Abstract
Both nuclear factor (NF)-κB–inducing kinase (NIK) and inhibitor of κB (IκB) kinase (IKK) have been implicated as essential components for NF-κB activation in response to many external stimuli. However, the exact roles of NIK and IKKα in cytokine signaling still remain controversial. With the use of in vivo mouse models, rather than with enforced gene-expression systems, we have investigated the role of NIK and IKKα in signaling through the type I tumor necrosis factor (TNF) receptor (TNFR-I) and the lymphotoxin β receptor (LTβR), a receptor essential for lymphoid organogenesis. TNF stimulation induced similar levels of phosphorylation and degradation of IκBα in embryonic fibroblasts from either wild-type or NIK-mutant mice. In contrast, LTβR stimulation induced NF-κB activation in wild-type mice, but the response was impaired in embryonic fibroblasts from NIK-mutant and IKKα-deficient mice. Consistent with the essential role of IKKα in LTβR signaling, we found that development of Peyer's patches was defective in IKKα-deficient mice. These results demonstrate that both NIK and IKKα are essential for the induction of NF-κB through LTβR, whereas the NIK–IKKα pathway is dispensable in TNFR-I signaling.
Keywords: alymphoplasia, cytokine signaling, IκB, Akt kinase, Peyer's patch
Introduction
The transcription factor nuclear factor (NF)-κB plays a pivotal role in the regulation of innate immunity, stress responses, inflammation, and the inhibition of apoptosis 1 2. The activity of NF-κB is tightly regulated by cytokines and other external stimuli. In most cell types, NF-κB is present as a heterodimer comprising 50-kD (p50) and 65-kD (p65) subunits and is sequestered in the cytoplasm by a member of the inhibitor of κB (IκB) family of inhibitory proteins. NF-κB activation requires the degradation of IκB proteins, and the mechanisms of IκB degradation and subsequent NF-κB activation have been the subject of intense investigation 3. Those studies have revealed two important classes of kinase involved in this pathway: mitogen-activated protein kinase kinase kinase (MAP3K) and its downstream target, IκB kinase (IKK) 4 5. NF-κB–inducing kinase (NIK) is structurally related to MAP3K and has been identified as a TNFR-associated factor (TRAF)2-interacting protein 6. On the basis of the finding that kinase-inactive mutants of NIK transfected into 293-EBNA cells abolished NF-κB activation in response to TNF or by cotransfection with type I TNF receptor (TNFR-I), NIK was considered to be involved in an NF-κB–inducing signaling cascade induced by TNF 6. Subsequently, NIK was demonstrated to phosphorylate IKKα and IKKβ, which directly associate with IκBα and specifically phosphorylate it on serines 32 and 36 4 5. These studies suggested that interaction of NIK and IKK constitutes an essential step for NF-κB activation. However, in vivo studies with mutant mice have thrown some doubt on the essential roles of NIK and IKKα in NF-κB activation, at least as induced by TNF. The alymphoplasia (aly) mouse is a natural strain with a mutated NIK 7. Despite the NIK mutation, upregulation of vascular cell adhesion molecule 1 (VCAM-1) after stimulation with TNF was present in aly mouse embryonic fibroblasts (EFs) 8. It was also reported that aly mice exhibited similar TNF-mediated endotoxin shock after generalized LPS administration 7. These observations suggested that NIK was not a critical element in the TNF signaling pathway to NF-κB activation. The in vivo role of IKK in NF-κB activation was also examined using gene-targeted mice. Although TNF-induced NF-κB activation was markedly reduced in IKKβ-deficient EFs 9 10, NF-κB activation from IKKα-deficient EFs was normal 11 12 or diminished but still present 13 after stimulation with TNF. Surprisingly, mice deficient in IKKα showed perinatal death associated with limb and skin abnormalities, suggesting that IKKα plays an essential role in the regulation of gene expression required for the development of limb and skin rather than for TNF signaling 11 12 13. Thus, the role of NIK–IKKα in NF-κB activation through TNFR signaling requires further investigation.
The lymphotoxin β receptor (LTβR) has emerged as a signaling system required for the development of lymphoid organs 14 15. Although LTβR has been shown to bind TRAF2, -3, -4, and -5, but not TRAF6 16 17, and to activate NF-κB after receptor ligation 18, the molecular mechanisms by which LTβR exerts its biological activities are still poorly understood. aly mice and LT ligand– or LTβR-deficient mice share a unique phenotype, which includes the lack of LN and Peyer's patches (PPs) and a disturbed splenic architecture 7 14 15. Therefore, we speculated that NIK plays a role in LTβR signaling. This hypothesis was supported by the demonstration that upregulation of VCAM-1 after stimulation with agonistic anti-LTβR mAb was absent from aly mouse EFs 8.
Phenotypic analyses of mutant and gene-targeted mice, as described above, have unveiled the essential roles of NIK in lymphoid organogenesis and of IKKα in limb and skin development. However, the roles of NIK and IKKα in cytokine signaling still remain controversial. We have approached this question with the use of in vivo mouse models. Here, we show that NIK–IKKα constitutes an essential pathway for the induction of NF-κB through LTβR, whereas this pathway is dispensable in TNFR-I signaling.
Materials and Methods
Mice.
aly/+, aly/aly, and C57BL/6J mice were purchased from CLEA Japan. The mice were maintained under pathogen-free conditions and were handled in accordance with the Guidelines for Animal Experimentation of Tokushima University School of Medicine. The experiments were initiated at 8–12 wk of age. IKKα-deficient mice were generated by gene targeting as described previously and maintained at Osaka University 11.
Use of EF to Assess Signaling through TNFR-I and LTβR.
EFs were established as described previously 8 11. EFs from aly/aly mice, IKKα-deficient mice, and C57BL/6J wild-type mice were cultured in DMEM (GIBCO BRL) supplemented with 10% heat-inactivated FCS (GIBCO BRL), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin at a density of 7 × 105 cells per 60-mm culture dish. After incubation with control mAb Ha4/8 (2 μg/ml), agonistic anti-LTβR mAb AC.H6 (2 μg/ml; reference 19), or recombinant human TNF (Genzyme, Inc.), whole cell lysates were harvested from the dish with a lysis buffer containing 1% NP-40 (Sigma-Aldrich) and subjected to Western blot analysis as described previously 20. The following Abs were used: rabbit antipeptide Ab directed against IκBα (cat. no. sc-371; Santa Cruz Biotechnology), phospho-specific IκBα (cat. no. 9241; New England Biolabs), and polyclonal rabbit Ab against actin (Biomedical Technologies). For blockade of the phosphatidylinositol-3′-OH kinase (PI[3]K)–Akt pathway, EFs were treated with 1 μM wortmannin (Calbiochem) for 30 min before stimulation with TNF. For blockade of proteasome activity, EFs were treated with 100 μM N-acetyl-Leu-Leu-norleucinal (ALLN; Nacalai Tesque) for 1 h.
Use of NF-κB Reporter Assay to Assess Signaling through TNFR-I and LTβR.
EFs cultured in a 35-mm culture dish (2 × 105 cells) were transfected with 2 μg of a reporter plasmid comprising three repeats of the NF-κB site upstream of a minimal thymidine kinase promoter and a luciferase gene in the pGL-2 vector (Promega), together with 2 μg of β-actin promoter–driven β-galactosidase expression plasmid. Transfected cells were incubated in the presence of recombinant human TNF (100 U/ml) or agonistic anti-LTβR mAb AC.H6 for 8 h. After 24 h, the cells were harvested in PBS and lysed in a luciferase lysis buffer, LC-β (Piccagene). Luciferase assays were performed with a luminometer (Lumat LB 9507; Berthold). Activity was normalized to β-galactosidase activity, and data were expressed as the fold activation compared with stimulation by control anti-KLH hamster mAb Ha4/8.
Assessment of LTβR Expression on EFs with Flow-cytometric Analysis.
EFs were incubated with anti-LTβR mAb AF.H6 19 or control mAb Ha4/8. After washing twice with PBS, cells were incubated with FITC-conjugated anti–hamster IgG mAb (clone G94-56; PharMingen). Cells were analyzed with a FACSCaliber™ flow cytometer (Becton Dickinson) with CELLQuest™ software. Mouse mAb–producing hybridoma cells were used as negative control.
Assessment of PP Formation with Whole-Mount Immunohistochemistry.
Whole-mount immunohistochemistry for the detection of PP was performed as described 21. In brief, 2% paraformaldehyde (pH 7.4)-fixed intestines from 18.5 days postcoitus (d.p.c.) embryos were incubated with mAb against VCAM-1 (PharMingen) and then with horseradish peroxidase (HRP)-conjugated anti–rat Ig (Tago Immunologicals). Color development for bound HRP was done with diaminobenzidine.
Assessment of Association between NIK and IKKα.
Proteins were expressed by transfecting expression constructs with the indicated cDNAs into COS-7 cells. Extracts were prepared 30 h after transfection. Immunoprecipitation and Western blot analysis was performed as described previously 20 22. Full-sized, Flag-tagged wild-type NIK 6, Flag-tagged aly-type NIK, and Myc-tagged IKKα 22 were expressed in pCR3 vectors (Invitrogen); aly-type NIK cDNA was generated by the introduction of an amino acid substitution (G860R) into the COOH-terminal region of human NIK 7 by site-directed mutagenesis. Anti-Flag mAb (clone M2) and anti-Myc mAb (clone 9E10) were from Sigma-Aldrich and Santa Cruz Biotechnology, respectively.
Results and Discussion
Retained TNFR-I Signaling in NIK-mutant EFs.
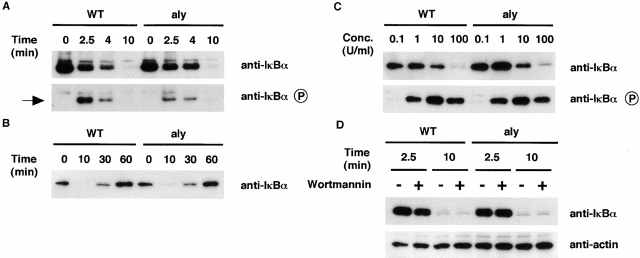
NIK was originally identified as a kinase that participates in an NF-κB–inducing signaling cascade induced by TNF, CD95, and IL-1 6. To assess the impact of NIK mutation in TNF responsiveness, we treated EFs from both wild-type and aly mice with human TNF, which signals only through mouse TNFR-I 23, and assessed NF-κB activation by Western blot analysis. Rapid IκBα degradation concomitant with the appearance of phosphorylated IκBα was observed with similar kinetics in EFs from both wild-type and aly mice (Fig. 1 A). 30 min after stimulation with TNF, IκBα started to recover similarly in both wild-type and aly mice (Fig. 1 B). IκBβ degradation in response to TNF was also indistinguishable between wild-type and aly mice (data not shown). We also tested TNF responsiveness by titrating the TNF concentration between 0.1 and 100 U/ml. In this range, TNF sensitivity assessed by IκBα degradation, and IκBα phosphorylation was indistinguishable between wild-type and aly mice (Fig. 1 C). Using an NF-κB–binding oligonucleotide probe in an electrophoretic mobility shift assay (EMSA), we also observed a very similar level of NF-κB activation between wild-type and aly mice in TNF-stimulated EFs or TNF-stimulated thymocytes (data not shown). Furthermore, IL-1 and IL-6 production from TNF-stimulated EFs was indistinguishable between wild-type and aly mice (data not shown). These results demonstrate that NF-κB activation through TNFR-I is not affected by the NIK mutation.
Figure 1.
NF-κB activation in response to TNF is retained in EFs from NIK-mutant mice. EFs from wild-type mice (WT) and aly mice (aly) were stimulated with TNF (100 U/ml), and cells were harvested at the indicated time points (A and B). IκBα degradation (detected by anti-IκBα Ab) and IκBα phosphorylation (detected by phospho-specific anti-IκBα Ab) was assessed by Western blot analysis. Arrow indicates phosphorylated IκBα (A). EFs were stimulated with different concentrations (Conc.) of TNF ranging from 0.1 to 100 U/ml as indicated, and cells were harvested 7 min after stimulation (C). EFs were stimulated with TNF (100 U/ml) with or without prior treatment with 1 μM wortmannin (D). The same blot was probed with anti-actin Ab (bottom).
Recently, it was demonstrated that Akt serine–threonine kinase is involved in the activation of NF-κB by TNF 24. Although the results described above do not suggest a role for NIK in TNFR-I signaling, it is possible that NIK plays an important role in NF-κB activation in combination with Akt. We therefore tested the combined effect of NIK and Akt in the NF-κB–inducing pathway downstream of the TNFR-I. IκBα degradation occurred 10 min after TNF stimulation in wild-type EFs, even in the presence of 1 μM wortmannin, a sufficient concentration for blockade of the PI(3)K–Akt pathway (references 24 and 25; Fig. 1 D). This suggests that Akt, the downstream target of PI(3)K, by itself has no major role in NF-κB activation by TNF. Additionally, no obvious effect of wortmannin on IκBα degradation was observed in aly mouse EFs, indicating that NF-κB activation by TNF can occur even when the functions of both NIK and Akt are inhibited. We failed to observe phosphorylation of Akt in response to TNF when we probed the same blot with phospho-specific anti-Akt Ab in this experimental setting (data not shown). These results suggest that neither NIK nor Akt is essential for NF-κB activation by TNF and that other IKK kinase(s) can substitute for NIK and Akt in NF-κB activation by TNF, at least in EFs. Elucidation of the IKK kinase(s) that activates IKK in response to TNF awaits further study.
Indispensable Role of NIK and IKKα in NF-κB Activation through LTβR.
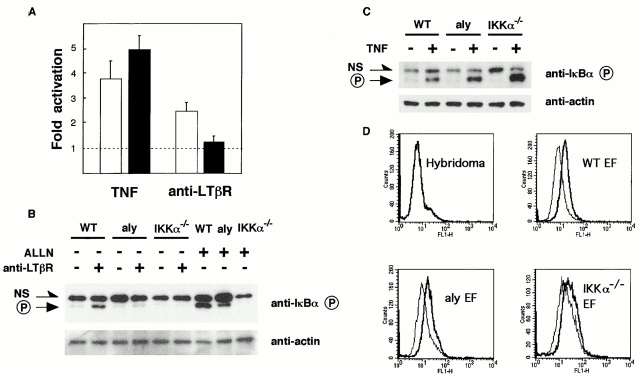
We intended to evaluate the role of NIK in TNFR-I and LTβR signaling with the use of in vivo mouse models, rather than introducing enforced gene-expression systems. To this end, we transfected EFs (which express both TNFR-I and LTβR) only with a reporter plasmid that has three repeats of the NF-κB site upstream of a minimal thymidine kinase promoter and a luciferase gene, and then stimulated the transfected EFs with TNF or agonistic anti-LTβR mAb (AC.H6). EFs from both wild-type and aly mice showed upregulation of luciferase activity in response to human TNF (Fig. 2 A). The normal level of TNF responsiveness in aly mice is consistent with the results shown above (Fig. 1). In contrast to TNF stimulation, NF-κB activation in response to agonistic anti-LTβR mAb was significantly reduced in aly mouse EFs, indicating that NIK is involved in LTβR signaling (Fig. 2 A).
Figure 2.
Impaired NF-κB activation in response to LTβR stimulation in NIK-mutant and IKKα-deficient EFs. Wild-type EFs and aly mouse EFs were transfected with NF-κB reporter plasmids and stimulated with TNF or agonistic anti-LTβR mAb. 8 h later, NF-κB activation was assessed by the measurement of luciferase activities. Data were expressed as fold activation compared with stimulation by control mAb, and the results were plotted as the mean ± SEM for a total of four independent experiments. White and black bars represent wild-type EFs and aly mouse EFs, respectively (A). EFs from wild-type, aly, and IKKα-deficient mice (IKKα−/−) were stimulated with agonistic anti-LTβR mAb for 1 h, and IκBα phosphorylation was assessed by Western blot analysis (B, top). For the assessment of the basal level of NF-κB activation, EFs were treated with ALLN alone. Phosphorylated IκBα is indicated as P. NS, nonspecific bands. The same blot was probed with anti-actin Ab (B, bottom). TNF stimulation induced IκBα phosphorylation in IKKα-deficient EFs as in wild-type and aly EFs (C). LTβR expression assessed by flow-cytometric analysis with anti-LTβR mAb (D, thick line) was similar among wild-type, aly, and IKKα-deficient EFs (D). Anti-KLH mAb Ha4/8 (D, thin line) and mouse hybridoma cells (top left) were used as negative control.
With the combination of EFs and agonistic anti-LTβR mAb, signals for NF-κB activation assessed by EMSA were not strong enough to evaluate the role of NIK in NF-κB activation through the LTβR (our unpublished observation). Involvement of NIK in LTβR signaling was therefore examined by detection of IκBα phosphorylation in response to agonistic anti-LTβR mAb. In wild-type EFs stimulated with agonistic anti-LTβR mAb for 1 h, IκBα phosphorylation was easily detected by Western blot analysis (Fig. 2 B). In contrast, aly mouse EFs showed minimal, if any, phosphorylated IκBα after LTβR stimulation. Taken together, these results demonstrate that NIK is essential for NF-κB activation in LTβR signaling, which accounts for the abnormal lymphoid organogenesis in aly mice.
We have previously demonstrated that EFs isolated from IKKα-deficient mice can activate NF-κB in response to TNF and IL-1, suggesting that IKKα is not essential for either the TNF or IL-1 signaling pathways 11. The dispensable role of IKKα in NF-κB activation through TNFR-I was also confirmed by the detection of phosphorylated IκBα in IKKα-deficient EFs after TNF stimulation (Fig. 2 C). Because NIK is essential for LTβR signaling, as demonstrated above, and NIK has been shown to phosphorylate IKKα 26, it is important to determine whether IKKα is also essential for LTβR signaling. We therefore treated EFs from IKKα-deficient mice with agonistic anti-LTβR mAb and assessed NF-κB activation by the detection of phosphorylated IκBα. We found that IKKα-deficient EFs showed no IκBα phosphorylation after LTβR stimulation, suggesting that NIK–IKKα constitutes an important pathway in LTβR signaling (Fig. 2 B). LTβR expression assessed by flow-cytometric analysis with anti-LTβR mAb (AF.H6) was similar among wild-type, aly, and IKKα-deficient EFs (Fig. 2 D). The basal level of NF-κB activation, assessed by the treatment of EFs with ALLN alone, which blocks the degradation of phosphorylated IκBα by proteasomes 3, was reduced in aly mice and reduced more profoundly in IKKα-deficient mice (Fig. 2 B).
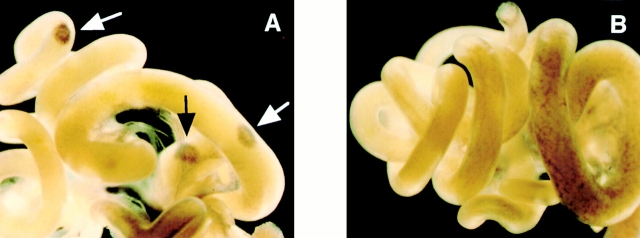
The essential role of IKKα in LTβR signaling was examined by investigation of lymphoid organogenesis in IKKα-deficient mice. Because IKKα-deficient mice show perinatal death associated with abnormal limb and skin development, lymphoid organogenesis in IKKα-deficient mice was assessed by the development of PP from 18.5 d.p.c. embryos. PP formation in control embryos (n = 8) was easily detected by whole-mount immunohistochemistry with mAb against VCAM-1 (Fig. 3 A); VCAM-1+ cells accumulate at the site of PP development starting from 15.5 d.p.c. and can be used as a stromal marker for PP formation 21. In contrast, no PP formation was detected in intestines isolated from IKKα-deficient embryos (n = 7; Fig. 3 B). A similar lack of VCAM-1+ cell accumulation in embryonic intestines has been demonstrated in aly mice 21. This result shows that LTβR signaling is fundamentally impaired in IKKα-deficient mice. Together, these findings are important in providing clear evidence that IKKα is involved in cytokine receptor signaling in vivo.
Figure 3.
Lack of PP development in IKKα-deficient mice. Embryonic intestines isolated from control littermates (A) and IKKα-deficient mice (B) were stained with anti–VCAM-1 mAb. Arrows indicate the sites of PPs. Original magnification, ×10.
It is important to note that abnormal lymphoid organogenesis in IKKα-deficient mice is not due to defective receptor activator of NF-κB (RANK) signaling, because mice deficient in RANK have PPs despite their lack of peripheral LNs 27; RANK activates NF-κB by recruiting TRAF6, which has not been observed to associate with LTβR 16 17. It remains possible, however, that there exist other undefined NIK–IKKα-activating receptor pathways involved in lymphoid organogenesis beyond LTβR.
The above data strongly suggest that NIK and IKKα together control LTβR signaling with a close mechanistic relationship in their pathway. We have therefore reasoned that impaired LTβR signaling in aly mice may be due to defective interaction between mutated NIK and IKKα. To investigate this, NIK and IKKα were coexpressed in COS-7 cells, and protein interactions were assessed by immunoprecipitation. Association of wild-type NIK with IKKα was easily detected (Fig. 4). In contrast, association of aly type NIK, which corresponds to a G855R substitution in mice, with IKKα was disrupted by the mutation, providing further support for the role of NIK–IKKα as an essential pathway for NF-κB activation in LTβR signaling. Despite this finding, the possibility remains that aly mice might have a different phenotype from NIK-null mutation mice.
Figure 4.
Disruption of interaction with IKKα by the aly-type NIK mutation. Protein extracts from COS-7 cells transfected with the indicated cDNAs were immunoprecipitated with anti-Myc mAb and detected with either anti-Flag mAb (top) or anti-Myc mAb (center). Expression of NIK was verified by Western blot analysis of total cell lysates with anti-Flag mAb (bottom). aly-type NIK (aly) has an amino acid substitution (G860R) in the COOH-terminal region. Minus (−) indicates transfection with empty vectors.
It is unclear whether TRAFs mediate all of the signaling activities of LTβR. In fact, mice deficient in TRAF2, -3, or -5 show LN development 28 29. In support of the dispensable role of TRAFs in LTβR signaling, recent mutational analyses of the cytoplasmic region of LTβR have demonstrated a TRAF-independent mechanism of NF-κB activation through LTβR 30. Consistent with this idea, TRAF–NIK interaction in COS-7 cells as assessed by immunoprecipitation was not affected by the aly mutation (our unpublished observation), although the aly mutation resides in a putative TRAF-binding domain of NIK 31. Elucidation of the molecular mechanisms by which NIK becomes activated after LTβR stimulation will be critical for understanding the biological nature of LTβR signaling.
Acknowledgments
We thank Drs. J.L. Browning, D. Wallach, T. Takemori, and K. Miyazono for providing us with reagents. We thank Drs. K. Honda, M. Shono, S. Noji, H. Ohuchi, T. Matsuzaki, K. Tsuchida, Z.-g. Liu, and C.F. Ware for valuable suggestions. We also thank Ms. M. Kimura for technical assistance.
This work was supported in part by Special Coordination Funds and Grant-in-Aid for Scientific Research of the Ministry of Education, Culture, Sports, Science, and Technology, the Japanese Government, and by the Japan Research Foundation for Clinical Pharmacology.
References
- Ghosh S., May M.J., Kopp E.B. NF-κB and Rel proteinsevolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Van Antwerp D.J., Martin S.J., Verma I.M., Green D.R. Inhibition of TNF-induced apoptosis by NF-κB. Trends Cell Biol. 1998;8:107–111. doi: 10.1016/s0962-8924(97)01215-4. [DOI] [PubMed] [Google Scholar]
- Baldwin A.S. The NF-κB and IκB proteinsnew discoveries and insights. Annu. Rev. Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Maniatis T. Catalysis by a multiprotein IκB kinase complex. Science. 1997;278:818–819. doi: 10.1126/science.278.5339.818. [DOI] [PubMed] [Google Scholar]
- Stancovski I., Baltimore D. NF-κB activationthe IκB kinase revealed? Cell. 1997;91:299–302. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- Malinin N.L., Boldin M.P., Kovalenko A.V., Wallach D. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- Shinkura R., Kitada K., Matsuda F., Tashiro K., Ikuta K., Suzuki M., Kogishi K., Serikawa T., Honjo T. Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-κb-inducing kinase. Nat. Genet. 1999;22:74–77. doi: 10.1038/8780. [DOI] [PubMed] [Google Scholar]
- Matsumoto M., Iwamasa K., Rennert P.D., Yamada T., Suzuki R., Matsushima A., Okabe M., Fujita S., Yokoyama M. Involvement of distinct cellular compartments in the abnormal lymphoid organogenesis in lymphotoxin-α-deficient mice and alymphoplasia (aly) mice defined by the chimeric analysis. J. Immunol. 1999;163:1584–1591. [PubMed] [Google Scholar]
- Li Q., Van Antwerp D., Mercurio F., Lee K.F., Verma I.M. Severe liver degeneration in mice lacking the IκB kinase 2 gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Fuentes M.E., Yamaguchi K., Durnin M.H., Dalrymple S.A., Hardy K.L., Goeddel D.V. Embryonic lethality, liver degeneration, and impaired NF-κB activation in IKK-β-deficient mice. Immunity. 1999;10:421–429. doi: 10.1016/s1074-7613(00)80042-4. [DOI] [PubMed] [Google Scholar]
- Takeda K., Takeuchi O., Tsujimura T., Itami S., Adachi O., Kawai T., Sanjo H., Yoshikawa K., Terada N., Akira S. Limb and skin abnormalities in mice lacking IKKα. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- Hu Y., Baud V., Delhase M., Zhang P., Deerinck T., Ellisman M., Johnson R., Karin M. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKα subunit of IκB kinase. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- Li Q., Lu Q., Hwang J.Y., Buscher D., Lee K.F., Izpisua-Belmonte J.C., Verma I.M. IKK1-deficient mice exhibit abnormal development of skin and skeleton. Genes Dev. 1999;13:1322–1328. doi: 10.1101/gad.13.10.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M., Fu Y.-X., Molina H., Chaplin D.D. Lymphotoxin-α-deficient and TNF receptor-I-deficient mice define developmental and functional characteristics of germinal centers. Immunol. Rev. 1997;156:137–144. doi: 10.1111/j.1600-065x.1997.tb00965.x. [DOI] [PubMed] [Google Scholar]
- Fütterer A., Mink K., Luz A., Kosco-Vilbois M.H., Pfeffer K. The lymphotoxin β receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- Nakano H., Oshima H., Chung W., Williams-Abbott L., Ware C.F., Yagita H., Okumura K. TRAF5, an activator of NF-κB and putative signal transducer for the lymphotoxin-β receptor. J. Biol. Chem. 1996;271:14661–14664. doi: 10.1074/jbc.271.25.14661. [DOI] [PubMed] [Google Scholar]
- VanArsdale T.L., VanArsdale S.L., Force W.R., Walter B.N., Mosialos G., Kieff E., Reed J.C., Ware C.F. Lymphotoxin-β receptor signaling complexrole of tumor necrosis factor receptor-associated factor 3 recruitment in cell death and activation of nuclear factor κB. Proc. Natl. Acad. Sci. USA. 1997;94:2460–2465. doi: 10.1073/pnas.94.6.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay F., Majeau G.R., Hochman P.S., Browning J.L. Lymphotoxin β receptor triggering induces activation of the nuclear factor κB transcription factor in some cell types. J. Biol. Chem. 1996;271:24934–24938. doi: 10.1074/jbc.271.40.24934. [DOI] [PubMed] [Google Scholar]
- Rennert P.D., James D., Mackay F., Browning J.L., Hochman P.S. Lymph node genesis is induced by signaling through the lymphotoxin β receptor. Immunity. 1998;9:71–79. doi: 10.1016/s1074-7613(00)80589-0. [DOI] [PubMed] [Google Scholar]
- Yamada T., Mitani T., Yorita K., Uchida D., Matsushima A., Iwamasa K., Fujita S., Matsumoto M. Abnormal immune function of hemopoietic cells from alymphoplasia (aly) mice, a natural strain with mutant NF-κB-inducing kinase. J. Immunol. 2000;165:804–812. doi: 10.4049/jimmunol.165.2.804. [DOI] [PubMed] [Google Scholar]
- Adachi S., Yoshida H., Honda K., Maki K., Saijo K., Ikuta K., Saito T., Nishikawa S.-I. Essential role of IL-7 receptor α in the formation of Peyer's patch anlage. Int. Immunol. 1998;10:1–6. doi: 10.1093/intimm/10.1.1. [DOI] [PubMed] [Google Scholar]
- Nakano H., Shindo M., Sakon S., Nishinaka S., Mihara M., Yagita H., Okumura K. Differential regulation of IκB kinase α and β by two upstream kinases, NF-κB-inducing kinase and mitogen-activated protein kinase/ERK kinase kinase-1. Proc. Natl. Acad. Sci. USA. 1998;95:3537–3542. doi: 10.1073/pnas.95.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M., Tartaglia L.A., Lee A., Bennett G.L., Rice G.C., Wong G.H., Chen E.Y., Goeddel D.V. Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. Proc. Natl. Acad. Sci. USA. 1991;88:2830–2834. doi: 10.1073/pnas.88.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozes O.N., Mayo L.D., Gustin J.A., Pfeffer S.R., Pfeffer L.M., Donner D.B. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- Delhase M., Li N., Karin M. Kinase regulation in inflammatory response. Nature. 2000;406:367–368. doi: 10.1038/35019154. [DOI] [PubMed] [Google Scholar]
- Ling L., Cao Z., Goeddel D.V. NF-κB-inducing kinase activates IKK-α by phosphorylation of Ser-176. Proc. Natl. Acad. Sci. USA. 1998;95:3792–3797. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougall W.C., Glaccum M., Charrier K., Rohrbach K., Brasel K., De Smedt T., Daro E., Smith J., Tometsko M.E., Maliszewski C.R. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh W.C., Shahinian A., Speiser D., Kraunus J., Billia F., Wakeham A., de la Pompa J.L., Ferrick D., Hum B., Iscove N. Early lethality, functional NF-κB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity. 1997;7:715–725. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- Nakano H., Sakon S., Koseki H., Takemori T., Tada K., Matsumoto M., Munechika E., Sakai T., Shirasawa T., Akiba H. Targeted disruption of Traf5 gene causes defects in CD40- and CD27-mediated lymphocyte activation. Proc. Natl. Acad. Sci. USA. 1999;96:9803–9808. doi: 10.1073/pnas.96.17.9803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Force W.R., Glass A.A., Benedict C.A., Cheung T.C., Lama J., Ware C.F. Discrete signaling regions in the lymphotoxin-β receptor for tumor necrosis factor receptor-associated factor binding, subcellular localization, and activation of cell death and NF-κB pathways. J. Biol. Chem. 2000;275:11121–11129. doi: 10.1074/jbc.275.15.11121. [DOI] [PubMed] [Google Scholar]
- Lin X., Mu Y., Cunningham E.T., Jr., Marcu K.B., Geleziunas R., Greene W.C. Molecular determinants of NF-κB-inducing kinase action. Mol. Cell Biol. 1998;18:5899–5907. doi: 10.1128/mcb.18.10.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]