Abstract
Gene targeting of the adaptor molecule DAP12 in mice caused abnormal distribution and impaired antigen presentation capacity of dendritic cells (DCs). However, the DAP12-associated receptors expressed on DCs and their functions have not been identified yet. Here we show that the triggering receptor expressed on myeloid cells-2 (TREM-2) is a cell surface receptor on human monocyte-derived DCs, which is associated with DAP12. TREM-2/DAP12 promotes upregulation of CC chemokine receptor 7, partial DC maturation, and DC survival through activation of protein tyrosine kinases and extracellular signal–regulated kinase. In contrast to Toll-like receptor-mediated signaling, TREM2/DAP12 stimulation is independent of nuclear factor-κB and p38 stress-activated protein kinase. This novel DC activation pathway may regulate DC homeostasis and amplify DC responses to pathogens, explaining the phenotype observed in DAP12-deficient mice.
Keywords: TREM, activation, human, survival, migration
Introduction
Dendritic cells (DCs) are a distinct population of bone marrow–derived leukocytes that initiate primary and secondary immune responses 1. DCs migrate from the blood to peripheral tissues, where they reside in an immature state, awaiting antigen encounter. Upon antigen capture, DCs process them into peptides, which are loaded onto MHC molecules for presentation to T cells. As a result of pathogen invasion, inflammation, and tissue damage, DCs receive additional activating signals, which induce a profound change in DC phenotype and functions, known as maturation 1 2 3. Mature DCs express the chemokine receptor CCR7, which interacts with the chemokines CCL19 (also known as EBI-1 ligand chemokine [ELC], or macrophage inflammatory protein 3 β [MIP-3β]) and CCL21 (also known as secondary lymphoid tissue chemokine [SLC], or 6-C-Kine) 4 5 6 7 8 9. These chemokines are crucial for guiding DCs from peripheral tissues to draining lymph nodes, as demonstrated in mice with natural or targeted genetic deletions of CCL19, CCL21, or CCR7 10 11 12 13 14. In addition, mature DCs express high levels of stable MHC-peptide complexes on the cell surface, upregulate costimulatory and adhesion molecules, and downregulate antigen-capturing molecules. Thus, mature DCs can efficiently present antigens and stimulate virgin-T cells located in the T cell–rich areas of lymph nodes 1 15. Here, DCs receive further activating signals from cognate Th cells, which express CD40 ligand (CD40L) 16, OX40 17 18, and TNF-related activation-induced cytokine (TRANCE) 19 20 21 22. These stimuli trigger IL-12 secretion by antigen presenting DCs thus promoting Th1 type T cell responses 20 23 24 25 26 27.
Activating signals induce DC maturation through several distinct signaling pathways. LPS, other bacterial and viral components, as well as products released by damaged tissues, activate DCs through Toll-like receptors (TLRs) 28 29. TLRs trigger downstream signaling pathways, which activate the nuclear factor (NF)-κB. NF-κB promotes the transcription of a variety of genes mediating maturation 30 31 32 33. In addition, TLRs activate mitogen-activated protein kinases (MAPKs), such as the stress-activated protein kinase p38 (P38/SAPK) and the extracellular signal–regulated kinase (ERK), which concur to DC activation 28 29. Inflammatory cytokines, such as IL-1, IL-18, and TNF-α, and T cell surface molecules, such as CD40L, OX40, and TRANCE, bind specific receptors that activate NF-κB as well 1 20 34 35 36 37. A second pathway of DC maturation is initiated by the receptors for the Fc portion of IgG (FcRs), which bind antibody-opsonized pathogens 38. FcRs lack intracellular signaling motifs, but display a charged residue in the transmembrane domain, which mediates association with the γ chain of FcR (FcRγ) 39. FcRγ contains a cytoplasmic immunoreceptor tyrosine-based activation motif (ITAM), which recruits protein tyrosine kinases (PTKs) of the src and syk families. PTKs trigger protein tyrosine phosphorylation, Ca2+ mobilization, and phosphorylation of several MAPKs 40 41. While FcRγ is essential for FcR-mediated DC maturation 42, the role of downstream PTKs and MAPKs is yet unknown.
Recent observations suggest that DC activation is controlled by yet another signaling pathway, which involves the adaptor molecule DAP12 (also called KARAP). DAP12 is associated with several NK and myeloid cells activating receptors 43 44 45 46 47 48 49 50 51 52 53 54. Like FcRγ, DAP12 contains a cytoplasmic ITAM, recruits the PTKs ZAP70 and p72/syk, and promotes activation of ERK 44 45 55 56. Knock-in mice bearing a nonfunctional mutation within the ITAM of DAP12 showed a dramatic accumulation of DCs in mucocutaneous epithelia and were resistant to hapten-specific contact sensitivity 57. In addition, DAP12-deficient mice were resistant to experimental autoimmune encephalomyelitis (EAE) induced by immunization with myelin oligodendrocyte glycoprotein peptide 58. These phenotypes suggested a role of DAP12 in regulating migration and antigen presentation capacity of DCs. Three DAP12-associated receptors have been identified in myeloid cells. One of these, myeloid DAP12-associating lectin-1 (MDL-1), is a member of the C-type lectin superfamily 50. The others, signal-regulatory protein β (SIRP-β) and triggering receptor expressed on myeloid cells-1 (TREM-1), belong to the Ig superfamily 53 59. TREM-1 is preferentially expressed on neutrophils and a subset of blood monocytes 53. SIRP-β and MDL-1 are mainly expressed on blood monocytes and macrophages 50 60. When monocytes are differentiated toward DCs by culturing them in vitro in the presence of GM-CSF and IL-4, expression of MDL-1, SIRP-β, and TREM-1 is completely downregulated 50 53 60.
Recently, we have cloned a cell surface receptor distantly related to TREM-1 called TREM-2. TREM-2 is a member of the Ig-superfamily characterized by a single V-type extracellular domain, a transmembrane region with a charged residue of lysine and a short cytoplasmic tail with no signaling motifs 53. Here we found that TREM-2 is associated with DAP12 and, in contrast to TREM-1, SIRP-β, and MDL-1, is not expressed on monocytes, but it is strongly upregulated on human DCs derived in vitro from monocytes. This observation provided the opportunity to investigate the role of TREM2/DAP12-mediated signaling pathways in DC migration and maturation.
Materials and Methods
Production of TREM-2 Human IgM Fusion Protein.
Soluble TREM-2 was produced as a chimeric protein consisting of TREM-2 extracellular domain and human IgM constant regions (TREM-2 human IgM [TREM-2-HuIgM]), as previously described 61. TREM-2 extracellular domain was amplified from the cloned full length cDNA by polymerase chain reaction using the following oligonucleotides: 5′-ACTCTGCTTCTGCCCTTGGCTGGGG, 3′-tagtagGTCGACATACTTACCGGGTGGGAAAGGGATTTCTCCTTCCAA. Purification of TREM-2-HuIgM from culture supernatants was performed by affinity chromatography on Sepharose-coupled mouse anti–human IgM mAb (Sigma-Aldrich) according to manufacturer's protocols.
Transfections.
293 cells were transiently transfected with a cDNA encoding human TREM-2 as a FLAG peptide NH2-terminal fusion protein (Eastman Kodak Co.) using cytofectene (Bio-Rad Laboratories).
Production and Modifications of Anti–TREM-2 and Control mAbs.
6-wk-old BALB/c mice (Iffa-Credo) were immunized with purified TREM-2-HuIgM. Spleen cells were fused with the SP2/0 myeloma cells and hybridoma supernatants were screened by ELISA using TREM-2-HuIgM as capturing protein and human-adsorbed horseradish peroxidase (HRP)-labeled goat anti–mouse IgG (BD PharMingen) as detecting Ab. ELISA-positive hybridoma supernatants were then tested by flow cytometry for staining 293 cells expressing FLAG-tagged TREM-2. mAb 29E3 (anti–TREM-2, IgG1,κ), mAb 21C7 (control IgG1, κ, anti–TREM-1) 53, and mAb 1B7.11 (control IgG1, κ, anti-2,4,6 TNP; American Type Culture Collection) were purified using GammaBind-Sepharose (Amersham Pharmacia Biotech). Purified mAbs were either biotinylated (Roche) or labeled with Cy5 (Amersham Pharmacia Biotech) according to manufacturer's protocols. F(ab′) or F(ab′)2 fragments of mAb 29E3 and mAb 21C7 were prepared using the F(ab′)/F(ab′)2 Kit (Pierce Chemical Co.). F(ab′) and F(ab′)2 were separated from the Fc portion by affinity chromatography on protein G-sepharose, followed by gel filtration on a Superdex 75 HR10/30 (Amersham Pharmacia Biotech). F(ab′) and F(ab′)2 preparations were tested for the absence of Fc fragments by immunoassay. F(ab′) and F(ab′)2 fragments were biotinylated allowing for crosslinking by ExtrAvidine (Sigma-Aldrich) or flow cytometry by Streptavidin-allophycocyanin (APC) or -PE (BD PharMingen). Alternatively, F(ab′)2 fragments were crosslinked using a goat anti–mouse IgG F(ab′)2 specific antibody (The Jackson Laboratory).
Cells.
PBMCs were purified from human blood by gradient density centrifugation on lymphocyte separation medium (LSM; ICN Biomedicals/Cappel). CD14+ monocytes were purified from PBMCs by magnetic cell sorting (MACS) using CD14 MicroBeads (Miltenyi Biotec). Monocyte-derived DCs were prepared from purified monocytes as described previously 62 63.
Antibodies and Flow Cytometry.
Before staining, all cells were preincubated with PBS-20% human serum for 1 h on ice to block Fc receptors (FcR). Monocytes cultured in M-CSF or GM-CSF and IL-4 were stained with either mAb 29E3, mAb 21C7, or mAb 1B7.11, followed by human-adsorbed PE-conjugated goat anti–mouse IgG (Southern Biotechnology Associates, Inc.). In three-color stainings, immature DCs cultured with LPS (100 ng/ml), TNF-α (10 ng/ml), or CD40L-transfected J558L cells 64 were incubated with Cy5-labeled mAbs 29E3 and FITC-conjugated anti-CD83 mAb (Immunotech). Cells were analyzed on a FACSCalibur™ cytometer using CELLQuest™ software (Becton Dickinson). Dead cells were excluded by gating on PI-negative cells.
Stimulation of DCs by LPS, F(ab′)2 Anti–TREM-2 mAb, or Human IgG in the Presence or Absence of Inhibitors.
Human IgG, F(ab′)2 29E3 (anti–TREM-2 mAb), or control F(ab′)2 (21C7 anti–TREM-1 mAb) were coated for 6 h at 37°C on 96-well flat-bottom plates with a final concentration of 20 μg/ml in PBS. LPS was used at a final concentration of 1 μg/ml. Immature DCs were plated at a concentration of 5 × 105 cells/well and simultaneous contact to the plate was induced by short centrifugation (400 g, 1 min, 25°C). Supernatants and cells were collected after 6, 12, 24, 36, 48, or 72 h and tested by ELISA or flow cytometry. In blocking experiments, inhibitors (PD98059 [20 μM], LY294002 [10 μM], SB203580 [2 μM], PP2 [1 μM]; all from Calbiochem), and TPCK (20 μM; Sigma-Aldrich) were added 60 min before stimulation.
Measurement of Cytokines, Chemokines, and Cell Surface Activation Markers.
To measure stimulation-dependent changes in the expression of cell surface markers and cytokine secretion, monocyte-derived DCs were stimulated as described above for 6, 12, 24, 48, and 72 h. Supernatants were collected and tested for production of IL-6, IL-8, IL-10, TGF-β, IL-12p40, IL-12p75, IL-13, IL-15, IL-18, IL-1α, IL-1β, TNF-α, and MCP-1 by ELISA (BD PharMingen). Cells were harvested and stained with anti–TREM-2, -MHC class I, -MHC class II, -CD1a, -CD11a, -CD11b, -CD11c, -CD29, -CD32, -CD35, CD38-, CD40-, -CD41, -CD54, -CD61, -CD64, -CD80, -CD83, -CD86, -CD89, -CD103, -CD115, -CD116, -CCR5, -CCR6, -CXCR4, or -Mannose receptor conjugated with Cy5-, PE-, or FITC (all from Immunotech and BD PharMingen). Anti-CCR7 mAb (BD PharMingen) was followed by F(ab′)2 PE-labeled goat anti–mouse IgM Ab (Southern Biotechnology Associates, Inc.). Stained cells were analyzed by flow cytometry.
Measurement of Cytosolic Ca2+.
Monocyte-derived DCs were loaded with Indo-1 AM (Sigma-Aldrich) for 30 min at 37°C, washed three times, and resuspended in RPMI/10 mM HEPES/5% FCS. Cytoplasmic Ca2+ levels were monitored in individual cells by measuring 405/525 spectral emission ratio of loaded Indo-1 dye by flow cytometry. After a baseline was acquired for at least 30 s, 29E3, 21C7, F(ab′) 29E3, F(ab′) 21C7, F(ab′)2 29E3, or F(ab′)2 21C7 were added to a final concentration of 1 μg/ml and analysis was continued up to 512 s. All antibodies and antibody fragments were biotinylated. In some experiments, ExtraAvidine (Sigma-Aldrich) was added as crosslinker together with the biotinylated primary antibodies or Ab fragments.
Determination of ERK, JNK, and p38/SAPK Activation.
Monocyte-derived DCs (106 cells per time point) were stimulated as described above. After 0 (unstimulated control), 1, 2, 5, 10, and 20 min cells were harvested and chilled on ice. Cells were spun down and lysed in reducing sample buffer. Specific induction of tyrosine phosphorylation and phosphorylation of ERK, p38/SAPK, and JNK was determined by reducing Western blot analysis using anti–phospho-ERK, anti-ERK, anti–phospho-p38/SAPK, anti-p38/SAPK, anti-phospho-JNK, and anti-JNK antibodies (all from New England Biolabs, Inc.).
Surface Biotinylation and Pervanadate Treatment.
Monocyte-derived DCs were washed three times in PBS followed by incubation with sulfo-NHS-biotin according to the manufacturer's protocol (Pierce Chemical Co.). For pervanadate treatment, cells were incubated with 200 μM pervanadate and 200 μM H2O2 at 37°C for 5 min. Biotinylation or pervanadate stimulation was stopped by washing the cells three times in PBS/10% FCS/200 μM pervanadate and one time with ice cold PBS/200 μM pervanadate, respectively.
Immunoprecipitations.
Surface-biotinylated cells were lysed in 1% digitonin, 100 mM Tris-HCl, pH 7.4, 150 mM NaCl, protease inhibitors (Complete; Roche Molecular Biochemicals). After overnight preclearing with normal mouse serum coupled to protein G Sepharose 4B (Amersham Pharmacia Biotech), lysates were subjected to immunoprecipitation with 5 μg/ml of 29E3, 21C7, or 1B7.11 at 4°C for 3 h. Immunocomplexes were precipitated by addition of protein-G-Sepharose 4B for 3 h at 4°C. Precipitates were washed four times with lysis buffer, followed by a final wash with 0.5% digitonin, 100 mM Tris-HCl, pH7.4, 150 mM NaCl. After separation by SDS-PAGE, precipitates were analyzed by Western blot with HRP-conjugated streptavidin. In deglycosylation experiments the precipitates were incubated for 18 h with or without N-Glycanase F (Roche) according to the manufacturer's protocol. Pervanadate-treated cells were subjected to immunoprecipitation as described above. Immunoprecipitates were analyzed by Western blot with antiphosphotyrosine PY20-HRP (Transduction Laboratories) or anti-DAP12 rabbit antiserum followed by human/mouse-adsorbed anti–rabbit IgG-HRP (Southern Biotechnology Associates, Inc.).
Chemotaxis Assay.
Monocyte-derived human DCs (107) were treated for 24 h with F(ab′)2 21C7, F(ab′)2 29E3 coated on plastic (20 μg/ml), or LPS (1 μg/ml). Cells (5 × 105 in 100 μl IMDM/0.5% BSA) were incubated for 1 h at 37°C. Cells were subsequently loaded into collagen-coated Transwells (Costar; 3-μm pore filter), which were placed onto 24-well plates containing 450 μl medium supplement with 100 ng/ml CCL19 (ELC/MIP-3β) or CCL20 (6-C-Kine/SLC) (both from PeproTech). After an incubation period of 4 h at 37°C, cells that had migrated to the lower chamber were collected and counted on a cytofluorimeter (FACSCalibur™, constant time acquisition; Becton Dickinson). In blocking experiments cells were preincubated with anti-CCR7 mAb (10 μg/ml) and added to the Transwell.
Detection of Apoptosis.
Determination of DNA fragmentation was performed as described previously 65. Inhibitors of kinases or serine proteases (PD98059 [20 μM], LY294002 [10 μM], TPCK [20 μM]) were added 60 min before stimulation. Inhibitors had no effect on cell viability or the rate of constitutive apoptosis at the indicated concentrations.
Nuclear Extracts and Electrophoretic Mobility Shift Assays.
Nuclear extracts were prepared according to the method of Schreiber et al. 66 with some modifications. Stimulation of monocyte-derived human DCs (107) with control or anti–TREM-2 antibody or with LPS was performed for 0.5 or 4 h at 37°C as described above. Cells were washed in PBS, resuspended in 10 ml of ice-cold buffer A (10 mM Tris-HCl, pH 7.9, 60 mM KCl, 1 mM EDTA, 0.75 mM spermidine, 0.15 mM spermine, 1 mM DTT, 0.5 mM PMSF, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 μg/ml pepstatin), and incubated for 15 min on ice. Nonidet P-40 was added from a 10% stock solution to a final concentration of 0.6%, and samples were vortexed for 10 s. After incubation for 3 min on ice, samples were centrifuged at 3,000 rpm for 10 min at 4°C. Nuclei were washed in 10 ml of ice-cold buffer A and resuspended in 30 ml of ice-cold buffer C (20 mM Tris-HCl, pH 8, 0.4 M NaCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM DTT, 0.5 mM PMSF, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, and 25% glycerol). Nuclei were incubated for 30 min at 4°C, and nuclear extracts were separated from debris by centrifugation at 15,000 g for 15 min at 4°C. Protein concentrations were determined by Bradford assay using Bio-Rad protein assay (Bio-Rad Laboratories). NF-κB consensus and mutant binding sites were 5′-AGTTGAGGGGACTTTCCCAGGC-3′ and 5′-AGTTGAGGCGACTTTCCCAGGC-3′, respectively. Annealed binding sites were radiolabeled using polynucleotide T4 kinase and γ[32P]-ATP. Radiolabeled oligonucleotides were purified by electrophoresis through an 8% polyacrylamide gel containing 22.5 mM Tris-borate and 0.5 mM EDTA, overnight elution from gel slices at 37°C, concentration using Elutip-d columns (Schleicher & Schuell), and ethanol precipitation. Electrophoretic mobility assays (EMSAs) were performed as described previously 67 with some modifications. Nuclear extracts (2 μg) were incubated with 1 μg of poly(dI-dC) carrier and 1 μg of BSA in a 25 μl reaction mix containing 10 mM Tris-HCl, pH 7.5, 50 mM NaCl, 1 mM DTT, 1 mM EDTA, and 5% glycerol for 10 min at 4°C in the presence or absence of 25-fold excess of unlabeled oligonucleotide competitors. Labeled binding-site probes (15 fmols, ∼5 × 104 cpm) were then added for an additional 20 min of incubation at 4°C. Samples were electrophoresed through a 4% polyacrylamide gel containing 22.5 mM Tris-borate and 0.5 mM EDTA at 4°C.
Results
Human Immature Monocyte-derived DCs Express TREM-2, a ∼40 kD Glycoprotein which Is Associated with DAP12.
In initial studies, TREM-2 transcript was selectively detected in monocyte-derived DCs by reverse transcriptase (RT)-PCR (data not shown). To precisely investigate the cellular distribution of TREM-2 as well as its biochemical characteristics and functions, we produced an anti–TREM-2 mAb (29E3). This antibody stained TREM-2–transfected 293 cells specifically, compared with control transfectants (Fig. 1 A). In agreement with RT-PCR data, TREM-2 was highly expressed on DCs derived from peripheral blood monocytes upon in vitro culture with GM-CSF and IL-4 (Fig. 1 B). DC maturation induced by LPS, TNFα, CD40L-expressing cells (Fig. 1 C), IL-1β, CpG oligonucleotides, or aggregated IgG (data not shown) led to complete downregulation of TREM-2. TREM-2 was undetectable on macrophages obtained by culturing monocytes up to 14 d with M-CSF (Fig. 1 B) and on primary DCs of peripheral blood (data not shown). Thus, TREM-2 is preferentially expressed on immature monocyte-derived DCs.
Figure 1.
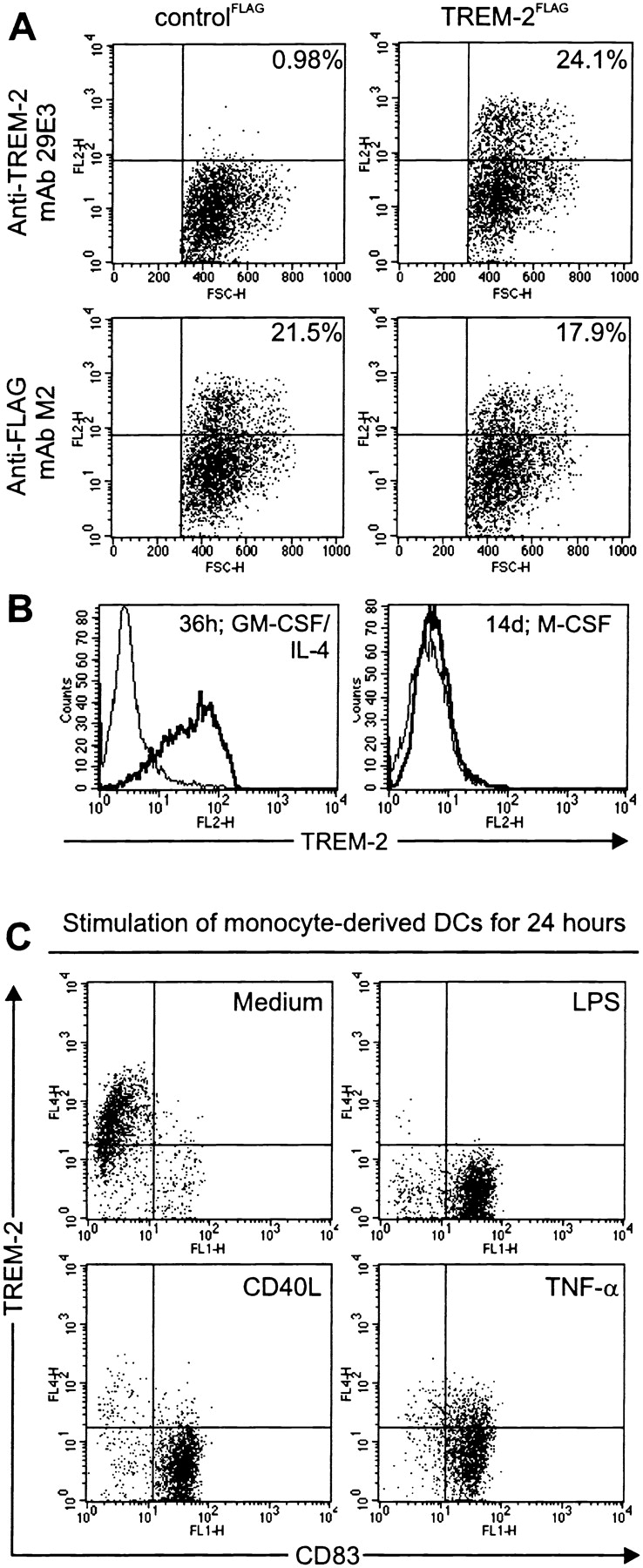
TREM-2 is selectively expressed on immature DCs. (A) mAb 29E3 recognizes selectively TREM-2. 293 cells transfected with a cDNA encoding FLAG-tagged TREM-2 (TREM-2FLAG; right panels) were stained with mAb 29E3 (top panel), compared with cells transfected with a control cDNA (controlFLAG; left panels). The percentages of positive cells (top right quadrants) are indicated. Expression of TREM-2FLAG and controlFLAG was confirmed using an anti-FLAG mAb (bottom panels). Cells stained with an isotype-matched control mAbs were comprised within the indicated bottom quadrant. (B) TREM-2 is strongly upregulated after stimulation of monocytes with GM-CSF and IL-4. Monocytes treated with GM-CSF/IL-4 (left panel) or M-CSF (right panel) were analyzed by flow cytometry for cell surface expression of TREM-2 (solid bold line) after 36 h or up to 14 d, respectively. Dashed profiles indicate background staining with a control IgG1 mAb. (C) TREM-2 is rapidly downregulated upon maturation of DCs. LPS- (top right panel), CD40L- (bottom left panel), TNFα-stimulated (bottom right panel), or unstimulated monocyte-derived DCs (top left panel) were analyzed by flow cytometry for cell surface expression of TREM-2 and CD83 after 36 h. Cells stained with an isotype-matched control mAbs were comprised within the indicated bottom quadrants.
Immunoprecipitation of TREM-2 from surface-biotinylated monocyte-derived DCs revealed that TREM-2 is a glycoprotein of ∼40 kD, that is reduced to 26 kD after N-deglycosylation (Fig. 2 A). This result is in agreement with the predicted molecular mass of TREM-2 53. As TREM-2 lacks known signaling motifs in the cytoplasmic domain and displays a charged residue of lysine in the transmembrane domain 53, it was likely to be associated with a separate adaptor molecule to transduce signals. Adaptor molecules, such as DAP12, DAP10, and FcRγ are tyrosine phosphorylated upon cell treatment with the phosphatase inhibitor pervanadate 44 68 69. Indeed, anti-phosphotyrosine blotting of TREM-2 immunoprecipitates from pervanadate-stimulated monocyte-derived DCs revealed a phosphorylated protein of ∼14 and ∼28 kD under reducing and nonreducing conditions, respectively (Fig. 2 B). This pattern was consistent with the association of TREM-2 with a tyrosine-phosphorylated protein that forms a disulfide-linked homodimer. Immunoblotting of TREM-2 immunoprecipitates with anti-DAP12, -DAP10, and -FcRγ antisera demonstrated that TREM-2, like TREM-1, associates only with DAP12 (Fig. 2 C, and data not shown). Thus, TREM-2 is capable of stimulating DAP12-linked signaling pathways in DCs.
Figure 2.
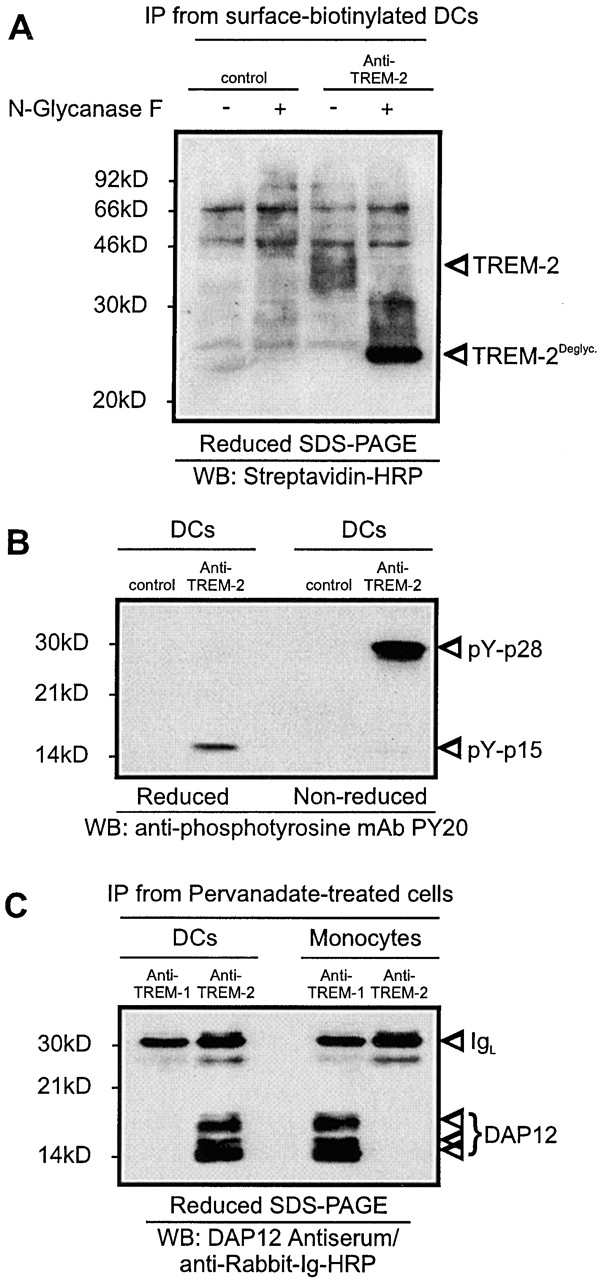
TREM-1 is a 40-kD glycoprotein associated with the adaptor protein DAP12. (A) Surface-biotinylated monocyte-derived DCs were lysed and subjected to immunoprecipitation with 29E3 anti–TREM-2 mAb (right lanes) or control IgG1 mAbs (21C7 anti–TREM-1 mAb). Immunoprecipitates (IP) were left untreated or treated with N-glycanase F and analyzed by Western blot analysis with Streptavidine-HRP. Molecular weight markers and specific protein bands are indicated. (B) Pervanadate-treated monocyte-derived DCs were subjected to immunoprecipitation with 29E3 anti–TREM-2 mAb or control IgG1 mAb (21C7 anti–TREM-1 mAb). The precipitates were analyzed by antiphosphotyrosine blot under reducing (left lanes) and nonreducing (right lanes) conditions. Tyrosine phosphorylated proteins are marked by arrows. Molecular weight markers are indicated. (C) Anti-DAP12 blot analysis of TREM-2 immunoprecipitate from monocyte-derived DCs (left lanes) and monocytes (right lanes) after pervanadate stimulation (reducing conditions). TREM-1 immunoprecipitates from monocytes and monocyte-derived DCs were included as positive and negative controls, respectively. Molecular weight markers and specific protein bands are indicated.
TREM-2 Induces ERK Activation and Survival of DCs.
To see whether the TREM-2/DAP12 complex transduces activating signals in DCs as other DAP12-associated receptors do in NK cells and neutrophils 43 44 45 47 48 49 50 51 52 53 54 56 70, we stimulated TREM-2 with 29E3 mAb or with its F(ab′)2 fragment. In both cases, ligation of TREM-2 elicited a rapid rise in intracellular Ca2+ concentration of DCs (Fig. 3 B). However, monovalent engagement of TREM-2 using F(ab′) 29E3 did not induce calcium mobilization, indicating that TREM-2–mediated activation needs at least two or more receptors crosslinked (data not shown). Cross-linking of TREM-2 with F(ab′)2 29E3 stimulated tyrosine phosphorylation of several proteins with approximate molecular masses of ∼110, ∼90, ∼60–70, and ∼30–40 kD (Fig. 3 C). The observed ∼40 kD tyrosine phosphorylated proteins corresponded to the ERK1/2, as demonstrated by antiphospho-ERK1/2 immunoblotting (Fig. 3 D). It was previously shown that survival of LPS-stimulated DCs is dependent on ERK 71 and phosphatidylinositol 3-kinase (PI-3K) 72, while maturation is mainly mediated through NF-κB. Therefore, we tested whether stimulation of TREM-2 leads to prolonged survival of DCs kept in culture in the absence of GM-CSF or IL-4. As shown in Fig. 3 E, crosslinking of TREM-2 with F(ab′)2 29E3 prolonged DC survival for almost 8 d. Treatment of TREM-2–stimulated DCs with the ERK inhibitor PD98059 blocked this survival effect. Inhibitors of PI-3K, IκB-phosphorylation, or IκB-degradation had no effect (Fig. 3 F, and data not shown). These observations indicate that TREM-2 induces survival of DCs through activation of the ERK pathway.
Figure 3.
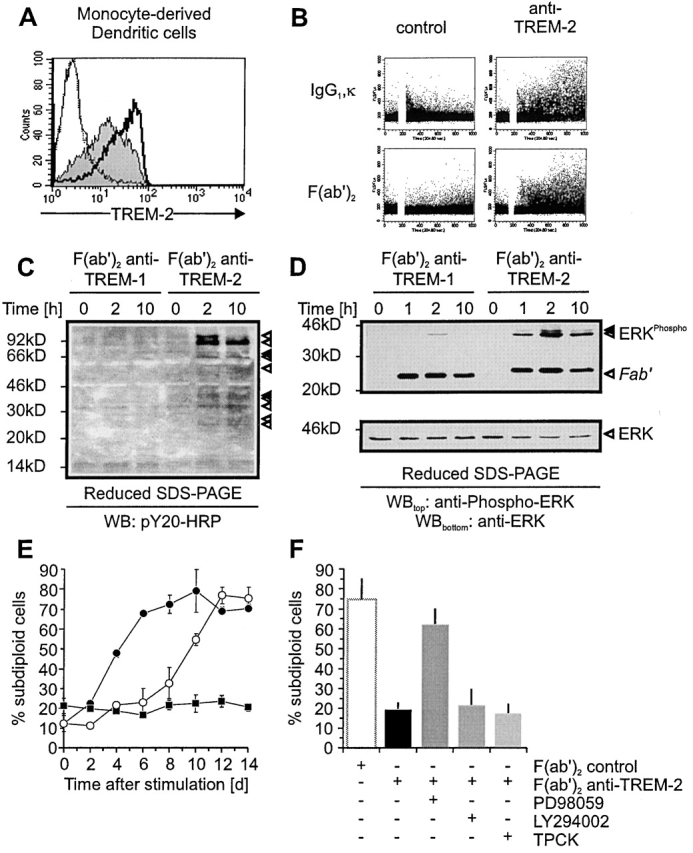
Stimulation of monocyte-derived DCs via TREM-2 induces calcium mobilization, tyrosine phosphorylation, and prolongs DC survival by an Erk-dependent pathway. (A) Functional characterization of F(ab′) and F(ab′)2 29E3. Monocyte-derived DCs were analyzed by flow cytometry for cell surface expression of TREM-2 using either biotinylated F(ab′)2 29E3 (solid bold profile), F(ab′) 29E3 (gray profile), control F(ab′) (dashed profile), or F(ab′)2 (solid profile) followed by Streptavidin-PE. (B) Stimulation of TREM-2 induces intracellular Ca2+ mobilization. Bivalent crosslinking of TREM-2 using IgG1, κ 29E3, or Fc-free F(ab′)2 29E3 induces intracellular Ca2+ mobilization in contrast to control mAbs (21C7 anti–TREM-1 mAb; left panels; 1A11 anti-MHC class I mAb; data not shown). (C) Antiphosphotyrosine blot of cell lysates from monocyte-derived DCs stimulated with F(ab′)2 29E3 (anti–TREM-2) or control F(ab′)2 (anti–TREM-1 mAb) for the indicated time periods. (D) Monocyte-derived DCs were stimulated as indicated in C and examined by Western blot analysis for anti-phospho-Erk1/2 (top panel) compared with anti-Erk 1/2 (bottom panel). Arrows indicate phosphorylated proteins in all panels. Molecular weight markers are shown. (E) Monocyte-derived DCs were washed five times to remove GM-CSF/IL-4 before stimulation with plastic-bound F(ab′)2 29E3 (open circles), control F(ab′)2 (21C7 anti–TREM-1 mAb) (filled circles), or GM-CSF (filled squares) for the indicated time periods. Apoptotic cell death was determined by measurement of DNA fragmentation. (F) Monocyte-derived DCs were stimulated plastic-bound F(ab′)2 29E3 in the presence or absence of Erk-inhibition (PD98059 [20 μM]), PI3K-inhibition (LY294002 [10 μM]), or inhibition of IκB-degradation (TPCK [20 μM]). Apoptotic cell death was determined after 8 d compared with DCs stimulated with control F(ab′)2 by measuring subdiploid DNA content.
TREM-2 Triggers Rapid Upregulation of CCR7 and Increased Expression of MHC Class II, CD86, and CD40.
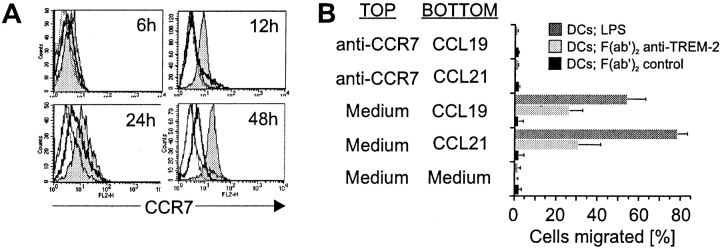
To examine whether TREM-2 can trigger migration of DCs and/or their maturation into efficient antigen-presenting cells, immature DCs were stimulated with F(ab′)2 29E3 mAb coated on plastic surface. These cells were tested for the expression of cell surface molecules involved in migration, antigen presentation, costimulation, and adhesion, as well as for the production of cytokines. Upon TREM-2 ligation, CCR7 surface expression was rapidly increased (Fig. 4 A). CCR7 was functionally competent, as TREM-2–stimulated DCs showed a specific chemotactic response towards the CCL19 and CCL21, which could be inhibited by anti-CCR7 mAb (Fig. 4 B). The amplitude and kinetics of CCR7 upregulation induced via TREM-2 were different compared with those induced by LPS stimulation (Fig. 4 A). While expression of CCR7 was detectable by 6 h after TREM-2 stimulation, LPS-induced upregulation of CCR7 was weaker and occurred only after 24 h of cell stimulation. Regardless of CCR7 surface levels, however, LPS-stimulated DCs displayed a stronger chemotactic response toward CCL19 and CCL21 than TREM-2–stimulated DCs. Therefore, DC mobility and chemotaxis to CCL19 and CCL21 is not solely related to CCR7 expression levels. Indeed, it has been shown that other receptors, such as the multidrug resistance-associated protein 1, can contribute to optimal mobilization of DCs from skin to secondary lymphoid organs 73.
Figure 4.
Stimulation of TREM-2 induces CCR7 expression and chemotactic response toward CCL19 and CCL21. (A) CCR7 is rapidly upregulated after stimulation of TREM-2 on DCs. DCs were stimulated with F(ab′)2 anti–TREM-2 mAb (gray profiles), control F(ab′)2 (21C7; anti–TREM-1; solid line profiles), or LPS (solid bold profiles). After the indicated time periods cells were harvested and analyzed by flow cytometry for cell surface expression of CCR7 by anti-CCR7 mAb (mouse IgM) followed by PE-labeled goat anti–mouse IgM. Dashed profiles indicate background staining with a control IgM mAb. (B) TREM-2 stimulation directs migration of DCs toward CCL19 and CCL21 by a CCR7-dependent pathway. DCs stimulated for 24 h with plastic-coated control F(ab′)2 (21C7, anti–TREM-1; black bars), F(ab′)2 anti–TREM-2 mAb (light gray bars), or LPS (dark gray bars) were tested in Transwell chemotaxis assays for the ability to migrate toward medium alone, medium supplemented with 100 ng/ml CCL19 or CCL21 (BOTTOM well). In control experiments, DCs were preincubated for 15 min with anti-CCR7 mAb before placing them in the TOP well for assessment of chemotaxis toward medium alone, medium supplemented with CCL19, or CCL21 placed in the BOTTOM well.
Ligation of TREM-2 also induced increased cell surface expression of several molecules involved in T cell stimulation, such as MHC class II, CD40, and CD86 (Table ). In contrast to LPS-activated DCs, CD83 and intracellular adhesion molecule (ICAM)-1 were not upregulated. Furthermore, antigen-capturing molecules, such as CD32, CD64, CD89, and the mannose receptor, were not downregulated (Table ). DCs activated through TREM-2 did not secrete either IL-12, or other cytokines (Table ). Thus, TREM-2 mediates a unique pattern of DC activation, characterized by upregulation of CCR7, expression of some T cell stimulatory molecules, and lack of cytokine secretion.
Table 1.
TREM-2–dependent Regulation of Cell Surface Activation Markers
| F(ab′)2 anti–TREM-1 | F(ab′)2 anti–TREM-2 | LPS | |
|---|---|---|---|
| TREM-2 | 112.23 | 3.45 | 7.1 |
| MHC class I | 67.8 | 65.3 | 107.1 |
| MHC class II | 119.12 | 278.65 | 454.67 |
| CD40 | 171.35 | 598.6 | 635.89 |
| CD80 | 32.1 | 39.4 | 104.6 |
| CD86/B7.2 | 14.04 | 387.91 | 683.56 |
| CCR5 | 12.95 | 13.56 | 3.12 |
| CCR6 | 3.68 | 3.45 | 4.01 |
| CCR7 | 6.82 | 21.98 | 12.45 |
| CXCR4 | 5.13 | 4.56 | 17.8 |
| CD11a | 10.92 | 6.78 | 13.72 |
| CD11b | 53.9 | 65.7 | 23.1 |
| CD11c | 91.1 | 65.7 | 123.5 |
| CD29 | 38.22 | 37.56 | 37.5 |
| CD41 | 4.54 | 4.67 | 4.39 |
| CD54/ICAM-1 | 56.87 | 54.78 | 271.45 |
| CD61 | 4.95 | 5.03 | 4.21 |
| CD103 | 3.63 | 3.96 | 3.26 |
| Mannose-R | 81.8 | 82.9 | 30.9 |
| CD64/FcγR I | 9.8 | 10.1 | 2.3 |
| CD32/FcγR II | 17.21 | 16.78 | 2.34 |
| CD89/FcαR | 4.54 | 4.75 | 4.96 |
| CD35/CR 1 | 3.94 | 4.23 | 3.67 |
| M-CSF-R | 14.6 | 4.23 | 5.21 |
| GM-CSF-R | 15.6 | 13.7 | 13.5 |
| CD38 | 2.5 | 2.2 | 43.5 |
| CD83 | 3.34 | 3.23 | 26.7 |
| CD1a | 106.76 | 134.9 | 87.54 |
DCs were cultured for 48 h in plates coated with control F(ab′)2, F(ab′)2 anti–TREM-2 mAb, or LPS as indicated in Fig. 6. Cells were subsequently analyzed by flow cytometry for the indicated cell surface molecules. Numerical values indicate specific mean fluorescence intensity after subtraction of the fluorescence detected with an isotype-matched control. The data shown are representative of four independent experiments.
Table 2.
Lack of Cytokine and Chemokine Secretion upon TREM-2 Engagement
| F(ab′)2 anti–TREM-1 | F(ab′)2 anti–TREM-2 | LPS | |
|---|---|---|---|
| IL-1α | N.D. | N.D. | 0.135 ± 0.026 |
| IL-1β | 0.027 ± 0.012 | N.D. | 0.162 ± 0.09 |
| TNF-α | 0.042 ± 0.005 | N.D. | 4.015 ± 0.078 |
| IL-18 | N.D. | N.D. | 2.56 ± 1.31 |
| IL-6 | N.D. | N.D. | 16.7 ± 5.43 |
| IL-10 | N.D. | N.D. | 2.03 ± 0.45 |
| TGF-β1 | N.D. | N.D. | N.D. |
| IL-12p40 | N.D. | N.D. | 3.48 ± 1.25 |
| IL-12p70 | N.D. | N.D. | 1.45 ± 0.09 |
| IL-13 | N.D. | N.D. | N.D. |
| IL-15 | N.D. | N.D. | N.D. |
| MCP-1 | 2.018 ± 0.875 | 0.449 ± 0.067 | 98.18 ± 35.86 |
| IL-8 | 1.23 ± 0.451 | 0.023 ± 0.01 | 124.76 ± 23.91 |
Cells were stimulated as described in Fig. 6. Cell supernatants were analyzed by ELISA for secretion of the indicated cytokines and chemokines. Data are representative for three independent experiments. Values are shown in pg/ml. N.D., not detectable.
TREM-2 Does Not Activate IκBα/NF-κB or p38/SAPK Signaling Pathways.
LPS-induced maturation of DCs requires the activity of NF-κB transcription factor 74 75 76. Prior to activation, NF-κB is retained in the cytoplasm through binding to the inhibitory IκB protein. Stimulation of cells with LPS leads to IκB kinase (IKK)-mediated phosphorylation of IκB. This is followed by rapid ubiquitination and proteolytic degradation of IκB, allowing nuclear translocation of NF-κB and binding to κB-promoter elements 30 31 32 33 77. To study whether TREM-2 activates the IκBα/NF-κB pathway, we stimulated DCs through TREM-2 and analyzed the phosphorylation of IκBα by Western blot analysis. In addition, we assessed the nuclear translocation of NF-κB by searching for NF-κB–containing complexes in EMSAs. In striking contrast to LPS 72, antibody-mediated ligation of TREM-2 did not lead to phosphorylation and degradation of IκBα (Fig. 5 A) or nuclear translocation of NF-κB (Fig. 5 B). It was previously shown that LPS-induced maturation of DCs is also mediated by activation of p38/SAPK 72 78. To see whether TREM-2 activates p38/SAPK, we crosslinked TREM-2 on DCs with F(ab′)2 29E3 and analyzed tyrosine phosphorylation of p38/SAPK by Western blot analysis. In contrast to LPS, TREM-2 did not induce p38/SAPK tyrosine phosphorylation (Fig. 5 C). Thus, the TREM-2–induced activation pathway is NF-κB- and p38/SAPK independent.
Figure 5.
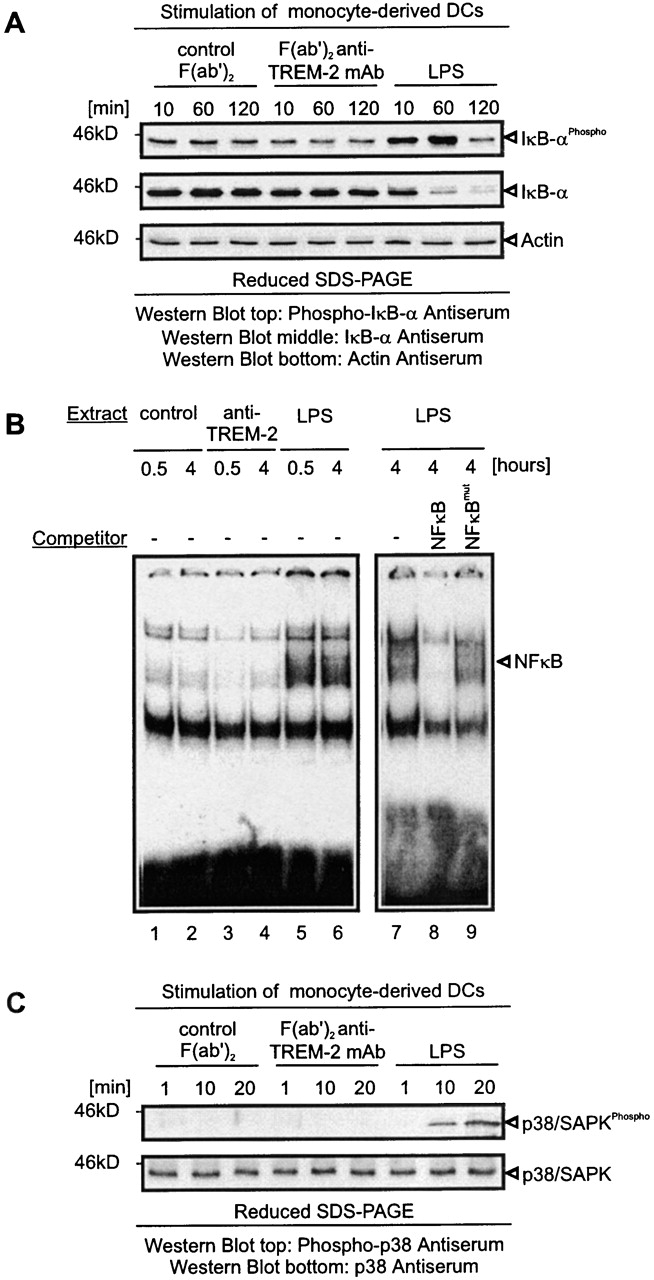
TREM-2 does not initiate IκBα/NF-κB- and p38/SAPK-dependent pathways. (A) Lack of IκBα phosphorylation and degradation upon TREM-2 stimulation of monocyte-derived DCs. Monocyte-derived DCs were stimulated by LPS or plastic-bound F(ab′)2 anti–TREM-2 mAb or control F(ab′)2 for the indicated times. Protein lysates were tested for IκBα phosphorylation and degradation by Western blot analysis. The same blot was sequentially stripped and reprobed with antiphospho-IκBα, anti-IκBα, and anti-actin (loading control) antibodies. (B) Lack of NF-κB translocation upon TREM-2 triggering. Monocyte-derived DCs were stimulated for the indicated time points as described in panel A and nuclear extracts were obtained. Radiolabeled NF-κB consensus double-stranded oligonucleotides were incubated with the indicated nuclear extracts in the absence of competing oligonucleotides or in the presence of a 25-fold molar excess of wild-type (NF-κB) or mutant (NF-κBmut) competing oligonucleotides. DNA–protein complexes were resolved by electrophoresis. The NF-κB–containing complex is marked. (C) Absence of p38/SAPK phosphorylation upon TREM-2 stimulation of monocyte-derived DCs. Monocyte-derived DCs were stimulated for the indicated times as described in panel A. Protein lysates were tested for p38/SAPK phosphorylation (top panel) by Western blot analysis. The same blot was sequentially stripped and reprobed with anti-p38/SAPK antibodies (bottom panel). Arrows indicate proteins in all panels. Molecular weight markers are shown. To confirm proper stimulation of TREM-2/DAP12 in all experimental settings an aliquot of stimulated DCs was kept and tested after 48 h for upregulation of MHC class II and CD86 (data not shown).
TREM-2 Induces DC Maturation through an ERK- and PTK-dependent, NF-κB, and p38/SAPK-independent Pathway.
To further characterize the signaling molecules involved in TREM-2–mediated DCs maturation, DCs were incubated with inhibitors of ERK (PD98049), NF-κB (TPCK), p38/SAPK (SB203580), and PTKs (PP2). Treated cells were stimulated with either F(ab′)2 anti–TREM-2 mAb, LPS, or immobilized IgG and subsequently analyzed for cell surface expression of maturation markers, such as CCR7, MHC class II, ICAM-1 CD83, CD40, and CD86. TREM-2 induced upregulation of CCR7, CD86, class II, and CD40, while cell surface expression of ICAM-1 and CD83 was not increased (Fig. 6, red bars). Remarkably, TREM-2–induced expression of CCR7, CD86, class II, and CD40 was completely blocked by a PTK inhibitor and partially blocked by an ERK inhibitor. This differential inhibitory capacity suggests that PTK may activate downstream signaling molecules other than ERK, which concur to TREM-2–mediated DC maturation. Incubation of DCs with NF-κB and p38/SAPK inhibitors had virtually no effect (Fig. 6, red bars).
Figure 6.
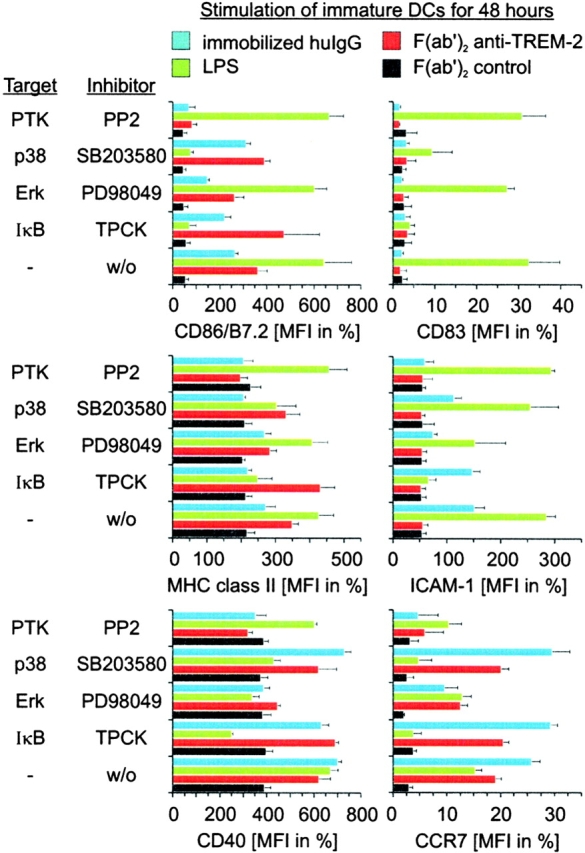
Comparison of TREM-2-, LPS/TLR-, and FcR-mediated maturation pathways. Monocyte-derived DCs were stimulated with plastic-bound control F(ab′)2 (black bars), F(ab′)2 anti–TREM-2 (red bars), human IgG (blue bars), or LPS (green bars) in the presence of inhibitors for Erk (PD98059), p38/SAPK (SB203580), PTK (PP2), IκBα degradation (TPCK), or an equal volume of DMSO as a control. After 48 h, cell surface expression of CD86 (top left panel), MHC class II (middle left panel), CD40 (bottom left panel), CD83 (top right panel), ICAM-1 (middle right panel), or CCR7 (bottom right panel) was determined by flow cytometry. Data shown are representative of four independent experiments and display the mean and standard deviation of three independent samples.
LPS-induced maturation pathway was totally distinct from that mediated by TREM-2. LPS-induced upregulation of CCR7, MHC class II, ICAM-1 CD83, CD40, and CD86 (Fig. 6, green bars) 1. Incubation of DCs with NF-κB and p38/SAPK inhibitors prevented LPS-induced maturation, whereas ERK inhibitor had a modest effect, as described previously (Fig. 6, green bars) 72 78. Finally, engagement of FcR by immobilized IgG induced a maturation pattern, which was similar to that induced by TREM-2, with the exception of an upregulation of ICAM-1 (Fig. 6, blue bars). Incubation of DCs with PTK and ERK inhibitors resulted in total and partial inhibition of FcR-induced maturation, respectively. These observations provide evidence that TREM-2 mediates DC maturation by PTK/ERK-dependent pathways. These pathways overlap with those initiated by FcRs, but are distinct from the IκBα/NF-κB and p38/SAPK-dependent pathways triggered by LPS.
Discussion
We have shown that TREM-2 is an activating receptor expressed on monocyte-derived DCs, which activates PTK and ERK signaling through the association with DAP12, an ITAM-containing adaptor molecule 44 45. TREM-2/DAP12-mediated signaling promotes survival of DCs and upregulation of CCR7, MHC class II, CD86, and CD40. Compared with the classical DC activation triggered by LPS, the TREM2/DAP12 pathway does not lead to upregulation of ICAM-1 and CD83 or secretion of IL-12, and is entirely independent of NF-κB and p38/SAPK signaling. TREM2/DAP12-induced DC maturation is more similar to that initiated by the FcRs, through the association FcRγ, another ITAM-containing adaptor molecule 42. Indeed, here we have shown that FcR-mediated maturation is dependent on PTK and ERK signaling.
This study is the first to show that a DAP12-mediated pathway can activate human DCs. What could be the physiological significance of this maturation pathway? TREM-2 or other DAP12-associated receptors could synergize with cell surface receptors, which activate DCs through NF-κB. This possibility is consistent with previous work on TREM-1, another DAP12-associated myeloid receptor 53, which is upregulated on neutrophils upon exposure to LPS and synergizes with LPS in promoting inflammatory responses to bacterial infections 79 80. Thus, DAP12-associated DC receptors could amplify maturation signals transduced by other receptors, allowing for optimal antigen presentation. DAP12-mediated DCs activation could also be important in the normal homeostasis of DCs. TREM-2 induces upregulation of CCR7, which plays a pivotal role in directing DCs from the periphery to the T cell rich areas of draining lymph nodes 10 11 12 13 14. Thus, in the absence of pathogens, DAP12-associated receptors could regulate the homeostatic circulation of DCs from the periphery to the lymph nodes, allowing for renewal of lymph node DCs. In addition, TREM-2 induces upregulation of some T cell stimulatory molecules, such as MHC class II, CD40, and CD86. Thus, DAP12-mediated maturation of DCs may promote partial activation of T cells in the absence of exogenous antigens. This activation presumably is critical for the survival of T cells and the homeostasis of T cell populations 81.
We have demonstrated that crosslinking of TREM-2 promotes DC survival. This observation is consistent with previous demonstration that ERK signaling prevents apoptosis of LPS-stimulated DCs 71. In addition, we have evidence that TREM-1/DAP12 complex promotes survival of neutrophils and monocytes (unpublished data). Together, these observations suggest that DAP12-mediated pathways are critical for myeloid cell survival. Based on our data and previous studies in other systems, DAP12-mediated ERK activation is likely to induce phosphorylation of Bad or other Bcl-2 inhibitors 82 83 84. Once released from inhibition, Bcl-2 could translocate into the mitochondria and inhibit DC apoptosis 85 86 87 88.
The physiological functions of DAP12-mediated DC activation are consistent with the reported phenotypes of knock-in mice bearing a nonfunctional DAP12 and DAP12-deficient mice 57 58. ITAM-deficient DAP12-knock-in mice showed an accumulation of DCs in mucocutaneous epithelia, associated with an impaired hapten-specific contact sensitivity 57. Our data suggest that this phenotype may be explained in part by a reduced ability of DCs to upregulate CCR7 expression and to respond to CCL19 and CCL21, affecting the migration of DCs to the T cell zone of draining lymph nodes. DAP12-deficient mice were resistant to EAE and resistance was associated with a strongly diminished production of IFN-γ by myelin-reactive CD4+ T cells 58. Our results suggest that this phenotype may be partly due to a reduced amplification of chemotactic and maturation signals by DAP12-associated receptors, resulting in an inadequate T cell priming in vivo.
Human TREM-2 is the first DAP12-associated receptor identified on DCs. TREM-2 is expressed on immature monocyte-derived DCs, but not on mature DCs or primary blood DCs. In addition, mouse TREM-2 transcripts were recently observed in cultured macrophage cell lines 54. Thus, more DAP12-associated receptors are likely to be expressed on blood and tissues DCs. In addition, such receptors might be expressed not only in immature DCs, but also in mature DCs, regulating critical functions, such as IL-12 secretion. To fully understand the physiological functions of DAP12 in DCs, it will be important to identify all the DAP12-associated DC receptors, their level of expression in different maturation stages, and their ligands.
Acknowledgments
We thank Rachel Ettinger, Greg Klein, and Fraser McBlane for reviewing the manuscript, and Lena Angman for technical assistance.
The Basel Institute for Immunology was founded and is supported by Hoffmann-LaRoche Ltd., CH-4002 Basel.
Footnotes
M. Cella's present address is Department of Pathology and Immunology, Washington University School of Medicine, 660 South Euclid Ave., St. Louis, MO 63110.
Abbreviations used in this paper: CCR7, CC chemokine receptor 7; DC, dendritic cell; EMSA, electrophoretic mobility assay; ERK, extracellular signal–regulated kinase; HRP, horseradish peroxidase; ITAM, immunoreceptor tyrosine-based activation motif; MAPK, mitogen-activated protein kinase; MDL-1, myeloid DAP12-associating lectin-1; NF, nuclear factor; PTK, protein tyrosine kinase; SAPK, stress-activated protein kinase; SIRP-β, signal-regulatory protein β; TLR, Toll-like receptor; TREM, triggering receptor expressed on myeloid cells.
References
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Gallucci S., Lolkema M., Matzinger P. Natural adjuvantsendogenous activators of dendritic cells. Nat. Med. 1999;11:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- Sauter B., Albert M.L., Francisco L., Larsson M., Somersan S., Bhardwaj N. Consequences of cell deathexposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieu M.C., Vanbervliet B., Vicari A., Bridon J.M., Oldham E., Ait-Yahia S., Briere F., Zlotnik A., Lebecque S., Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozzani S., Allavena P., D'Amico G., Luini W., Bianchi G., Kataura M., Imai T., Yoshie O., Bonecchi R., Mantovani A. Differential regulation of chemokine receptors during dendritic cell maturationa model for their trafficking properties. J. Immunol. 1998;161:1083–1086. [PubMed] [Google Scholar]
- Yanagihara S., Komura E., Nagafune J., Watarai H., Yamaguchi Y. EBI1/CCR7 is a new member of dendritic cell chemokine receptor that is up-regulated upon maturation. J. Immunol. 1998;161:3096–3102. [PubMed] [Google Scholar]
- Sallusto F., Schaerli P., Loetscher P., Schaniel C., Lenig D., Mackay C.R., Qin S., Lanzavecchia A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 1998;28:2760–2769. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Cyster J.G. Leukocyte migrationscent of the T zone. Curr. Biol. 2000;10:R30–R33. doi: 10.1016/s0960-9822(99)00253-5. [DOI] [PubMed] [Google Scholar]
- Zlotnik A., Yoshie O. Chemokinesa new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- Forster R., Schubel A., Breitfeld D., Kremmer E., Renner-Muller I., Wolf E., Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Nakano H., Mori S., Yonekawa H., Nariuchi H., Matsuzawa A., Kakiuchi T. A novel mutant gene involved in T-lymphocyte-specific homing into peripheral lymphoid organs on mouse chromosome 4. Blood. 1998;91:2886–2895. [PubMed] [Google Scholar]
- Saeki H., Moore A.M., Brown M.J., Hwang S.T. Cutting edgesecondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J. Immunol. 1999;162:2472–2475. [PubMed] [Google Scholar]
- Gunn M.D., Kyuwa S., Tam C., Kakiuchi T., Matsuzawa A., Williams L.T., Nakano H. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 1999;189:451–460. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo V.N., Korner H., Gunn M.D., Schmidt K.N., Riminton D.S., Cooper M.D., Browning J.L., Sedgwick J.D., Cyster J.G. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J. Exp. Med. 1999;189:403–412. doi: 10.1084/jem.189.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A. Immunology. Licence to kill. Nature. 1998;393:413–414. doi: 10.1038/30845. [DOI] [PubMed] [Google Scholar]
- Armitage R.J., Maliszewski C.R., Alderson M.R., Grabstein K.H., Spriggs M.K., Fanslow W.C. CD40La multi-functional ligand. Semin. Immunol. 1993;5:401–412. doi: 10.1006/smim.1993.1046. [DOI] [PubMed] [Google Scholar]
- Anderson D.M., Maraskovsky E., Billingsley W.L., Dougall W.C., Tometsko M.E., Roux E.R., Teepe M.C., DuBose R.F., Cosman D., Galibert L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- Murata K., Ishii N., Takano H., Miura S., Ndhlovu L.C., Nose M., Noda T., Sugamura K. Impairment of antigen-presenting cell function in mice lacking expression of OX40 ligand. J. Exp. Med. 2000;191:365–374. doi: 10.1084/jem.191.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M.F., Wong B.R., Josien R., Steinman R.M., Oxenius A., Choi Y. TRANCE, a tumor necrosis factor family member critical for CD40 ligand- independent T helper cell activation. J. Exp. Med. 1999;189:1025–1031. doi: 10.1084/jem.189.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josien R., Li H.L., Ingulli E., Sarma S., Wong B.R., Vologodskaia M., Steinman R.M., Choi Y. TRANCE, a tumor necrosis factor family member, enhances the longevity and adjuvant properties of dendritic cells in vivo. J. Exp. Med. 2000;191:495–502. doi: 10.1084/jem.191.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A.I., McAdam A.J., Buhlmann J.E., Scott S., Lupher M.L., Jr., Greenfield E.A., Baum P.R., Fanslow W.C., Calderhead D.M., Freeman G.J., Sharpe A.H. Ox40-ligand has a critical costimulatory role in dendritic cell:T cell interactions. Immunity. 1999;11:689–698. doi: 10.1016/s1074-7613(00)80143-0. [DOI] [PubMed] [Google Scholar]
- Kong Y.Y., Yoshida H., Sarosi I., Tan H.L., Timms E., Capparelli C., Morony S., Oliveira-dos-Santos A.J., Van G., Itie A. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- Macatonia S.E., Hosken N.A., Litton M., Vieira P., Hsieh C.S., Culpepper J.A., Wysocka M., Trinchieri G., Murphy K.M., O'Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J. Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- Cella M., Scheidegger D., Palmer-Lehmann K., Lane P., Lanzavecchia A., Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacityT-T help via APC activation. J. Exp. Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch F., Stanzl U., Jennewein P., Janke K., Heufler C., Kampgen E., Romani N., Schuler G. High level IL-12 production by murine dendritic cellsupregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J. Exp. Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heufler C., Koch F., Stanzl U., Topar G., Wysocka M., Trinchieri G., Enk A., Steinman R.M., Romani N., Schuler G. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur. J. Immunol. 1996;26:659–668. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- Ohshima Y., Tanaka Y., Tozawa H., Takahashi Y., Maliszewski C., Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J. Immunol. 1997;159:3838–3848. [PubMed] [Google Scholar]
- Aderem A., Ulevitch R.J. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- Medzhitov R., Janeway C., Jr. Innate immunity. N. Engl. J. Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- Baldwin A.S., Jr. Series introductionthe transcription factor NF-kappaB and human disease. J. Clin. Invest. 2001;107:3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., May M.J., Kopp E.B. NF-kappa B and Rel proteinsevolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Baeuerle P.A. IkappaB-NF-kappaB structuresat the interface of inflammation control. Cell. 1998;95:729–731. doi: 10.1016/s0092-8674(00)81694-3. [DOI] [PubMed] [Google Scholar]
- Karin M., Ben-Neriah Y. Phosphorylation meets ubiquitinationthe control of NF-[kappa]B activity. Annu. Rev. Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Tsuji-Takayama K., Aizawa Y., Koide K., Takeuchi M., Ohta T., Kurimoto M. Interleukin-18 activates NF-kappaB in murine T helper type 1 cells. Biochem. Biophys. Res. Commun. 1997;234:454–457. doi: 10.1006/bbrc.1997.6665. [DOI] [PubMed] [Google Scholar]
- Lalmanach-Girard A.C., Chiles T.C., Parker D.C., Rothstein T.L. T cell–dependent induction of NF-kappa B in B cells. J. Exp. Med. 1993;177:1215–1219. doi: 10.1084/jem.177.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata S., Hori T., Imura A., Takaori-Kondo A., Uchiyama T. Activation of OX40 signal transduction pathways leads to tumor necrosis factor receptor-associated factor (TRAF) 2- and TRAF5-mediated NF-kappaB activation. J. Biol. Chem. 1998;273:5808–5814. doi: 10.1074/jbc.273.10.5808. [DOI] [PubMed] [Google Scholar]
- Arch R.H., Thompson C.B. 4-1BB and Ox40 are members of a tumor necrosis factor (TNF)-nerve growth factor receptor subfamily that bind TNF receptor-associated factors and activate nuclear factor kappaB. Mol. Cell. Biol. 1998;18:558–565. doi: 10.1128/mcb.18.1.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnault A., Lankar D., Lacabanne V., Rodriguez A., Thery C., Rescigno M., Saito T., Verbeek S., Bonnerot C., Ricciardi-Castagnoli P., Amigorena S. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I–restricted antigen presentation after immune complex internalization. J. Exp. Med. 1999;189:371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J.V., Bolland S. IgG fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- Bonnerot C., Briken V., Brachet V., Lankar D., Cassard S., Jabri B., Amigorena S. syk protein tyrosine kinase regulates Fc receptor gamma-chain-mediated transport to lysosomes. EMBO J. 1998;17:4606–4616. doi: 10.1093/emboj/17.16.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis W., Harrison P.T., Hutchinson M.J., Allen J.M. Two distinct regions of FC gamma RI initiate separate signalling pathways involved in endocytosis and phagocytosis. EMBO J. 1995;14:432–441. doi: 10.1002/j.1460-2075.1995.tb07019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amigorena S., Bonnerot C. Fc receptor signaling and traffickinga connection for antigen processing. Immunol. Rev. 1999;172:279–284. doi: 10.1111/j.1600-065x.1999.tb01372.x. [DOI] [PubMed] [Google Scholar]
- Olcese L., Cambiaggi A., Semenzato G., Bottino C., Moretta A., Vivier E. Human killer cell activatory receptors for MHC class I molecules are included in a multimeric complex expressed by natural killer cells. J. Immunol. 1997;158:5083–5086. [PubMed] [Google Scholar]
- Lanier L.L., Corliss B.C., Wu J., Leong C., Phillips J.H. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391:703–707. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- Lanier L.L., Corliss B., Wu J., Phillips J.H. Association of DAP12 with activating CD94/NKG2C NK cell receptors. Immunity. 1998;8:693–701. doi: 10.1016/s1074-7613(00)80574-9. [DOI] [PubMed] [Google Scholar]
- Tomasello E., Olcese L., Vely F., Geourgeon C., Blery M., Moqrich A., Gautheret D., Djabali M., Mattei M.G., Vivier E. Gene structure, expression pattern, and biological activity of mouse killer cell activating receptor-associated protein (KARAP)/DAP-12. J. Biol. Chem. 1998;273:34115–34119. doi: 10.1074/jbc.273.51.34115. [DOI] [PubMed] [Google Scholar]
- Smith K.M., Wu J., Bakker A.B., Phillips J.H., Lanier L.L. Ly-49D and Ly-49H associate with mouse DAP12 and form activating receptors. J. Immunol. 1998;161:7–10. [PubMed] [Google Scholar]
- Cantoni C., Bottino C., Vitale M., Pessino A., Augugliaro R., Malaspina A., Parolini S., Moretta L., Moretta A., Biassoni R. NKp44, a triggering receptor involved in tumor cell lysis by activated human natural killer cells, is a novel member of the immunoglobulin superfamily. J. Exp. Med. 1999;189:787–796. doi: 10.1084/jem.189.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason L.H., Willette-Brown J., Mason A.T., McVicar D., Ortaldo J.R. Interaction of Ly-49D+ NK cells with H-2Dd target cells leads to Dap-12 phosphorylation and IFN-gamma secretion. J. Immunol. 2000;164:603–611. doi: 10.4049/jimmunol.164.2.603. [DOI] [PubMed] [Google Scholar]
- Bakker A.B., Baker E., Sutherland G.R., Phillips J.H., Lanier L.L. Myeloid DAP12-associating lectin (MDL)-1 is a cell surface receptor involved in the activation of myeloid cells. Proc. Natl. Acad. Sci. USA. 1999;96:9792–9796. doi: 10.1073/pnas.96.17.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J., Cella M., Seiffert M., Buhring H.J., Colonna M. Cutting edgesignal-regulatory protein beta 1 is a DAP12-associated activating receptor expressed in myeloid cells. J. Immunol. 2000;164:9–12. doi: 10.4049/jimmunol.164.1.9. [DOI] [PubMed] [Google Scholar]
- Tomasello E., Cant C., Buhring H.J., Vely F., Andre P., Seiffert M., Ullrich A., Vivier E. Association of signal-regulatory proteins beta with KARAP/DAP-12. Eur. J. Immunol. 2000;30:2147–2156. doi: 10.1002/1521-4141(2000)30:8<2147::AID-IMMU2147>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Bouchon A., Dietrich J., Colonna M. Cutting edgeinflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 2000;164:4991–4995. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- Daws M.R., Lanier L.L., Seaman W.E., Ryan J.C. Cloning and characterization of a novel mouse myeloid DAP12-associated receptor family. Eur. J. Immunol. 2001;31:783–791. doi: 10.1002/1521-4141(200103)31:3<783::aid-immu783>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- McVicar D.W., Taylor L.S., Gosselin P., Willette-Brown J., Mikhael A.I., Geahlen R.L., Nakamura M.C., Linnemeyer P., Seaman W.E., Anderson S.K. DAP12-mediated signal transduction in natural killer cells. A dominant role for the Syk protein-tyrosine kinase. J. Biol. Chem. 1998;273:32934–32942. doi: 10.1074/jbc.273.49.32934. [DOI] [PubMed] [Google Scholar]
- Campbell K.S., Cella M., Carretero M., Lopez-Botet M., Colonna M. Signaling through human killer cell activating receptors triggers tyrosine phosphorylation of an associated protein complex. Eur. J. Immunol. 1998;28:599–609. doi: 10.1002/(SICI)1521-4141(199802)28:02<599::AID-IMMU599>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Tomasello E., Desmoulins P.O., Chemin K., Guia S., Cremer H., Ortaldo J., Love P., Kaiserlian D., Vivier E. Combined natural killer cell and dendritic cell functional deficiency in KARAP/DAP12 loss-of-function mutant mice. Immunity. 2000;13:355–364. doi: 10.1016/s1074-7613(00)00035-2. [DOI] [PubMed] [Google Scholar]
- Bakker A.B., Hoek R.M., Cerwenka A., Blom B., Lucian L., McNeil T., Murray R., Phillips L.H., Sedgwick J.D., Lanier L.L. DAP12-deficient mice fail to develop autoimmunity due to impaired antigen priming. Immunity. 2000;13:345–353. doi: 10.1016/s1074-7613(00)00034-0. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A., Chen Z., Sures I., Wang H., Schilling J., Ullrich A. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature. 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- Seiffert M., Brossart P., Cant C., Cella M., Colonna M., Brugger W., Kanz L., Ullrich A., Buhring H.J. Signal-regulatory protein alpha (SIRPalpha) but not SIRPbeta is involved in T-cell activation, binds to CD47 with high affinity, and is expressed on immature CD34+CD38− hematopoietic cells. Blood. 2001;97:2741–2749. doi: 10.1182/blood.v97.9.2741. [DOI] [PubMed] [Google Scholar]
- Traunecker A., Oliveri F., Karjalainen K. Myeloma based expression system for production of large mammalian proteins. Trends Biotechnol. 1991;9:109–113. doi: 10.1016/0167-7799(91)90038-j. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A., Sapp M., Schuler G., Steinman R.M., Bhardwaj N. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J. Immunol. Methods. 1996;196:121–135. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- Cella M., Engering A., Pinet V., Pieters J., Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- Nicoletti I., Migliorati G., Pagliacci M.C., Grignani F., Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Muller M.M., Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Munain C., Krangel M.S. Regulation of the T-cell receptor delta enhancer by functional cooperation between c-Myb and core-binding factors. Mol. Cell. Biol. 1994;14:473–483. doi: 10.1128/mcb.14.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Song Y., Bakker A.B., Bauer S., Spies T., Lanier L.L., Phillips J.H. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285:730–732. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- Nakajima H., Samaridis J., Angman L., Colonna M. Human myeloid cells express an activating ILT receptor (ILT1) that associates with Fc receptor gamma-chain. J. Immunol. 1999;162:5–8. [PubMed] [Google Scholar]
- Makrigiannis A.P., Gosselin P., Mason L.H., Taylor L.S., McVicar D.W., Ortaldo J.R., Anderson S.K. Cloning and characterization of a novel activating Ly49 closely related to Ly49A. J. Immunol. 1999;163:4931–4938. [PubMed] [Google Scholar]
- Rescigno M., Martino M., Sutherland C.L., Gold M.R., Ricciardi-Castagnoli P. Dendritic cell survival and maturation are regulated by different signaling pathways. J. Exp. Med. 1998;188:2175–2180. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeshna K.M., Pizzey A.R., Devereux S., Khwaja A. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood. 2000;96:1039–1046. [PubMed] [Google Scholar]
- Robbiani D.F., Finch R.A., Jager D., Muller W.A., Sartorelli A.C., Randolph G.J. The leukotriene C(4) transporter MRP1 regulates CCL19 (MIP-3beta, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell. 2000;103:757–768. doi: 10.1016/s0092-8674(00)00179-3. [DOI] [PubMed] [Google Scholar]
- Doi T.S., Takahashi T., Taguchi O., Azuma T., Obata Y. NF-kappa B RelA-deficient lymphocytesnormal development of T cells and B cells, impaired production of IgA and IgG1 and reduced proliferative responses. J. Exp. Med. 1997;185:953–961. doi: 10.1084/jem.185.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontgen F., Grumont R.J., Strasser A., Metcalf D., Li R., Tarlinton D., Gerondakis S. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 1995;9:1965–1977. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- Sha W.C., Liou H.C., Tuomanen E.I., Baltimore D. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- Lin Y.C., Brown K., Siebenlist U. Activation of NF-kappa B requires proteolysis of the inhibitor I kappa B-alphasignal-induced phosphorylation of I kappa B-alpha alone does not release active NF-kappa B. Proc. Natl. Acad. Sci. USA. 1995;92:552–556. doi: 10.1073/pnas.92.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrighi J.F., Rebsamen M., Rousset F., Kindler V., Hauser C. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers. J. Immunol. 2001;166:3837–3845. doi: 10.4049/jimmunol.166.6.3837. [DOI] [PubMed] [Google Scholar]
- Bouchon A., Facchetti F., Weigand M.A., Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- Nathan C., Ding A. TREM-1a new regulator of innate immunity in sepsis syndrome. Nat. Med. 2001;7:530–532. doi: 10.1038/87846. [DOI] [PubMed] [Google Scholar]
- Tanchot C., Lemonnier F.A., Perarnau B., Freitas A.A., Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- Scheid M.P., Duronio V. Dissociation of cytokine-induced phosphorylation of Bad and activation of PKB/aktinvolvement of MEK upstream of Bad phosphorylation. Proc. Natl. Acad. Sci. USA. 1998;95:7439–7444. doi: 10.1073/pnas.95.13.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid M.P., Schubert K.M., Duronio V. Regulation of bad phosphorylation and association with Bcl-x(L) by the MAPK/Erk kinase. J. Biol. Chem. 1999;274:31108–31113. doi: 10.1074/jbc.274.43.31108. [DOI] [PubMed] [Google Scholar]
- Klein J.B., Rane M.J., Scherzer J.A., Coxon P.Y., Kettritz R., Mathiesen J.M., Buridi A., McLeish K.R. Granulocyte-macrophage colony-stimulating factor delays neutrophil constitutive apoptosis through phosphoinositide 3-kinase and extracellular signal-regulated kinase pathways. J. Immunol. 2000;164:4286–4291. doi: 10.4049/jimmunol.164.8.4286. [DOI] [PubMed] [Google Scholar]
- Adams J.M., Cory S. The Bcl-2 protein familyarbiters of cell survival. Science. 1998;28:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- Grenn D.R. Apoptotic pathwayspaper wraps stone blunts scissors. Cell. 2000;102:1–4. doi: 10.1016/s0092-8674(00)00003-9. [DOI] [PubMed] [Google Scholar]
- Huang D.C., Strasser A. BH3-only proteins-essential initiators of apoptotic cell death. Cell. 2000;103:839–842. doi: 10.1016/s0092-8674(00)00187-2. [DOI] [PubMed] [Google Scholar]
- Gross A., McDonnell J.M., Korsmeyer S.J. Bcl-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]