Abstract
Ultraviolet (UV) radiation plays a critical role in the induction of nonmelanoma skin cancer. UV radiation is also immune suppressive, and the immune suppression induced by UV irradiation has been identified as a major risk factor for skin cancer induction. Previously, we showed that UV exposure activates a cytokine cascade involving prostaglandin (PG)E2, interleukin (IL)-4, and IL-10 that induces immune suppression. However, the earliest molecular events that occur immediately after UV exposure, especially those upstream of PGE2, are not well defined. UV-irradiated keratinocytes secrete the inflammatory phospholipid mediator, platelet-activating factor (PAF). Because PAF upregulates the production of immunomodulatory compounds, including PGE2, we tested the hypothesis that UV-induced PAF activates cytokine production and initiates UV-induced immune suppression. Both UV and PAF activated cyclooxygenase (COX)-2 and IL-10 reporter gene construct transcription. PAF mimicked the effects of UV in vivo and suppressed delayed-type hypersensitivity (DTH). Furthermore, immune suppression was blocked when UV-irradiated mice were injected with PAF receptor antagonists. In addition to the well-known role of PAF as a proinflammatory lipid mediator, we propose that the PAF receptor senses cellular damage through the recognition of PAF and/or PAF-like molecules, such as oxidized phosphatidylcholine, which activates cytokine transcription and induces systemic immune suppression.
Keywords: inflammation, lipid mediators, delayed-type hypersensitivity, tolerance/suppression, immunomodulators
Introduction
Platelet-activating factor (PAF;*1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) is a phospholipid mediator with potent biological effects. As the name implies, PAF activates a wide variety of cells, including platelets, monocytes, mast cells, and polymorphonuclear leukocytes. It also promotes the migration of granulocytes to sites of inflammation and is mitotic for fibroblasts and lymphocytes (for a review, see reference 1). De novo synthesis of PAF occurs via a two step pathway. Phospholipase-A2 enzymatically cleaves arachidonic acid from the sn-2 position of cell membrane phosphatidylcholine. An acetyl residue is then transferred to the free hydroxyl from acetyl-CoA to form biologically active PAF (2). Cells that release PAF (endothelial cells, leukocytes, and monocytes) do not contain preformed stores of PAF, but rapidly synthesize PAF in response to stress. Cells responsive to PAF express a single specific receptor, a seven transmembrane spanning G-coupled protein. In addition to PAF, this receptor recognizes structural analogs of PAF, generated by oxidation of phosphatidylcholine. These fragmented phosphatidylcholine molecules (PAF-like lipids) bind to and activate the PAF receptor. Binding of the PAF-receptor stimulates a variety of downstream effects, including activation of the mitogen-activated protein (MAP) kinase pathway, activation of phospholipases, and the biosynthesis of a variety of cytokines and prostaglandins (for a review by Ishii and Shimizu, see reference 3). Platelet-activating factor and PAF-receptor binding has been shown to play a role in cellular communications in a variety of different organ systems, including the vascular system, the central nervous system, the endocrine system, and the gastrointestinal tract (1); however, a role for PAF in systemic immunosuppression is unknown.
The major focus of research in our laboratory is to determine the mechanisms underlying the immune suppression induced by the environmental immunotoxin, UV radiation. The primary cause of nonmelanoma skin cancer, the most prevalent form of human neoplasia is the UV radiation found in sunlight. The immune suppressive effects of UV radiation contribute to skin cancer induction by depressing cell-mediated immune reactions that normally serve to destroy the developing highly antigenic skin tumors. Epidemiological studies with immune suppressed renal transplant patients (4), experiments with laboratory mice (5), and immunologic studies with skin cancer patients (6), support the hypothesis that the immune suppression induced by UV exposure is a major risk factor for skin cancer induction.
In addition, UV exposure suppresses the immune responses to infectious organisms. This has been shown using both experimental animals (7) and human volunteers (8). In fact, the immune suppression induced by sunlight exposure is believed to play a major role in herpes viral recrudescence (9). In many of the studies examining the effects of UV radiation on the immune response to infectious agents, significant and substantial immune suppression is found after a single exposure to UV radiation. Further, the doses of UV radiation given in the experimental studies compare well to the amount of UV in sunlight that is received during normal occupational and recreational activities (8, 10). Because UV-induced immune suppression contributes to skin cancer induction, and in view of the fact that a single exposure to sunlight can suppress the immune response to microbial antigens, it is critically important to study the mechanism(s) underlying UV-induced immune suppression.
Considerable evidence exists supporting a role for UV-induced biological response modifiers in activating systemic immune suppression, including PGE2, cis-urocanic acid, histamine, IL-10, IL-4, and TNF-α (11). Although the interplay between these various UV-induced cytokines is complex and not completely understood, it does appear that a cytokine cascade is activated that ultimately induces immune suppression. Previous studies from our laboratory suggest that an early step in this cytokine cascade is UV-induced PGE2 production, which then causes downstream effects, including the secretion of IL-4 and IL-10 into the serum (12). The ultimate target of the these immunoregulatory cytokines is the dendritic cell, as one consequence of total body UV-irradiation on dendritic cell function is to suppress the secretion of IL-12p70 while at the same time promoting IL-12p40 homodimer production (13). Suppressed IL-12p70 secretion coupled with production of the IL-12p40 homodimer, a natural antagonist of biologically active IL-12, may explain why antigen-presenting cells isolated from the lymphoid organs of UV-irradiated mice fail to present antigen to Th1 clones (14).
Although our previous studies indicate an essential role for UV-induced keratinocyte-derived PGE2 in systemic immune suppression, the earliest molecular events that occur immediately after UV exposure are not well defined. Some have suggested that UV exposure can directly activate PGE2 synthesis in keratinocytes (15), whereas others have suggested that PGE2 secretion results only after a synergistic interaction between UV and endogenous mediators, such as histamine (16). This raises the possibility that the molecular targets of UV radiation in keratinocytes are upstream of cyclooxygenase (COX)-2 activation.
One potential candidate is PAF. Although PAF is not expressed in normal skin, keratinocytes and corneal stromal cells secrete PAF in response to UV exposure (17–19). Of particular interest are the observations that keratinocytes express PAF receptors on their surface (20) and that PAF upregulates COX-2 gene expression and PGE2 secretion by keratinocytes (21). Moreover, PAF receptor antagonists block UV-induced apoptosis (17). These studies suggest that UV-induced PAF may be upstream of PGE2 in the cascade of events that lead to UV-induced immune suppression. The focus of the experiments presented here is to test the hypothesis that PAF plays a role in systemic immune suppression.
Materials and Methods
Reagent and Cell Lines.
The metabolically stable analogue of PAF, carbamyl-PAF (cPAF), and the PAF receptor antagonists PCA-4248 (22), CV-3988 (23), and (±)trans-2,5-bis(3,4,5-trimethoxyphenyl)-1,3-dioxolane (hereafter referred to as dioxolane [24] were purchased from Biomol). Egg yolk phosphatidylcholine was purchased from Sigma-Aldrich. Dr. Peter Isakson (G.D. Searle & Co., St. Louis, MO) provided SC-236 the selective COX-2 inhibitor. Stock solutions of cPAF, PCA-4248, CV-3988, dioxolane, and SC-236 were prepared at 5 mM concentrations by dissolving each in a 50% DMSO/PBS buffer and diluted further in PBS before cell culture or injection into mice. A thin layer of phosphatidylcholine (5 mM in PBS) was spread into a polystyrene dish and irradiated with 200 J/m2 UVB under an FS-40 sunlamp. Irradiated solutions of phosphatidylcholine are referred to as UV-PC. The spontaneously transformed mouse keratinocyte cell line PAM-212 was obtained from Dr. Stuart Yuspa (National Cancer Institute, Bethesda, MD).
Mice.
Specific pathogen-free female C3H/HeNCr (MTV−) mice were obtained from the National Cancer Institute Frederick Cancer Research Facility Animal Production Area (Frederick, MD). The animals were maintained in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care International, in accordance with current regulations of the United States Department of Health and Human Services. All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee. Within each experiment all mice were age matched. The mice were 8–10 wk old at the start of each experiment.
Radiation Sources.
A bank of six FS-40 sunlamps (National Biological) was used to irradiate mice. These lamps emit a continuous spectrum from 270 to 390 nm; with peak emission at 313 nm; ∼65% of the irradiation is within the UVB range (280–320 nm) of the solar spectrum. The irradiance of the six bulbs averaged 10 W/m2, as measured by an IL-1700 research radiometer (International Light, Inc.). Keratinocyte cultures, or the phosphatidylcholine solution were suspended in PBS and placed under a single FS-40 sunlamp. The output of this lamp was ∼1.5 W/m2, at a tube to target distance of 23 cm.
Delayed-type Hypersensitivity.
The dorsal hair of the mice was removed with electric clippers, and the mice were exposed to 10 kJ/m2 of UVB radiation. Control mice were shaved but not exposed to UV. 5 d later the animals were immunized by injecting 107 formalin-fixed Candida albicans into each flank (subcutaneous injection, nonirradiated site). 9 d later, each hind footpad was measured with an engineer's micrometer (Swiss Precision Instruments), the thickness recorded and the animals were challenged by injecting 50 μl of Candida antigen (Antigen Supply House) into each hind footpad. Footpad thickness was measured again 18–24 h later and the mean footpad swelling for each mouse (left foot + right foot ÷ 2) swelling was recorded. The mean footpad swelling ± the standard error of the mean for each group (n = 5) was calculated. The specific footpad swelling was calculated by subtracting the mean footpad swelling found in control mice that were not immunized, but were challenged from the swelling seen in mice that were both immunized and challenged. Statistical differences between the controls and experimental groups were determined by use of a two-tailed Student's t test, with a probability of <0.05 considered significant (Prism Statistical Software; GraphPad Inc.).
Dual Luciferase Assay.
A dual luciferase assay was used to determine the effects of UV radiation, and/or cPAF on COX-2 or IL-10 promoter activity. The COX-2 promoter luciferase construct was generated from a PCR product comprising the −1012 to +12 nucleotides (relative to start of transcription) of the mouse COX-2 5′ promoter region ligated into the pGL3-luc firefly luciferase vector purchased from Promega. Dr. Sandra Gollnick (Roswell Park Cancer Institute, Buffalo, NY) provided the IL-10 promoter luciferase construct, which was originally prepared from a genomic library and ligated into the pGL3-luc firefly luciferase vector (25). The fidelity of the constructs was determined by sequencing. Dr. Menashe Bar-Eli (M.D. Anderson Cancer Center, Houston, TX) provided us with the control β-actin-luciferase construct (mouse β-actin promoter upstream of sea pansy luciferase). Pam 212 cells were plated at a concentration of 5 × 104 cells/well in 24-well tissue culture plates and incubated overnight at 37°C in 5% CO2. Plasmids containing the IL-10 promoter or the COX-2 were mixed with the β-actin sea pansy luciferase control plasmid, the cationic liposomal transfection reagent Tfx-20, base DMEM culture medium, and then incubated at 37°C for 15 min. 200 μl of the firefly/sea pansy mixture was added to the Pam 212 cells in triplicate wells. The plates were then incubated at 37°C. After 1 h each well was supplemented with 800 μl of complete DMEM with 10% BCS and incubated at 37°C. After 24 h, the cell monolayers were washed, overlaid with PBS, and exposed to UV radiation or cPAF. The plates were then washed, resuspended in complete DMEM, and incubated for 36 h at 37°C. At the end of the incubation, the cells were lysed, and firefly luciferase activity was determined using a luminometer. Immediately after the luciferase measurement the reaction was quenched, the substrate mix for the sea pansy luciferase was added, and a second reading was taken. The sea pansy luciferase reading was used to normalize transfection efficiency. The luminescence from UV and/or cPAF-activated cultures was compared with the luminescence obtained with control cultures (no UV and tissue culture medium devoid of cPAF) and the stimulation was expressed as fold increase.
Results
COX-2 and IL-10 Transcription Is Activated by UV Radiation and cPAF and Inhibited by a PAF Receptor Antagonist.
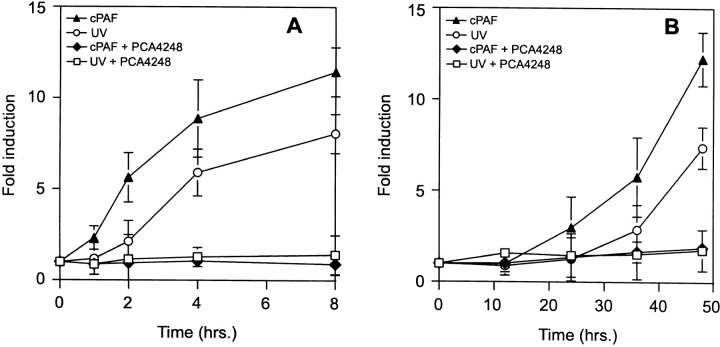
To determine a potential role for PAF in activating cytokine transcription, keratinocyte cultures transfected with COX-2 or IL-10 reporter constructs were cultured with 10 nM cPAF or exposed to 200 J/m2 of UVB radiation. Metabolically stable cPAF was used because of its stability and potency of action. At various times after the treatments, the cells were lysed and assayed for luciferase activity. UV irradiation and cPAF stimulated both the COX-2 (Fig. 1 A) and IL-10 promoter (Fig. 1 B) constructs. These data confirm that PAF and UV radiation can activate the COX-2 gene and demonstrate that PAF also activates IL-10 gene transcription. Furthermore, the similar kinetics imply that a shared responsive element is employed by PAF and UV radiation to turn on cytokine gene transcription.
Figure 1.
Upregulation of cytokine gene transcription by PAF. Murine keratinocytes were transfected with a COX-2 reporter construct (A) or an IL-10 reporter construct (B) and exposed to 200 J/m2 of UV radiation (○) or cultured with 10 nM cPAF (▴). The PAF receptor antagonist, PCA-4248, was added to the medium before UV radiation (□) or cPAF (♦). At the times indicated, the cells were lysed and the luciferase activity determined. The data is expressed as fold-induction by comparing the response of UV- or cPAF-treated cells to that of keratinocytes transfected with the reporter gene constructs and treated with tissue culture medium. Each point represents the mean ± SEM of three separate determinations.
Next, we employed a PAF receptor antagonist to establish the order of events. If UV triggers multiple, parallel, pathways for activating COX-2 and IL-10, then blocking PAF receptor signaling would be insufficient to halt the induction of both. Conversely, if these steps occur in a linear sequence, than a PAF receptor antagonist should prevent UV from activating COX-2 and IL-10 transcription. To test this hypothesis, we used the PAF-receptor antagonist, PCA-4248 (22). Prior to UV irradiation, the keratinocytes were cultured with 100 μM PCA-4248. The cells were then exposed to UV radiation, or treated with 10 nM cPAF (Fig. 1). UV-irradiated keratinocytes preloaded with PCA-4248 failed to upregulate the expression of COX-2 (Fig. 1 A), or IL-10 (Fig. 1 B). Inhibition of cPAF-induced COX-2 and IL-10 transcription demonstrated that the PAF-receptor antagonist was biologically active. These data indicate that UV-induced activation of cytokine transcription is mediated by PAF through the PAF receptor and suggest that UV-induced activation of PAF is upstream of PGE2 production.
PAF Suppresses Delayed-type Hypersensitivity In Vivo.
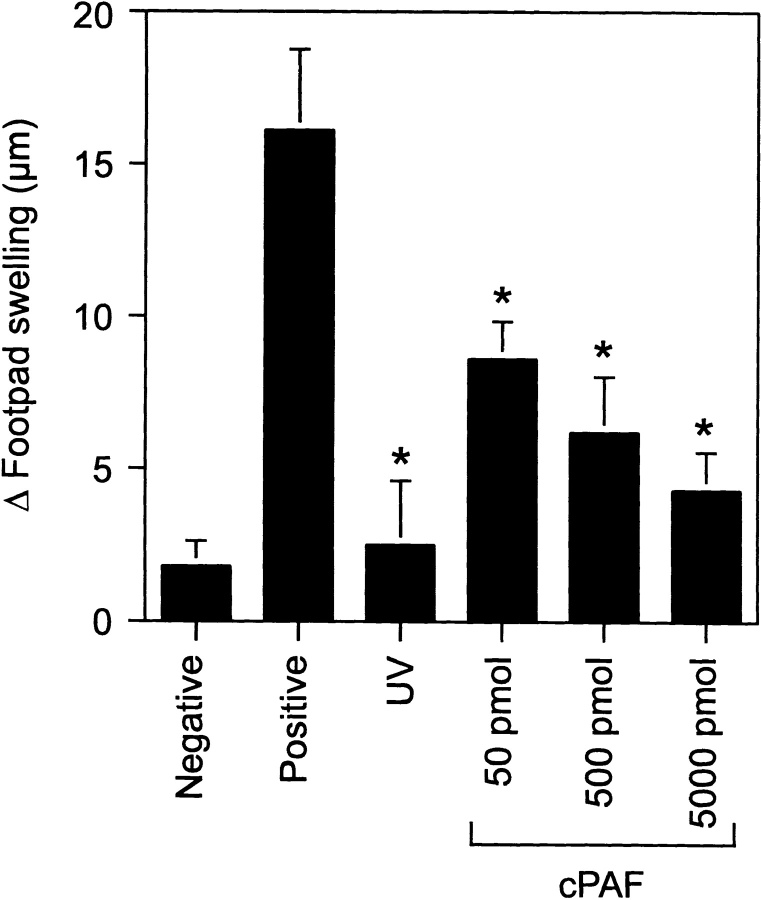
Next, we tested the hypothesis that PAF activates immune suppression. As shown in Fig. 2 , the intraperitoneal injection of cPAF induced significant immune suppression, in a dose-dependent manner. The immune suppression observed when the mice were injected with 500 pmol cPAF was similar to that seen when mice were exposed to 10 kJ/m2 of UVB radiation. These data indicate that cPAF mimics the effects of UV radiation and induces systemic immune suppression.
Figure 2.
PAF suppresses the induction of DTH. Mice were injected with cPAF (50 to 5,000 pmol) or exposed to UV radiation (10 kJ/m2) 5 d before immunization with C. albicans. 9 d later, the mice were challenged with Candida antigen. DTH was measured 24 h after challenge. The background response (negative control) was measured in mice that were not immunized but were challenged. The positive control was measured in mice that were immunized and challenged. Results are expressed as means ± SEM. An asterisk (*) indicates a statistically significant difference (P < 0.01) from the positive control (two-tailed Student's t test, n = 5). A representative experiment is shown; this experiment was repeated three times with similar results.
The PAF Receptor Antagonist PCA-4248 Blocks UV-induced Immune Suppression.
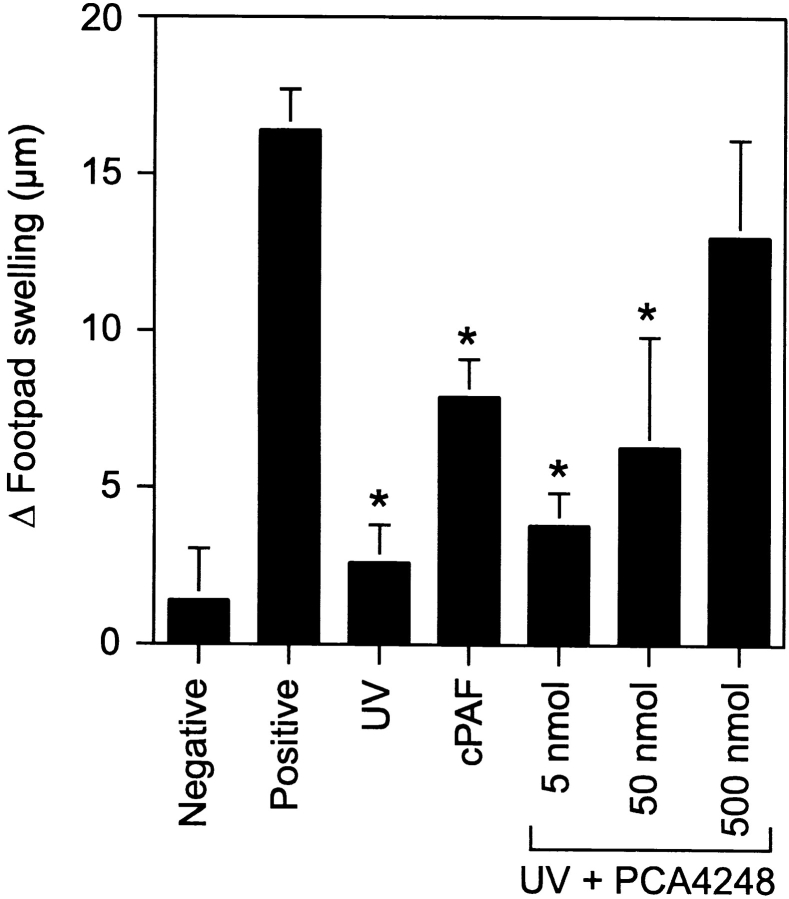
To further test the hypothesis that PAF is involved in UV-induced immune suppression, a PAF-receptor antagonist was used to block PAF signaling. Mice were first injected with various doses of PCA-4248 in PBS, and then irradiated with 10 kJ/m2 of UVB radiation. As the dose of PCA-4248 increased, so did protection against UV-induced immune suppression (Fig. 3) . In fact, at the highest dose of PCA-4248 used, we found that the response of UV-irradiated, receptor antagonist-injected mice was indistinguishable from the positive control (P > 0.05). These data indicate that blocking PAF receptor signaling blocks UV-induced immune suppression.
Figure 3.
Injecting a PAF receptor antagonist blocks UV-induced immune suppression. Mice were injected with 5 to 500 nmol PCA-4248, a PAF receptor antagonist 1 h before UV-irradiation (10 kJ/m2). 5 d later, the mice were immunized with C. albicans, and 9 d later, challenged with Candida antigen. DTH was measured 24 h after challenge. The background response (negative control) was measured in mice that were not immunized but were challenged. The positive control was measured in mice that were immunized and challenged. Results are expressed as means ± SEM. An asterisk (*) indicates a statistically significant difference (P < 0.01) from the positive control (two-tailed Student's t test, n= 5). A representative experiment is shown; this experiment was repeated three times with similar results.
Structurally Diverse PAF Receptor Antagonists Block UV-induced Immune Suppression.
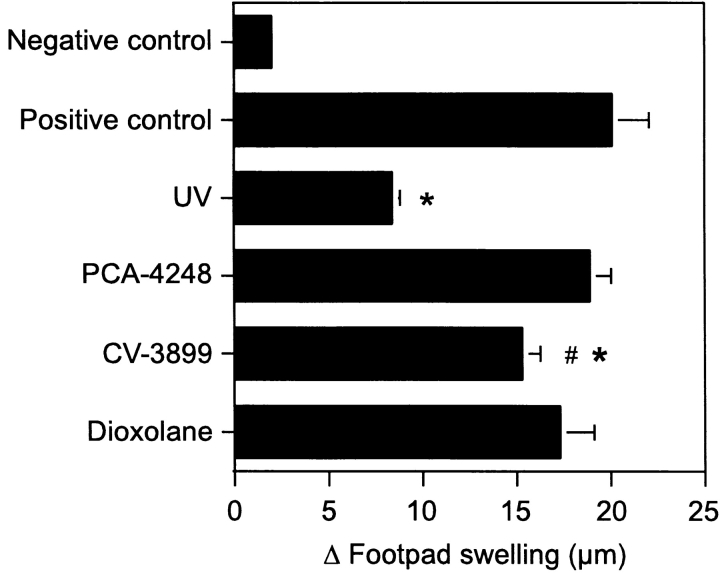
Lipid peroxidation causes the formation of other biologically active lipids, such as peroxisome proliferator activated receptor gamma agonists (26), that have been shown to mediate immunomodulatory effects (27). Our conclusion that immune suppression is mediated by PAF is based on inhibition studies using only one PAF receptor antagonist, PCA-4248. To ensure that the effects that we see are not unique to PCA-4248, and confirm the role of PAF receptor activation in the induction of immune suppression, we used two other structurally unrelated PAF receptor antagonists, CV-3988 (23) and dioxolane (24). Groups of mice were injected with 500 nmol of PCA-4248, CV-3988, or dioxolane and then exposed to UV radiation. All three of the PAF receptor antagonists blocked UV-induced immune suppression (Fig. 4) . When equal amounts of PCA-4248 and Dioxolane were injected into UV-irradiated mice, total immune restoration was noted. There was no significant difference between the response of the positive control and mice exposed to UV and treated with PCA-4248 and/or dioxolane. In addition a significant difference (P < 0.01) was noted between the response observed in UV-irradiated mice and those exposed to UV and injected with PCA-4248 and Dioxolane. In mice exposed to UV and injected with CV-3988 we noted a major reversal in immune suppression, in that there was a significant difference between the response found in mice exposed to UV and mice exposed to UV and injected with CV-3988 (P < 0.001). These data indicate that pharmacological blockade of the PAF receptor by structurally unrelated PAF-receptor antagonists block UV-induced immune suppression, thereby providing further support for the hypothesis that PAF receptor signaling is required for UV-induced immune suppression.
Figure 4.
Structurally diverse PAF receptor antagonists block UV-induced immune suppression. Mice were injected with 500 nmol PCA 4248, CV-3988 or dioxolane, and then exposed to 10 kJ/m2 UV radiation. 5 d later, the mice were immunized with C. albicans, and 9 d later, challenged with Candida antigen. DTH was measured 24 h after challenge. The background response (negative control) was measured in mice that were not immunized but were challenged. The positive control was measured in mice that were immunized and challenged. Results are expressed as means ± SEM. * indicates a statistically significant difference (P < 0.01) from the positive control (two-tailed Student's t test, n = 5). # indicates a statistically significant difference (P < 0.01) from the response found in UV-irradiated mice. A representative experiment is shown; this experiment was repeated three times with similar results.
UV-irradiated Phosphatidylcholine Induces Immune Suppression.
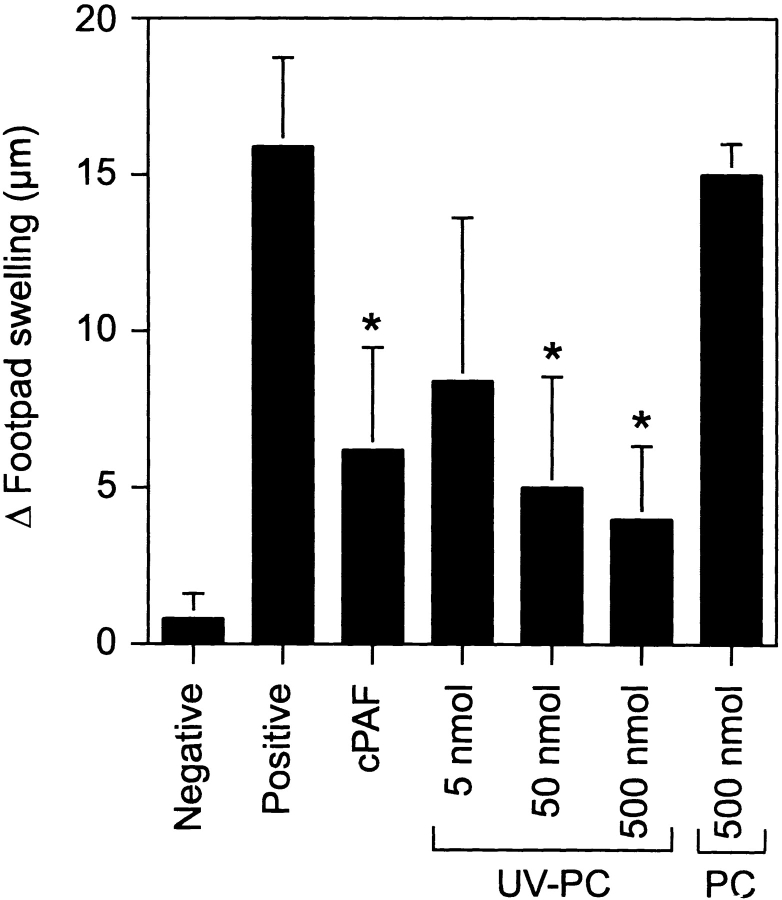
Data in the literature indicate that oxidative stress converts phosphatidylcholine into PAF-like receptor agonists (2, 28). Because UV radiation is a known activator of oxidative stress, we proposed that UV radiation oxidizes membrane lipids to become PAF-like receptor agonists, contributing to immune suppression. Egg yolk phosphatidylcholine was dissolved in PBS, at various concentrations, and spread thinly in polystyrene dishes to allow for complete exposure to UV and atmospheric oxygen. The solutions were exposed to 200 J/m2 UVB radiation. Groups of mice were then injected with the irradiated phosphatidylcholine (UV-PC). Control groups received normal unirradiated phosphatidylcholine. The mice were then immunized with C. albicans, and delayed-type hypersensitivity (DTH) was measured. We observed that UV-PC, but not normal unirradiated phosphatidylcholine induced immune suppression, in a dose-dependent manner (Fig. 5) . These findings suggest that UV-irradiation of plasma membranes can convert normal phosphatidylcholine into an endogenous inflammatory agent that activates immune suppression.
Figure 5.
UV-irradiated phosphatidylcholine induces immune suppression. Mice were injected with 500 pmol cPAF, 500 nmol normal phosphatidylcholine, (PC) or 5 to 500 nmol UV-PC, 5 d before immunization with C. albicans. 9 d later, the mice were challenged with Candida antigen. DTH was measured 24 h after challenge. The background response (negative control) was measured in mice that were not immunized but were challenged. The positive control was measured in mice that were immunized and challenged. Results are expressed as means ± SEM. An asterisk (*) indicates a statistically significant difference (P < 0.01) from the positive control (two-tailed Student's t test, n = 5). A representative experiment is shown; this experiment was repeated three times with similar results.
A Selective COX-2 Inhibitor Prevents Immune Suppression Caused by UV Radiation and PAF Receptor Agonists.
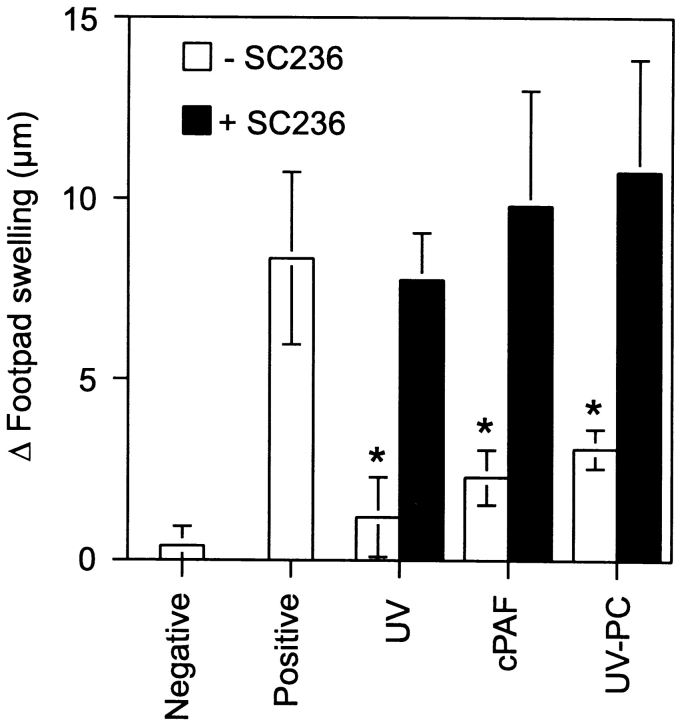
In an attempt to reconcile the data presented above with earlier findings where we demonstrated that PGE2 activated a cytokine cascade that ultimately lead to immune suppression (12), we used the selective COX-2 inhibitor SC-236 to inhibit prostaglandin synthesis in vivo. If PAF receptor signaling is upstream of PGE2 production, then SC-236 should inhibit PAF-induced immune suppression. If PAF receptor signaling acts via a parallel mechanism, then SC-236 should not reverse immune suppression. Immune suppression was induced by one of three methods: UV radiation, injecting cPAF, or injecting UV-PC. Duplicate groups of mice were injected with 0.2 μg SC-236 before UV radiation, cPAF, or UV-PC administration. The dose of SC-236 used here was based on previous studies in which 0.2 μg SC-236 totally blocked the immune suppression caused by exposing mice to 10 kJ/m2 of UVB radiation (12). The results from this experiment are shown in Fig. 6 . As demonstrated above, UV exposure, or injecting UV-PC and/or cPAF induced significant immune suppression. No immune suppression was observed in mice treated with SC-236. These results confirm that prostaglandin synthesis is essential for UV-induced immune suppression and indicate that the immune suppression induced by injecting cPAF and UV-PC work via a prostaglandin-dependent mechanism. Furthermore, they suggest that UV-induced activation of inflammatory lipid mediators is upstream of PGE2 synthesis in the cascade of events leading to systemic immune suppression.
Figure 6.
Blocking COX-2 activity in vivo abrogates PAF receptor agonist-induced immune suppression. Mice were exposed to UV radiation (10 kJ/m2), or injected with cPAF (500 pmol) or UV-PC (500 nmol) with (black bars) or without (white bars) the selective COX-2 inhibitor, SC 236. 5 d later, the mice were immunized with C. albicans. 9 d later, the mice were challenged with Candida antigen. DTH was measured 24 h after challenge. The background response (negative control) was measured in mice that were not immunized but were challenged. The positive control was measured in mice that were immunized and challenged. Results are expressed as means ± SEM. An asterisk (*) indicates a statistically significant difference (P < 0.01) from the positive control (two-tailed Student's t test, n = 5). A representative experiment is shown; this experiment was repeated three times with similar results.
Discussion
Because the systemic immune suppression induced by UV radiation is a well-known risk factor for skin cancer induction, it is important to understand the mechanisms involved. In addition, after a single exposure to an environmentally relevant dose of UV radiation the immune response to viral, fungal, and bacterial antigens is significantly suppressed. Ultraviolet radiation-induced, keratinocyte-derived cytokines and immune modulatory factors play an important role in the activation of systemic immune suppression. Previous findings indicated that PGE2 production was an early step in the cascade of events leading to immune suppression (12). In response to UV radiation, keratinocytes also produce and secrete the proinflammatory phospholipid mediator, PAF (17–19). Because PAF promotes cytokine synthesis and upregulates COX-2 biosynthesis, we tested that hypothesis that PAF is involved in the systemic immune suppression induced by UV radiation. Our findings support a role for PAF in the induction of immune suppression. PAF activates the transcription of COX-2 and IL-10, two important mediators of systemic immune suppression. Injecting PAF into mice mimics the effect of UV radiation in vivo by suppressing DTH, and injecting the PAF receptor antagonist blocks UV-induced immune suppression. These findings suggest that in addition to being sensors for cellular damage, proinflammatory oxidized phospholipids (PAF and PAF-like molecules) also activate immune suppressive mechanisms. To our knowledge, this is the first report demonstrating a systemic immunosuppressive role for PAF.
As mentioned above, synthesis of bona fide PAF occurs via the enzymatic removal of sn-2 side chains from phosphatidylcholine by phospholipase-A2, followed by acetylation of the free hydroxyl moiety (2). In addition, fatty acid side chains of phosphatidylcholine can become oxidized at points of unsaturation and undergo a radical scission leaving a shortened acyl chain at the sn-2 position and a hydroperoxide-alkyl byproduct. These fragmented phosphatidylcholine molecules (PAF-like lipids) signal through the PAF receptor, which amplifies and sustains the biological signal by inducing further PAF synthesis, arachidonic acid release, and PGE2 synthesis (21). Ultraviolet radiation can upregulate PAF production in vivo by affecting different points of this pathway. For example, UV radiation induces reactive oxygen species that have been shown to upregulate PAF production (29). Moreover, UV exposure upregulates phospholipase-A2 activity (30).
An important mechanism for generating PAF-like molecules from phosphatidylcholine involves degradation by reactive oxygen species. As UV radiation induces free radical formation under physiological conditions, this suggests that cell membranes are targeted by UV radiation to initiate the immunosuppressive pathway. Data from the experiments presented here, in which UV-irradiation of cell-free phosphatidylcholine converts it to an immunosuppressive compound, is consistent with this idea. Moreover, data in the literature indicating that UV-induced membrane effects activate gene expression (31, 32) provide further support for UV-induced lipid oxidation as an initial step in UV-induced immune suppression. On the other hand, a series of experiments published by Kripke and coworkers showed that repairing UV-induced DNA damage completely blocked immune suppression and restored immune function in UV-irradiated individuals (33–37), suggesting that DNA is the chromophore. How does one reconcile these apparently conflicting results? After genomic stress, a cell must progress through a series of checkpoints that determine whether that cell lives or dies. Ultraviolet exposure promotes arrest at the G2/M checkpoint to allow for DNA repair (38). If DNA repair is successful, progression through the cycle continues, if not, apoptosis and cell death results. As recently shown by Fornace and colleagues, MAP kinase activation plays a critical role in the initiation of G2/M delay after UV irradiation (39). Ultraviolet-induced DNA damage activates MAP kinase p38, which subsequently initiates a cascade of events that promotes G2/M cell cycle arrest. MAP kinase activation is also involved in the activation of phospholipase-A2, the first enzymatic step in PAF synthesis (40). Moreover, a feedback amplification loop may be involved because activated phospholipase-A2 has been shown to stimulate the appearance and activity of p38 (41). We propose that a side effect of the process used by cells to repair UV-induced DNA damage and maintain genomic integrity in vivo is the induction of immune suppression. The MAP kinase pathway controls G2/M check point delay and at the same time increases the enzymatic activity of phospholipase-A2. This results in the biosynthesis of PAF, and PGE2 thus driving systemic immune suppression. This may explain why in all of our previous experiments, repairing DNA damage completely reversed UV-induced immune suppression, regardless of the experimental system employed to measure immune function. Experiments are currently in progress to determine the molecular mechanisms underlying UV-induced PAF production.
There are a number of other observations in the literature supporting the suggestion that the maintenance of genomic integrity and the induction of UV-induced immune suppression are closely linked. First, reactive oxygen species, which are well known for their ability to induce DNA damage (42), induce PAF synthesis (29). Second, no induction of immune suppression was found in UV-irradiated apoptosis-deficient Fas and/or Fas-ligand–deficient mice (43, 44). Third, in the past we have been able to induce cytokine production and systemic immune suppression by inducing nonspecific DNA damage with HindIII-containing liposomes (45). We propose that the immune suppression induced by HindIII containing-liposomes works via a DNA damage-induced, MAP kinase-dependent, PAF-driven mechanism, and studies are in progress to directly test this hypothesis.
In addition to DNA, the other major chromophore for UV-induced immune suppression is urocanic acid. Naturally occurring trans-urocanic acid, found in the uppermost layers of the skin, is isomerized by UV radiation into the cis-isomer, which is a potent immune immunosuppressive agent (46). Although it is uncertain how UV-induced cis-urocanic acid fits into the pathway described above, preliminary results from our laboratory indicate that we can reverse cis-urocanic acid-induced suppression with PAF-receptor antagonists (unpublished data). Therefore, we suggest that cis-urocanic acid may be working in concert with UV-induced DNA damage to amplify PAF biosynthesis and contribute to immune suppression. This may explain why repairing UV-induced pyrimidine dimer formation in vivo will completely reverse immune suppression regardless of the immune parameter tested, (i.e., both DTH and contact hypersensitivity), whereas neutralization of cis-urocanic acid activity in vivo preferentially reverses some, but not all examples of UV-induced suppression (47, 48).
The immunoregulatory role of PAF may not be limited to UV-induced immune suppression. We propose that PAF release and PAF-receptor binding may play a role in the immune suppression induced by other dermal immunotoxins that suppress via PGE2-dependent pathways. One example is the induction of systemic immune suppression after dermal exposure to jet fuel (49). Jet fuels are lipophilic reagents that damage DNA (50) and suppress via the production of immune regulatory biological response modifiers, such as IL-10 and PGE2 (51). Studies are in progress to determine whether PAF and PAF-like molecules are involved in jet fuel–induced immune suppression.
In summary, our data support an immunosuppressive role for PAF. We suggest that UV-induced keratinocyte-derived PAF binds to PAF receptors on adjacent cells and drives cytokine transcription. This initiates a cytokine cascade that ultimately results in systemic immune suppression.
Acknowledgments
We thank Nasser Kazimi for his assistance with the experiments reported here; Dr. Peter Isakson, G.D. Searle & Co. for the selective COX-2 inhibitor; Dr. Stuart Yuspa, National Cancer Institute for the PAM 212 cells, and Dr. Sandra Gollnick, Roswell Park Cancer Institute for the IL-10 promoter constructs.
This work was supported by research grants from the National Cancer Institute (R01CA 75575 and R01CA 088943; SEU), a pre-doctoral training grant from the National Cancer Institute (T32-CA-09598-10, JPW), and pre-doctoral awards from the American Legion (J.P. Walterscheid), the Shell Foundation (D.X. Nghiem), and the R.E. “Bob” Smith Educational Fund (D.X. Nghiem) at the M.D. Anderson Cancer Center. The animal facilities at the M.D. Anderson Cancer Center are supported in part by a core grant from the National Cancer Institute (CA 16672).
Footnotes
Abbreviations used in this paper: COX, cyclooxygenase; cPAF, carbamyl-PAF; DTH, delayed-type hypersensitivity; MAP, mitogen-activated protein; PAF, platelet activating factor; UV-PC, UV-irradiated phosphatidylcholine.
References
- 1.Prescott, S.M., G.A. Zimmerman, D.M. Stafforini, and T.M. McIntyre. 2000. Platelet-activating factor and related lipid mediators. Annu. Rev. Biochem. 69:419–445. [DOI] [PubMed] [Google Scholar]
- 2.McIntyre, T.M., G.A. Zimmerman, and S.M. Prescott. 1999. Biologically active oxidized phospholipids. J. Biol. Chem. 274:25189–25192. [DOI] [PubMed] [Google Scholar]
- 3.Ishii, S., and T. Shimizu. 2000. Platelet-activating factor (PAF) receptor and genetically engineered PAF receptor mutant mice. Prog. Lipid Res. 39:41–82. [DOI] [PubMed] [Google Scholar]
- 4.Penn, I. 2000. Post-transplant malignancy: the role of immunosuppression. Drug Saf. 23:101–113. [DOI] [PubMed] [Google Scholar]
- 5.Fisher, M.S., and M.L. Kripke. 1982. Suppressor T lymphocytes control the development of primary skin cancers in UV-irradiated mice. Science. 216:1133–1134. [DOI] [PubMed] [Google Scholar]
- 6.Yoshikawa, T., V. Rae, W. Bruins-Slot, J.W. vand-den-Berg, J.R. Taylor, and J.W. Streilein. 1990. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J. Invest. Dermatol. 95:530-536. [DOI] [PubMed] [Google Scholar]
- 7.Jeevan, A., and M.L. Kripke. 1989. Effect of a single exposure to Ultraviolet radiation on Mycobacterium bovis Bacillus Calmette-Guerin infection in mice. J. Immunol. 143:2837–2843. [PubMed] [Google Scholar]
- 8.Goettsch, W., J. Garssen, W. Slob, F.R. de Gruijl, and H. Van Loveren. 1998. Risk assessment for the harmful effects of UVB radiation on the immunological resistance to infectious diseases. Environ. Health Perspect. 106:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norval, M., J. Garssen, H. Van Loveren, and A.A. el-Ghorr. 1999. UV-induced changes in the immune response to microbial infections in human subjects and animal models. J. Epidemiol. 9:S84–92. [DOI] [PubMed] [Google Scholar]
- 10.Nghiem, D.X., N. Kazimi, G. Clydesdale, H.N. Ananthaswamy, M.L. Kripke, and S.E. Ullrich. 2001. Ultraviolet A radiation suppresses an established immune response: Implications for sunscreen design. J. Invest. Dermatol. 117:1193–1199. [DOI] [PubMed] [Google Scholar]
- 11.Ullrich, S.E. 2000. The effects of ultraviolet radiation on the immune response. Biochemical Modulation of Skin Reactions: Transdermals, Topicals, Cosmetics. A.F. Kydonieus and J.J. Wille, editors. CRC Press, Boca Raton, FL. 281–300.
- 12.Shreedhar, V., T. Giese, V.W. Sung, and S.E. Ullrich. 1998. A cytokine cascade including prostaglandin E2, interleukin-4, and interleukin-10 is responsible for UV-induced systemic immune suppression. J. Immunol. 160:3783–3789. [PubMed] [Google Scholar]
- 13.Schmitt, D.A., and S.E. Ullrich. 2000. Exposure to ultraviolet radiation causes dendritic cells/macrophages to secrete immune suppressive IL-12p40 homodimers. J. Immunol. 165:3162–3167. [DOI] [PubMed] [Google Scholar]
- 14.Ullrich, S.E. 1994. Mechanism involved in the systemic suppression of antigen-presenting cell function by UV irradiation: Keratinocyte-derived IL-10 modulates antigen-presenting cell function of splenic adherent cells. J. Immunol. 152:3410–3416. [PubMed] [Google Scholar]
- 15.Buckman, S.Y., A. Gresham, P. Hale, G. Hruza, J. Anast, J. Masferrer, and A.P. Pentland. 1998. COX-2 expression is induced by UVB exposure in human skin: Implications for the development of skin cancer. Carcinogenesis. 19:723–729. [DOI] [PubMed] [Google Scholar]
- 16.Jaksic, A., J.J. Finlay-Jones, C.J. Watson, L.K. Spencer, I. Santucci, and P.H. Hart. 1995. Cis-urocanic acid synergizes with histamine for increased PGE2 production by human keratinocytes: Link to indomethacin-inhibitable UVB-induced immunosuppression. Photochem. Photobiol. 61:303–309. [DOI] [PubMed] [Google Scholar]
- 17.Barber, L.A., D.F. Spandau, S.C. Rathman, R.C. Murphy, C.A. Johnson, S.W. Kelley, S.A. Hurwitz, and J.B. Travers. 1998. Expression of the platelet-activating factor receptor results in enhanced ultraviolet B radiation-induced apoptosis in a human epidermal cell line. J. Biol. Chem. 273:18891–18897. [DOI] [PubMed] [Google Scholar]
- 18.Sheng, Y., and D.L. Birkle. 1995. Release of platelet activating factor (PAF) and eicosanoids in UVC-irradiated corneal stromal cells. Curr. Eye Res. 14:341–347. [DOI] [PubMed] [Google Scholar]
- 19.Calignano, A., G. Cirino, R. Meli, and P. Persico. 1988. Isolation and identification of platelet-activating factor in UV- irradiated guinea pig skin. J. Pharmacol. Methods. 19:89–91. [DOI] [PubMed] [Google Scholar]
- 20.Travers, J.B., J.C. Huff, M. Rola-Pleszczynski, E.W. Gelfand, J.G. Morelli, and R.C. Murphy. 1995. Identification of functional platelet-activating factor receptors on human keratinocytes. J. Invest. Dermatol. 105:816–823. [DOI] [PubMed] [Google Scholar]
- 21.Pei, Y., L.A. Barber, R.C. Murphy, C.A. Johnson, S.W. Kelley, L.C. Dy, R.H. Fertel, T.M. Nguyen, D.A. Williams, and J.B. Travers. 1998. Activation of the epidermal platelet-activating factor receptor results in cytokine and cyclooxygenase-2 biosynthesis. J. Immunol. 161:1954–1961. [PubMed] [Google Scholar]
- 22.Fernandez-Gallardo, S., M.P. Ortega, J.G. Priego, M.F. de Casa-Juana, C. Sunkel, and M. Sanchez Crespo. 1990. Pharmacological actions of PCA 4248, a new platelet-activating factor receptor antagonist: in vivo studies. J. Pharmacol. Exp. Ther. 255:34–39. [PubMed] [Google Scholar]
- 23.Terashita, Z., Y. Imura, and K. Nishikawa. 1985. Inhibition by CV-3988 of the binding of [3H]-platelet activating factor (PAF) to the platelet. Biochem. Pharmacol. 34:1491–1495. [DOI] [PubMed] [Google Scholar]
- 24.Corey, E.J., C.P. Chen, and M.J. Parry. 1988. Dual binding modes to the receptor for platelet activating factor (PAFF) of anti-PAF trans-2,5-diarylfurans. Tetrahedron Letters. 29:2899–2902. [Google Scholar]
- 25.Gollnick, S.O., B.Y. Lee, L. Vaughan, B. Owczarczak, and B.W. Henderson. 2001. Activation of the IL-10 gene promoter following photodynamic therapy of murine keratinocytes. Photochem. Photobiol. 73:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies, S.S., A.V. Pontsler, G.K. Marathe, K.A. Harrison, R.C. Murphy, J.C. Hinshaw, G.D. Prestwich, A.S. Hilaire, S.M. Prescott, G.A. Zimmerman, and T.M. McIntyre. 2001. Oxidized alkyl phospholipids are specific, high affinity peroxisome proliferator-activated receptor gamma ligands and agonists. J. Biol. Chem. 276:16015–16023. [DOI] [PubMed] [Google Scholar]
- 27.Zhang, X., J.M. Wang, W.H. Gong, N. Mukaida, and H.A. Young. 2001. Differential regulation of chemokine gene expression by 15-deoxy-delta 12,14 prostaglandin J2. J. Immunol. 166:7104–7111. [DOI] [PubMed] [Google Scholar]
- 28.Marathe, G.K., S.S. Davies, K.A. Harrison, A.R. Silva, R.C. Murphy, H. Castro-Faria-Neto, S.M. Prescott, G.A. Zimmerman, and T.M. McIntyre. 1999. Inflammatory platelet-activating factor-like phospholipids in oxidized low density lipoproteins are fragmented alkyl phosphatidylcholines. J. Biol. Chem. 274:28395–28404. [DOI] [PubMed] [Google Scholar]
- 29.Lewis, M.S., R.E. Whatley, P. Cain, T.M. McIntyre, S.M. Prescott, and G.A. Zimmerman. 1988. Hydrogen peroxide stimulates the synthesis of platelet-activating factor by endothelium and induces endothelial cell-dependent neutrophil adhesion. J. Clin. Invest. 82:2045–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gresham, A., J. Masferrer, X. Chen, S. Leal-Khouri, and A.L. Pentland. 1996. Increased synthesis of high molecular weight cPLA2 mediates early UV-induced PGE2 in human skin. Am. J. Physiol. 270:C1037–C1050. [DOI] [PubMed] [Google Scholar]
- 31.Simon, M.M., Y. Aragane, A. Schwarz, T.A. Luger, and T. Schwarz. 1994. UVB light induces a nuclear factor κB (NFκB) activity independently from chromosomal DNA damage in cell-free cytosolic extracts. J. Invest. Dermatol. 102:422–427. [DOI] [PubMed] [Google Scholar]
- 32.Devary, Y., R.A. Gottlieb, T. Smeal, and M. Karin. 1992. The mammalian ultraviolet response is triggered by activation of Src tyrosine kinases. Cell. 71:1081–1091. [DOI] [PubMed] [Google Scholar]
- 33.Applegate, L.A., R.D. Ley, J. Alcalay, and M.L. Kripke. 1989. Identification of the molecular target for the suppression of contact hypersensitivity by UV radiation. J. Exp. Med. 170:1117–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kripke, M.L., P.A. Cox, L.G. Alas, and D.B. Yarosh. 1992. Pyrimidine dimers in DNA initiate systemic immunosuppression in UV-irradiated mice. Proc. Natl. Acad. Sci. USA. 89:7516–7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vink, A.A., A.M. Moodycliffe, V. Shreedhar, S.E. Ullrich, L. Roza, D.B. Yarosh, and M.L. Kripke. 1997. The inhibition of antigen-presenting activity of dendritic cells resulting from UV irradiation of murine skin is restored by in vitro photorepair of cyclobutane pyrimidine dimers. Proc. Natl. Acad. Sci. USA. 94:5255–5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vink, A.A., F.M. Strickland, C. Bucana, P.A. Cox, L. Roza, D.B. Yarosh, and M.L. Kripke. 1996. Localization of DNA damage and its role in altered antigen-presenting cell function in ultraviolet-irradiated mice. J. Exp. Med. 183:1491–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishigori, C., D.B. Yarosh, S.E. Ullrich, A.A. Vink, C.D. Bucana, L. Roza, and M.L. Kripke. 1996. Evidence that DNA damage triggers interleukin 10 cytokine production in UV-irradiated murine keratinocytes. Proc. Natl. Acad. Sci. USA. 93:10354–10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gabrielli, B.G., J.M. Clark, A.K. McCormack, and K.A. Ellem. 1997. Ultraviolet light-induced G2 phase cell cycle checkpoint blocks cdc25- dependent progression into mitosis. Oncogene. 15:749–758. [DOI] [PubMed] [Google Scholar]
- 39.Bulavin, D.V., Y. Higashimoto, I.J. Popoff, W.A. Gaarde, V. Basrur, O. Potapova, E. Appella, and A.J. Fornace, Jr. 2001. Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase. Nature. 411:102–107. [DOI] [PubMed] [Google Scholar]
- 40.Lin, L.L., M. Wartmann, A.Y. Lin, J.L. Knopf, A. Seth, and R.J. Davis. 1993. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 72:269-278. [DOI] [PubMed] [Google Scholar]
- 41.Hii, C.S., Z.H. Huang, A. Bilney, M. Costabile, A.W. Murray, D.A. Rathjen, C.J. Der, and A. Ferrante. 1998. Stimulation of p38 phosphorylation and activity by arachidonic acid in HeLa cells, HL60 promyelocytic leukemic cells, and human neutrophils. Evidence for cell type-specific activation of mitogen-activated protein kinases. J. Biol. Chem. 273:19277–19282. [DOI] [PubMed] [Google Scholar]
- 42.Pourzand, C., and R.M. Tyrrell. 1999. Apoptosis, the role of oxidative stress and the example of solar UV radiation. Photochem. Photobiol. 70:380–390. [PubMed] [Google Scholar]
- 43.Schwarz, A., S. Grabbe, K. Grosse-Heitmeyer, B. Roters, H. Riemann, T.A. Luger, G. Trinchieri, and T. Schwarz. 1998. Ultraviolet light-induced immune tolerance is mediated via the Fas/Fas-ligand system. J. Immunol. 160:4262–4270. [PubMed] [Google Scholar]
- 44.Hill, L.L., V.K. Shreedhar, M.L. Kripke, and L.B. Owen-Schaub. 1999. A critical role for Fas ligand in the active suppression of systemic immune responses by ultraviolet radiation. J. Exp. Med. 189:1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishigori, C., D. Yarosh, A. O'Connor, V.K. Shreedhar, S.E. Ullrich, P. Cox, and M.L. Kripke. 1998. HindIII liposomes suppress delayed-type hypersensitivity responses in vivo and induce epidermal IL-10 in vitro. J. Immunol. 161:2684–2691. [PubMed] [Google Scholar]
- 46.De Fabo, E.C., and F.P. Noonan. 1983. Mechanism of immune suppression by ultraviolet irradiation in vivo. I. Evidence for the existence of a unique photoreceptor in skin and its role in photoimmunology. J. Exp. Med. 157:84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Ghorr, A.A., and M. Norval. 1995. A monoclonal antibody to cis-urocanic acid prevents the UV-induced changes in Langerhans cells and DTH responses in mice, although not preventing dendritic cell accumulation in lymph nodes draining the site of irradiation and contact hypersensitivity responses. J. Invest. Dermatol. 105:264–268. [DOI] [PubMed] [Google Scholar]
- 48.Moodycliffe, A.M., C.D. Bucana, M.L. Kripke, M. Norval, and S.E. Ullrich. 1996. Differential effects of a monoclonal antibody to cis-urocanic acid on the suppression of delayed and contact hypersensitivity following ultraviolet irradiation. J. Immunol. 157:2891–2899. [PubMed] [Google Scholar]
- 49.Ullrich, S.E. 1999. Dermal application of JP-8 jet fuel induces immune suppression. Toxicol. Sci. 52:61–67. [DOI] [PubMed] [Google Scholar]
- 50.Grant, G.M., S.M. Jackman, C.J. Kolanko, and D.A. Stenger. 2001. JP-8 jet fuel-induced DNA damage in H4IIE rat hepatoma cells. Mutat. Res. 490:67–75. [DOI] [PubMed] [Google Scholar]
- 51.Ullrich, S.E., and H.J. Lyons. 2000. Mechanisms involved in the immunotoxicity induced by dermal application of JP-8 jet fuel. Toxicol. Sci. 58:290–298. [DOI] [PubMed] [Google Scholar]