Abstract
T helper cell (Th)1-primed CD4 T cells from wild-type donors make little interleukin (IL)-4 when restimulated under Th2 conditions. However, such restimulation of Th1-primed cells from interferon (IFN)-γ2/− or IFN-γ receptor (IFN-γR)−/− mice resulted in substantial production of IL-4 and other Th2 cytokines. Adding IFN-γ to the priming culture markedly diminished the capacity of Th1-primed IFN-γ2/− cells to express IL-4. Even IFN-γ–producing cells from IFN-γR−/− mice could acquire IL-4–producing capacity. Thus, IFN-γ is not required for the development of IFN-γ–producing capacity, but it plays a critical role in suppressing the IL-4–producing potential of Th1 cells.
Keywords: IL-4, IL-12, differentiation, commitment, cell cloning
Introduction
Undesired immune responses mediated by polarized Th cells often mediate autoimmune, allergic, and infectious diseases 1. Altering the cytokine-producing potential of polarized Th cells has been proposed as a potential therapeutic intervention for many of these diseases. However, the phenotype of polarized Th cells is generally difficult to alter. In particular, well-polarized Th1 cells fail to produce IL-4 even after restimulation under Th2-polarizing conditions; such stability is seen for periods of months to years after initial priming 2 3 4 5. Understanding the factors that contribute to the inability of Th1 cells to express IL-4 may provide approaches to overcome this barrier.
Th1 and Th2 polarization in vitro have been well characterized. When TCRs of naive CD4+ T cells are first engaged, IL-4 is the major determinant of differentiation of these cells into Th2 cells 6 7 8 9. IL-4 mediates its functions through binding to and stimulating the IL-4 receptor (IL-4R). Upon interaction with IL-4, the IL-4Rα and γc-associated kinases, Janus kinase (Jak)1 and Jak3, are activated 10 11. Activated Jak1 and Jak3 then phosphorylate signal transducer and activator of transcription (STAT)6, which is essential for initiating IL-4–induced gene transcription and Th2 polarization 12.
Two STAT6-dependent, Th2-specific transcription factors, GATA3 and c-maf, have been cloned 13 14. GATA3 is expressed at low levels in naive CD4 T cells; its expression is greatly enhanced 2 d after initial priming in the presence of IL-4 and is suppressed in Th1 cells. Overexpression of GATA3 in naive CD4 T cells causes Th2 differentiation even in the absence of IL-4. However, it is not clear whether ectopic expression of GATA3 in committed Th1 cells is sufficient to induce competence to produce IL-4 15 16. c-maf expression is induced by IL-4 and antigen treatment. Once CD4 cells have been polarized to the Th2 phenotype, c-maf can be rapidly induced by antigen stimulation alone. Experiments using transgenic and gene targeting technologies have confirmed that c-maf is an important regulator of Th2 cell differentiation 17 18.
IL-12 is a key regulator for directing differentiation of naive CD4 T cells to the Th1 phenotype 19 20. IL-12 mediates its functions by binding to IL-12Rβ1 and β2 chains. IL-12Rβ2 is expressed at low levels in naive CD4 T cells; its expression can be upregulated by IFN-γ, IL-12, and antigen stimulation 21 22. Binding of IL-12 to its receptors activates Jak2 and Tyk2, leading to STAT4 activation. Activated STAT4 can induce IFN-γ expression by inducing STAT4-dependent, Th1-specific transcription factor(s), including T-bet 23. The in vivo role of IL-12, IL-12 receptors, and STAT4 in Th1 cell differentiation has been confirmed by genetic targeting experiments 24 25 26.
IFN-γ has been reported to play an indirect role in Th1 cell differentiation. It upregulates IL-12Rβ2 chain expression in naive CD4+ T cells and enhances IL-12 production by activated macrophages 27. The direct action of IFN-γ in Th1 cell differentiation has not been clearly documented 28.
Here we report that although IFN-γ2/− and IFN-γ receptor (IFN-γR)−/− naive CD4+ T cells appear to differentiate into Th1 cells in the presence of IL-12 and anti–IL-4, they retain the potential to express IL-4 if restimulated in the presence of IL-4. Our study strongly suggests that IFN-γ is essential for completion or stabilization of Th1 polarization.
Materials and Methods
Animals and Cell Cultures.
C57Bl/6, IFN-γ knockout (IFN-γ2/−), or IFN-γR knockout (IFN-γR−/−) mice (on 129 × C57Bl/6 background) were purchased from The Jackson Laboratory. Lymph node cells were depleted of CD8+, B220+, and IAb+ cells by negative selection using magnetic beads. The purified CD4 cells were then centrifuged on a discontinuous 50, 60, and 70% Percoll gradient. Cells with a density of >70% Percoll were collected and used for priming 29. Primary stimulation of CD4 T cells was carried out by culturing 106 CD4 T cells in the presence of 107 irradiated T cell–depleted spleen cells from C57Bl/6, IFN-γ2/−, or IFN-γR−/− mice with anti-CD3 (3 μg/ml 2C11), 3 μg/ml anti-CD28, and 10 U/ml IL-2 for 7 d. For differentiation of Th1 cells (Th1-inducing conditions), monoclonal anti–IL-4 antibody (10 μg/ml 11B11) and 10 ng/ml IL-12 (R&D Systems) were also added to the culture; for differentiation of Th2 cells (Th2-inducing conditions), 5 ng/ml IL-4 (BD PharMingen) and anti–IL-12 antibody (10 μg/ml C17.8) were added. Th1 cells were primed two or three times before switching to Th2-inducing conditions.
For single cell cloning experiments, naive CD4 T cells were deposited at one cell per well into a 96-well plate. Cells were cultured for 2 wk under Th1-inducing conditions. Clones were screened for IFN-γ production by ELISA. IFN-γ–producing clones were divided into two portions; one was cultured under Th1-inducing conditions and the other under Th2-inducing conditions for 1 wk. They were then assayed for IFN-γ or IL-4 by ELISA, RNase protection assay (RPA), or intracellular staining. Clones that produced >450 pg/ml of IFN-γ were considered IFN-γ producing; clones that produced >300 pg/ml of IL-4 were considered IL-4 producing.
Intracellular Staining.
Primed cells were stimulated with PMA and ionomycin in the presence of 2 μM monensin (Calbiochem). After 6 h of stimulation, cells were treated with 20 μg/ml of DNase 1 (Boehringer) for 5 min at 37°C, washed with cold PBS, fixed with 4% paraformaldehyde for 5 min at 37°C, washed with buffer containing 0.1% saponin and 0.1% BSA, and stained with FITC-labeled anti–IFN-γ and/or PE-labeled anti–IL-4 (11B11) monoclonal antibody (BD PharMingen). Samples were analyzed using a FACScan™ (Becton Dickinson).
RPA.
Total RNA was isolated with the guanidinium method. Multiple cytokine RNA probes were synthesized with RiboQuant RNase Protection kit (BD PharMingen) following the manufacturer's instructions. GATA3 template was made by ligating GATA3 PCR product into PCR2.1 Topo vector (Invitrogen). Total RNA (2 μg) was hybridized with multiple RNA probes overnight. These reactions were then treated with DNase-free RNase. Protected RNA was separated in a 5% acrylamine gel.
Affinity Matrix Assay and ELISA.
To detect living IFN-γ–secreting cells, cells were stimulated with immobilized anti-CD3 and anti-CD28 for 3 d. The treated cells were then incubated with a capture antibody (dual specific against CD45 and IFN-γ; Miltenyi Biotec) for 5 min on ice. Stained cells were allowed to secrete IFN-γ at 37°C for 30–45 min before staining with the detection antibody (PE-conjugated anti–IFN-γ; Miltenyi Biotec). For capture and intracellular double staining, FITC-labeled anti–IFN-γ antibodies (BD PharMingen) were incubated in the presence or absence of saponin with cells that were stained with capture antibody. IFN-γ–positive cells were sorted by a FACStar™ (Becton Dickinson). Sorted IFN-γ–secreting cells were further cultured under either Th1- or Th2-inducing conditions for 7 d. At the end of cultures, cells were washed extensively and restimulated with 20 ng/ml PMA and 1 μm ionomycin overnight. IL-4 and IFN-γ production were measured using commercial ELISA detection kits (Endogen).
Results
IFN-γ2 /− CD4 T Cells Primed under Th1 Conditions Can Be Stimulated to Produce IL-4.
To investigate the role of IFN-γ in Th1 commitment, we primed IFN-γ2/− CD4 T cells with anti-CD3, anti-CD28, IL-2, IL-12, and anti–IL-4 in the presence of irradiated T cell–depleted spleen cells from IFN-γ2/− mice as APC (Th1 conditions). This Th1-priming regimen was carried out for two periods of 7 d. These cells were then subjected to a third round of stimulation either under the same conditions as the initial two rounds 1 or under Th2 conditions (i.e., in the presence of IL-4 and anti–IL-12; 1-2). While only 3.5% the IFN-γ2/− cells restimulated under Th1 conditions produced IL-4 upon challenge, 50.2% of those cells that had been restimulated under Th2 conditions did so. By contrast, IL-4 was produced by only 2.4% of wild-type (WT) cells that had been primed twice under Th1 conditions and restimulated under Th2 conditions (Fig. 1).
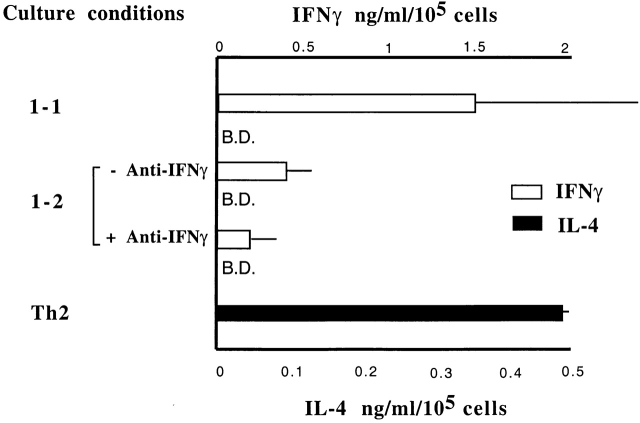
Figure 1.
IFN-γ2/− CD4 T cells primed under Th1 conditions can be restimulated to acquire IL-4–producing capacity. Naive CD4 T cells prepared from WT mice and IFN-γ2/− mice were cultured under Th1- or Th2-inducing conditions in the presence or absence of IFN-γ (10 ng/ml) for 7 d. Priming under Th1-inducing conditions was repeated once. At the end of the priming, Th1 cells were washed and further cultured under either Th1-inducing conditions 1 in the presence or absence of IFN-γ or under Th2-inducing conditions 1 2 for 7 d. Th2-primed cells were also restimulated under Th1- (2-1) or Th2- 2 inducing conditions. Cells were then washed and restimulated with PMA and ionomycin for 6 h. Cytosolic cytokine content was measured by staining with anti–IFN-γ and anti–IL-4 antibodies.
Addition of IFN-γ during the first two rounds of Th1 priming markedly inhibited the capacity of IFN-γ2/− cells to acquire IL-4–producing capacity when switched to Th2 conditions (4.4% IL-4 producers; Fig. 1). This supports the conclusion that the effect of IFN-γ in preventing Th1-primed cells from acquiring IL-4–producing cell phenotype is a direct one rather than an indirect one caused by lack of IFN-γ during T cell development. One caveat regarding experiments in which IFN-γ is added to the priming cultures is that cell yields are diminished by the addition of this cytokine. However, the addition of IFN-γ to Th1-inducing cultures of WT cells or to Th2-inducing cultures of IFN-γ2/− cells did not diminish Th1 polarization and diminished Th2 polarization only moderately. This suggests that diminished cell yield does not explain the inability of IFN-γ2/− cells primed under Th1 conditions, with the addition of IFN-γ, to acquire IL-4–producing capacity. Adding IFN-γ to the restimulation culture (i.e., IL-4, anti–IL-12, and IFN-γ) caused a much less striking diminution in the frequency of IFN-γ2/− cells that produced IL-4 (to 36.2%).
To further determine whether the presence of IFN-γ is only required early in the priming period to suppress IL-4–producing potential in Th1 cells, naive CD4 T cells were primed under Th1 conditions and then switched to Th2 conditions in the presence or absence of anti-IFN-γ antibody. Th1 cells that were switched in the presence of anti–IFN-γ failed to produce detectable IL-4 as measured by ELISA. The amount of IFN-γ produced by “switched” Th1 cells was reduced 4–5-fold compared with cells restimulated in IL-12 plus anti–IL-4 (Fig. 2). Collectively, these data indicate that suppression of IL-4–producing potential by IFN-γ is determined early in priming phase and is thereafter independent of IFN-γ.
Figure 2.
Maintenance of IL-4 silence in Th1 cells is independent of IFN-γ. Naive CD4 T cells from C57Bl6 mice were primed under Th1 conditions for 7 d plus 7 d of resting in IL-2 medium. The resulting cultures were washed and restimulated under Th1 conditions or Th2 conditions in the presence or absence of anti–IFN-γ antibody for 7 d. For control, naive CD4 T cells were primed under Th2 conditions for 7 d. At the end of culture period, cells were washed extensively and restimulated with PMA and ionomycin overnight. Supernatants were collected and measured for IL-4 and IFN-γ proteins by ELISA. B.D., below detection.
Switched IFN-γ2 /− Cells Express mRNA for Th2 Cytokines.
IFN-γ2/− CD4 T cells were subjected to three rounds of Th1 priming and then switched to Th2 conditions for an additional round. Upon stimulation, these cells expressed mRNA for IL-4, IL-5, and IL-13, as detected by a RPA (Fig. 3). By contrast, WT cells primed for three rounds under Th1 conditions failed to develop the capacity to express mRNA for any of these cytokines when restimulated under Th2 conditions.
Figure 3.

Switched IFN-γ2/− Th1 cells can express mRNA for Th2 cytokines. Naive CD4 T cells from C57Bl6 mice and IFN-γ2/− mice were incubated under Th1-inducing conditions for three rounds of 7 d each. At the end of third round of differentiation, the cells were further differentiated under Th1-inducing conditions 1 or Th2-inducing conditions 1 2 for 7 d. Cells were then restimulated with anti-CD3, anti-CD28, and irradiated T cell–depleted APCs for 4 h. Total RNA was analyzed for cytokine gene expression by RPA.
IFN-γR− /− Th1 Cells Can Be Switched to Expression of Th2 Cytokines.
Naive CD4 T cells from IFN-γR−/− mice were subjected to three rounds of priming under Th1 conditions. When restimulated under Th1 conditions and then challenged, they expressed IFN-γ mRNA, but no IL-4, IL-5, or IL-13 mRNA (Fig. 4 A). When analyzed after two and three rounds of Th1 priming, CD4 T cells from IFN-γR−/− mice produced 50 and 70%, respectively, as much IFN-γ as did similarly primed WT cells (Fig. 4 B). Consistent with previous studies, these data suggest that IFN-γ is not essential for CD4 T cells to acquire IFN-γ–producing capacity 28.
Figure 4.

Switched IFN-γR−/− Th1 cells can express Th2 cytokines. (A) WT and IFN-γR−/− CD4 T cells were cultured under Th1 conditions for three rounds. For the fourth round, the cells were cultured under Th1-inducing conditions 1 or Th2-inducing conditions 1 2 for 7 d. As control, naive CD4 T cells were primed under Th2-inducing conditions for 7 d. Cells were restimulated with PMA and ionomycin for 4 h. Total RNA was analyzed for cytokine gene expression by RPA. (B) Naive CD4 T cells from WT and IFN-γR−/− were primed under Th1-inducing conditions for various rounds of priming (2×, 2 rounds; 3×, 3 rounds). Cells were stimulated with PMA and ionomycin overnight. IFN-γ content of supernatants was measured by ELISA. (C) Three round–primed WT and IFN-γR−/− Th1 cells were cultured under Th1- or Th2-inducing conditions for 1 wk. Cells were challenged with PMA and ionomycin for 4 h. IL-4 and IFN-γ were detected by intracellular staining.
Despite their acquisition of IFN-γ–producing capacity, Th1-primed IFN-γR−/− cells could develop into IL-4 producers upon restimulation under Th2 conditions. These cells expressed IL-4 mRNA and large amounts of IL-5 and IL-13 mRNA when compared with one round–primed WT Th2 cells (Fig. 4 A). Thus, cells that are insensitive to IFN-γ can be primed to express IFN-γ but retain the capacity to express Th2 cytokines upon appropriate stimulatory conditions. In these experiments, the APCs used were obtained from WT donors; thus, the effect of IFN-γ in preventing Th1-primed cells from acquiring IL-4–producing capacity must be on the developing Th1 cells.
Intracellular staining for IL-4 or IFN-γ showed that 47.7% of IFN-γR−/− Th1 cells demonstrated the capacity to produce IL-4 when stimulated under Th2-inducing conditions for a week, whereas IFN-γ–producing capacity of these cells was diminished markedly (from 40.9 to 14.6%). Conversely, WT Th1 cells even stimulated under Th2-inducing conditions failed to acquire IL-4-producing capacity. The percentage of IFN-γ–producing cells decreased only moderately (from 40.7 to 39.6%) after stimulation under Th2-inducing conditions (Fig. 4 C).
To determine whether the capacity to develop into Th1 cells that could be switched was the property of rare or common naive IFN-γR−/− CD4 T cells, single dense CD4 T cells from IFN-γR−/− mice were sorted onto a 96-well plate. The cells were cultured under Th1-inducing conditions for one round (2 wk), two rounds, or three rounds. Clones that produced IFN-γ were identified by ELISA. On average, they produced 4.8 ng/ml of IFN-γ (range: 0.45–15 ng/ml) and no detectable IL-4. IFN-γ–producing clones were divided into two aliquots. One was restimulated under Th1-inducing conditions and the other under Th2-inducing conditions for 1 wk. At the end of culture period, cells were washed extensively and restimulated with PMA and ionomycin. IFN-γ and IL-4 expression were measured by ELISA, intracellular staining, or RPA. We found that 50, 40, and 50% of IFN-γR−/− Th1 cell clones could become IL-4 producers after being primed under Th1-inducing conditions for one round, two rounds, and three rounds, respectively. On average, IL-4–producing clones produced 1.9 ng/ml of IL-4 (range: 0.3–6.1 ng/ml). Conversely, none of the WT Th1 clones retained the ability to differentiate into Th2 cells, even those primed just after one round (Fig. 5). Thus, switched IFN-γR−/− IL-4 producers were not the result of the expansion of rare precommitted Th2 cells that might have been present in the naive CD4 T cell preparations. These results indicate that IFN-γR−/− Th1 cells retain IL-4–producing potential.
Figure 5.
IFN-γR−/− Th1 cell clones retain the capacity to become IL-4 producers. Single dense naive CD4 T cells from WT and IFN-γR−/− mice were automatically deposited into a 96-well plate. Cells were further cultured for the time periods indicated under Th1-inducing conditions. Clones were assayed for IFN-γ production and divided into cultures under Th1- or Th2-inducing conditions for 1 wk. At the end of culture, cells were washed and restimulated with PMA and ionomycin. Supernatants were measured for IFN-γ and IL-4 production by ELISA. Data were representative of two independent experiments.
IFN-γ Producers from IFN-γR− /− Mice Retain the Potential to Develop IL-4–producing Capacity.
The previous experiments showed that a cell population that produces IFN-γ can be stimulated to produce IL-4. However, they do not establish that individual IFN-γ–producing cells can acquire the capacity to produce IL-4 upon restimulation under Th2 conditions. To determine whether such IFN-γ–producing cells could become IL-4 producers, we primed WT and IFN-γR−/− naive CD4+ T cells under Th1 conditions with WT APCs for three rounds. IFN-γ producers in those cultures were then purified by the affinity matrix technology and electronic cell sorting. We stimulated IFN-γR−/− Th1 cells with anti-CD3 and anti-CD28 plus irradiated APCs for 3 d. IFN-γ–secreting cells were stained with bispecific antibody against CD45 and IFN-γ, and then the captured IFN-γ was detected with PE-labeled anti–IFN-γ antibody. The authenticity of these IFN-γ producers was confirmed by intracellular staining with an FITC-labeled, anti–IFN-γ antibody (Fig. 6 A). Sorted IFN-γ producers from both IFN-γR−/− and WT donors were restimulated under either Th1 or Th2 conditions with WT APCs. IFN-γ producers from the IFN-γR−/− donors produced IL-4 after Th2 restimulation, whereas WT IFN-γ producers did not (Fig. 6b and Fig. c). The amount of IL-4 produced per 106 switched IFN-γR−/− cells was ∼1/2 that made by a comparable number of one round–primed Th2 cells from WT donors.
Figure 6.
IFN-γ producers from Th1-primed IFN-γR−/− cells can produce IL-4. (A) IFN-γR−/− Th1 cells were stimulated with anti-CD3 and anti-CD28 for 3 d. IFN-γ–producing cells were detected by affinity matrix assay (IFN-γ–PE). Cells were double stained with FITC-labeled anti-CD4 (CD4–FITC) or FITC-labeled anti–IFN-γ antibody (IFN-γ–FITC) in the presence or absence of saponin. (B) Three round–primed WT and IFN-γR−/− Th1 cells were stimulated with anti-CD3 and anti-CD28 for 3 d. IFN-γ–secreting cells were detected by the affinity matrix assay. IFN-γ1 cells were purified by a FACStar™. Purity for WT and IFN-γR−/− IFN-γ1 cells were 99 and 96%, respectively. (C) Purified WT and IFN-γR−/− IFN-γ producers were restimulated under Th1-inducing conditions or Th2-inducing conditions for 7 d. Cells were then washed extensively and stimulated with PMA and ionomycin at 106 cells per milliliter density. IL-4 content of supernatants was measured by ELISA.
Induction of GATA3 mRNA Is Enhanced in IFN-γR− /− Th1 Cells.
To study possible mechanism(s) by which IFN-γ silences Th2 cytokine gene transcription in committed Th1 cells, we measured GATA3 mRNA levels in Th1-primed IFN-γR−/− and WT cells that had been primed under Th1 conditions for three rounds and then restimulated under Th2 conditions. The IFN-γR−/− Th1 cells showed dramatic upregulation of GATA3 mRNA expression when exposed to Th2-inducing conditions for 48 h (data not shown) and 7 d (Fig. 7), as measured by RPA, while similarly treated WT cells failed to express GATA3 mRNA. These data suggest that IFN-γ represses the GATA3 transcription that normally occurs in response to IL-4 stimulation.
Figure 7.
Induction of GATA3 is enhanced in IFN-γR−/− Th1 cells. Three round Th1-primed WT and IFN-γR−/− cells were further differentiated under either Th1- or Th2-inducing conditions for 7 d. Total RNA (2 μg) was used to detect GATA3 mRNA by RPA.
Discussion
Our data indicate that development of IFN-γ–producing capacity of naive T cells and the suppression of the potential to acquire IL-4–producing activity are two separate events in the development of a polarized Th1 phenotype. In the presence of IL-12 and appropriate TCR signals, naive CD4 T cells can develop IFN-γ–producing capacity. This process is STAT4 dependent 26. Regulation of IL-12Rβ2 chain expression by IFN-γ, IL-12, and antigen stimulation also contribute to the polarization of Th1 cells 21 22. However, IL-12 alone is not sufficient to suppress the IL-4–producing potential of cells that produce IFN-γ. The IFN-γ–producing cells from the IFN-γR−/− donors retained the capacity to develop into IL-4 producers even after three or more rounds of priming in the presence of IL-12.
The suppressive effect of IFN-γ on the IL-4–producing potential of Th1 cells appears to be a direct effect rather than a reflection of developmental abnormalities due to lack of IFN-γ or IFN-γ responsiveness. Addition of IFN-γ to IFN-γ2/− Th1 cell cultures during the initial Th1 priming period suppressed the ability of IFN-γ Th1 cells to acquire IL-4–producing capacity. Furthermore, the immune systems of IFN-γ2/− and IFN-γR−/− mice appear to develop normally 30 31.
IFN-γ–mediated suppression of IL-4–producing potential appears to occur principally during the initial priming phase. This result is consistent with previous reports that demonstrated IFN-γRβ chain was downregulated in differentiated Th1 cells that would result in insensitivity to IFN-γ 32 33. Furthermore, addition of anti–IFN-γ antibody to one round–primed WT Th1 cells that were restimulated under Th2 conditions failed to develop IL-4–producing capacity. Thus, these data indicate that once IFN-γ exerts its effects on suppressing the IL-4–producing potential in the initial priming, it is no longer required to maintain IL-4 “silencing.”
It has previously been concluded that a key factor in stabilizing Th1 phenotype was the absence of IL-4. Nakamura et. al. 34 reported that even in the presence of anti–IL-4 antibody, Th1 cells that were primed twice could retain the ability to respond to IL-4 with upregulated CD30 expression and 90 U/ml of IL-5, whereas similarly primed IL-4−/− Th1 cells lost this IL-4 responsiveness. Our data demonstrate that priming naive WT CD4 T cells in the presence of IL-12 and anti–IL-4 results in a cell population that cannot acquire the capacity to produce IL-4, IL-5, or IL-13 even if restimulated in the presence of anti–IL-12 and IL-4. The presence of small amounts of IL-4 during the priming period might allow “Th1” cells to switch or might prime a population of cells to differentiate partially along the Th2 pathway so that the switching culture could complete such differentiation. In any case, the inability of the cells that we have primed in the presence of IL-12 and anti–IL-4 suggests that the concentration of anti–IL-4 we have used is sufficient to completely neutralize IL-4 and thus to reveal the critical role IFN-γ plays in stabilization of the Th1 phenotype.
How does IFN-γ mediate its suppressive effects on IL-4–producing potential in WT Th1 cells? Inhibition of IL-4–induced GATA3 transcription appears to be one key mechanism by which IFN-γ suppresses the potential of CD4 T cells to acquire IL-4–producing capacity and thus maintains a stable Th1 phenotype. IFN-γ may suppress GATA3 transcription through mechanism(s) not involving suppressor of cytokine signaling (SOCS)1–3. It has been reported that overexpression of SOCS1, but not SOCS2, can suppress STAT6 functions in B cells 35. Similar findings in human monocytes and hepatocytes were also reported 36. SOCS1 is rapidly induced by IFN-γ treatment. Surprisingly, we found that IFN-γR−/− Th1 cells expressed the same amount of SOCS1–3 mRNA as WT Th1 cells (data not shown).
Our study raises the question of how to define the Th1 phenotype. Simple capacity to produce IFN-γ may not be sufficient, since it has been shown that well-polarized Th2 cells can develop IFN-γ–producing capacity if restimulated in the presence of anti–IL-4 and IL-12 5. Thus, one might require that Th1 cells both have the capacity to produce IFN-γ and have lost the capacity to become IL-4 producers. On the other hand, one could argue that the acquisition of IFN-γ–producing capacity by nonIL-4 producers marks acquisition of a Th1 phenotype, and inability to switch to IL-4 production denotes stabilization of that phenotype. Recently, Grogan et. al. 37 have reported that the IL-4 gene is found in centromeric heterochromatin in Th1 cells but not in naive or Th2 cells. This suggests that silencing of the IL-4 gene is an active process during Th1 differentiation. The capacity of cells that have been primed under Th1 conditions but did not receive an IFN-γ signal to retain the potential to activate the IL-4 gene suggests that such silencing has not occurred and that IFN-γ may play a role in the silencing process.
Acknowledgments
We thank Pat Simms for excellent cell sorting and Drs. John Clancy, Jr., Daniel Quinn, Martin Kast, and Phong Le for critically reading the manuscript, and Dr. Baltazar Espiritu for allowing us to use his laboratory equipment.
This study is funded in part by a National Institutes of Health K22 grant (AI01663-02), Lydia Schweppe Immunology Career Development Award, and the Potts and Earl M. Bane Trust Funds of Loyola Medical School.
Footnotes
Abbreviations used in this paper: Jak, Janus kinase; RPA, RNase protection assay; SOCS, suppressor of cytokine signaling; STAT, signal transducer and activator of transcription; WT, wild-type.
References
- Romagnani S. Th1 and Th2 in human diseases. Clin. Immunol. Immunopathol. 1996;80:225–235. doi: 10.1006/clin.1996.0118. [DOI] [PubMed] [Google Scholar]
- Murphy E., Shibuya K., Hosken N., Openshaw P., Maino V., Davis K., Murphy K., O'Garra A. Reversibility of T helper 1 and 2 populations is lost after long term stimulation. J. Exp. Med. 1996;183:901–913. doi: 10.1084/jem.183.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sornasse T., Larenas P.V., Davis K.A., deVries J., Yssel H. Differentiation and stability of T helper 1 and 2 cells derived from naive human neonatal CD4+ T cells, analyzed at the single-cell level. J. Exp. Med. 1996;184:473–483. doi: 10.1084/jem.184.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez V.L., Lederer J.A., Lichtman A.H., Abbas A.K. Stability of Th1 and Th2 populations. Int. Immunol. 1995;7:869–875. doi: 10.1093/intimm/7.5.869. [DOI] [PubMed] [Google Scholar]
- Hu L.J., Huang H., Ryan J., Paul W.E. In differentiated CD4+ T cells, interleukin 4 production is cytokine-autonomous, whereas interferon γ production is cytokine-dependent. Proc. Natl. Acad. Sci. USA. 1997;94:3189–3194. doi: 10.1073/pnas.94.7.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGros G., Ben S.S., Seder R., Finkelman F.D., Paul W.E. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitroIL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J. Exp. Med. 1990;172:921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder R.A., Paul W.E., Davis M.M., Fazekas D.S., Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J. Exp. Med. 1992;176:1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C.S., Heimberger A.B., Gold J.S., O'Garra A., Murphy K.M. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an αβ T-cell-receptor transgenic system. Proc. Natl. Acad. Sci. USA. 1992;89:6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S.L., Weinberg A.D., English M., Huston G. IL-4 directs the development of Th2-like helper effectors. J. Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- Johnston J.A., Kawamura M., Kirken R.A., Chen Y.Q., Blake T.B., Shibuya K., Ortaldo J.R., McVicar D.W., O'Shea J.J. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature. 1994;370:151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- Witthuhn B.A., Silvennoinen O., Miura O., Lai K.S., Cwik C., Liu E.T., Ihle J.N. Involvement of the Jak-3 Janus kinase in signalling by interleukins 2 and 4 in lymphoid and myeloid cells. Nature. 1994;370:153–157. doi: 10.1038/370153a0. [DOI] [PubMed] [Google Scholar]
- Shimoda K., van Deursen J., Sangster M.Y., Sarawar S.R., Carson R.T., Tripp R.A., Chu C., Quelle F.W., Nosaka T., Vignali D.A. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- Zheng W., Flavell R.A. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- Ho I.C., Hodge M.R., Rooney J.W., Glimcher L.H. The proto-oncogene c-maf is responsible for tissue-specific expression of interleukin-4. Cell. 1996;85:973–983. doi: 10.1016/s0092-8674(00)81299-4. [DOI] [PubMed] [Google Scholar]
- Ouyang W., Ranganath S.H., Weindel K., Bhattacharya D., Murphy T.L., Sha W.C., Murphy K.M. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9:745–755. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- Lee H.J., Takemoto N., Kurata H., Kamogawa Y., Miyatake S., O'Garra A., Arai N. GATA-3 induces T helper type 2 (Th2) cytokine expression and chromatin remodeling in committed Th1 cells. J. Exp. Med. 2000;192:105–115. doi: 10.1084/jem.192.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.I., Ho I.C., Grusby M.J., Glimcher L.H. The transcription factor c Maf controls the production of interleukin-4 but not other Th2 cytokines. Immunity. 1999;10:745–751. doi: 10.1016/s1074-7613(00)80073-4. [DOI] [PubMed] [Google Scholar]
- Ho I.C., Lo D., Glimcher L.H. c-maf promotes T helper cell type 2 (Th2) and attenuates Th1 differentiation by both interleukin 4-dependent and -independent mechanisms. J. Exp. Med. 1998;188:1859–1866. doi: 10.1084/jem.188.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder R.A., Gazzinelli R., Sher A., Paul W.E. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon γ production and diminishes interleukin 4 inhibition of such priming. Proc. Natl. Acad. Sci. USA. 1993;90:10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C.S., Macatonia S.E., Tripp C.S., Wolf S.F., O'Garra A., Murphy K.M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Chang J.T., Shevach E.M., Segal B.M. Regulation of interleukin (IL)-12 receptor β2 subunit expression by endogenous IL-12a critical step in the differentiation of pathogenic autoreactive T cells. J. Exp. Med. 1999;189:969–978. doi: 10.1084/jem.189.6.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo S.J., Dighe A.S., Gubler U., Murphy K.M. Regulation of the interleukin (IL)-12R β2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J. Exp. Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo S.J., Kim S.T., Costa G.L., Zhang X., Fathman C.G., Glimcher L.H. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Magram J., Connaughton S.E., Warrier R.R., Carvajal D.M., Wu C.Y., Ferrante J., Stewart C., Sarmiento U., Faherty D.A., Gately M.K. IL-12-deficient mice are defective in IFN γ production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- Wu C., Wang X., Gadina M., O'Shea J.J., Presky D.H., Magram J. IL-12 receptor ss2 (IL-12Rss2)-deficient mice are defective in IL-12-mediated signaling despite the presence of high affinity IL-12 binding sites. J. Immunol. 2000;165:6221–6228. doi: 10.4049/jimmunol.165.11.6221. [DOI] [PubMed] [Google Scholar]
- Kaplan M.H., Sun Y.L., Hoey T., Grusby M.J. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- Yoshida A., Koide Y., Uchijima M., Yoshida T.O. IFN-γ induces IL-12 mRNA expression by a murine macrophage cell line, J774. Biochem. Biophys. Res. Commun. 1994;198:857–861. doi: 10.1006/bbrc.1994.1122. [DOI] [PubMed] [Google Scholar]
- Wenner C.A., Guler M.L., Macatonia S.E., O'Garra A., Murphy K.M. Roles of IFN-γ and IFN-α in IL-12-induced T helper cell-1 development. J. Immunol. 1996;156:1442–1447. [PubMed] [Google Scholar]
- Zhu J., Huang H., Guo L., Stonehouse T., Watson C.J., Hu-Li J., Paul W.E. Transient inhibition of interleukin 4 signaling by T cell receptor ligation. J. Exp. Med. 2000;192:1125–1134. doi: 10.1084/jem.192.8.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton D.K., Pitts-Meek S., Keshav S., Figari I.S., Bradley A., Stewart T.A. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- Huang S., Hendriks W., Althage A., Hemmi S., Bluethmann H., Kamijo R., Vilcek J., Zinkernagel R.M., Aguet M. Immune response in mice that lack the interferon-γ receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- Bach E.A., Szabo S.J., Dighe A.S., Ashkenazi A., Aguet M., Murphy K.M., Schreiber R.D. Ligand-induced autoregulation of IFN-γ receptor β chain expression in T helper cell subsets. Science. 1995;270:1215–1218. doi: 10.1126/science.270.5239.1215. [DOI] [PubMed] [Google Scholar]
- Pernis A., Gupta S., Gollob K.J., Garfein E., Coffman R.L., Schindler C., Rothman P. Lack of interferon γ receptor β chain and the prevention of interferon γ signaling in TH1 cells. Science. 1995;269:245–247. doi: 10.1126/science.7618088. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Lee R.K., Nam S.Y., Podack E.R., Bottomly K., Flavell R.A. Roles of IL-4 and IFN-γ in stabilizing the T helper cell type 1 and 2 phenotype. J. Immunol. 1997;158:2648–2653. [PubMed] [Google Scholar]
- Dickensheets H.L., Venkataraman C., Schindler U., Donnelly R.P. Interferons inhibit activation of STAT6 by interleukin 4 in human monocytes by inducing SOCS-1 gene expression. Proc. Natl. Acad. Sci. USA. 1999;96:10800–10805. doi: 10.1073/pnas.96.19.10800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losman J.A., Chen X.P., Hilton D., Rothman P. Cutting edgeSOCS-1 is a potent inhibitor of IL-4 signal transduction. J. Immunol. 1999;162:3770–3774. [PMC free article] [PubMed] [Google Scholar]
- Grogan J.L., Mohrs M., Harmon B., Lacy D.A., Sedat J.W., Locksley R.M. Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity. 2001;14:205–215. doi: 10.1016/s1074-7613(01)00103-0. [DOI] [PubMed] [Google Scholar]