24gag, had been absorbed with mouse and horse serum. To block the unoccupied binding sites, sections were incubated with a sheep IgG Fab fragment (Rockland Inc.).
The second combination was for TRAIL and TUNEL. Cy5-LSAB method was performed with anti-TRAIL Ab (K-18, goat polyclonal IgG; Santa Cruz Biotechnology, Inc.). An indirect method was used with TRITC-conjugated antidigoxigenin Ab for TUNEL. Biotinylated donkey anti–goat IgG Ab (Polysciences, Inc.), which was used as the secondary Ab, had been absorbed with sheep serum.
The third combination was for human Fas, FasL, and TUNEL. Cy5-LSAB method was performed using anti–human Fas mAb (clone APO-1, mouse IgG; Dako), and an indirect method was performed with FITC-conjugated anti–rabbit IgG Ab for anti-FasL Ab (C-20, rabbit polyclonal IgG; Santa Cruz Biotechnology, Inc.). To block the unoccupied binding sites of biotinylated anti–mouse IgG Ab, sections were incubated with nonimmunized goat serum before FITC-conjugated anti–rabbit IgG Ab incubation.
The fourth combination was for human TNF-α, TNFR1, and TUNEL. Cy5-LSAB method was performed with anti-TNFR1 Ab (C-20, goat polyclonal IgG; Santa Cruz Biotechnology, Inc.). TSA™-Direct method was performed with anti-TNF-α mAb (clone C6-H6, mouse IgG; NeoMarkers), FITC-conjugated horse anti–mouse IgG Ab (Vector laboratories), HRP-conjugated goat anti-FITC Ab (NEN Life Science Products), and FITC-conjugated tyramide (NEN Life Science Products). To block the unoccupied binding sites of biotinylated anti–goat IgG Ab, sections were incubated with nonimmunized goat serum before HRP-conjugated anti-FITC Ab incubation.
The fifth combination was for human CD4, TRAIL, and TUNEL. Cy5-LSAB method was performed with anti-TRAIL Ab. An indirect method was performed using anti–human CD4 mAb (clone MT310) and FITC-conjugated donkey anti–mouse IgG Ab (Chemicon International Inc.). TUNEL was carried out by the indirect method as above. Biotinylated anti–goat IgG Ab (Polysciences, Inc.), which was used as the secondary Ab for TRAIL, had been absorbed with mouse and sheep serum. FITC-conjugated donkey anti-mouse IgG Ab, which was used as the secondary Ab for human CD4, did not react with goat and sheep IgG.
The sixth combination was for human CD3, TRAIL, and TUNEL. Immunostaining for TRAIL and TUNEL was carried out as in the fifth combination. Subsequently, an indirect method using anti–human CD3 Ab (rabbit polyclonal IgG; Dako) and FITC-conjugated goat anti-rabbit IgG Ab (Zymed Laboratories) was performed. To avoid cross-reaction of secondary antibodies, sections were treated with nonimmunized goat IgG and sheep IgG Fab fragment before incubation with FITC-conjugated goat anti–rabbit IgG Ab.
The absence of primary Abs or digoxigenin-labeled dUTP served as negative controls for these combinational staining methods. Sections were then covered with VECTASHIELD® (Vector Laboratories) and examined under a Carl Zeiss LSM 310 or a Leica TCS NT confocal laser scanning microscope. Single beams (488 nm for FITC, 542 nm for TRITC, and 600 nm for Cy5) from an argon–krypton laser were used for excitation. Emissions from FITC were detected through a long pass filter (<540 nm) and displayed in green. Emissions from TRITC were detected through a band pass filter (600 ± 10 nm) and displayed in red. Emissions from Cy5 were detected through a band pass filter (740 ± 70 nm) and displayed in blue or green.
Results
Reconstitution of Human Lymphoid Organ–like Structure in the Spleens of hu-PBL-NOD-SCID Mice and Apoptosis in the HIV-1–infected Spleen.
Splenomegaly was consistently observed in the hu-PBL-NOD-SCID mice, reaching a maximum size within 2 wk that was three to five times larger than the spleens of NOD-SCID mice (data not shown). This splenomegaly was maintained up to 4–5 wk after transplantation. Immunostaining of spleen sections with Abs specific for human leukocyte surface markers revealed that many human CD3+ T cells, including both CD4+ and CD8+ T cells, human CD20+ B cells, and a few human CD68+ macrophages, efficiently migrated to the spleen from the peritoneal cavity (data not shown). Human CD20+ B cells were distributed close to the small arteries within 2–4 wk. Human CD3+ T cells were found in the periarterial region within 2 wk of transplantation and subsequently spread to the region distal to the small arteries by 4 wk. Among the CD3+ T cells, CD4+ T cells were found predominantly in the proximal area, whereas CD8+ T cells were generally found in the distal area. In the CD8+ T cell–rich regions, scattered human CD68+ macrophages and human CD1a+ DCs were found. In the spleens of untransplanted NOD-SCID mice, no specific immunostaining was found.
A similar extent of splenomegaly was observed in HIV-1–infected mice 2 wk after human PBL transplantation. However, the splenomegaly in infected mice regressed more rapidly than that of uninfected mice at 4 wk (data not shown). A marked decrease in the number of human CD4+ T cells was observed in infected mice with immunostaining analysis (data not shown). HIV-1 p24gag–expressing cells were found in the human CD4+ T cell–rich region; however, the number of p24gag+ cells was far lower than the number of human CD4+ T cells. In contrast, the number and distribution of human CD8+ T cells, human CD20+ B cells, and human CD68+ macrophages was apparently unchanged. TUNEL staining showed a large number of apoptotic cells around the small arteries in the spleen 2 wk after HIV-1 infection (Fig. 1 a). In contrast, only a few TUNEL+ apoptotic cells were observed in the spleens of uninfected hu-PBL-NOD-SCID mice at the same time point (data not shown). Furthermore, a NOD-SCID mouse section without human PBL transplantation showed no staining. The specificity of the TUNEL staining was confirmed as described in Materials and Methods.
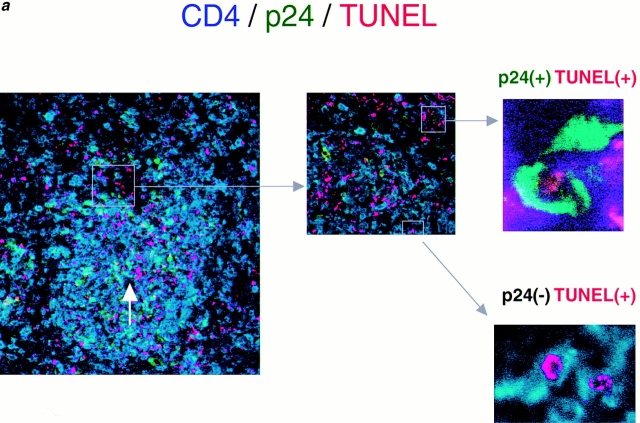
Figure 1.
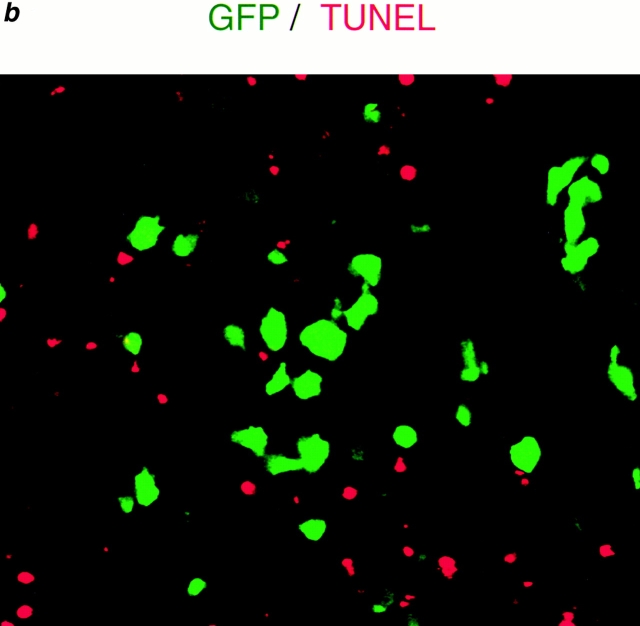
Apoptosis occurs predominantly in HIV-1–uninfected CD4+ T cells. (a) Triple immunofluorescent staining for HIV p24gag (FITC, green), human CD4 (Cy5, blue), and TUNEL (TRITC, red) of spleens from HIV-1–infected hu-PBL-NOD-SCID mice 2 wk after inoculation of human PBMCs (9 d after infection). Original magnification ×25 (left panel), ×100 (middle panel), and ×1,000 (right two panels). TUNEL (red) and CD4 (blue) double-positive cells are shown as pink in lower magnification (left panel). CD4 (blue) and HIV p24gag (green) double-positive cells are shown as light green. Arrow shows a small artery. Right upper panel indicates TUNEL+, CD4+, and p24gag+ cells, which represented <2% of both TUNEL+ and CD4+ cells. Right lower panel indicates TUNEL+, CD4+, and p24gag− cells, which accounted for >90% of both TUNEL+ and CD4+ cells. (b) Dual color detection for GFP-expressing HIV-1 (GFP, green) and TUNEL staining (TRITC, red) of spleens from GFP/HIV-1–infected hu-PBL-NOD-SCID mice 2 wk after human PBL transplantation (9 d after infection). The majority of TUNEL+ cells were GFP/HIV-1 negative. Original magnification ×100.
Predominant Apoptosis of HIV-1–uninfected Human CD4+ T Cells.
To determine whether only HIV-1–infected cells undergo apoptosis, multicolor immunostaining using Abs against various molecules was employed together with the TUNEL method. Triple-color staining for TUNEL, CD4, and p24gag showed that >90% of the TUNEL+ apoptotic cells in the spleen were human CD4+ cells 9 d after infection (Fig. 1 a). Furthermore, the majority of the apoptotic CD4+ cells did not express detectable levels of p24. To confirm the apoptosis of HIV-1–negative cells, hu-PBL-NOD-SCID mice were inoculated with GFP-expressing recombinant HIV-1, and their spleens were examined 9 d after infection. As shown in Fig. 1 b, almost all TUNEL+ apoptotic cells were GFP−. These results suggested that the abundant depletion of human CD4+ T cells in the spleens of HIV-1–infected hu-PBL-NOD-SCID mice was mostly due to apoptosis of HIV-1–uninfected CD4+ T cells.
Critical Contribution of TRAIL to Human CD4+ T Cell Apoptosis.
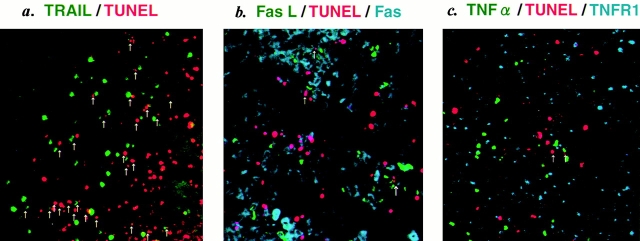
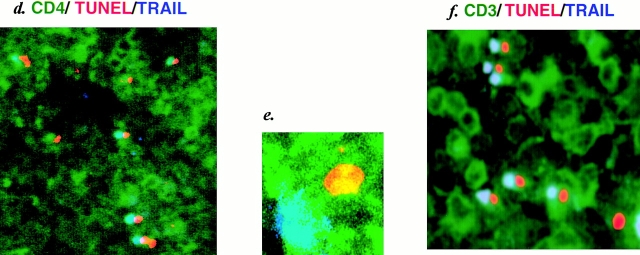
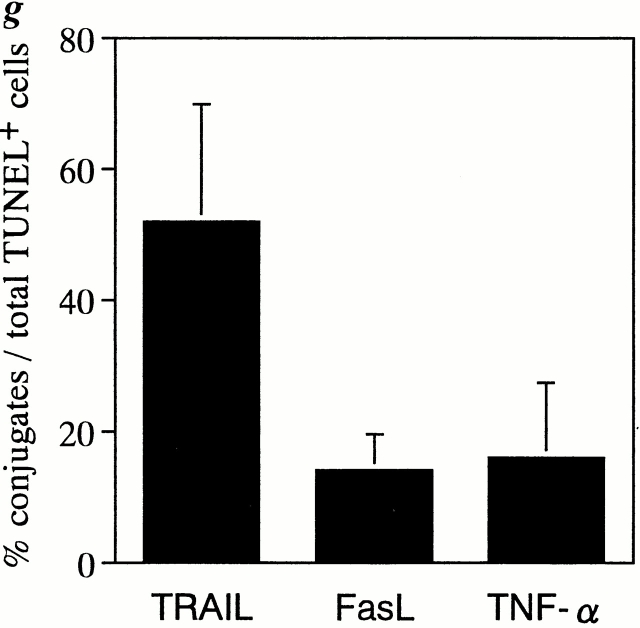
To explore the possible involvement of TRAIL, FasL, and TNF-α in human CD4+ T cell apoptosis in the spleens of HIV-1–infected hu-PBL-NOD-SCID mice, we examined the expression of these death-inducing ligands and their receptors by multicolor immunostaining along with TUNEL staining. Since tissue slices were 10 μm thick, a depth of only one to two cells was anticipated in each section, thus enabling the identification of ligand-positive cells conjugated with TUNEL+ apoptotic cells. Strikingly, dual color staining for TRAIL and TUNEL showed that >50% of apoptotic cells were conjugated with TRAIL+ cells (Fig. 2, a and g). These TUNEL+ cell–conjugated TRAIL+ cells were human CD3+CD4+ T cells revealed by triple staining of TUNEL, TRAIL, and either CD4 or CD3 (Fig. 2, d–f). Similar results were obtained by staining with anti–human TRAIL mAb RIK-2. Only a few TRAIL+ cells were observed in the spleens of uninfected mice (data not shown). In contrast, <15% of TUNEL+ apoptotic cells were conjugated with FasL+ or TNF-α+ cells (Fig. 2, b, c, and g). These findings suggested that TRAIL might play a major role in human CD4+ T cell apoptosis.
Figure 2.
Immunofluorescent analysis for death-inducing ligands and their receptors in spleens from HIV-1–infected hu-PBL-NOD-SCID mice. (a) Double staining for TRAIL (Cy5, green) and TUNEL (TRITC, red) (magnification ×50). (b) Triple staining for FasL (FITC, green), TUNEL (TRITC, red), and Fas (Cy5, blue) (magnification ×50). (c) Triple staining for TNF-α (FITC, green), TUNEL (TRITC, red), and TNFR1 (Cy5, blue) (magnification ×50). (d) Triple staining for human CD4 (FITC, green), TUNEL (TRITC, red), and TRAIL (Cy5, blue) (magnification ×100). Merging of green and blue is shown as light blue. Merging of green and red is shown as orange. (e) High magnification (×500) of d showing a TUNEL+CD4+ T cell conjugated with a TRAIL+CD4+ T cell (blue). Light blue indicates merging of green and blue. (f ) Triple immunostaining for human CD3 (FITC, green), TUNEL (TRITC, red), and TRAIL (Cy5, blue) (magnification ×200). Merging of green and blue is shown as light blue. Merging of green and red is shown as orange. (g) Quantification of TUNEL+ cells conjugated with death-inducing ligand–positive cells. TUNEL+ cells conjugated with TRAIL+, FasL+, or TNF-α+ cells (indicated by arrows in a, b, and c) were counted in five randomly selected visual fields at magnification of 100. Data are expressed as percentage of conjugates among total TUNEL+ cells (mean ± SD of five fields).
To further determine the contribution of TRAIL or FasL to apoptosis of CD4+ T cells, we administered neutralizing anti-TRAIL or anti-FasL mAb to HIV-1–infected hu-PBL-NOD-SCID mice 9 d after infection and counted TUNEL+ cells in the spleen 3 d later. The number of TUNEL+ apoptotic cells in anti-TRAIL mAb–treated mice was markedly decreased as compared with the number in untreated or control IgG–treated mice (Fig. 3 and Table ). In contrast, no significant difference was observed between anti-FasL mAb–treated mice and control IgG–treated mice. These results indicated that TRAIL played a critical role in the apoptotic depletion of human CD4+ T cells in the spleens of HIV-1–infected hu-PBL-NOD-SCID mice.
Figure 3.
Inhibition of apoptosis by anti-TRAIL mAb. HIV-1–infected hu-PBL-NOD-SCID mice were injected intraperitoneally with 1 mg of anti-TRAIL mAb (a), anti-FasL mAb (b), or control mouse IgG (c) 9 d after infection. After 3 d (12 d after infection), spleen sections were stained for apoptotic cells by TUNEL (TRITC, red). Original magnification ×100.
Table 1.
Quantification of TUNEL+ Cells in the Spleens of HIV-1–infected hu-PBL-NOD-SCID Mice after Treatment with Anti–Human TRAIL mAb or Anti–Human FasL mAb
| Mouse | Donor | Treatment | No. TUNEL+ cells |
|---|---|---|---|
| R1 | S | RIK-2 | 42.4 ± 11.2 |
| R2 | S | RIK-2 | 19 ± 3.86 |
| R3 | S | RIK-2 | 17.6 ± 4.01 |
| R4 | S | RIK-2 | 23.8 ± 8.22 |
| R5 | A | RIK-2 | 35.7 ± 10.8 |
| R6 | W | RIK-2 | 27.2 ± 3.71 |
| R7 | W | RIK-2 | 26.3 ± 4.88 |
| R8 | W | RIK-2 | 32.5 ± 3.87 |
| R9 | W | RIK-2 | 35.2 ± 9.89 |
| N1 | S | NOK-1 | 65.9 ± 14.5 |
| N2 | S | NOK-1 | 80.9 ± 8.74 |
| N3 | A | NOK-1 | 65.1 ± 10.5 |
| N4 | A | NOK-1 | 70.6 ± 9.96 |
| N5 | A | NOK-1 | 63.7 ± 9.76 |
| N6 | W | NOK-1 | 77.8 ± 12.8 |
| N7 | W | NOK-1 | 83.1 ± 14.7 |
| N8 | W | NOK-1 | 62.2 ± 14.3 |
| IG1 | S | Control IgG | 78.4 ± 10 |
| IG2 | S | Control IgG | 72.3 ± 9.18 |
| IN1 | A | None | 81.4 ± 10.2 |
| IN2 | A | None | 89.9 ± 15.8 |
Discussion
The mechanism of CD4+ T cell depletion in HIV-1–infected individuals has not been fully elucidated 29. Our study demonstrated that massive apoptosis of HIV-1–uninfected human CD4+ T cells in the spleens of HIV-1–infected hu-PBL-NOD-SCID mice was mainly mediated by TRAIL expressed on adjacent human CD3+CD4+ T cells. As apoptotic depletion of HIV-uninfected CD4+ T cells has also been observed in lymph nodes of HIV-1–infected humans 3, a similar mechanism may be operative in the pathogenesis of AIDS.
An unresolved issue critical for understanding the CD4+ T cell depletion in HIV-1–infected individuals is that the number of HIV-1–infected cells in vivo is significantly low. Previous studies using quantitative PCR analysis have estimated the presence of only one provirus-positive cell among 1,000–20,000 PBLs from HIV-1–infected individuals 30. One reason for the low number of infected cells and severe CD4+ T cell depletion may be the rapid dynamics of HIV-1 replication in infected individuals, in which productively HIV-1–infected CD4+ T cells have a very short half-life and ∼2 × 109 cells die per day 31 32. In addition, it has been postulated that HIV-1 infection strongly forces uninfected CD4+ T cells to die, and a bystander cell killing mechanism has been suggested by histopathological analyses of lymph nodes obtained from HIV-1–infected individuals and SIV-infected monkeys 3. However, the molecular processes of this bystander mechanism are controversial.
It has been reported that peripheral blood CD4+ and CD8+ T cells from HIV-1–infected individuals were highly susceptible to Fas-mediated apoptosis 6 7. Moreover, in vitro exposure of monocytes to HIV-1 has been reported to enhance FasL expression 33 34. However, subsequent studies showed that biological activity of FasL was deficient in freshly isolated PBMCs from HIV-1–infected individuals 11. In this study, we observed substantial numbers of both Fas+ and FasL+ cells in the spleens of HIV-1–infected hu-PBL-NOD-SCID mice, but only a few of these were associated with apoptotic cells (Fig. 2 b). More importantly, administration of neutralizing anti-FasL mAb did not significantly inhibit CD4+ T cell apoptosis (Fig. 3 and Table ). Thus, in our model, FasL was not the principal mediator of the apoptosis.
Based on our findings, it is likely that TRAIL is primarily responsible for apoptosis of bystander CD4+ T cells in HIV-infected lymphoid organs. There remain several issues to be resolved in further studies. First, the mechanism by which HIV-1 infection induces TRAIL expression in CD3+CD4+ T cells remains to be determined. In this study, we found that the number of TRAIL+ cells was consistently higher in HIV-1–infected mice than in uninfected mice. It has been shown that the expression of TRAIL on T cells was induced by a variety of stimuli, including type I IFNs and TCR-mediated signals 13 35 36. Thus, it can be postulated that TRAIL was induced on HIV-1–uninfected CD4+ T cells either by viral or cellular factors produced from HIV-1–infected or uninfected cells in HIV-1–infected lymphoid organs. Second, it is necessary to determine the exact receptor involved in TRAIL-mediated apoptosis. TRAIL has been shown to interact with at least four cell surface receptors on target cells, including two death-inducing receptors (DR4 and DR5) and two decoy receptors (DcR1 and DcR2) 37 38. It has been suggested that the expression of decoy receptors may be responsible for the protection of normal cells from TRAIL-mediated apoptosis 37 39. Therefore, certain factor(s) arising from the virus-infected cells might upregulate the expression of death receptors or downregulate the expression of decoy receptors on uninfected human CD4+ T cells. In addition, certain intracellular mechanism(s) that regulate TRAIL-induced apoptosis, such as the expression of cFLIP (cellular FLICE-inhibitory protein), might also be affected 40. Further studies are necessary to address these questions.
Some experimental advantages were recognized in the hu-PBL-NOD-SCID mouse model of HIV infection used in this study. First, a lymphoid organ–like structure could be reconstituted in the spleens of NOD-SCID mice by CD4+ T cells, CD8+ T cells, B cells, macrophages, and DCs of human origin. Second, massive apoptosis of bystander CD4+ T cells could be reproduced in the spleen after systemic HIV-1 infection. Indeed, this model was appropriate for better analysis of apoptotic events, probably due to macrophage dysfunction in NOD-SCID mice 41. As apoptotic cells are rapidly eliminated by phagocytic function of macrophages in normal animals 42, it is generally difficult to quantify the number of apoptotic cells in vivo by histological methods. As the NOD-SCID mouse has a low level of phagocyte activity, we were able to find many TUNEL+ cells in hu-PBL-NOD-SCID mice. Third, the hu-PBL-NOD-SCID mouse system was suitable for immunohistological analysis of human cells with mouse-derived mAb because of the lack of endogenous mouse Igs. Furthermore, Fc receptor–positive cells in NOD mice have a low affinity for mouse IgG 43, resulting in little nonspecific staining. Finally, the possible contribution of various molecules of interest could be easily tested by the administration of neutralizing mAbs. These advantages of the hu-PBL-NOD-SCID mouse model of HIV-1 infection will aid further elucidation of the mechanisms of CD4+ T cell depletion by HIV-1 infection in infected individuals.
In conclusion, this study has shown that TRAIL-mediated apoptosis is the major mechanism of human CD4+ T cell destruction in the spleens of hu-PBL-NOD-SCID mice after HIV-1 infection. Although this mechanism may not fully account for the loss of CD4+ T cells in HIV-1–infected humans, TRAIL may be a significant target for preventing the progression to AIDS.
Acknowledgments
We thank Professors K. Sugamura, T. Kitamoto, and H. Kudou (Tohoku University) for helpful discussion; H. Tanaka, T. Ohwada, and S. Numata (Tokyo Medical and Dental University) for technical support; and T. Uchihara (Tokyo Metropolitan Institute for Neuroscience), H. Ohtani (Tohoku University School of Medicine), K. Ishikawa and T. Miake (Tokyo Medical and Dental University), and T. Mogi (University of Tsukuba) for critical advice. We thank F.G. Issa for reading and editing the manuscript and M. Miyasaka for providing TMβ-1 mAb.
This work was supported by grants from the Ministry of Health, Labor, and Welfare of Japan. Y. Koyanagi, Y. Tanaka, and N. Yamamoto were sponsored by Priority Areas from the Ministry of Education, Culture, Sports, Science, and Technology, and CREST (Core Research for Evolutional Science and Technology) of Japan Science and Technology Corporation. Y. Koyanagi and N. Yamamoto were also supported by the Program for Promotion of Fundamental Studies in Health Sciences of the Organization for Drug ADR Relief, R&D Promotion and Product Review of Japan.
References
- Douek D.C., McFarland R.D., Keiser P.H., Gage E.A., Massey J.M., Haynes B.F., Polis M.A., Haase A.T., Feinberg M.B., Sullivan J.L. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- Muro-Cacho C.A., Pantaleo G., Fauci A.S. Analysis of apoptosis in lymph nodes of HIV-infected persons. Intensity of apoptosis correlates with the general state of activation of the lymphoid tissue and not with stage of disease or viral burden. J. Immunol. 1995;154:5555–5566. [PubMed] [Google Scholar]
- Finkel T.H., Tudor-Williams G., Banda N.K., Cotton M.F., Curiel T., Monks C., Baba T.W., Ruprecht R.M., Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat. Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- Groux H., Torpier G., Monte D., Mouton Y., Capron A., Ameisen J.C. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus–infected asymptomatic individuals. J. Exp. Med. 1992;175:331–340. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyaard L., Otto S.A., Jonker R.R., Mijnster M.J., Keet R.P., Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992;257:217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- Katsikis P.D., Wunderlich E.S., Smith C.A., Herzenberg L.A., Herzenberg L.A. Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus–infected individuals. J. Exp. Med. 1995;181:2029–2036. doi: 10.1084/jem.181.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestris F., Cafforio P., Frassanito M.A., Tucci M., Romito A., Nagata S., Dammacco F. Overexpression of Fas antigen on T cells in advanced HIV-1 infectiondifferential ligation constantly induces apoptosis. AIDS. 1996;10:131–141. doi: 10.1097/00002030-199602000-00002. [DOI] [PubMed] [Google Scholar]
- Baumler C.B., Bohler T., Herr I., Benner A., Krammer P.H., Debatin K.M. Activation of the CD95 (APO-1/Fas) system in T cells from human immunodeficiency virus type-1-infected children. Blood. 1996;88:1741–1746. [PubMed] [Google Scholar]
- Estaquier J., Tanaka M., Suda T., Nagata S., Golstein P., Ameisen J.C. Fas-mediated apoptosis of CD4+ and CD8+ T cells from human immunodeficiency virus-infected personsdifferential in vitro preventive effect of cytokines and protease antagonists. Blood. 1996;87:4959–4966. [PubMed] [Google Scholar]
- Katsikis P.D., Garcia-Ojeda M.E., Wunderlich E.S., Smith C.A., Yagita H., Okumura K., Kayagaki N., Alderson M., Herzenberg L.A., Herzenberg L.A. Activation-induced peripheral blood T cell apoptosis is Fas independent in HIV-infected individuals. Int. Immunol. 1996;8:1311–1317. doi: 10.1093/intimm/8.8.1311. [DOI] [PubMed] [Google Scholar]
- Sieg S., Smith D., Yildirim Z., Kaplan D. Fas ligand deficiency in HIV disease. Proc. Natl. Acad. Sci. USA. 1997;94:5860–5865. doi: 10.1073/pnas.94.11.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley S.R., Schooley K., Smolak P.J., Din W.S., Huang C.P., Nicholl J.K., Sutherland G.R., Smith T.D., Rauch C., Smith C.A. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- Kayagaki N., Yamaguchi N., Nakayama M., Eto H., Okumura K., Yagita H. Type I interferons (IFNs) regulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression on human T cellsa novel mechanism for the antitumor effects of type I IFNs. J. Exp. Med. 1999;189:1451–1460. doi: 10.1084/jem.189.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith T.S., Wiley S.R., Kubin M.Z., Sedger L.M., Maliszewski C.R., Fanger N.A. Monocyte-mediated tumoricidal activity via the tumor necrosis factor–related cytokine, TRAIL. J. Exp. Med. 1999;189:1343–1353. doi: 10.1084/jem.189.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedger L.M., Shows D.M., Blanton R.A., Peschon J.J., Goodwin R.G., Cosman D., Wiley S.R. IFN-gamma mediates a novel antiviral activity through dynamic modulation of TRAIL and TRAIL receptor expression. J. Immunol. 1999;163:920–926. [PubMed] [Google Scholar]
- Vidalain P.O., Azocar O., Lamouille B., Astier A., Rabourdin-Combe C., Servet-Delprat C. Measles virus induces functional TRAIL production by human dendritic cells. J. Virol. 2000;74:556–559. doi: 10.1128/jvi.74.1.556-559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsikis P.D., Garcia-Ojeda M.E., Torres-Roca J.F., Tijoe I.M., Smith C.A., Herzenberg L.A., Herzenberg L.A. Interleukin-1 beta converting enzyme–like protease involvement in Fas-induced and activation-induced peripheral blood T cell apoptosis in HIV infection. TNF-related apoptosis-inducing ligand can mediate activation-induced T cell death in HIV infection. J. Exp. Med. 1997;186:1365–1372. doi: 10.1084/jem.186.8.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeremias I., Herr I., Boehler T., Debatin K.M. TRAIL/Apo-2-ligand-induced apoptosis in human T cells. Eur. J. Immunol. 1998;28:143–152. doi: 10.1002/(SICI)1521-4141(199801)28:01<143::AID-IMMU143>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Ribas J.L., Burke A., Racz P., Tenner-Racz K., Haase A.T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993;362:359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Demarest J.F., Butini L., Montroni M., Fox C.H., Orenstein J.M., Kotler D.P., Fauci A.S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- Koyanagi Y., Tanaka Y., Kira J., Ito M., Hioki K., Misawa N., Kawano Y., Yamasaki K., Tanaka R., Suzuki Y. Primary human immunodeficiency virus type 1 viremia and central nervous system invasion in a novel hu-PBL-immunodeficient mouse strain. J. Virol. 1997;71:2417–2424. doi: 10.1128/jvi.71.3.2417-2424.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y., Tanaka Y., Misawa N., Tanaka R., Kira J., Kimura T., Fukushi M., Sano K., Goto T., Nakai M. Mutational analysis of human immunodeficiency virus type 1 accessory genesrequirement of a site in the nef gene for HIV-1 replication in activated CD4+ T cells in vitro and in vivo. J. Virol. 1997;71:8456–8466. doi: 10.1128/jvi.71.11.8456-8466.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N., Yamaguchi N., Nakayama M., Kawasaki A., Akiba H., Okumura K., Yagita H. Involvement of TNF-related apoptosis-inducing ligand in human CD4+ T cell-mediated cytotoxicity. J. Immunol. 1999;162:2639–2647. [PubMed] [Google Scholar]
- Seino K., Iwabuchi K., Kayagaki N., Miyata R., Nagaoka I., Matsuzawa A., Fukao K., Yagita H., Okumura K. Chemotactic activity of soluble Fas ligand against phagocytes. J. Immunol. 1998;161:4484–4488. [PubMed] [Google Scholar]
- McLean I.W., Nakane P.K. Periodate-lysine-paraformaldehyde fixative. A new fixative for immunoelectron microscopy. J. Histochem. Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Gendelman H.E., Moench T.R., Narayan O., Griffin D.E. Selection of a fixative for identifying T cell subsets, B cells, and macrophages in paraffin-embedded mouse spleen. J. Immunol. Methods. 1983;65:137–145. doi: 10.1016/0022-1759(83)90310-1. [DOI] [PubMed] [Google Scholar]
- Sabattini E., Bisgaard K., Ascani S., Poggi S., Piccioli M., Ceccarelli C., Pieri F., Fraternali-Orcioni G., Pileri S.A. The EnVision++ systema new immunohistochemical method for diagnostics and research. Critical comparison with the APAAP, ChemMate, CSA, LABC, and SABC techniques. J. Clin. Pathol. 1998;51:506–511. doi: 10.1136/jcp.51.7.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strappe P.M., Wang T.H., McKenzie C.A., Lowrie S., Simmonds P., Bell J.E. Enhancement of immunohistochemical detection of HIV-1 p24 antigen in brain by tyramide signal amplification. J. Virol. Methods. 1997;67:103–112. doi: 10.1016/s0166-0934(97)00083-9. [DOI] [PubMed] [Google Scholar]
- Fauci A.S., Desrosiers R.C. Pathogenesis of HIV and SIV. In: Coffin J.M., Hughes S.H., Varmus H.E., editors. Retroviruses. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1997. pp. 587–636. [PubMed] [Google Scholar]
- Schnittman S.M., Psallidopoulos M.C., Lane H.C., Thompson L., Baseler M., Massari F., Fox C.H., Salzman N.P., Fauci A.S. The reservoir for HIV-1 in human peripheral blood is a T cell that maintains expression of CD4. Science. 1989;245:305–308. doi: 10.1126/science.2665081. [DOI] [PubMed] [Google Scholar]
- Ho D.D., Neumann A.U., Perelson A.S., Chen W., Leonard J.M., Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- Wei X., Ghosh S.K., Taylor M.E., Johnson V.A., Emini E.A., Deutsch P., Lifson J.D., Bonhoeffer S., Nowak M.A., Hahn B.H. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- Badley A.D., McElhinny J.A., Leibson P.J., Lynch D.H., Alderson M.R., Paya C.V. Upregulation of Fas ligand expression by human immunodeficiency virus in human macrophages mediates apoptosis of uninfected T lymphocytes. J. Virol. 1996;70:199–206. doi: 10.1128/jvi.70.1.199-206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorp M.O., Frank R., Ochsenbauer C., Stricker K., Dhein J., Walczak H., Debatin K.M., Krammer P.H. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- Martinez-Lorenzo M.J., Anel A., Gamen S., Monleón I., Lasierra P., Larrad L., Pineiro A., Alava M.A., Naval J. Activated human T cells release bioactive Fas ligand and APO2 ligand in microvesicles. J. Immunol. 1999;163:1274–1281. [PubMed] [Google Scholar]
- Musgrave B.L., Phu T., Butler J.J., Makrigiannis A.P., Hoskin D.W. Murine TRAIL (TNF-related apoptosis inducing ligand) expression induced by T cell activation is blocked by rapamycin, cyclosporin A, and inhibitors of phosphatidylinositol 3-kinase, protein kinase C, and protein tyrosine kinasesevidence for TRAIL induction via the T cell receptor signaling pathway. Exp. Cell. Res. 1999;252:96–103. doi: 10.1006/excr.1999.4631. [DOI] [PubMed] [Google Scholar]
- Pan G., Ni J., Wei Y.-F., Yu G.-L., Gentz R., Dixit V.M. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- Walczak H., Degli-Esposti M.A., Johnson R.S., Smolak P.J., Waugh J.Y., Boiani N., Timour M.S., Gerhart M.J., Schooley K.A., Smith C.A. TRAIL-R2a novel apoptosis-mediating receptor for TRAIL. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:5386–5397. doi: 10.1093/emboj/16.17.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan J.P., Marsters S.A., Pitti R.M., Gurney A., Skubatch M., Baldwin D., Ramakrishnan L., Gray C.L., Baker K., Wood W.I. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- Leverkus M., Neumann M., Mengling T., Rauch C.T., Bröcker E.B., Krammer P.H., Walczak H. Regulation of TRAIL sensitivity in primary and transformed human keratinocytes. Cancer Res. 2000;60:553–559. [PubMed] [Google Scholar]
- Serreze D.V., Gaedeke J.W., Leiter E.H. Hematopoietic stem-cell defects underlying abnormal macrophage development and maturation in NOD/Lt micedefective regulation of cytokine receptors and protein kinase C. Proc. Natl. Acad. Sci. USA. 1993;90:9625–9629. doi: 10.1073/pnas.90.20.9625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok V.A., Voelker D.R., Campbell P.A., Cohen J.J., Bratton D.L., Henson P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- Prins J.B., Todd J.A., Rodrigues N.R., Ghosh S., Hogarth P.M., Wicker L.S., Gaffney E., Podolin P.L., Fischer P.A., Sirotina A. Linkage on chromosome 3 of autoimmune diabetes and defective Fc receptor for IgG in NOD mice. Science. 1993;260:695–698. doi: 10.1126/science.8480181. [DOI] [PubMed] [Google Scholar]