Abstract
Developing pea cotyledons contain functionally different vacuoles, a protein storage vacuole and a lytic vacuole. Lumenal as well as membrane proteins of the protein storage vacuole exit the Golgi apparatus in dense vesicles rather than in clathrin-coated vesicles (CCVs). Although the sorting receptor for vacuolar hydrolases BP-80 is present in CCVs, it is not detectable in dense vesicles. To localize these different vacuolar sorting events in the Golgi, we have compared the distribution of vacuolar storage proteins and of α-TIP, a membrane protein of the protein storage vacuole, with the distribution of the vacuolar sorting receptor BP-80 across the Golgi stack. Analysis of immunogold labeling from cryosections and from high pressure frozen samples has revealed a steep gradient in the distribution of the storage proteins within the Golgi stack. Intense labeling for storage proteins was registered for the cis-cisternae, contrasting with very low labeling for these antigens in the trans-cisternae. The distribution of BP-80 was the reverse, showing a peak in the trans-Golgi network with very low labeling of the cis-cisternae. These results indicate a spatial separation of different vacuolar sorting events in the Golgi apparatus of developing pea cotyledons.
Keywords: Golgi apparatus, clathrin-coated vesicle, dense vesicles, storage protein, vacuolar sorting receptor
Introduction
It is now well established that plants are unique in harboring functionally different vacuoles in a single cell (Hoh et al. 1995; Paris et al. 1996; Di Sansebastiano et al. 1998; Swanson et al. 1998). These vacuoles are characterized by the presence of distinct aquaporins, members of the family of major intrinsic proteins (MIPs; Weig et al. 1997; Jauh et al. 1998, Jauh et al. 1999) in their membranes, and by the differential distribution of hydrolases and storage proteins in their lumen (Hara-Nishimura and Maeshima 2000). Lumenal vacuolar proteins appear to reach the protein storage vacuole (PSV) in one of three ways. The first route is through autophagy of ER-released storage protein aggregates (Levanony et al. 1992). The second route, which also bypasses the Golgi apparatus, entails the release of vesicles from the ER. This happens with storage proteins during PSV formation in pumpkin cotyledons. Here, 200-nm diameter so-called precursor-accumulating vesicles are formed, which also receive an additional vesicular contribution from the Golgi apparatus en route to the PSV (Hara-Nishimura et al. 1998). It can also occur with proteinases during PSV to lytic vacuole transformation in germinating seeds (Toyooka et al. 2000). The third route includes transport through the Golgi apparatus where they become sorted from other proteins of the secretory pathway. This is typical of proteins stored in legumes (Robinson and Hinz 1999).
Receptor-mediated sorting of lysomal/vacuolar acid hydrolases in the Golgi apparatus of mammalian/yeast cells occurs in a late Golgi compartment and culminates in their packaging into clathrin-coated vesicles (CCVs) (for reviews see Conibear and Stevens 1998; Le Borgne and Hoflack 1998). Recently, a family of vacuolar sorting receptor (VSR) proteins has been identified from widely differing plant species (Kirsch et al. 1994; Paris and Rogers 1996; Ahmed et al. 1997; Paris et al. 1997; Shimada et al. 1997; Laval et al. 1999; Miller et al. 1999). Both the pea and the Arabidopsis homologues localize to the CCV (Ahmed et al. 1997; Robinson et al. 1998b; Sanderfoot et al. 1998; Hinz et al. 1999), and it has been shown that their cytoplasmic domains bind to mammalian Golgi AP-1 adaptins (Robinson et al. 1998b; Sanderfoot et al. 1998). Thus, it would seem that receptor–CCV adaptin interactions are common to the Golgi apparatus of all eukaryotic cells.
The lumenal domain of the pea cotyledon VSR, known as BP-80, has been shown to bind in vitro with high affinity to the NH2-terminal sorting sequence NPIR of the thiol hydrolase barley aleurain (Kirsch et al. 1994). In addition, Ahmed et al. 2000 identified an endogenous cargo protein in Arabidopsis, Arabidopsis thaliana Aleu strain (AtALEU), which interacts with the Arabidopsis VSR homologue Arabidopsis thaliana Elp (AtELP). However, controversy surrounds the question as to whether the VSR interacts additionally with COOH-terminal sorting sequences (Matsuoka and Neuhaus 1999). According to recent results by Ahmed et al. 2000, AtELP does not bind to COOH-terminal sorting motifs. In contrast, Cao et al. 2000 demonstrated that the BP-80 seems to possess two separate ligand binding sites, each recognizing distinct ligands. This supports the observation that the BP-80 class of VSR also recognizes COOH-terminal sorting motives in other vacuolar proteins (Kirsch et al. 1994, Kirsch et al. 1996; Shimada et al. 1997; Miller et al. 1999). However, globally, these motifs represent only a subset of the sorting signals found to be present in plant vacuolar proteins. Therefore, it has been suggested that this may reflect the presence of different sorting mechanisms for vacuolar proteins within the plant Golgi apparatus (Matsuoka and Neuhaus 1999).
Support for this contention is based on biochemical and morphological observations. On the one hand, Matsuoka et al. 1995 have shown that wortmannin, an inhibitor of the enzyme phosphatidylinositol 3-kinase, which plays an essential role in CCV budding in yeast and mammals (Stack et al. 1995), prevents the transport of barley lectin (possessing a complex COOH-terminal targeting signal and not binding to BP-80) but not of sporamin (a protein from sweet potato with an NH2-terminal NPIR BP-80 binding motif) to the vacuole. On the other hand, previous investigations from our group on developing pea cotyledons have established that the storage globulins legumin and vicilin, which do not contain the NPIR motif (Saalbach et al. 1991), enter the Golgi apparatus and are packaged into special transport vesicles called “dense vesicles” (DVs), rather than CCVs (Hohl et al. 1996; Robinson et al. 1997; Hinz et al. 1999). DVs and CCVs are visible at the same Golgi stack, but although CCVs are restricted to the TGN, DVs show an increasing amount of electron opacity in a cis to trans direction (Hohl et al. 1996; Robinson et al. 1997).
The latter observations raise the question as to where the sorting of storage proteins in the pea cotyledon actually occurs and how this is related to CCV-mediated sorting events. To address this problem, we have employed the techniques of high pressure freezing and cryosectioning, which have allowed us to detect nonaggregated storage proteins as well as BP-80 in the various subcompartments of the pea cotyledon Golgi apparatus. Our results clearly show that the sorting of vacuolar storage proteins occurs immediately upon their entrance into the Golgi apparatus. Such a cis-driven protein sorting mechanism has so far not been described in eukaryotes.
Materials and Methods
Plant Materials
Pea (Pisum sativum L., var. Haubner's Exzellenz) plants were grown hydroponically in a greenhouse. Seeds with a 8–9-mm-long axis diameter (∼20–22 d after anthesis) were collected and the testa removed (Hoh et al. 1995).
Cryosectioning
Small blocks of tissue close to the epidermis were cut from the cotyledons and immediately immersed either in a mixture of 1.5%(wt/vol) paraformaldehyde and 0.2%(vol/vol) glutaraldehyde, or in 1%(vol/vol) glutaraldehyde, both in 100 mM phosphate buffer, pH 7.0. After evacuation for 15 min at room temperature, the samples were transferred to 4°C, where the fixation continued for another 16 h. The blocks were then washed in phosphate buffer followed by stepwise infiltration with increasing concentrations of sucrose for 24 h at 4°C. During infiltration, the samples were constantly rotated while the concentration of sucrose was increased to 2.3 M (Tokuyasu 1980). The blocks were then transferred to specimen stubs and, after removal of surplus sucrose, were frozen in liquid nitrogen. Ultrathin sections were cut at −120°C (UCT; Leica) and the sections were picked up using the method of Liou et al. 1996.
High Pressure Freezing
Subepidermal tissue was excised, briefly immersed in hexadecene, and frozen immediately in a high pressure freezer (HPM 010; Leica).
Afterwards, the specimens were freeze substituted in dry acetone over a 4-d period (AFS; Leica) before the temperature was raised slowly to −35°C. At this temperature, the medium was replaced with acetone/0.5% wt/vol osmium tetroxide and maintained at this temperature for 4 h. The samples were then washed three times with dry ethanol, slowly warmed to room temperature, and infiltrated stepwise in London-Resin-White hard grade (Plano) before polymerizing for 24 h at 60°C in a vacuum oven.
Antibodies and Immunogold Labeling
Labeling of cryosections was performed as described previously (Hinz et al. 1999). The polyclonal antibodies used in this study were as follows: affinity-purified antibodies raised in rabbits against pea (P. sativum) legumin (Casey, 1979a; diluted 1:200 for high pressure frozen [HPF] and 1:100 for cryosectioned material); affinity-purified antibodies raised in mice against the 50-kD subunit of broad bean (Vicia faba) vicilin (Hohl et al. 1996; diluted 1:200 for HPF and 1:100 for cryosectioned material); antibodies raised in rabbits against the complex glycan of carrot (Daucus carota) β-fructosidase (βF1) (Laurière et al. 1989; diluted 1:400 for HPF and 1:200 for cryosectioned material); antibodies raised in rabbits against bean (Phaseolus vulgaris) α-TIP (Johnson et al. 1989; diluted 1:400 for cryosectioned material); antibodies raised in rabbits against the NH2-terminal 10 amino acids of pea (P. sativum) BP-80 (Paris et al. 1997; diluted 1:200 for cryosectioned material); and protein A–purified antibodies raised in rabbits against Arabidopsis Sec21p homologue (Atγ-COP; Movafeghi et al. 1999; diluted 1:200). Goat secondary antibodies (anti–mouse and anti–rabbit IgG) coupled to 10-nm colloidal gold were obtained from Biocell and used at a dilution of 1:30 in TBS (50 mM Tris-HCl, pH 7.4, 0.9% NaCl supplemented with 1% [wt/vol] BSA and 0.01% [wt/vol] acetylated BSA [BSA-C; Aurion]).
Results
The Morphology of the Golgi Apparatus in Pea Cotyledons and the Distribution of Storage Globulins
The principal storage globulins of pea seeds, legumin and vicilin, are encoded for by multigene families (Casey 1979b; Müntz 1998). The affinity-purified legumin antibodies used in this investigation were raised against the major mature 20- and 40-kD polypeptides (Casey 1979a), but they also recognize the other legumin polypeptides (Hohl et al. 1996; Hinz et al. 1997). To detect vicilin polypeptides, we have employed an affinity-purified antiserum generated against the mature 50-kD subunit of broad bean vicilin (Hohl et al. 1996). As shown by Chrispeels et al. 1982, vicilin precursor polypeptides oligomerize to 7S trimers in the ER. Trimer formation is unspecific with respect to the multiple vicilin polypeptides, and only trimers get exported out of the ER (Müntz 1998). Therefore, the distribution of gold particles in the Golgi stack can be understood as being representative for all of the different vicilin polypeptides.
The Golgi apparatus of pea cotyledon storage parenchyma cells is composed of numerous dictyosomes, each with four to five cisternae and a TGN (Hohl et al. 1996; Robinson et al. 1997, Robinson et al. 1998c). Although the morphological differences between the cis- and trans-cisternae are clearly visible after aldehyde/osmium fixation, the membranes of the Golgi stacks were only weakly pronounced in both cryosectioned and HPF samples (Fig. 1, Fig. 2, and Fig. 3). Nevertheless, cis-, median, trans-cisternae, and TGN were still distinguishable in both HPF and cryosectioned cotyledons. DVs were also recognized in both types of cryosamples. However, the Golgi stacks in HPF material had a much more compact appearance than in cryosections.
Figure 1.
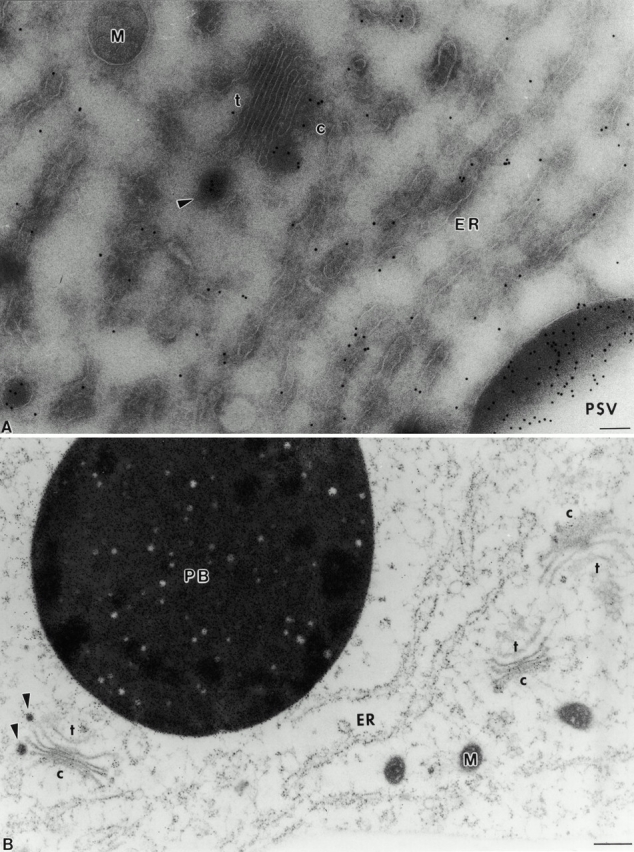
Distribution of the storage protein vicilin in the endomembrane system of pea cotyledons. (A) Cryosectioned sample. (B) HPF sample. M, Mitochondria; PB, protein body; ER, endoplasmic reticulum. DVs are marked by arrowheads. The cis and trans side of the Golgi are marked by c and t, respectively. c, cis; t, trans. Bars: (A) 100 nm; (B) 250 nm.
Figure 2.
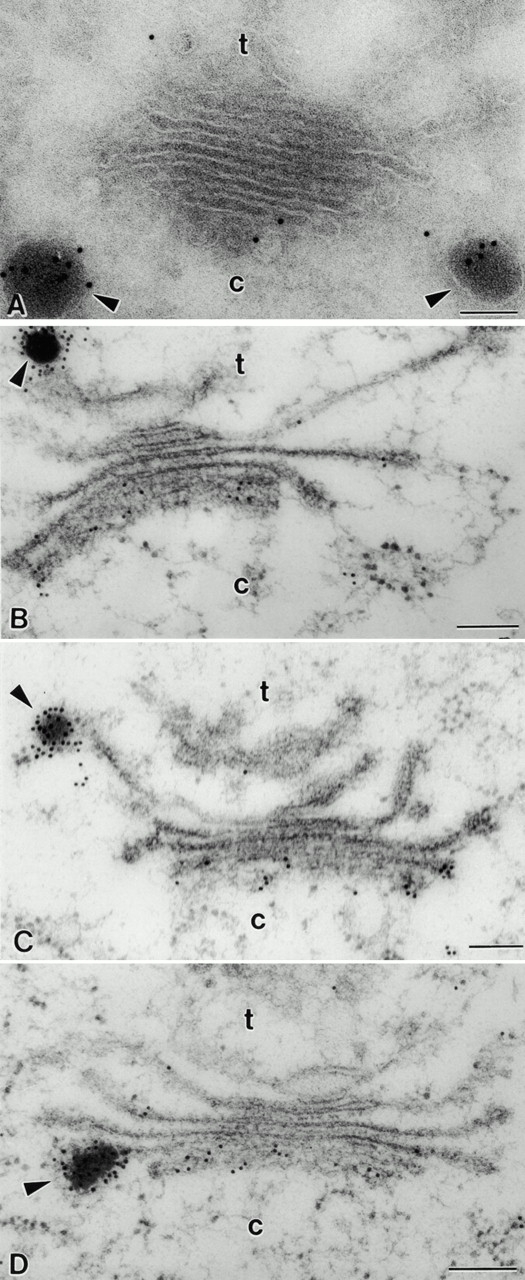
Immunogold staining of pea cotyledon Golgi stacks with antibodies against vicilin. (A) Cryosectioned sample. Background labeling is very low. DVs are heavily labeled, and the label in the Golgi stack is restricted to the cis-cisternae. (B, C, and D) HPF sample. Labeling of the cisternae reveals a steep gradient from the cis to the trans side of the Golgi. DVs are seen attached to the TGN (B), the trans-cisternae (C), and median cisternae (D). c, cis; t, trans. Bars, 100 nm.
Figure 3.
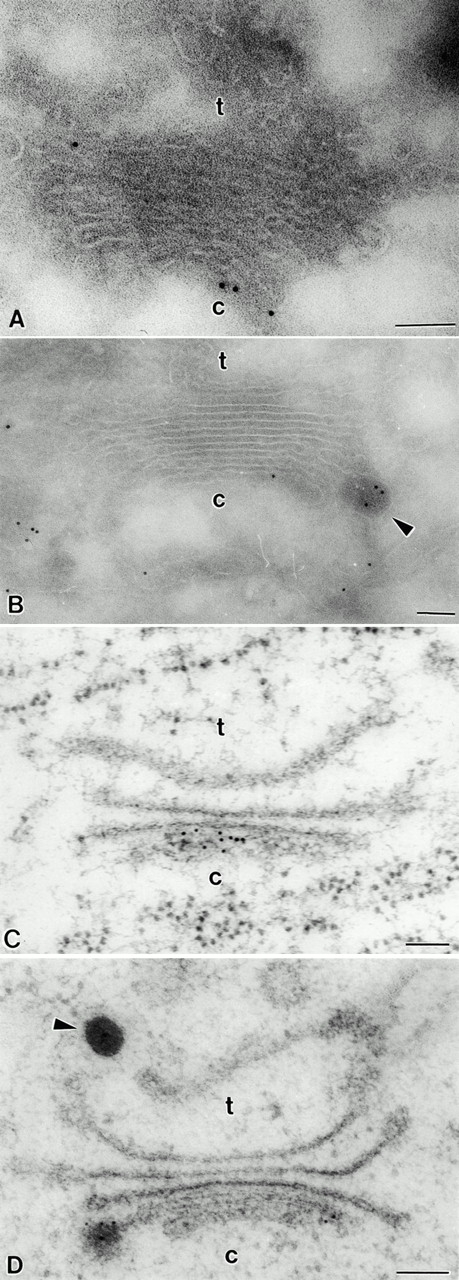
Immunogold staining of pea cotyledon Golgi stacks with antibodies against legumin. (A and B) Cryosection samples. Background labeling is very low. DVs are significantly labeled; the label is restricted to the cis-cisternae of the Golgi stack. (C and D) HPF sample. The distribution of gold particles is similar to that in A and B. c, cis; t, trans. Bars, 100 nm.
In previous studies on pea cotyledons where the tissue was fixed with aldehyde/osmium and resin embedded, immunogold staining with legumin and vicilin antibodies was restricted to DVs and the PSVs, i.e., only storage globulins in a condensed form were detected (Craig et al. 1980; Hoh et al. 1995, 1996). In contrast, a significant increase in the sensitivity of immunogold labeling was obtained when using the Tokuyasu 1980 method of cryosectioning. Although background labeling was still very low (Fig. 1 A, 2 A, and 3, A and B) and no label was found in the cell wall (data not shown), PSVs were intensely labeled (Fig. 1 A). However, as seen in Fig. 1 A, it now became possible to detect nonaggregated vicilin in the lumen of the ER, and legumin and vicilin in the cisternae of the Golgi stack as well (Fig. 1 A, 2 A, and 3, A and B). This type of labeling was not uniformly distributed throughout the stack: nonaggregated legumin and vicilin was restricted to the cis- and first median cisterna, with virtually no gold particles over the trans-cisterna and TGN (Fig. 1 A, 2 A, and 3, A and B).
Despite the improvement in antigen detection through cryosectioning, the slow aldehyde fixation and sucrose infiltration steps that precede freezing represent possible sources of artifact, which might lead to a redistribution of storage polypeptides within the lumen of the Golgi cisternae. For this reason, we have also examined the distribution of legumin and vicilin in HPF samples. Due to the instantaneous immobilization of cellular contents, a possible relocation of storage polypeptides can be ruled out with this technique. As shown in Fig. 1 B, background labeling is low; the protein bodies and the ER are significantly labeled. Nevertheless, the distribution of both antigens in the Golgi stack of pea cotyledons remained the same (Fig. 1 B, 2, B, C, and D, and 3, C and D). Additionally, as shown in Fig. 1 B and 2, B, C, and D, DVs were labeled with vicilin antibodies irrespective to their position in the Golgi stack.
A quantitative analysis of the distribution of legumin and vicilin (aggregated and nonaggregated forms) across the Golgi stack in HPF specimens is given in Table . Approximately 1/3 (31.7%) of the vicilin label was located to DVs, and another 40% (42.6%) was found over the cis-cisterna. The density of gold label decreased from 12.5% over the first median cisterna to 3.3% over the trans-cisterna and 2.3% over the TGN. Labeling with legumin antibodies revealed an even more pronounced differential distribution in the Golgi stack for this storage globulin. About half of the label (50.9%) was present over DVs, and 40.9% was found over the cis-cisterna. By comparison, the trans-cisterna and the TGN together represented <2% of the total Golgi-based label. Expressed in another way, although ∼25% of the vicilin label is localized to post–cis-compartments in the pea cotyledon Golgi apparatus, only ∼8% of the legumin label shares this distribution.
Table 1.
Distribution of Immunogold Labeling with Antibodies against Legumin, Vicilin, Xylose, α-TIP, and BP-80 across Dictyosomes of Developing Pea Cotyledons
| Antibody | cis | Median 1 | Median 2 | trans | TGN | DV | Gp (%) |
|---|---|---|---|---|---|---|---|
| βF1 (xylose) | 157 (16.4) | 172 (18) | 184 (19.3) | 208 (21.8) | 208 (21.8) | 25 (2.6) | 954 (100) |
| Vicilin | 255 (42.6) | 75 (12.5) | 45 (7.5) | 20 (3.3) | 14 (2.3) | 190 (31.7) | 599 (100) |
| Legumin | 70 (40.9) | 7 (4.1) | 3 (1.8) | 3 (1.8) | 1 (0.6) | 87 (50.9) | 171 (100) |
| BP-80 | 5 (6.5) | 14 (18.4) | 23 (30.3) | 29 (38) | 5 (6.5) | 0 (0) | 76 (100) |
| α-TIP | 4 (4) | 9 (9) | 12 (12) | 8 (8) | 7 (7) | 60 (60) | 100 (100) |
To elucidate the distribution of the respective antigens in the Golgi, photographs of Golgi stacks with distinguishable cisternae were taken and the number of gold particles was counted for each individual cisterna and for the DVs attached to them, irrespective of their positions in the stack. 7 Golgi stacks from different fixations were evaluated for the βF1 label, 22 for the vicilin label, 20 for the legumin label, 20 for the BP-80 label, and 13 for the α-TIP label. Gp, number of gold particles.
The Distribution of Complex Glycoproteins in the Pea Cotyledon Golgi Apparatus
To assess the extent of artifacts caused by specimen preparation, the distribution of complex glycans in the Golgi stack was determined in both HPF and cryosectioned specimens. In the past, antibodies against xylose residues of complex glycosylated proteins have been successfully used to characterize the functional compartmentalization of the plant Golgi apparatus (Zhang and Staehelin 1992; Fitchette-Lainé et al. 1994). βF1 antibodies, raised against carrot β-fructosidase, are specific for xylose-containing processed glycan side chains; they do not recognize the deglycosylated polypeptide backbone (Laurière et al. 1989). Background labeling with βF1 antibodies was very low (Fig. 4A and Fig. B) with both methods of cryofixation. The cell wall was prominently labeled, as were, to a certain extent, the PSVs (data not shown). Golgi stacks, but no other endomembranes (including the ER), were also conspicuously labeled. Although embedded in resin, the labeling density with the βF1 antibody was significantly higher on sections from HPF material than on cryosections. This indicates that antigen–βF1 antibody coupling is very sensitive towards aldehydes, which are used at low concentrations at the beginning of the cryosectioning method. However, an equally plausible explanation for the reduced labeling density of the βF1 antibody in cryosections is that a portion of the lightly fixed glycoproteins may be leached from the surface of the cryosections upon thawing.
Figure 4.
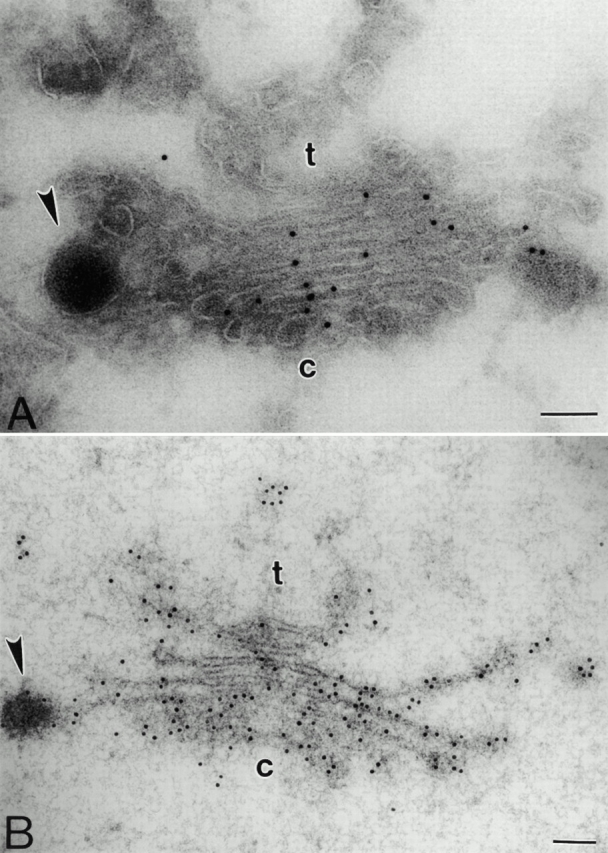
Immunogold staining of pea cotyledon Golgi stacks with antibodies directed against xylose residues (βF1). (A) Labeling of the Golgi cisternae and DVs on cryosections. Background labeling is very low. Although the Golgi cisternae are more or less uniformly labeled at a high density, labeling of the DVs is rather low. (B) Labeling of the Golgi cisternae and DVs in HPF samples. The distribution of gold particles is the same as for cryosections, but labeling density is considerably higher. c, cis; t, trans. Bars, 100 nm.
The distribution of gold particles over the Golgi stack did not differ between HPF and cryosectioned specimens, with >100 gold particles being visible per stack in the HPF specimens. Golgi-based βF1 label in HPF specimens was quantitated (Table ) and revealed the median cisternae as being the major location for xylose residues. Together they represent roughly 40% of the label, with 20% in each of the cis- and trans-cisternae, about 16% in the TGN, and the rest (2.5 %) in the DVs. This distribution is in agreement with previous findings on other plant Golgi stacks (Zhang and Staehelin 1992; Fitchette-Lainé et al. 1994), and confirms the observations of Hohl et al. 1996, who also reported a rather low βF1 labeling of DVs.
Distribution of COPI Coat Proteins in the Golgi Stack
Intra-Golgi protein transport is mediated by COPI-coated vesicles (reviewed in Lowe and Kreis 1998; Robinson et al. 1998a), which have also been identified in plant cells (Pimpl et al. 2000). Because DVs are to be seen attached to all cisternae of the pea Golgi stack (Hohl et al. 1996; and see Fig. 2b, Fig. c, and Fig. d), the question arises as to whether these vesicles might be related to COPI vesicles. To examine this possibility, the distribution of Atγ-COP (Movafeghi et al. 1999; Pimpl et al. 2000) in the pea Golgi stack and associated vesicles has been determined on cryosections. As shown in Fig. 5, the Atγ-COP antibody clearly labeled small (∼60 nm in diameter) COPI-like vesicles budding from the Golgi cisternae, DVs remained unlabeled. This observation was supported by a statistical analysis of the distribution of Atγ-COP labeling in the Golgi apparatus as shown in Table . Although the Golgi apparatus was labeled with an average of 10.5 gold particles per stack, no DV labeling was detected with the COPI antibodies.
Figure 5.
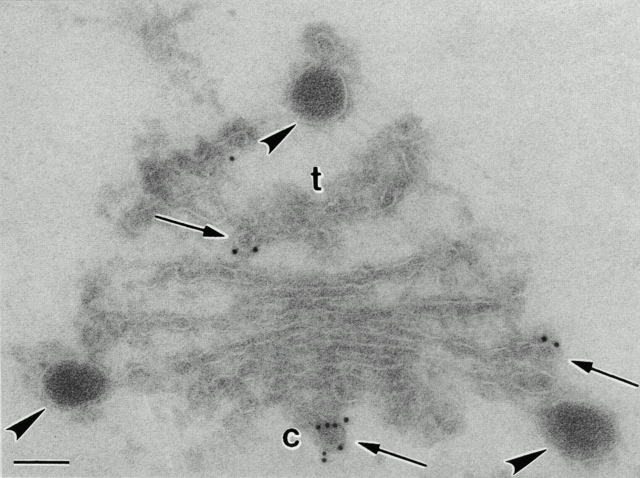
Immunogold staining of cryosectioned pea cotyledon Golgi stacks with antibodies against Atγ-COP. Small (∼60 nm in diameter) budding COPI vesicles (arrows) are significantly labeled with the antibodies. The lumen of the cisternae is not labeled. DVs attached to the cis- and the trans-Golgi cisternae are also not labeled. c, cis; t, trans. Bar, 100 nm.
Table 2.
Distribution of Immunogold Labeling with Antibodies against Atγ-COP across the Golgi Stack of Developing Pea Cotyledons
| Golgi stacks | DVs | ||||
|---|---|---|---|---|---|
| Total | Labeled | Gold particles/stack | Total | Labeled | Gold particles/DV |
| 15 | 15 | 10.5 | 28 | 0 | 0 |
To elucidate the distribution of Atγ-COP, micrographs of 15 cryosectioned Golgi stacks with distinguishable cis- and trans-cisternae and attached DVs were taken, and the number of gold particles was counted.
Distribution of α-TIP and BP-80 in the Golgi Apparatus
We have also investigated the distribution of α-TIP, the characteristic aquaporin of the PSV, and the VSR BP-80 as additional parameters of vacuolar protein transport through the pea cotyledon Golgi apparatus. Although immunogold labeling for α-TIP can be performed on chemically fixed, resin-embedded specimens (e.g., Hoh et al. 1995), we have not been able to localize BP-80 under these conditions (Hinz et al. 1999). Similarly, because of the resin embedment, HPF also cannot be employed for the detection of BP-80. Therefore, we have resorted to cryosectioning as a means of determining the distribution of these two antigens in the pea Golgi apparatus.
Cryosectioned pea Golgi apparatus can be significantly labeled with α-TIP antibodies (Hinz et al. 1999; Robinson and Hinz 1999). Quantitation of this labeling indicates that α-TIP is concentrated in the DVs (Table ). Accounting for 60% of the total label, this is even more pronounced than was the case with the two storage globulins. However, in contrast to legumin and vicilin, α-TIP was more or less equally distributed across the Golgi stack: 4% in the cis-cisterna, 9% in the first median cisterna, 12% in the second median cisterna, 8% in the trans-cisterna, and 7% in the TGN. Because we have not been able to localize precisely α-TIP–labeled DVs within the stack, we could not determine whether α-TIP is sorted into DVs throughout the stack or exclusively at the TGN.
In confirmation of earlier data (Hinz et al. 1999; Robinson and Hinz 1999), BP-80 is readily detectable in cryosections of pea Golgi and gold label is absent from the DVs. A quantitative analysis of BP-80 immunogold labeling now reveals a distribution across the stack that is the reverse of that visualized for the storage globulins (Table ). Out of 75 gold particles present on 20 Golgi stacks, 5 were present over the cis-cisternae, 14 over the first median cisternae, 23 over the second median cisternae, 29 over the trans-cisternae, and 5 over the TGN.
Discussion
Differential Distribution of Vacuolar Sorting Events across the Pea Cotyledon Golgi Stack
The mannosyl 6-phosphate receptor (MPR) is responsible for sorting lysosome-destined acid hydrolases in the mammalian Golgi apparatus (Le Borgne and Hoflack 1998). It has been localized throughout the stack (Brown and Farquhar 1987), predominantly in the TGN (Geuze et al. 1985), as well as in budding CCVs at the TGN (Klumpermann et al. 1993), depending on the cell type under investigation. MPRs have also been detected in isolated CCVs (e.g., Le Borgne and Hoflack 1997). Although morphologically not present as a stack of cisternae, a functional compartmentalization for the Golgi apparatus in baker's yeast has been resolved in biochemical terms (Conibear and Stevens 1998; Brigance et al. 2000). As a result, the analogous receptor for vacuolar acid hydrolases, vps10p, has also been localized to a late Golgi compartment (Graham and Emr 1991). Members of the plant BP-80 VSR family also seem to have a similar distribution to the MPR and vps10p receptors: they have been localized to the Golgi apparatus in pea roots (Paris et al. 1997) and to the TGN in Arabidopsis roots and pea cotyledons (Sanderfoot et al. 1998; Hinz et al. 1999; this report). They also appear to be highly enriched in CCVs (Sanderfoot et al. 1998; Hinz et al. 1999). Thus, in analogy to mammalian and yeast cells, it is not unreasonable to assume that BP-80–mediated sorting in plants is also a late Golgi event. Our current immunogold localization data support this conclusion.
Although storage protein–containing transport vesicles (i.e., DVs) have been seen attached to Golgi cisternae in developing pea (Hohl et al. 1996) and garden bean (Baumgarten et al. 1980; Greenwood and Chrispeels 1985) cotyledons, the exact site where these proteins are sorted within the plant Golgi apparatus has been made uncertain due to contradictory reports on immunogold localizations. On the one hand, a more or less even distribution for storage proteins throughout the Golgi stack of the field bean has been claimed on the basis of plastic sections (zur Nieden et al. 1984). A similar distribution for phaseolin (a vicilin homologue) and the lectin phytohemagglutinin in the Golgi apparatus of garden beans was given by Greenwood and Chrispeels 1985, who used cryosections. On the other hand, Craig and Goodchild 1984 and Craig and Miller 1984, using periodate-etched plastic sections, claimed an exclusive localization of vicilin in the trans-cisternae of the pea cotyledon Golgi stack, whereas legumin was restricted to the cis-cisternae.
Using a combination of the most sensitive antigen detection methods currently available for electron microscopy (HPF and cryosectioning), we have shown that there are very steep gradients in the distribution of nonaggregated forms of vicilin and legumin precursor polypeptides across the Golgi stack of pea cotyledons. Both types of storage protein are highly enriched in the cis-cisterna and in DVs but are markedly reduced in the median and virtually absent in trans-Golgi compartments. Thus, receptor-mediated sorting of vacuolar acid hydrolases and the sorting of storage proteins into DVs are spatially separated in the pea cotyledon Golgi apparatus (summarized in Fig. 6).
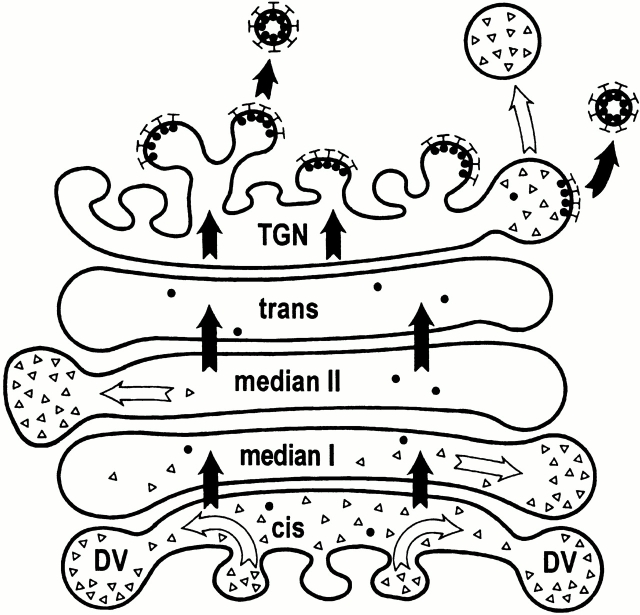
Figure 6.
Scheme for the localization of the sorting events in the pea cotyledon Golgi stack. Vacuolar hydrolases (filled circles) are first transported through the Golgi stack (black arrows) into the TGN where they are sorted by the VSR BP-80 into CCV (closed bars). Proteins of the PSV (open circles), by contrast, are sorted mainly at the cis-half of the Golgi stack into developing DVs (white arrows). Finally, after maturation, DVs are released from the TGN.
Golgi Processing Events, Sites of DV Formation, and Intra-Golgi Transport
The majority of vacuolar and secretory proteins in mammalian and yeast cells are posttranslationally modified as they pass through the Golgi apparatus (e.g., Rothman and Orci 1992). Some of these events—e.g., proteolytic processing, terminal glycosylation with sialic acid residues, and sulfation—occur in the trans-compartments (Burgess and Kelly 1987; Graham and Emr 1991). In plants, although glycoprotein processing and polysaccharide synthesis is compartmentalized in the plant Golgi apparatus, neither sialysation nor sulfation occurs (Staehelin and Moore 1995; Dupree and Sherrier 1998), nor is there any evidence for the condensation of secretory proteins in the TGN (Robinson and Hinz 1999). However, as pointed out by Vitale and Raikhel 1999, the aggregation/condensation of secretory or storage proteins is an event that could severely interfere with glycoprotein processing. Of the two major storage proteins of pea cotyledons, only a small proportion of the vicilin polypeptides become complex glycosylated (Davey and Dudman 1979; Müntz 1998). Therefore, glycosylated vicilin probably enters the DVs downstream of the cis-cisternae, where storage protein aggregation begins. This interpretation finds support in the slight, but significant, difference in the distribution of legumin and vicilin across the Golgi stack. In the case of other legumes, e.g., common bean (P. vulgaris), where the degree of complex glycosylation is higher (Müntz 1998), we would predict that the formation of DVs is delayed in the Golgi stack.
The increase in electron opacity of the DVs in a cis–trans gradient across the Golgi stack (Robinson et al. 1997; Robinson and Hinz 1999) indicates that sorting and condensation of storage proteins is an ongoing event as these proteins pass through the stack. The question is whether this maturation of the DVs is achieved by cisternal progression or by the sequential release and fusion of DVs. The answer is of direct relevance to the current controversy surrounding mechanisms for intra-Golgi transport (for reviews see Glick et al. 1997; Mironov et al. 1998; Pelham and Rothman 2000). At the two extremes are two models: according to one, intra-Golgi transport in the anterograde direction is mediated by COPI-coated vesicles. At the other end of the scale, anterograde transport through the Golgi stack is thought to be mediated by cisternal progression. In an attempt to resolve this problem, Volchuk et al. 2000 have developed a transgenic model system that allows for the specific induction of large aggregates of secretory protein in the Golgi apparatus of human fibrosarcoma cells. Whereas aggregates of procollagen have been shown to be transported via cisternal progression rather than via anterograde vesicle transport in vivo (Bonfanti et al. 1998), the synthetically induced aggregates of Volchuk et al. 2000, in turn, promote the formation of megavesicles with a diameter of ∼400 nm. Because ∼20% of the megavesicles present were not connected with Golgi cisternae, as judged by quantitative analysis of serial sections, Volchuk et al. 2000 concluded that released megavesicles mediate the transport of the protein aggregates through the stack by a budding and fusion process. Like DVs, these megavesicles are present at all cisternae of the Golgi. However, whereas DVs are only to be seen at the cisternal rim ∼10% of the megavesicles were formed in the central part of a cisternae. Moreover, these megavesicles were characterized by the presence of a distinct COPI coat. In contrast, DVs are without a visible coat when attached to the Golgi cisternae and they do not label with COPI-specific antibodies. In addition, CCVs have been shown to bud from DVs at the pea cotyledon TGN (Hohl et al. 1996; Robinson and Hinz 1997; Robinson et al. 1998a). Thus, the formation and passage of DVs through the pea cotyledon Golgi stack is not a special type of anterograde COPI transport. However, because data for the movement of secreted proteins or cell wall polysaccharides in pea cotyledons are not yet available, we cannot exclude divergent transport kinetics through the pea cotyledon Golgi. In the event that such differences do exist, one must assume that cisternal maturation and anterograde vesicle transport are not mutually exclusive models for intra-Golgi trafficking as suggested by Pelham and Rothman 2000.
Storage Protein Segregation in the Pea Cotyledon Golgi Apparatus
Two major questions arise out of the observations made in this study. First, why do legumin and vicilin first form aggregates in the Golgi apparatus rather than in the ER? In this regard, we draw attention to the fact that there are numerous cases where storage proteins condense in the ER, particularly in cereal grains (Müntz 1998). There are also examples in which ER-derived vesicles with condensed storage proteins appear to bypass the Golgi apparatus (Hara-Nishimura et al. 1998) on their way to the PSV, even in the presence of a KDEL retrieval signal (Toyooka et al. 2000). Although in some cases the interacting domains on the proteins have been identified, and thereby a molecular basis for the aggregation of the storage proteins in the ER has been provided (Geli et al. 1994), the most important parameter nevertheless is concentration, as has been demonstrated for plants (Wandelt et al. 1992) as well as mammals (Tooze et al. 1989). Obviously, despite high rates of storage protein translation in pea cotyledons we must assume that the critical concentration for aggregation is never, or seldom (see Robinson et al. 1995), reached in the ER of the pea cotyledon.
Second, why does the condensation of legumin and nonglycosylated vicilin occur immediately upon entry into the Golgi apparatus of pea cotyledons, rather than at the TGN as it occurs in mammalian cells showing regulated secretion (Tooze 1998)? In this respect we also draw attention to the morphological similarity between DVs and immature secretory granules in terms of CCV budding (compare Hohl et al. 1996; Robinson and Hinz 1997; Robinson et al. 1997 with Kuliawat et al. 1997). Clearly, the physiological make-up of the cis-cisternae of the pea Golgi apparatus is such that the critical concentration is already achieved in this subcompartment. However, this concentration is reached only at the periphery of the cisternae, where the DVs are formed. Thus, a highly efficient centrifugal segregation of storage polypeptides within the cis-cisternae must precede the condensation process. This might be achieved by one of two ways. First, via an osmotic mechanism postulated on the basis of studies on the effects of ionophores on the plant Golgi apparatus (e.g., Griffing and Ray 1985). According to this hypothesis, acidification of the cisternal lumen leads to a decrease in internal osmolarity with a concomitant collapse of the cisternae. This would result in a squeezing of secretory molecules into vesicle buds at the rim of the cisternae (Staehelin et al. 1990). However, for two reasons it is doubtful whether the lateral segregation of storage proteins in the pea cotyledon Golgi apparatus could be explained in this manner. The hypothesis depends on the presence of a cisternal-based proton pumping activity; but, although there are numerous reports in the literature of mammalian and plant cells concerning the presence of V-H-ATPase and H-PPase activities and polypeptides at the trans-Golgi, we know of no proven example for the acidification of cis-cisternae. In addition, a pH-dependent osmotic mechanism would not be able to differentiate between lumenal cargo molecules, but it is clear from our findings that glycoproteins are somehow prevented from entering the DVs. Nevertheless, pH may be an important factor in promoting conditions under which storage proteins can aggregate. Certainly, the pI of pea legumin indicates that at least mature legumin is less soluble at low rather than neutral pHs (Casey 1979b). The second possibility is that lateral sorting of storage proteins is a receptor-mediated event, although sorting receptors for pea legumin and/or vicilin remain to be identified. In this respect, it is worthwhile noting that prolegumin is a much more hydrophobic protein compared with mature legumin and, contrary to the mature protein, is also resistant to sodium carbonate extraction (Hinz et al. 1997).
Acknowledgments
We thank Christel Rühling for technical assistance and Bernd Raufeisen for the drawing. We gratefully acknowledge the Institute of Plant Genetics and Plant Research in Gatersleben for allowing us the use of their high pressure freezer and the excellent technical assistance by Bernhard Claus. We are indebted to Drs. Roderick C. Casey, Maarten J. Chrispeels, Renate Manteuffel, and John C. Rogers, who provided us with the necessary antibodies for this study.
This research was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 523, Teilprojekte A7, B2, and Z2), and the Human Frontier Science Program (RG0018/2000-M). A. Movafeghi was a recipient of a scholarship from the Ministry of Culture and Education of Iran.
Footnotes
S. Hillmer, A. Movafeghi, and D. Robinson's present address is Zellenlehre, Universität Heidelberg, Im Neuenheimer Feld 230, 69120 Heidelberg, Germany.
Abbreviations used in this paper: AtALEU, Arabidopsis thaliana Aleu; AtELP, Arabidopsis thaliana Elp; βF1, β-fructosidase; CCV, clathrin-coated vesicle; DV, dense vesicle; HPF, high pressure frozen; MPR, mannosyl 6-phosphate receptor; PSV, protein storage vacuole; VSR, vacuolar sorting receptor.
References
- Ahmed S.U., Bar-Peled M., Raikhel N.V. Cloning and subcellular location of an Arabidopsis receptor-like protein that shares common features with protein-sorting receptors of eukaryotic cells. Plant Physiol. 1997;114:326–336. doi: 10.1104/pp.114.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S.U., Rojo E., Kovaleva V., Venkataram S., Dombrowski J.E., Matsuoka K., Raikhel N.V. The plant vacuolar sorting receptor AtELP is involved in the transport of NH2-terminal propeptide–containing vacuolar proteins in Arabidopsis thaliana . J. Cell Biol. 2000;149:1335–1344. doi: 10.1083/jcb.149.7.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten B., Tokuyasu K.T., Chrispeels M.J. Immunocytochemical localization of reserve protein in the endoplasmic reticulum of developing bean (Phaseolus vulgaris) cotyledons. Planta. 1980;150:419–425. doi: 10.1007/BF00390179. [DOI] [PubMed] [Google Scholar]
- Bonfanti L., Mironov A.A., Jr., Martinez-Menarguez J.A., Martella O., Fusella A., Baldassarre M., Buccione R., Geuze H.J., Mironov A.A., Luini A. Procollagen traverses the Golgi stack without leaving the lumen of the cisternaeevidence for cisternal maturation. Cell. 1998;95:993–1003. doi: 10.1016/s0092-8674(00)81723-7. [DOI] [PubMed] [Google Scholar]
- Brigance W.T., Barlowe C., Graham T.R. Organization of the yeast Golgi complex into at least four functionally distinct compartments. Mol. Biol. Cell. 2000;11:171–182. doi: 10.1091/mbc.11.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W.J., Farquhar M.G. The distribution of 215-kilodalton mannose 6-phosphate receptors within cis (heavy) and trans (light) Golgi subfractions varies in different cell types. Proc. Natl. Acad. Sci. USA. 1987;84:9001–9005. doi: 10.1073/pnas.84.24.9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess T.L., Kelly R.B. Constitutive and regulated secretion of proteins. Annu. Rev. Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- Cao X., Rogers S.W., Butler J., Beevers L., Rogers J.C. Structural requirements for ligand binding by a probable plant vacuolar sorting receptor. Plant Cell. 2000;12:493–506. doi: 10.1105/tpc.12.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey R.D. Immunoaffinity chromatography as a means of purifying legumin from Pisum (pea) seeds Biochem. J. 177 1979. 509 520a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey R.D. Genetic variability in the structure of the α-subunits of legumin from Pisum—a two-dimensional electrophoresis study Heredity. 43 1979. 265 272b [Google Scholar]
- Chrispeels M.J., Higgins T.J.V., Craig S., Spencer D. Assembly of storage protein oligomers in the endoplasmic reticulum and processing of the polypeptides in protein bodies of developing pea cotyledons. J. Cell Biol. 1982;93:306–313. doi: 10.1083/jcb.93.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conibear E., Stevens T.H. Multiple sorting pathways between the late Golgi and the vacuole in yeast. Biochim. Biophys. Acta. 1998;1404:211–230. doi: 10.1016/s0167-4889(98)00058-5. [DOI] [PubMed] [Google Scholar]
- Craig S., Goodchild D.J. Periodate-acid treatment of sections permits on-grid immuno-gold localisation of pea seed vicilin in ER and Golgi. Protoplasma. 1984;122:35–44. [Google Scholar]
- Craig S., Miller C. LR-white resin and improved on-grid immunogold detection of vicilin, a pea seed storage protein. Cell Biol. Int. Rep. 1984;8:879–886. doi: 10.1016/0309-1651(84)90072-9. [DOI] [PubMed] [Google Scholar]
- Craig S., Millerd A., Goodchild D.J. Structural aspects of protein accumulation in developing pea cotyledons IIIimmunocytochemical localization of legumin and vicilin using antibodies shown to be specific by the enzyme-linked immunoabsorbend assay (ELISA) Aust. J. Plant Physiol. 1980;7:339–351. [Google Scholar]
- Davey R.A., Dudman W.F. The carbohydrate of storage glycoproteins from seeds of Pisum sativumcharacterization and distribution of component polypeptides. Aust. J. Plant Physiol. 1979;6:435–447. [Google Scholar]
- Di Sansebastiano G.P., Paris N., Marc-Martin S., Neuhaus J.M. Specific accumulation of GFP in a non-acidic vacuolar compartment via a C-terminal propeptide-mediated sorting pathway. Plant J. 1998;15:449–457. doi: 10.1046/j.1365-313x.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- Dupree P., Sherrier D.J. The plant Golgi apparatus. Biochim. Biophys. Acta. 1998;1404:259–270. doi: 10.1016/s0167-4889(98)00061-5. [DOI] [PubMed] [Google Scholar]
- Fitchette-Lainé A.-C., Gomord V., Chekkafi A., Faye L. Distribution of xylosylation and fucosylation in the plant Golgi apparatus. Plant J. 1994;5:673–682. [Google Scholar]
- Geli M.I., Torrent M., Ludevid D. Two structural domains mediate two sequential events in γ-zein targetingprotein endoplasmic retention and protein body formation. Plant Cell. 1994;6:1911–1922. doi: 10.1105/tpc.6.12.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H.J., Slot J.W., Strous G.J., Hasilik A., von Figura K. Possible pathways for lysosomal enzyme delivery. J. Cell Biol. 1985;101:2253–2262. doi: 10.1083/jcb.101.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B.S., Elston T., Oster G. A cisternal maturation mechanism can explain the asymmetry of the Golgi stack. FEBS Lett. 1997;414:177–181. doi: 10.1016/s0014-5793(97)00984-8. [DOI] [PubMed] [Google Scholar]
- Graham T.R., Emr S.D. Compartmental organization of Golgi-specific protein modification and vacuolar protein sorting events defined in a yeast sec18 (NSF) mutant. J. Cell Biol. 1991;114:207–218. doi: 10.1083/jcb.114.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood J.S., Chrispeels M.J. Immunocytochemical localization of phaseolin and phytohemagglutinin in the endoplasmic reticulum and Golgi complex of developing bean cotyledons. Planta. 1985;164:295–302. doi: 10.1007/BF00402940. [DOI] [PubMed] [Google Scholar]
- Griffing L.R., Ray P.M. Involvement of monovalent cations in Golgi secretion by plant cells. Eur. J. Cell Biol. 1985;36:24–31. [Google Scholar]
- Hara-Nishimura I., Maeshima M. Vacuolar processing enzymes and aquaporins. In: Robinson D.G., Rogers J.C., editors. Vacuolar Compartments. Sheffield Academic Press; Sheffield, UK: 2000. [Google Scholar]
- Hara-Nishimura I., Shimada T., Hatano T., Takeuchi Y., Nishimura M. Transport of storage proteins to protein storage vacuoles is mediated by large precursor-accumulating vesicles. Plant Cell. 1998;10:825–836. doi: 10.1105/tpc.10.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz G., Menze A., Hohl I., Vaux D. Isolation of prolegumin from developing pea seedsits binding to endomembranes and assembly into prolegumin hexamers in the protein storage vacuole. J. Exp. Bot. 1997;48:139–149. [Google Scholar]
- Hinz G., Hillmer S., Bäumer M., Hohl I. Vacuolar storage proteins and the putative vacuolar sorting receptor BP-80 exit the Golgi apparatus of developing pea cotyledons in different transport vesicles. Plant Cell. 1999;11:1509–1524. doi: 10.1105/tpc.11.8.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoh B., Hinz G., Joeng B.-K., Robinson D.G. Protein storage vacuoles form de novo during pea cotyledon development. J. Cell Sci. 1995;108:299–310. doi: 10.1242/jcs.108.1.299. [DOI] [PubMed] [Google Scholar]
- Hohl I., Robinson D.G., Chrispeels M.J., Hinz G. Transport of storage proteins to the vacuole is mediated by vesicles without a clathrin coat. J. Cell Sci. 1996;109:2539–2550. doi: 10.1242/jcs.109.10.2539. [DOI] [PubMed] [Google Scholar]
- Jauh G.-Y., Fischer A.M., Grimes H.D., Ryan C.A., Jr., Rogers J.C. α-Tonoplast intrinsic protein defines unique vacuole functions. Proc. Natl. Acad. Sci. USA. 1998;95:12995–12999. doi: 10.1073/pnas.95.22.12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauh G.-Y., Phillips T.E., Rogers J.C. Tonoplast intrinsic protein isoforms as markers for vacuolar function. Plant Cell. 1999;11:1867–1882. doi: 10.1105/tpc.11.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K.D., Herman E.M., Chrispeels M.J. An abundant, highly conserved tonoplast protein in seeds. Plant Physiol. 1989;91:1006–1013. doi: 10.1104/pp.91.3.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch T., Paris N., Butler J.M., Beevers L., Rogers J.C. Purification and initial characterization of a potential plant vacuolar targeting receptor. Proc. Natl. Acad. Sci. USA. 1994;91:3403–3407. doi: 10.1073/pnas.91.8.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch T., Saalbach G., Raikhel N.V., Beevers L. Interaction of a potential targeting receptor with amino- and carboxyl-terminal targeting determinants. Plant Physiol. 1996;111:469–474. doi: 10.1104/pp.111.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpermann J., Hille A., Veenendaal T., Oorschot V., Stoorvogel W., von Figura K., Geuze H. Differences in the endosomal distributions of the two mannose 6-phosphate receptors. J. Cell Biol. 1993;121:997–1010. doi: 10.1083/jcb.121.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuliawat R., Klumperman J., Ludwig T., Arvan P. Differential sorting of lysosomal enzymes out of the regulated secretory pathway in pancreatic beta cells. J. Cell Biol. 1997;137:595–608. doi: 10.1083/jcb.137.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurière M., Laurière C., Chrispeels M.J., Johnson K.D., Sturm A. Characterization of a xylose-specific antiserum that reacts with complex asparagine-linked glycans of extracellular and vacuolar glycoproteins. Plant Physiol. 1989;90:1182–1188. doi: 10.1104/pp.90.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laval V., Chabannes M., Carrière M., Canut H., Barre A., Rougé R., Pont-Lezica R., Galaud J.P. A family of Arabidopsis plasma membrane receptors presenting animal β-integrin domains. Biochim. Biophys. Acta. 1999;1435:61–70. doi: 10.1016/s0005-2728(99)00087-0. [DOI] [PubMed] [Google Scholar]
- Le Borgne R., Hoflack B. Mannose 6-phosphate receptors regulate the formation of clathrin coated vesicles in the TGN. J. Cell Biol. 1997;137:335–345. doi: 10.1083/jcb.137.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne R., Hoflack B. Protein transport from the secretory to the endocytic pathway in mammalian cells. Biochim. Biophys. Acta. 1998;1404:195–210. doi: 10.1016/s0167-4889(98)00057-3. [DOI] [PubMed] [Google Scholar]
- Levanony H., Rubin R., Altschuler Y., Galili G. Evidence for a novel route of wheat storage proteins to vacuoles. J. Cell Biol. 1992;119:1117–1128. doi: 10.1083/jcb.119.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou W., Geuze H.J., Slot J.W. Improving structural integrity of cryosections for immunogold labeling. Histochem. Cell Biol. 1996;106:41–58. doi: 10.1007/BF02473201. [DOI] [PubMed] [Google Scholar]
- Lowe M., Kreis T.E. Regulation of membrane traffic in animal cells by COPI. Biochim. Biophys. Acta. 1998;1404:53–66. doi: 10.1016/s0167-4889(98)00046-9. [DOI] [PubMed] [Google Scholar]
- Matsuoka K., Neuhaus J.-M. Cis-elements of protein transport to the plant vacuoles. J. Exp. Bot. 1999;50:165–174. [Google Scholar]
- Matsuoka K., Bassham D.C., Raikhel N.V., Nakamura K. Different sensitivity to wortmannin of two vacuolar sorting signals indicates the presence of distinct sorting machineries in tobacco cells. J. Cell Biol. 1995;130:1307–1318. doi: 10.1083/jcb.130.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E.A., Lee M.C.S., Anderson M.A. Identification and characterization of a prevacuolar compartment in stigmas of Nicotiana alata . Plant Cell. 1999;11:1499–1508. doi: 10.1105/tpc.11.8.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov A., Jr., Luini A., Mironov A. A synthetic model of intra-Golgi traffic. FASEB J. 1998;12:249–252. doi: 10.1096/fasebj.12.2.249. [DOI] [PubMed] [Google Scholar]
- Movafeghi A., Happel N., Pimpl P., Tai G.-H., Robinson D.G. Arabidopsis Sec21p and Sec23p homologs. Probable coat proteins of plant COP-coated vesicles. Plant Physiol. 1999;119:1437–1446. doi: 10.1104/pp.119.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müntz K. Deposition of storage proteins. Plant Mol. Biol. 1998;38:77–99. [PubMed] [Google Scholar]
- Paris N., Rogers J.C. The role of receptors in targeting soluble proteins from the secretory pathway to the vacuole. Plant Physiol. Biochem. 1996;34:223–227. [Google Scholar]
- Paris N., Stanley M.C., Jones R.L., Rogers J.C. Plant cells contain two functionally distinct vacuolar compartments. Cell. 1996;85:563–572. doi: 10.1016/s0092-8674(00)81256-8. [DOI] [PubMed] [Google Scholar]
- Paris N., Rogers S.W., Jiang L., Kirsch T., Beevers L., Phillips T.E., Rogers J.C. Molecular cloning and further characterization of a probable plant vacuolar sorting receptor. Plant Physiol. 1997;115:9–39. doi: 10.1104/pp.115.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H.R.B., Rothman J.E. The debate about transport in the Golgi—two sides of the same coin? Cell. 2000;102:713–719. doi: 10.1016/s0092-8674(00)00060-x. [DOI] [PubMed] [Google Scholar]
- Pimpl P., Movafeghi A., Coughlan S., Denecke J., Hillmer S., Robinson D.G. In situ localization and in vitro induction of plant COPI-coated vesicles. Plant Cell. 2000;12:2219–2236. doi: 10.1105/tpc.12.11.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D.G., Hinz G. Vacuole biogenesis and protein transport to the plant vacuolea comparison with the yeast vacuole and the mammalian lysosome. Protoplasma. 1997;197:1–25. [Google Scholar]
- Robinson D.G., Hinz G. Golgi mediated transport of seed storage proteins. Seed Sci. Res. 1999;9:267–283. [Google Scholar]
- Robinson D.G., Hoh B., Hinz G., Joeng B.-K. One vacuole or two vacuolesdo protein storage vacuoles arise de novo during pea cotyledon development? J. Plant Physiol. 1995;145:654–664. doi: 10.1242/jcs.108.1.299. [DOI] [PubMed] [Google Scholar]
- Robinson D.G., Bäumer M., Hinz G., Hohl I. Ultrastucture of pea cotyledon Golgi apparatusorigin of dense vesicles and the action of brefeldin A. Protoplasma. 1997;200:198–209. [Google Scholar]
- Robinson D.G., Hinz G., Holstein S.E.H. The molecular characterization of transport vesicles Plant Mol. Biol. 38 1998. 49 76a [PubMed] [Google Scholar]
- Robinson, D.G., T. Braulke, J. Denecke, N. Happel, A. Movafeghi, and P. Pimpl. 1998b. New developments in plant coated vesicle research. In Progress in Botanical Research. Proceedings of the First Balkan Botanical Congress. I. Tsekos and M. Moustakas, editors. Kluwer Academic Publishers, Dordrecht, The Netherlands. 399–406.
- Robinson D.G., Bäumer M., Hinz G., Hohl I. Vesicle transfer of storage proteins to the vacuolethe role of the Golgi apparatus and multivesicular bodies J. Plant Physiol. 152 1998. 659 667c [Google Scholar]
- Rothman J.E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992;355:409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Saalbach G., Jung R., Kunze G., Saalbach I., Adler K., Müntz K. Different legumin domains act as vacuolar targeting signals. Plant Cell. 1991;3:695–708. doi: 10.1105/tpc.3.7.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderfoot A.A., Ahmed S.U., Marty-Mazars F., Rapoport I., Kirchhausen T., Marty F., Raikhel N.V. A putative vacuolar cargo receptor partially colocalizes with AtPEP12p on a prevacuolar compartment in Arabidopsis roots. Proc. Natl. Acad. Sci. USA. 1998;95:9920–9925. doi: 10.1073/pnas.95.17.9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T., Kuroyanagi M., Mishimura M., Hara-Nishimura I. A pumpkin 72-kDa membrane protein of precursor-accumulating vesicles has characteristics of a vacuolar sorting receptor. Plant Cell Physiol. 1997;38:1414–1420. doi: 10.1093/oxfordjournals.pcp.a029138. [DOI] [PubMed] [Google Scholar]
- Stack J.H., Horazdovsky B., Emr S.D. Receptor-mediated protein sorting to the vacuole in yeastroles for a protein kinase, a lipid kinase and GTP-binding proteins. Annu. Rev. Cell Dev. Biol. 1995;11:1–33. doi: 10.1146/annurev.cb.11.110195.000245. [DOI] [PubMed] [Google Scholar]
- Staehelin L.A., Moore I. The plant Golgi apparatusstructure, functional organization and trafficking mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995;46:261–288. [Google Scholar]
- Staehelin L.A., Giddings T.H., Jr., Kiss J.Z., Sack F.D. Macromolecular differentiation of Golgi stacks in root tips of Arabidopsis and Nicotiana seedlings as visualized in high pressure frozen and freeze-substituted samples. Protoplasma. 1990;157:75–91. doi: 10.1007/BF01322640. [DOI] [PubMed] [Google Scholar]
- Swanson S.J., Bethke P.C., Jones R.L. Barley aleuron cells contain two types of vacuolescharacterization of lytic organelles by use of fluorescent probes. Plant Cell. 1998;10:685–698. doi: 10.1105/tpc.10.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K.T. Immunocytochemistry on ultrathin frozen sections. Histochem. J. 1980;12:381–403. doi: 10.1007/BF01011956. [DOI] [PubMed] [Google Scholar]
- Tooze S.A. Biogenesis of secretory granules in the trans-Golgi network neuroendocrine and endocrine cells. Biochim. Biophys. Acta. 1998;1404:231–244. doi: 10.1016/S0167-4889(98)00059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J., Kern H.F., Fuller S.F., Howell K.E. Condensation-sorting events in the rough endoplasmic reticulum of exocrine pancreatic cells. J. Cell Biol. 1989;109:35–50. doi: 10.1083/jcb.109.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyooka K., Okamoto T., Minamikawa T. Mass transport of proform of a KDEL-tailed cysteine proteinase (SH-EP) to protein storage vacuoles by endoplasmic reticulum–derived vesicles is involved in protein mobilization in germinating seeds. J. Cell Biol. 2000;148:453–463. doi: 10.1083/jcb.148.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale A., Raikhel N.V. What do proteins need to reach different vacuoles? Trends Plant Sci. 1999;4:149–155. doi: 10.1016/s1360-1385(99)01389-8. [DOI] [PubMed] [Google Scholar]
- Volchuk A., Amherdt M., Ravazzola M., Brügger B., Rivera V.M., Clackson T., Perrelet A., Söllner T.H., Rothman J.E., Orci L. Megavesicles implicated in the rapid transport of intracisternal aggregates across the Golgi stack. Cell. 2000;102:335–348. doi: 10.1016/s0092-8674(00)00039-8. [DOI] [PubMed] [Google Scholar]
- Wandelt C., Khan M.R.I., Craig S., Schroeder H.E., Higgins T.J.V. Vicilin with carboxy-terminal KDEL is retained in the endoplasmic reticulum and accumulates in high levels in leaves of transgenic plants. Plant J. 1992;2:181–192. doi: 10.1046/j.1365-313x.1992.t01-41-00999.x. [DOI] [PubMed] [Google Scholar]
- Weig A., Deswarte C., Chrispeels M.J. The major intrinsic protein family of Arabidopsis has 23 members that form three distinct groups with functional aquaporins in each group. Plant Physiol. 1997;114:1347–1357. doi: 10.1104/pp.114.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.F., Staehelin L.A. Functional compartimentation of the Golgi apparatus of plant cells. Plant Physiol. 1992;99:1070–1083. doi: 10.1104/pp.99.3.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Nieden U., Manteuffel R., Weber E., Neumann D. Dictyosomes participate in the intracellular pathway of storage proteins in developing Vicia faba cotyledons. Eur. J. Cell Biol. 1984;34:9–17. [PubMed] [Google Scholar]