Abstract
We generated T cell receptor transgenic mice specific for the liver stages of the rodent malaria parasite Plasmodium yoelii and studied the early events in the development of in vivo effector functions in antigen-specific CD8+ T cells. Differently to activated/memory cells, naive CD8+ T cells are not capable of exerting antiparasitic activity unless previously primed by parasite immunization. While naive cells need to differentiate before achieving effector status, the time required for this process is very short. Indeed, interferon (IFN)-γ and perforin mRNA are detectable 24 h after immunization and IFN-γ secretion and cytotoxic activity are detected ex vivo 24 and 48 h after immunization, respectively. In contrast, the proliferation of CD8+ T cells begins after 24 h and an increase in the total number of antigen-specific cells is detected only after 48 h. Remarkably, a strong CD8+ T cell–mediated inhibition of parasite development is observed in mice challenged with viable parasites only 24 h after immunization with attenuated parasites. These results indicate that differentiation of naive CD8+ T cells does not begin only after extensive cell division, rather this process precedes or occurs simultaneously with proliferation.
Keywords: malaria, CD8+ T cells, TCR transgenic mouse, effector T cell, in vivo differentiation
Introduction
Naive and memory CD8+ T cells differ greatly with regards to their capacity to respond to antigenic stimulation. Activated/memory T cells secrete cytokines and proliferate immediately after antigen recognition. In contrast, naive CD8+ T cells undergo a series of phenotypic changes before differentiating into effector cells 1 2. The mechanisms involved in the in vivo development of effector functions in naive CD8+ T cells still remain poorly understood. Indeed, the basic features of this T cell response, such as the time frame and sequence of events involved in the differentiation and proliferation of naive cells activated after infection by intracellular pathogens, have yet to be defined.
In rodent malaria models, it is well established that CD8+ T cells induced after immunization with attenuated or viable malaria sporozoites play an important role in protection against liver stages of this parasite. This has been demonstrated in adoptive transfer experiments using CD8+ T cell clones specific for defined malaria epitopes that inhibit parasite development in the liver, thus preventing the onset of blood-stage infection 3 4. Moreover, immunization with subunit vaccines based on recombinant viruses or DNA-expressing MHC class I–restricted malaria epitopes induces a strong parasite-specific CD8+ T cell response that protects against parasite challenge 5 6 7 8. Overall, these studies demonstrate that in the malaria system, unlike most infectious models, CD8+ T cells exert such a strong antiparasitic effect that T cell responses against a single epitope can eliminate infection.
The induction of CD8+ T cells against the liver stages of malaria parasites differs from T cell responses to most infectious organisms. Soon after Plasmodium yoelii sporozoites invade hepatocytes, they undergo extensive transformation and about 44 h later, a distinct parasite stage, displaying a different antigenic make-up, is released from the liver and invade red blood cells. Since parasites from erythrocytic stages do not reinvade hepatocytes, the CD8+ T cell response against the liver stages is the result of a single and short-lived infectious event. In contrast, the CD8+ T cell responses to bacterial and viral infections are “shaped” by repeated overlapping cycles of infection and reinfection, generating a mixture of cell populations in different stages of differentiation.
A major limitation in analyzing the early in vivo events leading to the development of effector CD8+ T cells against malaria is the low frequency of precursors for specific epitopes. To overcome this limitation, we generated transgenic mice expressing a TCR specific for the H2Kd-restricted epitope, SYVPSAEQI, located in the P. yoelii circumsporozoite protein (amino acids 252–260). This epitope is recognized by CD8+ but not by CD4+ T cells 5. Using this transgenic system, we sought to define the early events involved in the development of effector functions and acquisition of protective antiparasitic activity in naive malaria-specific CD8+ T cells.
Materials and Methods
Parasites and Mice.
P. yoelii (17X NL strain) and P. berghei (NK65) sporozoites were obtained as described previously 4. Normal BALB/c, C57Bl/6, or CB6/F1 mice were purchased from the National Cancer Institute.
Transgenic mice were generated based on the rearranged V(D)J segments of the TCR-α and -β genes of the CD8+ T cell clone YA26, which is specific for SYVPSAEQI, a CD8+ T cell epitope located in the P. yoelii circumsporozoite protein 4. A cDNA library was constructed (Express cDNA Gigapack II Gold; Stratagene) from YA26 and the TCR genes were screened using 32[P]-labeled probes from each conserved regions: 5′-TGTTCACCG-ACTTTGACTCC-3′; 5′-TGGCGTTGGTCTCTTTGAAG-3′ (for Cα); 5′-TGAGAAATGTGACTCCACCC-3′; and 5′-CTGCTCAGGCAGTAGCTATA-3′ (for Cβ). After genomic amplification of the positive clones, the rearranged genes were determined to be Vα10 and Vβ8.1. The TCR-α and -β transgenes were constructed by inserting the identified rearranged genomic sequences into TCR-α and -β cassette vectors 9 using the following primers: 5′-CCGCCCGGGCCACAGCCCAGGGAC-TGGTTACTTGC-3′; 5′-TCCCCGCGGTGTGGTCGTCTG-TGTGATAAAGGCTATGAG-3′ (for α); 5′-CCGCTCGAGGAGAAGTGGTGGAGTGTCTTAACTGTGCAG-3′; and 5′-TCCCCGCGGTCCTTAGCCTGGGAAATGCTCCC-3′ (for β). The underlined nucleotides represent restriction enzyme sites used for cloning. The transgenes were coinjected into C57BL/6 oocytes and the resulting transgenic mice were identified by PCR screening of genomic DNA isolated from tails. Since the SYVPSAEQI epitope is H2d-restricted, TCR transgenic C57BL/6 mice were crossed with BALB/c mice and the resulting CB6/F1–expressing H2Kb/d were used for subsequent experiments.
Tetramers, Antibodies, and 5-Carboxyfluorescein Diacetate Succinimidyl Ester Staining.
SYVPSAEQI-specific H2Kd tetramers were prepared as described previously 10. Antibodies to mouse CD8 (53-6.7), CD11a (M17/4), CD44 (IM-7), and CD122 (TM-β1) were obtained from BD PharMingen. 5-carboxyfluorescein diacetate succinimidyl ester (CFSE) staining was performed based on the manufacturer's instructions (Molecular Probes).
Adoptive Transfers, Immunizations, and Parasite Challenge.
Spleen cells from transgenic mice containing 1–2 × 106 CD8+ tetramer+ cells, unless otherwise specified, were used for adoptive transfers. The total number of transgenic cells used in the experiments was determined by FACS® (Becton Dickinson) analysis after staining spleen cells for CD8 and with SYVPSAEQI-tetramers.
Sporozoite immunizations, done 24 h after adoptive transfer, were performed by intravenous injection of 5 × 104 radiation-attenuated sporozoites (γ-source, 20 Krad). Other mice were immunized intraperitoneally with 106 plaque forming units of a recombinant vaccinia virus expressing the 9-mer SYVPSAEQI epitope 5.
Challenge of mice was performed by intravenous injection of 5 × 104 viable (nonirradiated) P. yoelii sporozoites. Quantification of parasite load in the livers of mice after challenge was performed as described previously 11. This competitive reverse transcription (RT)-PCR is based on the use of primers specific for Plasmodium 18s rRNA that amplify a 393-bp fragment in total parasite RNA but not in mouse RNA. As competitor, a truncated cloned 333-bp Plasmodium rRNA fragment, was used in all assays.
ELISPOT and Cytotoxic Assay.
The determination of individual SYVPSAEQI-specific IFN-γ–secreting CD8+ T cells by ELISPOT was performed as described previously 12. The ex vivo cytotoxic assay was performed using a standard 51Cr release assay as described previously 10.
Quantification of Mouse IFN-γ and Perforin mRNA Transcripts.
SYVPSAEQI-specific tetramer+ cells were purified from the spleens of mice receiving transgenic CD8+ T cells using anti-PE antibodies bound to magnetic beads following the manufacturer's instructions (Miltenyi Biotec). Total RNA was extracted using standard guanidinium thiocyanate phenol/chloroform extraction. mRNA transcripts for mouse IFN-γ and perforin were amplified using specific primers in quantitative RT-PCR as described previously 13 14 15. The total amounts of IFN-γ and perforin mRNA were determined by densitometry using a GelDoc 2000 system and Quantity One® software (Bio-Rad Laboratories).
Results and Discussion
Specificity of TCR Transgenic Mice.
To assess the specificity of the TCR transgenic CD8+ T cells, we analyzed spleen cells by FACS®, after staining for CD8 and with SYVPSAEQI-specific H2Kd tetramers 10. As shown in Fig. 1 A, a large number of CD8+ T cells from the transgenic mice bind the SYVPSAEQI-tetramers. As expected, CD4+ T cells from these mice do not bind the tetramers, in agreement with previous studies indicating that CD4+ T cells do not recognize this epitope 5.
Figure 1.
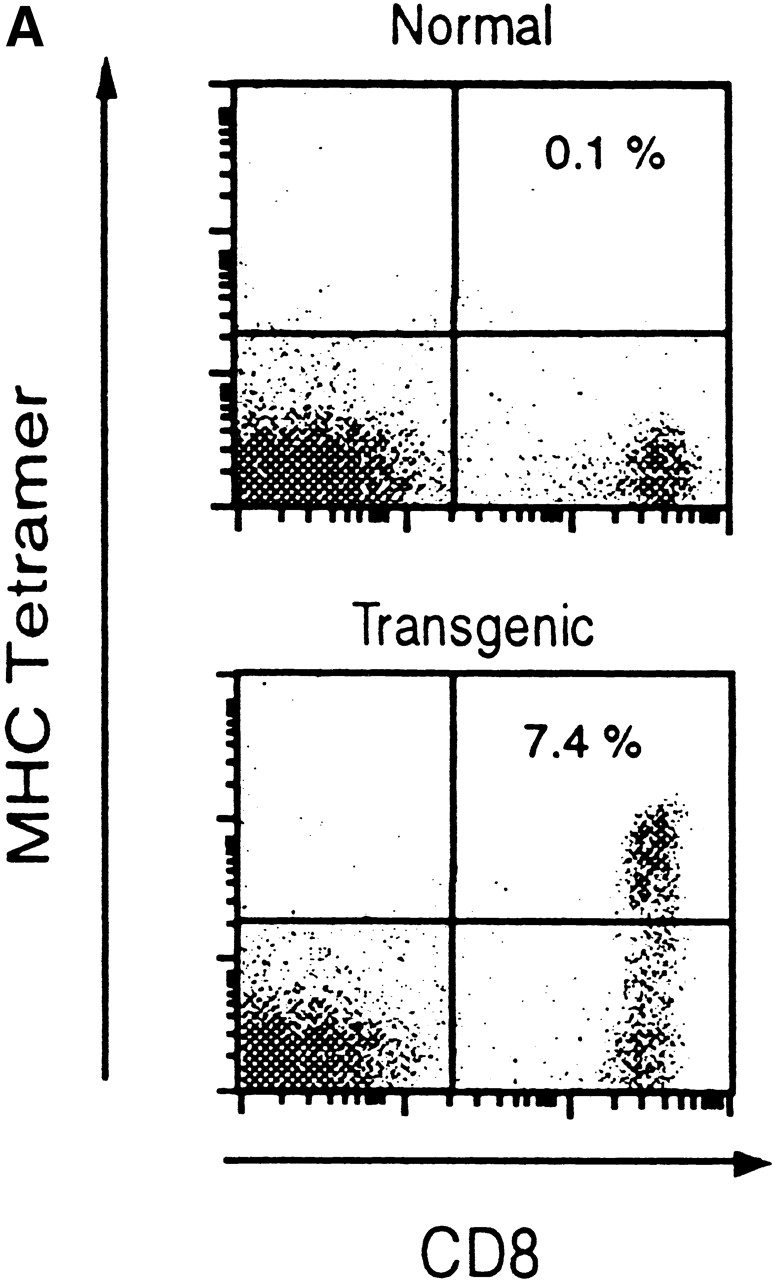
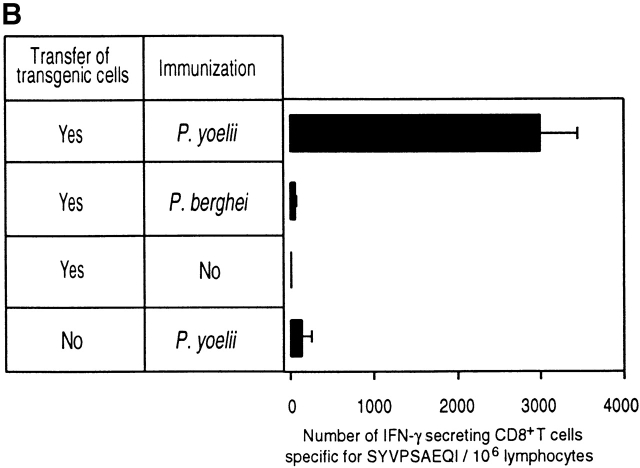
Phenotypic and functional specificity of the transgenic CD8+ T cells. (A) CD8+ T cells from transgenic CB6/F1 mice bind to SYVPSAEQI-tetramers. Plots were analyzed on live lymphocytes and the numbers indicated the frequency of CD8+ tetramer+ cells in total lymphocytes. (B) Adoptively transferred transgenic CD8+ T cells are activated only after immunization with radiation-attenuated P. yoelii sporozoites. The frequencies of SYVPSAEQI-specific IFN-γ–secreting cells in the spleen were determined by ELISPOT, 8 d after immunization, in the presence of SYVPSAEQI-coated target cells. Nonimmunized recipient mice and immunized normal mice were also used as controls. Results are expressed as average ± SD of duplicate cultures. Negligible numbers of spots are obtained using control target cells (data not shown).
The specificity of the transgenic CD8+ T cells was further studied after adoptive transfer into normal mice followed by immunization with radiation-attenuated P. yoelii sporozoites. The CD8+ T cell response was evaluated using ELISPOT to detect IFN-γ secretion using SYVPSAEQI-coated target cells 12. As shown in Fig. 1 B, in mice receiving transgenic cells, a large number of IFN-γ–secreting cells are detected after immunization with P. yoelii sporozoites. In contrast, negligible numbers of cells are detected in recipient mice which were not immunized or immunized with P. berghei sporozoites, a related malaria parasite expressing a noncross reacting CD8+ T cell epitope, SYIPSAEKI 4. The IFN-γ–secreting cells detected in mice that did not receive transgenic cells but were immunized with P. yoelii sporozoites represent the response of endogenous anti-SYVPSAEQI specific CD8+ T cells (Fig. 1 B).
Induction of In Vivo Effector Functions and Proliferation of CD8+ T Cells.
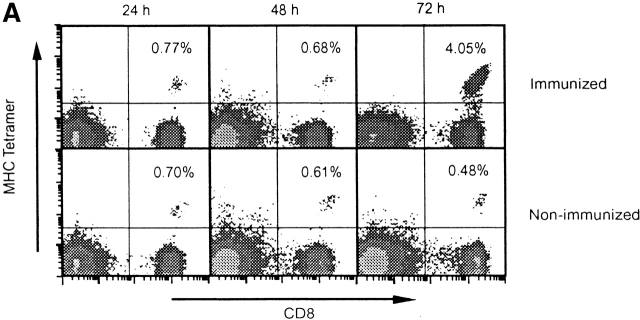
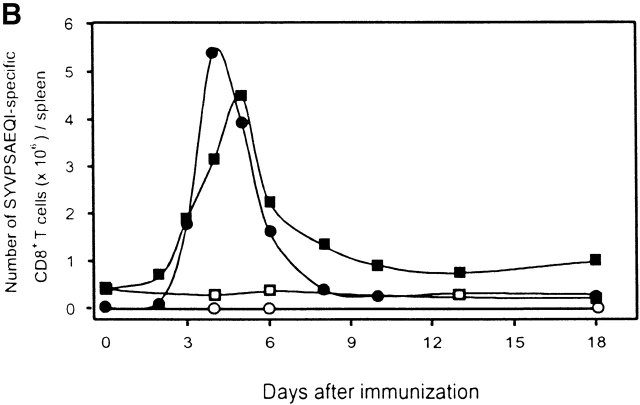
As determined by FACS® after tetramer staining of CD8+ T cells from spleens of immunized recipient mice, a significant increase in the frequency of SYVPSAEQI-specific CD8+ T cells is evident 72 h after immunization (Fig. 2 A). The evolution of this response, monitored by FACS® and ELISPOT, shows that it peaks at days 4–5 and decreases rapidly thereafter. After day 8, the magnitude of the CD8+ T cell response is stabilized and remains unchanged for several weeks (Fig. 2 B). The sudden decrease in the number of antigen-specific T cells after the initial antigen-driven expansion has been observed in other infectious systems and has been suggested to be caused by programmed cell death 16 17 18 19. In spite of the dramatic decrease in the frequency of CD8+ T cells, those cells that remain after day 15 are fully capable of mediating protection against challenge with P. yoelii sporozoites (data not shown).
Figure 2.
Activation kinetics of antigen-activated naive CD8+ T cells. (A) Spleen cells from immunized and nonimmunized recipient mice were isolated at 24, 48, and 72 h and stained for CD8 and with SYVPSAEQI-specific tetramers. Plots were analyzed on live lymphocytes and the numbers indicated represent the frequency of CD8+ tetramer+ T cells in total lymphocytes. (B) Spleen cells from immunized and nonimmunized recipient mice were analyzed by ELISPOT and tetramer staining on days 0, 2, 3, 4, 5, 6, 8, 10, 13, and 18 after immunization. Results are expressed as absolute number of SYVPSAEQI-specific CD8+ T cells per spleen calculated as the frequencies obtained by either ELISPOT (•, ○) or tetramer staining (▪, □ ) multiplied by the total number of cells obtained after spleen excision. Filled symbols represent data from immunized mice and open symbols represent data from nonimmunized mice.
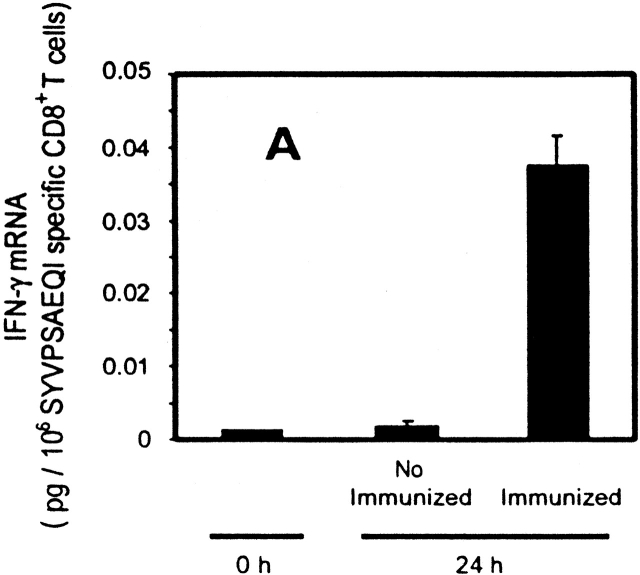
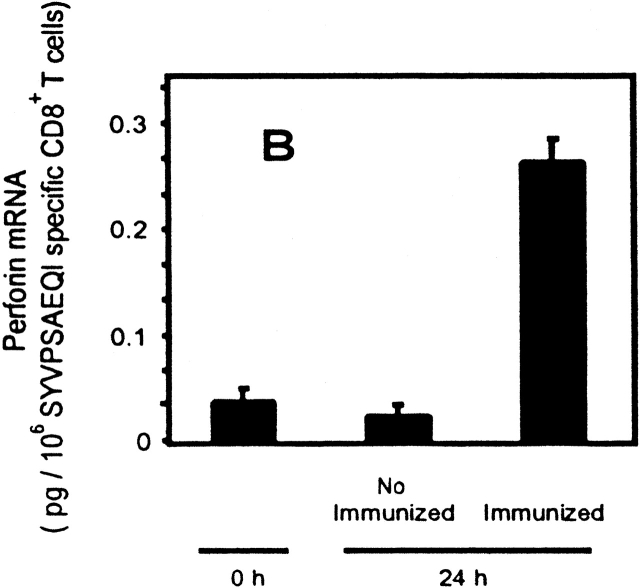
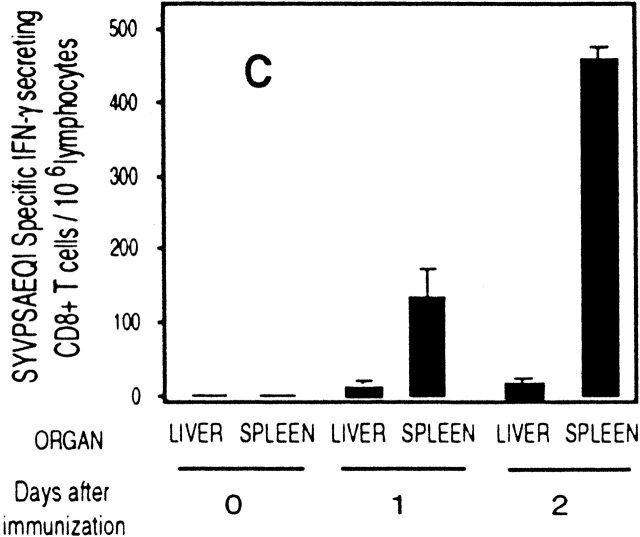
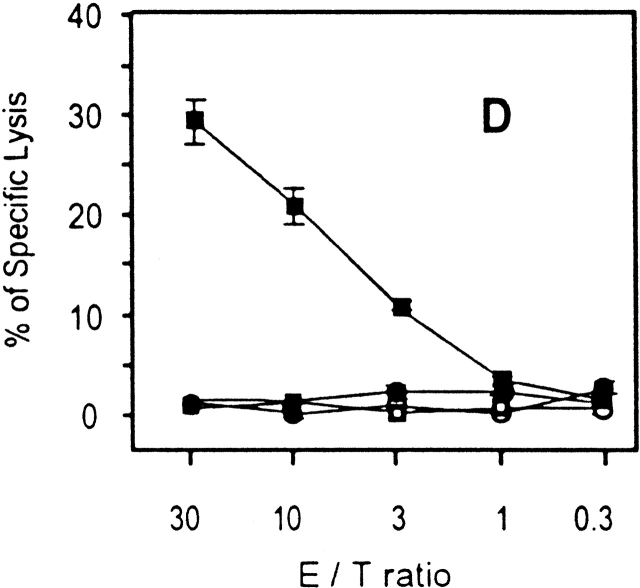
To further study the differentiation of naive CD8+ T cells to effector cells, we determined the transcriptional changes in the levels of both IFN-γ and perforin in naive cells after immunization. Total RNA was isolated from tetramer+ cells purified by magnetic beads 24 h after immunization. As analyzed by RT-PCR (Fig. 3a and Fig. b), a significant induction of IFN-γ and perforin mRNA is detected in SYVPSAEQI-specific CD8+ T cells at 24 h as compared with controls. Moreover, antigen-specific secretion is clearly detectable in an ex vivo ELISPOT assay using spleen cells of recipient mice immunized 24 h earlier (Fig. 3 C). Based on the frequency of tetramer+ cells determined by FACS® and the results of ELISPOT, we estimate that the population of cells secreting INF-γ at 24 h represents ∼2–4% of the SYVPSAEQI-specific T cells. Furthermore, ex vivo cytotoxic activity by SYVPSAEQI-specific CD8+ T cells is detectable 48 h immunization, as assessed by an ex vivo 51Cr release assay (Fig. 3 D). CD8+ T cells obtained from recipient mice that were not immunized did not secrete INF-γ and did not display cytotoxic activity. Since malaria sporozoites invade and develop in hepatocytes, we also studied the presence of activated CD8+ T cells in the liver during the course of immunization 20. We found that these cells are also detected in the liver 24 h after immunization, although their frequency is much lower than in the spleen (Fig. 3 C).
Figure 3.
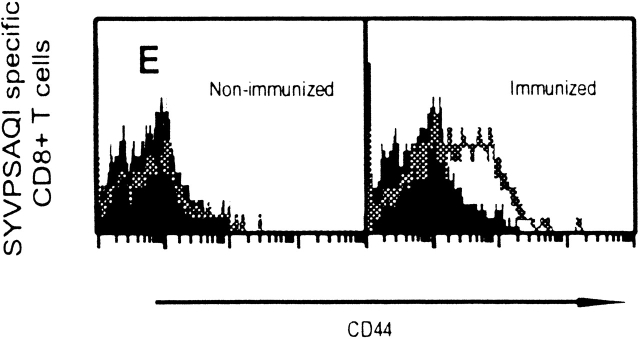
Rapid acquisition of effector functions and phenotypic activation in antigen-activated naive CD8+ T cells. (A and B) Tetramer+ spleen cells from immunized and nonimmunized recipient mice were isolated at 24 h and total mRNA was processed for RT-PCR to measure IFN-γ perforin and hypoxanthine ribosyltransferase (HPRT) as internal control (data not shown, and reference 15). The results are expressed as average ± SD of the amount of respective transcript per 106 tetramer+ cells. (C) Spleen and intrahepatic cells from immunized and nonimmunized (data not shown) recipient mice were isolated at 0, 24, and 48 h and analyzed by ELISPOT. Results are expressed as average ± SD of duplicate cultures. (D) 51Cr release assay using spleen cells from recipient mice obtained 48 h after immunization. Results are expressed as specific lysis at different target ratios of CD8+ T cells to SYVPSAEQI-coated target cells. Cells from immunized mice (▪, □) and nonimmunized mice (•, ○) were incubated 6 h with peptide-coated target cells (filled symbols) or target cells without peptide (open symbols). (E) Spleen cells from immunized and nonimmunized recipient mice stained for CD8 and CD44, and with SYVPSAEQI tetramers. Dark lines represent cells obtained at 0 h, while light lines represent cells obtained at 24 h. Histograms are gated on live CD8+ tetramer+ T cells.
Antigen-activated cells also undergo early surface phenotype changes. We determined by FACS® that the CD8+ tetramer+ T cells used for adoptive transfer display CD11alo, CD122lo, and CD44lo surface phenotype (data not shown), characteristic of naive cells 21 22 23. 24 h after immunization, ∼30% of the CD8+ tetramer+ cells already express higher levels of CD44 (Fig. 3 E), and 48–72 h later most of these T cells become CD11ahi CD122hi (data not shown).
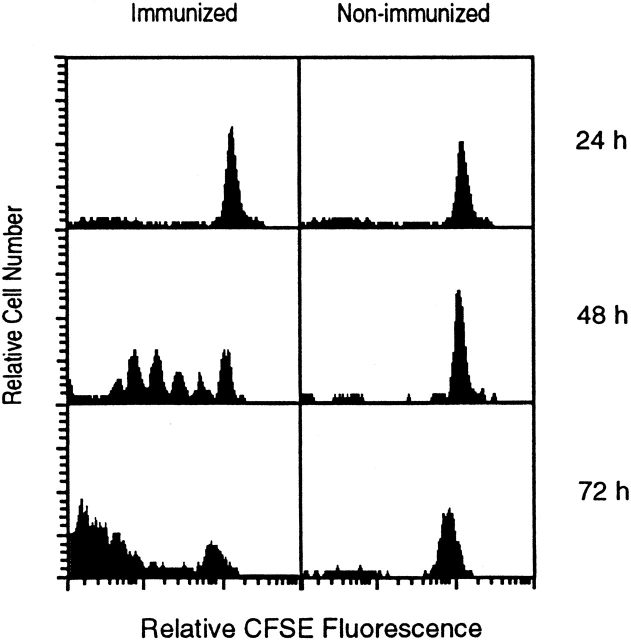
To determine the relationship between the proliferation of activated CD8+ T cells and the differentiation pattern described above, transgenic CD8+ T cells were labeled with CFSE 1 and transferred into normal mice that were immunized with radiation-attenuated sporozoites. Every 24 h, mice were killed and the proliferative status of the labeled CD8+ tetramer+ T cells was determined by evaluating the dilution of CFSE stain. As shown in Fig. 4, a massive proliferative activity of activated CD8+ tetramer+ cells is observed 48 h after immunization and it proceeds rapidly over the next 24 h. In the absence of immunization, the proliferation of these cells is negligible. It is worth noting that, as determined by FACS® (Fig. 2 A), a significant increase in the total number of SYVPSAEQI-specific CD8+ T cells is detected only after 48 h, thus suggesting that the proliferative activity initiated between 24 and 48 h involve a small number of cells.
Figure 4.
In vivo proliferation of antigen-activated naive CD8+ T cells. CFSE-labeled spleen cells from transgenic mice were transferred into normal mice and immunized with P. yoelii sporozoites. Spleen cells were isolated 24, 48, and 72 h after immunization and stained for CD8 and with SYVPSAEQI-tetramers. Spleen cells from nonimmunized mice were used as controls. Histograms are shown as CFSE dilution patterns gated on live lymphocytes and CD8+ tetramer+ T cells.
In Vivo Protective Activity of CD8+ T Cells.
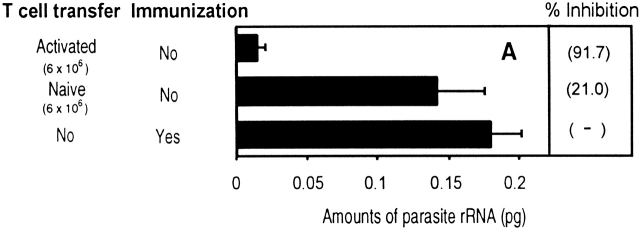
In view of these results, we compared the in vivo antiparasitic activity of naive and activated T cells. Activated/memory CD8+ T cells were obtained from recipient mice immunized with a recombinant vaccinia virus expressing the 9-mer epitope SYVPSAEQI 5. Equal numbers of naive or activated/memory CD8+ tetramer+ T cells were transferred into normal mice that were immediately challenged with viable P. yoelii sporozoites. After 40 h, the livers were excised and the parasite load determined using RT-PCR that measures parasite rRNA 11. As shown in Fig. 5 A, only mice which received activated/memory T cells were capable of strongly inhibiting parasite development.
Figure 5.
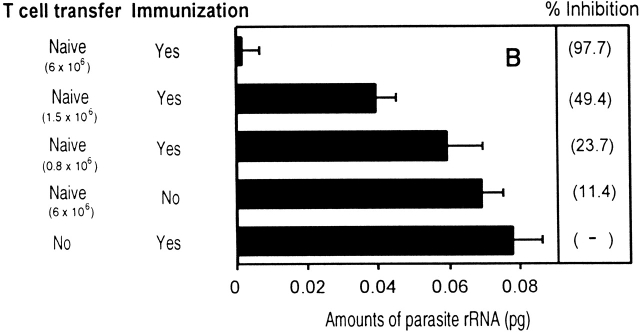
In vivo antiparasitic activity of CD8+ T cells. (A) Equal numbers of naive or activated/memory CD8+ tetramer+ T cells were transferred into normal mice and challenged with viable sporozoites. Activated/memory cells were obtained from recipient mice immunized with a recombinant vaccinia virus expressing the 9-mer SYVPSAEQI epitope at day 7. The parasite loads in the livers were measured by RT-PCR 40 h after challenge. The results are expressed as the average amount of parasite rRNA ± SD of three mice calculated based on competitor values. Numbers in parenthesis indicate the average percentage of inhibition compared with parasite load detected in normal mice. (B) The antiparasitic activity of activated CD8+ T cells is detectable in mice challenged 24 h after immunization. Mice receiving varying amounts of naive CD8+ tetramer+ T cells were immunized with attenuated sporozoites and 24 h later, challenged with viable sporozoites. The parasite loads in the livers were determined as described previously.
These results clearly demonstrate that naive CD8+ T cells are incapable of exerting antiparasitic activity unless they are previously activated by antigen. Surprisingly however, this activation process is very rapid. This was determined in experiments designed to define the minimal time required for naive CD8+ T cells to become capable of eliminating parasites. For this purpose, mice receiving naive cells were immunized with attenuated P. yoelii sporozoites and at various time points after immunization, mice were challenged with viable sporozoites and the parasite loads were measured 40 h later 11. Parasite development was not affected in mice challenged 12 h after immunization (data not shown). In contrast, a strong protective antiparasitic response was observed in mice challenged 24 h after immunization (Fig. 5 B). This protection closely depends on the number of cells transferred into mice, as the transfer 6 × 106 CD8+ tetramer+ T cells exerts a strong antiparasitic effect while four and eight times less T cells conferred a reduced or a nonsignificant degree of protection, respectively.
Taken together, the present results highlight the striking differences between naive and activated/memory CD8+ T cells. Naive cells require antigen priming and a differentiation period before developing the mechanisms allowing them to exert in vivo antiparasitic activity. This is in sharp contrast with activated/memory cells, which secrete IFN-γ only 6 h after incubation with antigen 17, and as demonstrated in this study, exert their protective activity immediately after parasite infection. These results support the notion that there are fundamental differences between naive and memory T cells regarding their pattern of activation and differentiation 1 2 23.
However, it was surprising that the time required for the generation of effector CD8+ T cells is rather short, as IFN-γ and perforin mRNA are detected 24 h after immunization, and IFN-γ secretion and cytotoxic activity are detected ex vivo 24 and 48 h after immunization, respectively. The speed at which these effector mechanisms are developed is not a result of T cells already activated before transfer, due to cross-reactivity with peptides of environmental origin or to nonspecific activation as observed in lymphopenic mice 24 25 26. In our system, we have clearly shown that transgenic T cells transferred into normal mice are naive as defined by their surface phenotype and their inability to produce IFN-γ or exert cytotoxic activity. Furthermore, we showed that transferred CD8+ T cells can be activated and proliferate in vivo only when recipient mice are immunized with P. yoelii sporozoites expressing the epitope SYVPSAEQI, but not with P. berghei sporozoites.
Our results indicating that the development of effector functions in naive CD8+ T cells begins in the absence of detectable proliferation suggests that differentiation of these cells is a process that precedes or develops simultaneously with cell proliferation. These findings challenge the current notion that CD8+ T cell differentiation occurs only after eight or more cell divisions 27. These also indicate that the limited efficacy of primary CD8+ T cell responses against malaria parasites or other pathogens is not due to the time it takes for naive cells to achieve an effector status. In fact, although activated/memory CD8+ T cells appear to exert their protective activity immediately after antigen recognition, naive CD8+ T cells are capable of developing this capacity only 24 h later. Therefore, it appears that a more important factor limiting the protective activity of primary CD8+ T cells responses is the low frequency of antigen-specific precursors present before immunization. This is indicated by the experiments in which we determined that while CD8+ T cell develop protective effector mechanisms 24 h after immunization, their antiparasitic activity strictly depended on the number of naive CD8+ T cell precursors transferred into mice before immunization.
Thus, as CD8+ T cell differentiation is induced rapidly, the magnitude and efficacy of primary and memory CD8+ T cell responses appears to be more closely determined by the total number of effectors cells capable of inhibiting parasite development. The number of these effector cells is greatly increased by the proliferation induced after antigen recognition, while it is severely reduced by mechanisms inducing massive cell death after the CD8+ T cell response reaches a peak. A better understanding of these mechanisms with opposite functional effects should facilitate their manipulation and thus, open new avenues for the design and development of vaccines against malaria and other intracellular pathogens.
Acknowledgments
We thank Drs. Ruth and Victor Nussenzweig for their support and encouragement. We thank Drs. Byron Waksman and Maria Curotto de Lafaille for their helpful critical discussions. We also thank Alex Chen for technical support.
Tetramers were provided by the Tetramer Core Facility of the National Institutes of Health (NIH)-National Institute of Allergic and Infectious Diseases. This work was supported by NIH grant AI44375.
Footnotes
Abbreviations used in this paper: CFSE, 5-carboxyfluorescein diacetate succinimidyl ester; RT, reverse transcription.
References
- Veiga-Fernandes H., Walter U., Bourgeois C., McLean A., Rocha B. Response of naïve and memory CD8+ T cells to antigen stimulation in vivo . Nat. Immunol. 2000;1:47–53. doi: 10.1038/76907. [DOI] [PubMed] [Google Scholar]
- Iezzi G., Karjalainen K., Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- Romero P., Maryanski J.L., Corradin G., Nussenzweig R.S., Nussenzweig V., Zavala F. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature. 1989;341:323–326. doi: 10.1038/341323a0. [DOI] [PubMed] [Google Scholar]
- Rodrigues M.M., Cordey A.S., Arreaza G., Corradin G., Romero P., Maryanski J.L., Nussenzweig R.S., Zavala F. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int. Immunol. 1991;3:579–585. doi: 10.1093/intimm/3.6.579. [DOI] [PubMed] [Google Scholar]
- Rodrigues M., Li S., Murata K., Rodriguez D., Rodriguez J.R., Bacik I., Bennink J.R., Yewdell J.W., Garcia-Sastre A., Nussenzweig R.S. Influenza and vaccinia viruses expressing malaria CD8+ T and B cell epitopes. Comparison of their immunogenicity and capacity to induce protective immunity. J. Immunol. 1994;153:4636–4648. [PubMed] [Google Scholar]
- Rodrigues E.G., Zavala F., Eichinger D., Wilson J.M., Tsuji M. Single immunizing dose of recombinant adenovirus efficiently induces CD8+ T cell-mediated protective immunity against malaria. J. Immunol. 1997;158:1268–1274. [PubMed] [Google Scholar]
- Tsuji M., Bergmann C.C., Takita-Sonoda Y., Murata K., Rodrigues E.G., Nussenzweig R.S., Zavala F. Recombinant Sindbis viruses expressing a cytotoxic T-lymphocyte epitope of a malaria parasite or of influenza virus elicit protection against the corresponding pathogen in mice. J. Virol. 1998;72:6907–6910. doi: 10.1128/jvi.72.8.6907-6910.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedegah M., Hedstrom R., Hobart P., Hoffman S.L. Protection against malaria by immunization with plasmid DNA encoding circumsporozoite protein. Proc. Natl. Acad. Sci. USA. 1994;91:9866–9870. doi: 10.1073/pnas.91.21.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouskoff V., Signorelli K., Benoist C., Mathis D. Cassette vectors directing expression of T cell receptor genes in transgenic mice. J. Immunol. Methods. 1995;180:273–280. doi: 10.1016/0022-1759(95)00002-r. [DOI] [PubMed] [Google Scholar]
- Altman J.D., Moss P.A.H., Goulder P.J.R., Barouch D.H., McHeyzer-Williams M.G., Bell J.I., McMichael A.J., Davis M.M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- Briones M.R.S., Tsuji M., Nussenzweig V. The large difference in infectivity for mice of Plasmodium berghei and Plasmodium yoelii sporozoites cannot be correlated with their ability to enter into hepatocytes. Mol. Biochem. Parasitol. 1996;77:7–17. doi: 10.1016/0166-6851(96)02574-1. [DOI] [PubMed] [Google Scholar]
- Miyahira Y., Murata K., Rodriguez D., Rodriguez J.R., Esteban M., Rodrigues M.M., Zavala F. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J. Immunol. Methods. 1995;181:45–54. doi: 10.1016/0022-1759(94)00327-s. [DOI] [PubMed] [Google Scholar]
- Prud'homme G.J., Kono D.H., Theofilopoulos A.N. Quantitative polymerase chain reaction analysis reveals marked overexpression of interleukin-1β, interleukin-1 and interferon-γ mRNA in the lymph nodes of lupus-prone mice. Mol. Immunol. 1995;32:495–503. doi: 10.1016/0161-5890(95)00024-9. [DOI] [PubMed] [Google Scholar]
- Borson N.D., Strausbauch M.A., Kennedy R.B., Oda R.P., Landers J.P., Wettstein P.J. Temporal sequence of transcription of perforin, Fas ligand, and tumor necrosis factor-α genes in rejecting skin allografts. Transplantation. 1999;67:672–680. doi: 10.1097/00007890-199903150-00006. [DOI] [PubMed] [Google Scholar]
- Svetic A., Finkelman F.D., Jian Y.C., Dieffenbach C.W., Scott D.E., McCarthy K.F., Steinberg A.D., Gause W.C. Cytokine gene expression after in vivo primary immunization with goat antibody to mouse IgD antibody. J. Immunol. 1991;147:2391–2397. [PubMed] [Google Scholar]
- Zimmerman C., Brduscha-Riem K., Blaser C., Zinkernagel R.M., Pircher H. Visualization, characterization, and turnover of CD8+ memory T cells in virus-infected hosts. J. Exp. Med. 1996;183:1367–1375. doi: 10.1084/jem.183.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali-Krishna K., Altman J.D., Suresh M., Sourdive D.J.D., Zajac A.J., Miller J.D., Slansky J., Ahmed R. Counting antigen-specific CD8 T cellsa re-evaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- Busch D.H., Pilip I., Pamer E.G. Evolution of a complex T cell receptor repertoire during primary and recall bacterial infection. J. Exp. Med. 1998;188:61–70. doi: 10.1084/jem.188.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belz G.T., Altman J.D., Doherty P.C. Characteristics of virus-specific CD8+ T cells in the liver during the control and resolution phases of influenza pneumonia. Proc. Natl. Acad. Sci. USA. 1998;95:13812–13817. doi: 10.1073/pnas.95.23.13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens P.L., Jouin H., Marchal G., Milon G. Isolation and flow cytometric analysis of the free lymphomyeloid cells present in murine liver. J. Immunol. Methods. 1990;132:137–144. doi: 10.1016/0022-1759(90)90407-m. [DOI] [PubMed] [Google Scholar]
- Zhang X., Sun S., Hwang I., Tough D.F., Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- Shen H., Miller J.F., Fan X., Kolwyck D., Ahmed R., Harty J.T. Compartmentalization of bacterial antigensdifferential effects on priming of CD8 T cells and protective immunity. Cell. 1998;92:535–545. doi: 10.1016/s0092-8674(00)80946-0. [DOI] [PubMed] [Google Scholar]
- Cho B.K., Wang C., Sugawa S., Eisen H.N., Chen J. Functional differences between memory and naive CD8 T cells. Proc. Natl. Acad. Sci. USA. 1999;96:2976–2981. doi: 10.1073/pnas.96.6.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldrath A.W., Bogatzki L.Y., Bevan M.J. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J. Exp. Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho B.K., Rao V.P., Ge Q., Eisen H.N., Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J. Exp. Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali-Krishna K., Ahmed R. Cutting edgenaive T cells masquerading as memory cells. J. Immunol. 2000;165:1733–1737. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- Bevan M.J., Fink P.J. The CD8 response on autopilot. Nat. Immunol. 2001;2:381–382. doi: 10.1038/87676. [DOI] [PubMed] [Google Scholar]