Abstract
Phagosomes are key organelles for the innate ability of macrophages to participate in tissue remodeling, clear apoptotic cells, and restrict the spread of intracellular pathogens. To understand the functions of phagosomes, we initiated the systematic identification of their proteins. Using a proteomic approach, we identified >140 proteins associated with latex bead–containing phagosomes. Among these were hydrolases, proton pump ATPase subunits, and proteins of the fusion machinery, validating our approach. A series of unexpected proteins not previously described along the endocytic/phagocytic pathways were also identified, including the apoptotic proteins galectin3, Alix, and TRAIL, the anti-apoptotic protein 14-3-3, the lipid raft-enriched flotillin-1, the anti-microbial molecule lactadherin, and the small GTPase rab14. In addition, 24 spots from which the peptide masses could not be matched to entries in any database potentially represent new phagosomal proteins. The elaboration of a two-dimensional gel database of >160 identified spots allowed us to analyze how phagosome composition is modulated during phagolysosome biogenesis. Remarkably, during this process, hydrolases are not delivered in bulk to phagosomes, but are instead acquired sequentially. The systematic characterization of phagosome proteins provided new insights into phagosome functions and the protein or groups of proteins involved in and regulating these functions.
Keywords: phagosome, proteome, matrix-assisted laser desorption/ionization–time-of-flight–mass spectrometry, membrane fusion, apoptosis
Introduction
Professional phagocytes, such as macrophages, internalize large particulate material by phagocytosis. This constitutive process, initiated by binding of the particle to cell surface receptors, triggers a reorganization of the plasma membrane and its cortical cytoskeletal elements, leading to particle engulfment and formation of the phagosome. Phagosomes are pivotal organelles in the ability of macrophages to perform several of their key functions, such as the handling of apoptotic cells, tissue remodeling, and the restriction of the establishment and spread of intracellular pathogens (see Méresse et al. 1999). The functional properties of phagosomes are acquired through a complex remodeling process involving regulated interactions with a series of endovacuolar organelles. Indeed, during phagolysosome biogenesis, phagosomes intersect the biosynthetic pathway and fuse sequentially with early endosomes, late endosomes, and lysosomes (Pitt et al. 1992; Desjardins et al. 1997; Jahraus et al. 1998). These interactions, facilitated by phagosome binding and movement along cytoskeletal elements (Desjardins et al. 1994a; Defacque et al. 2000), and controlled in part by small GTPases of the rab family (Alvarez-Dominguez and Stahl 1999; Roberts et al. 1999; Duclos et al. 2000), allow the acquisition of groups of molecules conferring new functions to maturing phagosomes. Despite our current knowledge, the molecules and mechanisms coordinating the various steps involved in phagolysosome biogenesis are still mostly unknown. A significant part of our ignorance originates from the fact that few of the phagosome proteins are currently known or studied, despite the presence of hundreds of polypeptides on this compartment (Desjardins et al. 1994b; Haas 1998). Identification of these molecules is likely to provide insights into the complex mechanisms governing phagosome functions.
In this study, we present a comprehensive analysis of phagosomes from which >140 proteins have been identified. Furthermore, we built a two-dimensional (2-D) phagosome map that could be used to monitor complex series of modifications occurring during phagosome maturation. This systematic characterization of phagosomes has allowed us to extend greatly our understanding of this organelle and to propose new concepts regarding its biogenesis.
Materials and Methods
Cell Culture, Phagosome Isolation and Sample Preparation
The J774 mouse macrophage-like cell line was cultured and the phagosome formed and isolated as described previously (Desjardins et al. 1994a). Protease inhibitors were present in the samples and sucrose solutions throughout the procedure. In some cases, phagosomes were treated with pronase (Sigma-Aldrich) (a mixture of proteases from Streptomyces griseus with broad proteolytic activities, digesting proteins down to single amino acids; Narahashi and Yanagita 1967), added to a final concentration of 3 μg/ml. This mixture was incubated at 37°C for 1 h, with occasional mixing. After this treatment, the phagosomes were pelleted as usual in a large volume of PBS containing the protease inhibitors. This step allowed us to keep in the supernatant the material released from phagosomes after the pronase treatment.
In a third set of experiments, the phagosomes were lysed in 1% Triton X-114 to analyze their membrane-associated proteins. Triton X-114 partitioning of phagosome membrane proteins was performed by the method of Bordier 1981. The proteins present in the detergent phase were then separated by SDS-PAGE using standard procedures.
High Resolution 2-D Gel Electrophoresis
Total phagosome proteins were first separated according to their isoelectric point along linear immobilized pH-gradient strips of 18 cm (Amersham Pharmacia Biotech). Sample loading in the first dimension was performed by in-gel reswelling (Pasquali et al. 1997). The strips were then equilibrated in a solution containing 13 mM DTT for 10 min, and then in a solution containing 2.5% iodoacetamide for 5 min. The proteins were then separated according to their molecular mass using standard SDS-PAGE. The large gels (18 × 20 cm) were either silver stained for protein patterns analysis or processed for mass spectrometry (MS) analysis.
For MS analysis, unfixed gels were first incubated in a 1% sodium carbonate solution for 5 min followed by incubation in 0.2 M imidazole/0.1% SDS for 15 min. Gels were then rinsed in ultra pure water for 15 s and incubated in a 0.2-M zinc acetate solution for 45 s. The reaction was then stopped with several washes of ultra pure water.
Protein Digestion
The protein spots of interest were excised from 2-D gels and further washed and analyzed essentially as previously described (Shevchenko et al. 1996). In brief, gel pieces were washed in 25 mM ammonium hydrogenocarbonate (NH4HCO3), pH 8.0, for 30 min, and then in 50% acetonitrile 25 mM NH4HCO3 for another 30 min, and finally with ultra pure water before complete dehydration in a vacuum centrifuge. The 2-D gel pieces were reswollen with a minimum amount of sequenced grade modified porcine trypsin (Promega) solution containing from 0.25 to 0.5 μg of protease, depending on the amount of protein (typically 10 μl of a 0.05-μg trypsin/μl solution, in 25 mM NH4HCO3 containing 10% acetonitrile). When necessary, NH4HCO3 buffer was further added until the gel piece was completely rehydrated. Digestion was performed at 37°C for 3–5 h.
Matrix-assisted Laser Desorption/Ionization-MS Analysis
Mass spectra of the tryptic digests were acquired on a Biflex (Bruker-Franzen Analytik) matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometer equipped with a gridless delayed extraction. The instrument was operated in the reflector mode. 0.5 μl of each digest solution (in 25 mM NH4HCO3/10% acetonitrile) was deposited directly onto the sample probe on a dry thin layer of matrix made of α-cyano-4-hydroxy-trans-cinnamic acid (CCA) mixed with nitrocellulose (mixture 4:3 vol/vol, of a saturated solution of CCA in acetone, and a solution consisting of 5 mg nitrocellulose dissolved in 1 ml isopropanol/acetone, 1:1 vol/vol). Deposits were washed with 5 μl of 0.1% trifluoroacetic acid before the analysis. A mass list of peptides was obtained for each protein digest. This peptide mass fingerprint was then submitted to an appropriate software to identify the proteins (MS-FIT, available online at http://prospector.ucsf.edu/ucsfhtml3.4/msfit.htm, or ProFound, available online at http://129.85.19.192/prowl-cgi/ProFound.exe).
When a protein could not be identified from its tryptic peptide mass map, the tryptic digest was extracted twice with a 50% acetonitrile-25 mM NH4HCO3 solution. The digest solution and the extracts were then pooled, dried in a vacuum centrifuge, and desalted with ZipTip C18 (Millipore) before the nanospray tandem MS analysis.
Nanospray-MS/MS
A quadrupole time-of-flight (Q-TOF) instrument (Micromass) was used with a Z-Spray ion-source working in the nanospray mode. Approximately 3–5 μl of the desalted sample was introduced into a needle (medium sample needle; PROTANA Inc.) to run MS and MS/MS experiments. The capillary voltage was set to an average of 1,000 V, and the sample cone to 50 V. Glufibrinopeptide was used to calibrate the instrument in the MS/MS mode. MS/MS spectra were transformed using MaxEnt3 (MassLynx; Micromass Ltd.), and amino acid sequences were analyzed using PepSeq (BioLynx; Micromass Ltd.). Amino acid sequences, sequence tags, or peptide ion fragments that could be determined were used to screen the protein and expression sequence tag (EST) databases with dedicated software: Pepfrag (http://prowl1.rockefeller.edu/prowl/pepfragch.html), Scan (http://dna.stanford.edu/scan), or BLAST for searching homologies (http://www.ncbi.nlm.nih.gov/blast/blast.cgi).
Analysis of 2-D Gel Spot Patterns
Gels to be compared were always processed in parallel. The same number of phagosomes, determined by the number of latex beads, was loaded in the first dimension. All gels were made in a similar way and stained (silver or Coomassie blue) for the same period of time. For the analysis and comparison of protein patterns, the 2-D gels were scanned using the same scanner set ups. Spot detection and gel alignment were performed using the software package PD-Quest (Bio-Rad Laboratories).
Antibody Preparation and Immunofluorescence Analysis
A full-length cDNA encoding mouse Alix (Missotten et al. 1999) was cloned into the pGEX-6P-2 (Amersham Pharmacia Biotech) using SmaI-XhoI. This allowed the fusion of Alix with glutathione-S-transferase (GST). Alix-GST fusion protein was affinity purified using glutathione sepharose; Alix was cleaved from the GST using the “precision protease.” Purified Alix was dialyzed against PBS and used to immunize rabbits. The antibody, purified on Protein G-sepharose, recognizes one single band migrating at 96 kD in Hek cells transfected with an expression vector encoding Alix.
In addition to Alix, some of the newly identified proteins were further studied by immunofluorescence to confirm their association to phagosomes using standard procedures. Antibodies against Rab7 and the cytoplasmic tail of LAMP1 were kind gifts from Stéphane Méresse (Centre d'lmmunologie de Marsaille, Luminy, France). Anti–flotillin-1 was a kind gift from Rob Parton (University of Queensland, Brisbane, Australia). Anti–14-3-3 was from Santa Cruz Biotechnology, Inc. Anti–GAPDH was from Chemicon.
Results
Latex bead–containing phagosomes can be formed and isolated at various time points after phagocytosis using a very simple flotation technique. This system provides tremendous advantages to study the full range of transformations occurring during phagolysosome biogenesis and constitutes a unique system to follow the dynamic process of membrane trafficking. Remarkably, these phagosome preparations were shown to be devoid of major contaminants from other organelles (Desjardins et al. 1994a,Desjardins et al. 1994b). More importantly, latex bead–containing phagosomes display several of the functional properties required to generate a microbicidal environment: they (a) fuse with endocytic organelles (Desjardins et al. 1994a, Desjardins et al. 1997), (b) mature into phagolysosomes (Desjardins 1995), and (c) display degradative molecules such as hydrolases (Claus et al. 1998).
With the migration conditions used in the present study, the phagosome preparations loaded on 2-D gels yielded patterns displaying a few hundred spots between 15 and 100 kD in size with pI values ranging from 3.0 to ∼9.0. Most of these protein spots were not observed in 2-D gels of total cell lysates, demonstrating the ability of our approach to enrich phagosome proteins (not shown). A representative silver-stained gel of phagosomal proteins was used to display the identified proteins and build our database (Fig. 1). Since only a handful of the spots present on phagosome 2-D gels were known at the beginning of the present study (see Desjardins et al. 1994b), we systematically excised the visible spots from zinc acetate–stained gels for mass spectrometry analysis. These analyses allowed the recognition of >140 phagosomal proteins identified, for the most part, by peptide mass fingerprinting (PMF) (Table ).
Figure 1.
Phagosome protein 2-D gel map. Latex beads were internalized by J774 macrophages for 60 min followed by a 60-min incubation without bead. After cell breakage, phagosomes were isolated on sucrose gradients and their proteins separated by high-resolution 2-D gel electrophoresis. Proteins were separated according to their isoelectric point on immobilized pH-gradients 3-10, and then by standard SDS-PAGE. The major spots were excised and analyzed by mass spectrometry. The image used here is a representative gel stained with silver.
Table 1.
Identified Phagosomal Proteins
| Protein | Accession number* | Prot Param | Observed | Remarks | MALDI-TOF-MS | MS/MS | Triton X-114 | Endo Phago | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptides | Coverage | |||||||||||
| Mol Wt | pI | Mr | pI | |||||||||
| 14-3-3 | P35215 | 27771.1 | 4.73 | 31600 | 4.2 | Involved in exocytosis through actin interaction. | × | |||||
| Acid ceramidase | Q9WV54 | 44669.5 | 8.68 | 40000 | ND | Lysosomal. | 13% | × | × | |||
| A-X actin | AAA37170 | 41693.7 | 5.21 | MF | Cytoskeletal proteins. | 20% | × | × | ||||
| β-actin | P02570 | 41736.7 | 5.29 | 41000 | 5.1 | Cytoskeletal proteins. | 21% | × | × | |||
| γ-actin | P02571 | 41792.8 | 5.31 | 40500 | 5.0 | Cytoskeletal proteins. | 41% | × | ||||
| Alix | O88695 | 96010.2 | 6.15 | 94600 | 6.1 | Programmed cell death 6-interacting protein. Implicated in apoptosis, with ALG-2. | 7/7 | 13% | ||||
| Annexin 5 | P48036 | 35752.4 | 4.83 | 34600 | 4.4 | Amount bound to phagosomes stays approximately the same as the phagosome matures. | 41% | × | ||||
| Apolipoprotein D | P51910 | 21529.7 | 4.82 | 33000 | ND | Transports a variety of ligands in a number of different contexts. | × | × | ||||
| ARP3 | P32391 | 47371.1 | 5.61 | 47600 | 5.4 | Actin-like protein 3. Cytoskeletal protein. | 9/10 | 19% | ||||
| Arylsulfatase B | P50429 | 31726.9 | 7.32 | 41300 | 6.5 | Lysosomal. | 20% | × | ||||
| Ash | Q63059 | 25206.3 | 5.89 | MF | 41% | |||||||
| ATP synth. β | P10719 | 56353.5 | 5.18 | 47900 | 4.7 | Mitochondrial protein. | 14% | × | × | |||
| CABP1 | Q63081 | 47220.1 | 4.95 | 49800 | 4.8 | Calcium-binding protein 1. Probable PDI P5 precursor. | ||||||
| Calnexin | P35564 | 67277.9 | 4.5 | 120000 | ND | Retains incorrectly folded glycoproteins in the ER. | × | × | × | |||
| Calreticulin | P14211 | 47994.5 | 4.33 | 58700 | 3.8 | ER chaperone. Also found in T cell lytic granules. | 31% | × | ||||
| CAP 1 | P40124 | 51574.8 | 7.16 | 57000 | 7.0 | Adenylyl cyclase-associated protein 1. Located on the cell membrane. | 6/9 | 12% | ||||
| CAPZ (α-actinin) | P47757 | 31345.4 | 5.47 | 32100 | 5.3 | F-actin capping protein β subunit isoform 2. | 39% | |||||
| Cathepsin A | P16675 | 53844.1 | 5.56 | MF | Lysosomal protective protein; carboxypeptidase C. | 10/11 | 21% | × | × | |||
| Cathepsin B | P10605 | 37279.8 | 5.57 | MF | Lysosomal cysteine protease. | 13/18 | 32% | × | ||||
| Cathepsin D | P18242 | 44953.8 | 6.71 | MF | Lysosomal aspartic protease. | 26% | × | × | ||||
| Cathepsin L | P06797 | 37547.3 | 6.37 | MF | Lysosomal cysteine proteases. | 15% | × | × | ||||
| Cathepsin S | O70370 | 36909.6 | 6.16 | MF | Lysosomal cysteine proteases. | 29% | × | |||||
| Cathepsin Z | Q9WUU7 | 33996.2 | 6.13 | MF | Lysosomal cysteine proteases. | 30% | × | × | ||||
| Coronin | P31146 | 51026.2 | 6.25 | MF | Shared homology with TACO. | 12/32 | 20% | |||||
| CyCAP | O35649 | 64055.5 | 4.91 | MF | Cyclophilin C-associated protein. Lysosomal. | 10/13 | 20% | × | ||||
| cytochrome P450 | Q06766 | 15681.8 | 6.71 | MF | ER membrane-bound protein. | 2/4 | 20% | |||||
| EEA1 | Q14221 | 162496.5 | 5.51 | 126600 | 5.4 | Rab5 effector. Early endosome/phagosome. | 10% | × | ||||
| Elongation factor 1-a 1 | P10126 | 50163.9 | 9.10 | 44700 | 8.6 | EF-TU | ||||||
| Endoplasmin | P08113 | 92475.7 | 4.74 | 100300 | 4.3 | ER protein. | 13% | |||||
| α-enolase | P17182 | 47124.7 | 6.37 | MF | 2-phospho-d-glycerate hydro-lyase. Cytoplasmic. | 30% | × | |||||
| Epididymal secretory protein | Q9Z0J0 | 16442 | 7.58 | 23300 | 4.6 | Unknown function. | × | |||||
| ERP29 | P52555 | 28574.8 | 6.23 | 31300 | 5.9 | Found in the lumen of the ER. | 6/8 | 17% | ||||
| Ferritin heavy chain | P09528 | 21066.6 | 5.53 | MF | Stores iron in a soluble, nontoxic, readily available form. | 51% | × | × | ||||
| Ferritin light chain 1 | P29391 | 20802.3 | 5.66 | MF | 8/8 | 48% | × | |||||
| Flotillin | O08917 | 47513.4 | 6.71 | 42700 | 6.8 | Present in lipid rafts. | 35% | |||||
| Galectin-3 | P16110 | 27514.8 | 8.47 | 32200 | 8.3 | MAC-2, laminin-binding protein. Galactose-specific lectin that binds IgE. Highest levels in activated macrophages. Involved in apoptosis. | 9/10 | 35% | × | |||
| GAPDH | P16858 | 35810 | 8.44 | 34100 | 8.4 | Glycolysis. | 19% | × | ||||
| GILT | Q9UL08 | 29148.9 | 4.88 | MF | IF-γ inducible lysosomal thiol reductase. May be involved in MHC class II–restricted antigen processing. | × | ||||||
| Mol Wt | pI | Mr | pI | |||||||||
| Glucosylceramidase | P17439 | 57621.8 | 7.64 | 27000 | ND | β-glucocerebrosidase. Lysosomal. Membrane bound. | 9% | × | × | |||
| β-glucuronidase | P12265 | 74239.2 | 6.16 | MF | Lysosomal. | 16% | × | × | ||||
| GRB2 | P29354 | 25206.3 | 5.89 | MF | Associates with tyrosine-phosphorylated proteins. Also interacts with Ras in the signaling pathway leading to DNA synthesis. | 25% | ||||||
| GRP 78 | P20029 | 72422 | 5.07 | 77000 | 4.6 | BIP. An ER chaperone. | 37% | × | × | |||
| β-hexosaminidase α | P29416 | 60599 | 6.09 | MF | N-acetyl-β-glucosaminidase, β-N-acetylhexosaminidase. Lysosomal. | 14% | × | |||||
| β-hexosaminidase β | P20060 | 61115.7 | 8.28 | MF | 19% | × | ||||||
| HSC70T | P16627 | 70695.3 | 5.82 | MF | Heat shock-related protein. Usually an ER or mitochondrial protein. | |||||||
| HSC71 | P08109 | 70804.9 | 5.24 | MF | Heat shock cognate protein | 30% | × | |||||
| HSP-60 | P19226 | 60955.4 | 5.91 | MF | Mitochondrial matrix protein P1. Chaperonin. Interacts with P21RAS. Usually an ER or mitochondrial protein. | 6/9 | 27% | × | ||||
| HSP-70 | A45935 | 70837 | 5.37 | MF | Cytoplasmic chaperone. | 27% | × | |||||
| HSP-70 precursor | A48127 | 73461.2 | 5.81 | MF | Cytoplasmic. | 31% | ||||||
| HSP-70 protein2 | P17156 | 69740.8 | 5.58 | MF | 23% | |||||||
| HSP-73 | P08109 | 70871 | 5.37 | 70900 | 5.0 | Heat shock cognate 71 kD protein. | 24/32 | 46% | ||||
| HSP-90b (HSP-84) | P11499 | 83194 | 4.97 | 90000 | 4.0 | Molecular chaperone. Has ATPase activity. Cytoplasmic. Interacts with the cytoskeleton as well. | 13% | × | × | |||
| Lactadherin | P21956 | 51465.2 | 6.02 | MF | Milk fat globule-EGF factor 8 (MFG-E8). Antiviral activity. | 17% | × | |||||
| Lamin B1 | P14733 | 66884.7 | 5.11 | MF | Component of the nuclear lamina. | 13/13 | 25% | |||||
| LAMP-1 | P11438 | 43865.1 | 8.66 | 135000 | ND | Lysosome-associated membrane glycoprotein 1. Type I membrane protein. | × | × | × | |||
| “LAMP-2, type B” | P17047 | 45647.0 | 7.05 | 120000 | ND | Lysosome-associated membrane glycoprotein 2. | × | × | × | |||
| Legumain | O89017 | 49372.9 | 5.92 | 37600 | 6 | Lysosomal cysteine endopeptidase. | 14% | × | × | |||
| LIMP II | P27615 | 53959.7 | 4.91 | 95000 | ND | Lysosome membrane protein II. May act as a lysosomal receptor. Type II membrane protein. Lysosomal. Belongs to the CD36 family. | 18% | × | × | |||
| Lysosomal acid lipase/cholesteryl ester hydrolase | P38571 | 45415 | 6.42 | 43000 | ND | Crucial for the intracellular hydrolysis of cholesteryl esters and triglycerides. Lysosomal. | 15% | × | × | × | ||
| Lysosomal membrane glycoprotein-type B | P17046 | 43127.2 | 7.16 | 120000 | ND | Very similar to LAMP2 (P17047). | × | × | × | |||
| lysozyme C, type M (LYCM) | P08905 | 16688.9 | 9.11 | 17000 | ND | 1,4-β-N-acetylmuraminidase C. Bacteriolytic. Enhances the activity of immunoagents. | 25% | × | × | |||
| Macrophage capping protein | P24452 | 39240.4 | 6.73 | MF | Actin-capping protein GCAP39; myc basic motif homolog-1. | 6/10 | 17% | |||||
| MHC class Ib, mature α chain | CAA06194 | 33419 | 5.08 | 27000 | ND | Expected to be secreted in soluble form due to absence of exon 5, which encodes the transmembrane domain. | 18% | × | × | |||
| MPS1 | I52603 | 73166.6 | 5.91 | MF | Macrophage-specific protein. Upregulated during monocyte to macrophage differentiation. | 13%‡ | ||||||
| Myosin heavy chain-A | O89055 | 18177.4 | 5.34 | 155000 | ND | Non muscle form. | × | × | ||||
| Napsin | CAB82907 | 45544.3 | 7.13 | 55000 | ND | Membrane-anchored aspartyl protease. | × | × | ||||
| NDK B | Q01768 | 17363 | 6.97 | 21100 | 6.9 | Nucleotide diphosphate kinase B. | 61% | |||||
| NSF | P46460 | 82565.4 | 6.52 | 77000 | 6.4 | Vesicular-fusion protein. N-ethylmaleimide-sensitive factor. | 29% | × | × | |||
| Mol Wt | pI | Mr | pI | |||||||||
| ORP150 | Q63617 | 111289.1 | 5.11 | 125700 | 4.8 | Oxygen regulated protein. | ||||||
| Palmitoyl-protein thioesterase | O88531 | 34621.3 | 8.26 | MF | Removes thioester-like fatty acyl groups from modified cysteine residues in proteins or peptides during lysosomal degradation. | 23% | × | × | ||||
| PDI (ER-59) | P09103 | 57143.6 | 4.79 | 55500 | 4.3 | Protein disulfide isomerase. | 6/11 | 22% | × | |||
| PDI (ER-60) | P27773 | 56621.3 | 5.99 | MF | Involved in MHC class I assembly. | × | ||||||
| PGAM-B | P25113 | 28514.4 | 6.21 | 31500 | 6.6 | Phosphoglycerate mutase, brain form. | 40% | |||||
| Prohibitin | P24142 | 29820.1 | 5.57 | 31000 | 5.2 | Present in lipid rafts. | 51% | |||||
| RAB2 | P53994 | 23547.5 | 6.08 | 23000 | ND | Vesicular traffic. Associated with an intermediate compartment between the ER and Golgi apparatus. | 30% | × | ||||
| RAB3C | Q63482 | 24890.9 | 4.98 | 25000 | ND | Protein transport and vesicular traffic. | 14% | × | ||||
| RAB5C | P35278 | 5496.1 | 4.83 | 26500 | ND | Regulates early endocytic/phagocytic trafficking. | 36% | × | × | |||
| RAB7 | P51150 | 23558.8 | 7.53 | 24000 | ND | Regulates late endocytic/phagocytic trafficking. | 44% | × | × | |||
| RAB10 | O88386 | 22540.9 | 8.58 | 23200 | ND | Vesicular traffic and neurotransmitter release. | 30% | × | ||||
| RAB11B | P46638 | 24489.4 | 5.64 | 25000 | ND | Involved in membrane recycling. | 17% | × | × | |||
| RAB14 | P35287 | 23926.9 | 5.85 | 26500 | ND | Vesicular traffic and neurotransmitter release. | 40% | × | ||||
| RAP1B | P09526 | 20824.7 | 5.65 | 22000 | ND | Involved in initiation of oxidative burst in neutrophils. | 39% | × | × | |||
| RHO GDI α | P19803 | 23421.4 | 5.12 | 27500 | 4.8 | RHO GDP-dissociation inhibitor 1 | × | |||||
| RSP4 | P08865 | 32854 | 4.79 | MF | 40S ribosomal protein SA. Cytoplasmic. | 18% | × | |||||
| SNAP-α | P54921 | 33194.9 | 5.16 | 34600 | 5.0 | Soluble NSF attachment protein. Required to prepare intracellular membranes for fusion. | 76% | × | ||||
| SNAP-γ | Q99747 | 34746.2 | 5.30 | 37400 | 5.1 | 30% | × | |||||
| Stomatin | P54116 | 31403.3 | 6.46 | MF | ND | Found in lipid rafts, exposed on the cytoplasmic surface of the membrane. | 43% | × | ||||
| Syntenin | O88601 | 32263.2 | 6.66 | 33500 | ND | Localized in early endocytic compartments. | × | × | × | |||
| TCBP-49 | O70341 | 37454.9 | 4.26 | 47200 | 3.6 | Taipoxin-associated calcium binding protein 49. | 36% | |||||
| TCP-1α | P11983 | 60448.6 | 5.82 | 55500 | 5.7 | T-complex protein 1. Molecular chaperone. Known to play a role, in vitro, in the folding of actin and tubulin. Cytoplasmic. | 5/6 | 10% | × | |||
| TCP-1β | P80314 | 57447.2 | 5.97 | 55500 | 6.9 | 20% | ||||||
| TCP-1ε | P80316 | 59624 | 5.72 | 60600 | 5.6 | 18% | ||||||
| Thioreductase peroxidase 2 | P35700 | 22176.5 | 8.26 | 26800 | 8.4 | Cytoplasmic. | 4/4 | 20% | ||||
| Ti-225 (ubiquitin C) | Q62317 | 14175.4 | 9.33 | 14200 | 5.7 | Similar to human ubiquitin. | 42% | |||||
| TMP21 | Q63584 | 23276.8 | 6.03 | 23200 | ND | Transmembrane protein. Vesicular protein trafficking. Type I membrane protein. Present in Golgi cisternae. | 26% | × | ||||
| TRAIL | P50592 | 33477.3 | 8.21 | 31300 | 5.9 | TNF-related apoptosis inducing ligand. | ||||||
| Trimeric G α2 | P08752 | 40470.9 | 5.28 | 37400 | 4.9 | Guanine nucleotide-binding protein G(I)/G(S)/G(T). Adenylate cyclase-inhibiting Ga protein. Regulatory G-proteins of signaling cascades. | 13/16 | 30% | × | × | ||
| Trimeric G β1 | P04901 | 37376.9 | 5.60 | 35900 | 5.2 | 28% | × | |||||
| Trimeric G β2 | P54312 | 37333 | 5.60 | 35700 | 5.2 | 34% | × | × | ||||
| Tropomyosin 5 | P21107 | 29020.6 | 4.75 | 33600 | 4.2 | Cytoskeletal type. | 30% | |||||
| Tubulin α-6 | P05216 | 49909.3 | 4.96 | 54700 | 4.8 | Microtubule proteins. | 14% | |||||
| Tubulin β-5 | P05218 | 49670.8 | 4.78 | 49500 | 4.5 | 13/24 | 38% | × | ||||
| UDPGT | Q64550 | 59662.7 | 8.77 | 27000 | ND | UDP-glucuronosyltransferase. E.R. protein. | 22% | × | ||||
| VAP33 | Q9QY77 | 27271.5 | 7.66 | 83000 | ND | Vesicle-associated membrane protein, associated protein A. Associated with ER and microtubules. | 40% | × | ||||
| Mol Wt | pI | Mr | pI | |||||||||
| v-ATPase α (catalytic subunit) | P50516 | 68268 | 5.62 | MF | Involved in phagosome acidification. | 44% | × | |||||
| v-ATPase β | P50517 | 56584.9 | 5.57 | MF | 9/15 | 28% | × | |||||
| v-ATPase ε | P50518 | 26588 | 9.28 | MF | 34% | × | ||||||
| VDAC1 | Q60932 | 30624.3 | 8.63 | 28500 | ND | Voltage-dependent anion-selective channel protein 1. Mitochondrial. Also found on the plasma membrane and endosomes. | 25% | × | × | |||
| Vimentin | P20152 | 53687.6 | 5.06 | MF | Class III intermediate filaments. | 54% | ||||||
| Antitrypsin | P34955 | 46103.9 | 6.05 | MF | Serum proteins adsorbed on latex beads during internalization. Highly enriched in our preparations. Endocytic cargo. | 18% | ||||||
| Apolipoprotein A-I | P15497 | 30276.3 | 5.71 | 29300 | 5.0 | |||||||
| BSA | P02769 | 69293.4 | 5.82 | MF | 16/22 | 32% | ||||||
| α-S1 caseine | P02662 | 24528.9 | 4.98 | 20600 | 4.3 | 35% | ||||||
| Hemoglobin α | P01966 | 15053.1 | 8.19 | 16300 | 7.4 | 50% | ||||||
| Hemoglobin β | P02081 | 15859.2 | 6.51 | MF | 6/11 | 46% | ||||||
| Putative gag | P23090 | 60511.7 | 7.63 | MF | Duplan murine leukaemia virus. | |||||||
(continues)
(continues)
(continues)
Proteomic analysis of phagosome proteins. The 1- and 2-D gel methods combined allowed the identification of 116 phagosomal proteins directly from existing databases (including seven cargo molecules probably originating from the serum). In addition, 17 spots analyzed could not be matched to any entry in databases, while seven corresponded to ESTs (not shown), suggesting that they are novel proteins.
*Whenever possible, the SWISS-PROT or TrEMBL accession numbers are listed. Otherwise, NCBI entries are used. MF, multiple forms. Observed pI values could not be evaluated for proteins identified by the 1-D gel method.
‡MPS1 coverage is only NH2 terminal, and when this region is considered alone, the match percentage is very satisfactory; MALDI-TOF-MS, peptides, ratio of peptides that matched the expected mass of the theoretical trypsin digests. Coverage, percentage of the full-length sequence covered by the matching peptides; MS/MS, proteins further identified my MS/MS; Triton X-114, proteins that were identified from the detergent phase samples; Endo/phago, proteins previously reported to be present in endosomal/phagosomal compartments.
Protein Identification by Mass Spectrometry
To identify a protein from its PMF, several criteria were considered. The most important of these was the coverage of the full-length protein. When exceeding 15%, the coverage was considered to be sufficient unless there was some obvious conflicts such as discrepancies between the experimental molecular weight and that of the identified protein. At between 10 and 15% of coverage, the identification was validated when the matching peptides versus input peptides ratio exceeded 75% and when the molecular weight and species were not too distant. The mass accuracy of our analyses was always set at 100 ppm, but was usually better than 50 ppm. Matching peptides with one missed cleavage were validated only when there were two consecutive basic residues (KK, KR, RK, or RR) or an RP sequence inside the peptide amino acid sequence.
MS/MS analyses were conducted on proteins not identified by the PMF approach. In most cases, several peptide sequence tags were easily obtained and the protein was identified by mining protein databases with Pepfrag (PROWL). As an example, the lysosomal acid lipase (10% coverage) was confirmed by tandem MS. In other cases, when the mouse protein was not present in the protein and EST databases, we had to generate amino acid sequences by MS/MS and use BLAST software to look for proteins exhibiting high amino acid sequence homology (for example, GILT was identified from human databases). We could also reach some EST sequences using peptide sequence tags (e.g., ESTAA445025, which shows an homology with cathepsin A) or peptide mass fingerprints (only in the case of low molecular weight proteins; e.g., EST 1447369, similar to CU-Zn SOD), and even reconstruct one protein amino acid sequence by matching a first EST, and then clustering several ESTs [e.g., an ADP-ribosylation factor (ARF)–6 isoform].
Based on these different criteria, we identified 116 proteins by the PMF approach and 7 ESTs. 19 were further analyzed by MS/MS to get unambiguous identifications. All of these were confirmed. Furthermore, 17 protein spots yielded good MS spectra that could not be matched to any entries in current databases. Identification of these potentially novel proteins will require further analysis and sequencing.
Phagosome Membrane-associated Proteins
Separation of membrane proteins is still problematic with 2-D gels (Santoni et al. 2000). Although some of the proteins identified on 2-D gels from our samples are clearly membrane associated, such as flotillin-1, other expected proteins, such as those of the LAMP family, were not identified. Accordingly, to identify as many membrane-associated proteins as possible, we complemented our 2-D gel studies with SDS-PAGE analysis of phagosome membrane proteins obtained after phase partition in Triton X-114. Using this approach, we resolved 24 major bands visualized after zinc acetate staining (Fig. 2). From these bands, we were able to identify 36 proteins, 5 of which had previously been identified from the 2-D gels. Among these proteins were seven members of the ras superfamily: rab2, 3c, 5c, 7, 10, 11, and rab14, as well as rap1b. Interestingly, these included the small GTPases previously shown to be associated to phagosomes; namely, rab5, 7, 11, and rap1 (Desjardins et al. 1994a; Pizon et al. 1994; Cox et al. 2000). Transmembrane proteins were also identified in our phagosome preparations, including LAMP1, LAMP2, lgp110, and LIMPII. LAMP1 was also identified by MS/MS analysis in a band at ∼44 kD. Other identified membrane proteins included stomatin, Tmp21, and VDAC1.
Figure 2.
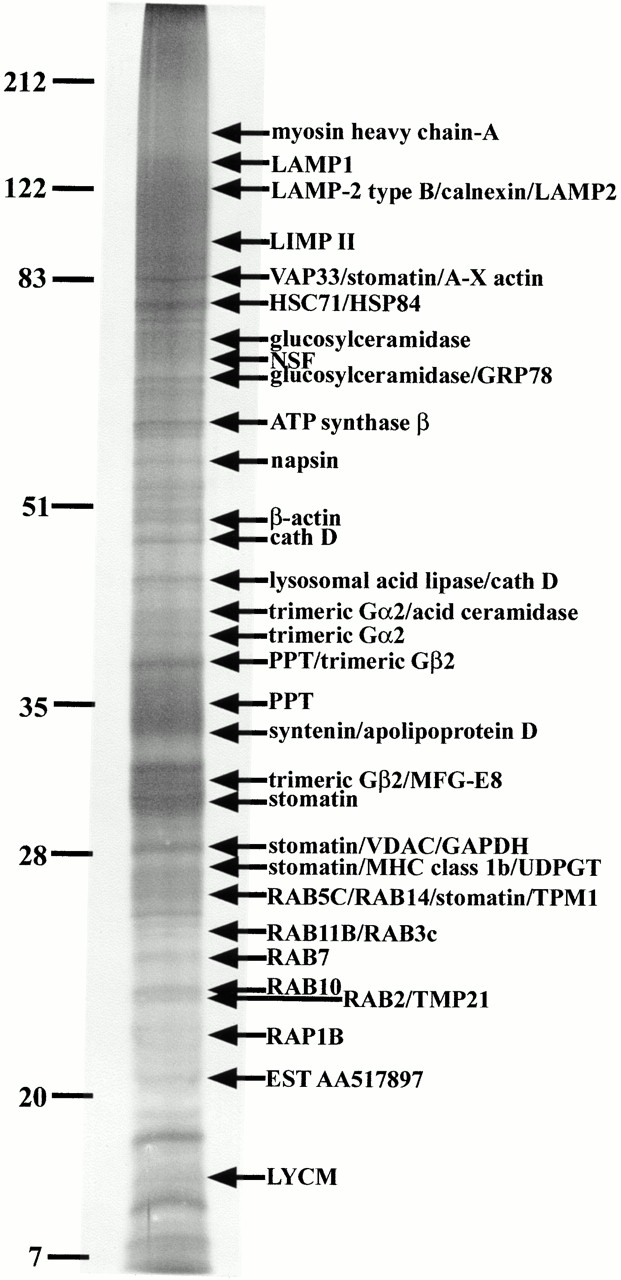
Isolation of phagosome membrane-associated proteins by Triton X-114 extraction. Latex beads were internalized by J774 macrophages for 60 min followed by a 60-min incubation without bead. Isolated phagosomes were then treated with Triton X-114 to separate membrane-associated proteins from soluble proteins. Proteins present in the detergent phase were then separated by SDS-PAGE. The gels were stained with zinc acetate and the major bands excised for mass spectrometry analysis. In some cases, several proteins were identified by mass fingerprinting in the same band.
Western Blotting and Immunofluorescence Analysis
Several proteins not previously reported to be associated to phagosomes were identified in our preparations. It was impossible to systematically test all the new proteins by Western blotting or immunofluorescence to confirm their enrichment on phagosomes and their localization to this organelle since antibodies against these molecules were often not available. Nevertheless, we were able to further investigate some of these proteins and demonstrate the association of LAMP1, a well-known phagosome protein used here as a control, alix, flotillin-1, GAPDH, and 14-3-3 to phagosomes (Fig. 3). Western blot analysis indicated that all these proteins are enriched in phagosomes compared with total cell lysates. Furthermore, immunofluorescence analysis showed that the staining of these molecules is localized to the periphery of latex beads, as well as to other cellular organelles not further investigated in the present study. GAPDH, a cytosolic enzyme involved in glycolysis and the production of ATP, was shown to associate with actin filaments (Masters 1984). The recent finding of its enrichment in pseudopodia (Nguyen et al. 2000), suggests that GAPDH might associate to phagosomes very early during the internalization process, providing a local source of energy for actin-based phagosome formation and functions.
Figure 3.

Immunolocalization of novel proteins to phagosomes. To demonstrate the phagosomal association of the new proteins identified in our study, we performed Western blot and immunofluorescence analyses of some of these proteins in macrophages that had internalized latex beads for 60 min, followed by a 60-min chase. LAMP1, a well known phagosomal protein, was used as a control. For Western blot analysis, the same amount of protein from total cell lysates or isolated phagosomes was loaded on SDS-PAGE. A clear enrichment of each protein on the phagosomes is observed. Immunofluorescence analysis clearly demonstrate the localization of the protein around compartments containing latex beads. In the case of 14-3-3 and GAPDH, cells were permeabilized before the labeling to get rid of most of the cytosolic proteins. Note that one of the cells has not been permeabilized in the case of 14-3-3. For calnexin, an antibody against the cytosolic portion of the molecule was used. The same amount of protein was loaded for total cell lysate (TCL), the phagosomes (Phagos), and a microsome preparation (ER) as a positive control.
Lumenal Versus Peripheral Phagosome Proteins
To determine which proteins are present within the lumen of phagosomes and which are associated with the cytoplasmic side of this compartment, we performed enzymatic degradation of intact phagosomes recovered on sucrose gradients. By treating phagosomes with pronase, we were able to induce the degradation of the proteins exposed partially or totally on the cytoplasmic side of phagosomes. Silver staining of phagosome proteins separated by SDS-PAGE showed that several spots were no longer detected after pronase treatment (not shown). On the other hand, the proteins present within the lumen of the organelle were protected from pronase attack by the phagosome membrane. To demonstrate the selectivity of proteolysis towards proteins exposed at the cytoplasmic side of the phagosome membrane, we studied the effect of pronase on LAMP1, a transmembrane protein with a short cytoplasmic tail. Using a monoclonal antibody against a lumenal epitope of the LAMP1 molecule, we were able to show the presence of this molecule in both control and pronase-treated samples (Fig. 4, top left). In contrast, when a polyclonal antibody against the cytoplasmic tail of LAMP1 was used, LAMP1 was observed in the control, but not in the pronase-treated sample, indicating that this part of the molecule had been efficiently degraded by the treatment. Furthermore, the pronase treatment had no effect on cathepsin D, a known lumenal molecule, but induced the disappearance of the peripheral protein Rab7.
Figure 4.
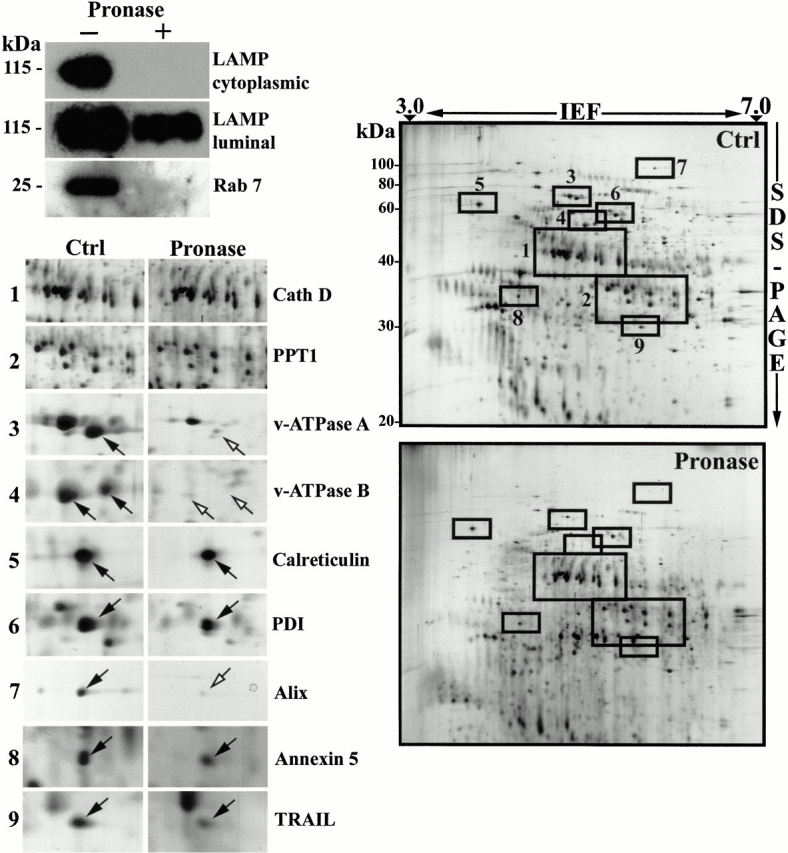
Sensitivity of phagosome proteins to protease treatment: lumenal versus cytoplasmic proteins. (A) Phagosomes isolated on sucrose gradients were treated with pronase to digest proteins exposed on the cytoplasmic side of phagosomes. Western blot analyses showed that the lumenal part of LAMP1, a transmembrane protein with a short cytoplasmic tail, was not affected by the pronase treatment, while the cytoplasmic tail was degraded. Rab7, a protein associated with the cytoplasmic side of phagosomes was efficiently degraded (or released) by the treatment. (B) Comparison of the protein patterns between phagosomes treated or not with pronase shows that several protein spots are no longer present on phagosomes after the pronase treatment (arrows), indicating they have been digested by the proteases. (C) Insets of selected regions of the gel show that hydrolases present within the lumen of phagosomes, such as cathepsins A and D, are not affected by the pronase treatment. In contrast, proteins like the A and B subunits of the vacuolar proton pump, or proteins associated with cytoskeletal elements, which are exposed on the cytoplasmic side of phagosomes, are degraded and almost absent on the 2-D gel. Interestingly, proteins of the ER, such as calreticulin and PDI, are not degraded, indicating that these ER elements are within the lumen of phagosomes and are thus unlikely to constitute contaminants trapped by cytoskeletal elements on the outside of phagosomes, which would be released by the treatment. Another interesting observation is the presence of TRAIL and Annexin5 in the lumen of the phagosome, in contrast to Alix on the cytoplasmic side, as expected from proteins involved in apoptotic signaling.
These results clearly demonstrate the usefulness of this approach to distinguish lumenal proteins from those present on the cytoplasmic side of phagosomes. 2-D gel analysis of pronase-treated and non–treated preparations clearly showed differences between the two samples (Fig. 4, right). For example, the A and B subunits of the proton pump ATPase, which are on the cytoplasmic side of endovacuolar organelles (Forgac 1989), are missing from the pronase-treated samples (Fig. 4, bottom left). None of the cathepsins are modified by the treatment, demonstrating their lumenal localization (Fig. 4, bottom left). Interestingly, the spots corresponding to the various ER proteins identified in our phagosome preparations, such as endoplasmin, calreticulin, and protein disulfide isomerase (PDI), are not affected by the pronase treatment (Fig. 4, bottom left). Contrary to what one might have originally postulated, these results strongly suggest that ER elements are probably not contaminants trapped in a mesh of filaments (actin or microtubules) floated during the phagosome isolation, which would be released by the pronase treatment. Instead, the pronase resistance suggests that ER components might indeed be present within the lumen of phagosomes or recruited to form part of the phagosome membrane.
Modulation of Phagosome Composition during Phagolysosome Biogenesis
Our 2-D database is a powerful tool that can be used to follow modifications of phagosome composition in various conditions. To illustrate the usefulness of this database, we isolated phagosomes at different time points after their formation in order to monitor the changes occurring to phagosomes during phagolysosome biogenesis. Several proteins were shown to be modulated during this process (Fig. 5, arrowheads), including a variety of hydrolases (Table ). Our results clearly indicate that hydrolases are not acquired simultaneously by phagosomes. While some hydrolases, such as cathepsin A and β-hexosaminidase are already present in a high amount in early phagosomes, others, such as cathepsin S and the cleaved form of cathepsin D, appear at later time points during phagolysosome biogenesis (Fig. 6). Others, such as the recently cloned cathepsin Z (Santamaría et al. 1998), are present at early time points but disappear during phagosome maturation, suggesting that they are either recycled or degraded and that they play a role in the processing of peptides at the early stages of phagolysosome biogenesis.
Figure 5.

Modulation of phagosome composition during maturation. Latex bead–containing phagosomes were isolated at different stages of maturation and their proteins separated by 2-D gel electrophoresis. The various gels were then compared with each other using the software PD-Quest. Arrows point to peaking proteins at their respective time points.
Table 2.
Sequential Acquisition of Hydrolases
| Phagosome age | ||||
|---|---|---|---|---|
| 30′/0′ | 1 h/1 h | 1 h/6 h | 1 h/24 h | |
| Cath A | +++ | +++ | +++ | +++ |
| β-Hexosaminidase α | +++ | +++ | +++ | +++ |
| Cath B | + | +++ | +++ | +++ |
| Carboxypeptidase | + | +++ | +++ | +++ |
| Arylsulfatase B | + | ++ | +++ | +++ |
| Legumain | + | + | +++ | +++ |
| Superoxide dismutase | + | + | +++ | +++ |
| Cath D | + | ++ | ++ | +++ |
| Cath L | + | ++ | ++ | +++ |
| Cath S | + | ++ | ++ | +++ |
| GILT | + | ++ | ++ | +++ |
| Palmitoyl thioesterase | − | ++ | +++ | +++ |
| β-glucuronidase | + | +++ | +++ | ++ |
| Cath Z | + | ++ | +++ | − |
Sequential acquisition of hydrolases during phagosome maturation. Each identified hydrolase was monitored by 2-D gel electrophoresis after isolation of latex bead–containing phagosomes at different time points during phagolysosome biogenesis. The level of each protein was evaluated by measuring the density of the spots and their surface using PD-Quest. The results clearly indicate that phagolysosome biogenesis is characterized by the sequential acquisition of hydrolases. Note that some hydrolases, such as LAP and napsin, could not be listed here, as they were only identified by the 1-D gel method.
Figure 6.
Sequential acquisition of hydrolases during phagosome maturation. Phagosomes were formed by the internalization of latex beads for 30 or 60 min, followed by increasing periods of chase to allow phagosome maturation. After 2-D gel electrophoresis, the protein patterns were analyzed using the phagosome 2-D database (Fig. 1). These analyses indicated that hydrolases are not acquired simultaneously by phagosomes, but rather appear sequentially during phagolysosome biogenesis.
Discussion
At the subcellular level, the observation through high-resolution 2-D gel electrophoresis that organelles are made up of hundreds of proteins, several of which are extensively post-translationally modified, highlights the complexity of the mechanisms associated with the functions of these compartments (Scianimanico et al. 1997; Rabilloud et al. 1998). It also emphasizes the overwhelming task of understanding how organelles work using conventional approaches consisting of studying each of their proteins, one by one. Hopefully, the refinement of technical approaches enabling us to separate thousands of proteins by 2-D gel electrophoresis, and analyze them from minute amounts of material by mass spectrometry, has allowed us to envisage the possibility of identifying most, if not all, proteins associated with a given organelle and understand how they are modulated in various conditions (Lamond and Mann 1997). This holistic approach is likely to provide new insights into the function of complex organelles and their regulation.
In the present study, we identified >140 of the proteins present on or within latex bead–containing phagosomes. Several of these proteins are expected constituents of an organelle that moves along cytoskeletal elements, interacts and fuses with endovacuolar organelles, acidifies its lumen, and degrades its content. Among these are a series of hydrolases, subunits of the vacuolar proton pump ATPase, molecules of the fusion/fission machinery, various GTPases of the Ras superfamily, as well as coat proteins and cytoskeletal-related proteins. Remarkably, a series of novel proteins, not previously reported to be associated with phagosomes were also identified. The confirmation of the enrichment of several of these proteins on phagosomes by various approaches, including differential 2-D gel display against a macrophage total cell lysate, Western blot analysis, and immunofluorescence localization, allowed us to propose new concepts regarding the molecular mechanisms of phagolysosome biogenesis.
Membrane Fusion and Small GTPases
Phagosomes were shown to fuse sequentially with subsets of endosomes (Desjardins et al. 1997). Thus, it was not surprising to find SNARE molecules and regulatory small GTPases, including Rab4, Rab5, Rab7, Rab11, and Rap1b, previously described on phagosomes (Desjardins et al. 1994a; Pizon et al. 1994; Hackam et al. 1996; Cox et al. 2000). Rab5 and Rab7 allow phagosomes to interact with early and late endosomes, respectively (see Méresse et al. 1999), while the association of Rab4 and Rab11 with recycling endosomes (Sheff et al. 1999; Sonnichsen et al. 2000) suggests that recycling processes might also be important for phagolysosome biogenesis. Three other Rab proteins, Rab 3c, Rab10, and Rab14, have been identified on phagosomes in the present study. Rab3, a small GTPase involved in Ca2+-dependent exocytosis (see Geppert and Sudhof 1998), was also localized to endosomes (Slembrouck et al. 1999). The functions for Rab10 and Rab14 still remain to be elucidated. A novel GTPase of the ARF family was also identified by tandem MS and searches in EST databases (AI006608). Based on the alignment of two overlapping ESTs, this sequence was predicted to code for a 21.6-kD protein (189 amino acids) displaying 64% homology to human and mouse ARF6. Western blot analysis using a polyclonal antibody raised against the COOH-terminal region of the protein indicated that this ARF6-like protein is highly enriched on phagosomes compared with a total cell lysate of macrophages (not shown). Previous studies have shown that ARF6 is involved in actin cytoskeleton organization and recycling process (D'Souza-Schorey et al. 1998; Franco et al. 1999). A similar role on phagosomes remains to be established.
Maturation of Phagosomes Is Accompanied by the Sequential Acquisition of Hydrolases
We have highlighted in the present study the presence of several hydrolases in phagosomes, including six members of the cathepsin family as well as some of their cleaved forms. In addition, 12 other hydrolases have been identified, including legumain, shown to be involved in the processing of microbial peptides with affinity for major histocompatibility complex (MHC) class II molecules (Manoury et al. 1998), and GILT, a gamma interferon-induced lysosomal thioesterase (Arunachalam et al. 2000).
Using our 2-D gel database, we were able to show that phagosome composition is modulated during phagolysosome biogenesis (Fig. 5). An interesting observation was that hydrolases are not delivered simultaneously to phagosomes (Fig. 6). Instead, they appear sequentially, at different time points during phagosome maturation. Cathepsin A, an exopeptidase required for the activity of other hydrolases, is already present at its highest detectable level in early phagosomes, at a time point when cathepsins D and S are barely detectable. The appearance of the 46-kD form of cathepsin D in phagosomes occurs before the detection of the 32-kD cleaved form of this protein. While most of the hydrolases seem to reach their highest level 6 h after phagosome formation, the levels of few others, such as cathepsin S, continue to increase as late as 24 h after phagosome formation. Since phagolysosome biogenesis is accompanied by the sequential fusion of phagosomes with early endosomes, late endosomes, and lysosomes (Desjardins et al. 1997), our results support the hypothesis that hydrolases are heterogeneously distributed along the degradative pathway (Munier-Lehmann et al. 1996). The sequential appearance of hydrolases in phagosomes might regulate the correct processing and presentation of antigens by MHC class II molecules (Villadangos and Ploegh 2000). These results indicate that our 2-D gel database is a unique tool to monitor changes occurring to phagosome proteins in various conditions.
Contaminants or Genuine Phagosomal Proteins?
Several proteins not previously shown to be associated with endocytic or phagocytic compartments were also identified in our phagosome preparations. These proteins were rather localized to other organelles, including the plasma membrane (flotillin-1), mitochondria (ATP synthase, prohibitin and VDAC-1), the cytoplasm (GAPDH, 14-3-3), and the endoplasmic reticulum (calreticulin, calnexin, GRP78, endoplasmin, Erp29). The simplest explanation for the presence of these proteins is that our phagosome preparations are contaminated by other organelles, a limitation of most subcellular fractionation approaches. However, unlike most approaches relying on the isolation of organelles based on their intrinsic density, isolation of latex bead–containing phagosomes is facilitated by the low buoyant density of latex. Thus, phagosomes are floated in a region of the sucrose gradient where other cellular organelles are not detected. Previous morphological and biochemical analysis of latex bead phagosome preparations indicated the virtual absence of contamination by mitochondria, Golgi vesicles, endosomes, and the plasma membrane, while low levels of ER elements were found (Desjardins et al. 1994b). Based on all these observations, it is worthwhile to consider that the presence in our preparations of molecules previously identified in other compartments is perhaps representative of more than a simple contamination.
Four mitochondrial proteins were identified in our phagosome preparations, which are ATP synthase, prohibitin, HSP60, and VDAC-1. Interestingly, two of these proteins have been shown to be present on other compartments in the cell. Prohibitin was found to be associated with receptors at the cell surface (see below), while VDAC-1, a type 1 porin molecule of the outer mitochondrial membrane, was shown to be present on endosomes by immunofluorescence and immunogold electron microscopy (Reymann et al. 1998). VDAC-1 was also shown to be present at the cell surface, where it is targeted through the expression of an alternative first exon (Buettner et al. 2000). The potential roles of these molecules on extramitochondrial compartments are not known. An additional finding suggesting that the detection of prohibitin and VDAC-1 in our phagosome preparation is not due to mitochondrial contamination is the fact that these proteins were not identified as major constituents of mitochondria in a recent in-depth proteomic study (Rabilloud et al. 1998). Furthermore, at least two of the most prominent spots present on 2-D gels of mitochondria, fumarate hydratase and aconitase, are not present at their migration coordinates in our phagosome gels. If mitochondria were contaminating our preparations, one would assume that these spots would also be present in our gels. These data support the concept that at least some of the mitochondrial proteins present in our preparations might be genuine constituents of phagosomes.
Insights into New Phagosome Functions
ER Recruitment to Phagosomes.
Our phagosome analysis allowed us to identify several molecules normally associated with the endoplasmic reticulum. Although this might represent a contamination, further data suggest that ER elements could interact directly with phagosomes. The pronase experiments have shown that all the lumenal ER proteins identified in our 2-D gels were not affected by the pronase treatment, indicating, at least, that they are not simply associated with ER elements entrapped in the cytoskeletal matrix associated to the phagosomes and floated during the isolation procedure. A further indication of the close association of ER with phagosomes came from Western blot analysis showing the enrichment of calnexin in our phagosome preparations compared with the total cell lysate. Furthermore, pre-embedding immunocytochemical analysis using an antibody against the cytosolic portion of calnexin demonstrated the presence of this protein on the phagosomal membrane, while an antibody against the lumenal portion of the molecule gave no signal (Gagnon, E., and M. Desjardins, manuscript in preparation). The presence of ER molecules on phagosomes is not surprising. Recent studies have shown that microorganisms such as Legionella and Brucella reside within their host cells in compartments displaying ER features (see Méresse et al. 1999). In our experiments, it was not uncommon to observe macrophages that had engulfed >25 latex beads. Despite the important surface of plasma membrane needed for the engulfment of these particles, the cell does not consume itself, but rather maintains a relatively stable size. This suggests that while plasma membrane recycles rapidly back to the cell surface (Pitt et al. 1992), membrane from the ER could be recruited to keep the particles within closed compartments.
Another interesting link between phagolysosomes and ER elements comes from the finding that calreticulin, a chaperone of the ER identified in our phagosome preparations, is also present in the lysosome-like lytic granules of T lymphocytes (Dupuis et al. 1993). There, it controls the lytic activity of perforin by stabilizing membranes to prevent polyperforin pore formation (Fraser et al. 2000). Although perforin has not been identified in our phagosome preparations, a newly identified protein, MPS1, was shown to display a certain homology with perforin (Spilsbury et al. 1995). The MPS1 gene was first identified as being upregulated during monocyte-to-macrophage differentiation (Spilsbury et al. 1995), as well as during prion infection, along with other lysosomal hydrolases (HEXA and HEXB) (Kopacek et al. 2000). The coded protein appears to share distant ancestry to perforin, although its property to form pores, yet alone to polymerize or span a membrane, has not been shown. Further analyses should clarify whether the ER is a contaminant or if it is recruited to form phagosomes.
Lipid Rafts.
Among the new phagosomal proteins found in our study is flotillin-1. This protein was reported to be associated with caveolae or other subdomains of the plasma membrane (Bickel et al. 1997; Lang et al. 1998). Its identification by mass spectrometry on phagosomes, further confirmed by Western blot and immunofluorescence analyses, suggests that lipid rafts could also be present on phagosomes, and not solely on the Golgi apparatus or the plasma membrane (Simons and Ikonen 1997). A second molecule recently shown to be associated to lipid rafts, stomatin (Snyers et al. 1999), is also present on phagosomes. Although stomatin was first proposed to be involved in the maintenance of the structural integrity of the red blood cell membrane, this function was ruled out after the observation that red blood cells from stomatin knockout mice were not altered (Zhu et al. 1999). Of further interest, flotillin-1 and stomatin share a homologous domain with prohibitin, a molecule also present on lipid rafts (Terashima et al. 1994), identified in our phagosome preparations. Although prohibitin is present in mitochondria (Ikonen et al. 1995), reports indicate that it may associate with certain receptors present at the cell surface (Terashima et al. 1994), a phenomenon that could explain its presence on phagosomes. The potential involvement of prohibitin in the regulation of mitochondrial membrane protein degradation (Steglich et al. 1999) may be relevant to some of the phagolysosome degradative functions. Altogether, these results strongly suggest that specialized subdomains or lipid rafts might be present on the phagosome membrane. Lipid rafts have been implicated in many important cellular processes, such as polarized sorting of apical membrane proteins in epithelial cells and signal transduction (for review, see Kurzchalia and Parton 1999). Interestingly, molecules involved in signal transduction, such as the α, β1, and β2 subunits of trimeric G-protein, as well as annexin II, are present on phagosomes (Desjardins et al. 1994b; Berón et al. 1995).
Phagosomes and Apoptosis.
Using immunofluorescence analysis, we were able to demonstrate that 14-3-3 is effectively associated with phagosomes. In adrenal chromaffin cells, 14-3-3 proteins were shown to regulate secretory vesicle exocytosis by reorganizing the cortical actin barrier (Morgan and Burgoyne 1992), possibly by interacting with annexin II (Roth et al. 1993), a protein also present on phagosomes (Desjardins et al. 1994b). Recently, dominant-negative forms of 14-3-3 were used to disrupt 14-3-3 function in cultured cells and transgenic animals. Fibroblasts transfected with these mutants exhibited markedly increased apoptosis, suggesting that a primary function of mammalian 14-3-3 proteins is to inhibit apoptosis (Xing et al. 2000). Interestingly, other molecules related to apoptosis have been identified in our study. These are Alix, annexin 5, TRAIL, and galectin3. All of these proteins are prominent spots in 2-D gels of phagosomes, but not detectable in gels from total cell lysates, demonstrating their enrichment on phagosomes (not shown). Intracellular TRAIL/Apo2L has been demonstrated in macrophages, but, to our knowledge, this is the first report of its enrichment within phagosomes. This finding is noteworthy in view of the recent report that thymocytes from ICAD-Sdm mice only develop oligonucleosomal DNA degradation inside macrophages (McIlroy et al. 2000), and that the emergence of TUNEL-positive cells is prevented in Caenorhabditis elegans Ced-7 mutants, in which engulfment is impaired (Wu et al. 2000). These observations suggest that phagolysosomes may induce apoptosis within macrophages through an unknown mechanism. Intraphagosomal TRAIL binding to its receptor on the phagocytosed cell could be part of such a mechanism. A role for lysosomes in cell-autonomous apoptosis has also been suggested since autophagy or cathepsin D translocation to nonlysosomal structures can be instrumental during the death of nonphagocytic cells (see Ferri and Kroemer 2000). Our findings showing that proteins with a demonstrated function in apoptosis like galectin 3, Alix, VDAC1, GAPDH, or 14-3-3 are enriched in phagosomes reinforces this hypothesis and may open new avenues in understanding the role of phagolysosomal compartments in apoptosis.
Phagolysosome biogenesis is obviously a complex process made possible by the contribution of a large number of molecules. In the last few years, in-depth studies of some of the phagosome proteins have allowed us to significantly increase our knowledge of this organelle's functional properties. In the present study, we used a proteomic approach to gain a global view of the composition of phagosomes and their potential functions. The confidence level attained by the identification of several key phagosomal components and the immunofluorescent localization of some of the unexpected proteins to phagosomes, together with the low contamination of our preparations by other organelles, allows us to consider most of the proteins identified in this study as genuine constituents of phagosomes. This wide body of data provides new insights into the molecular mechanisms governing phagosome functions and phagolysosome biogenesis (Fig. 7). This proteomic approach is likely to become extremely powerful as we learn to fully use all of its strengths.
Figure 7.
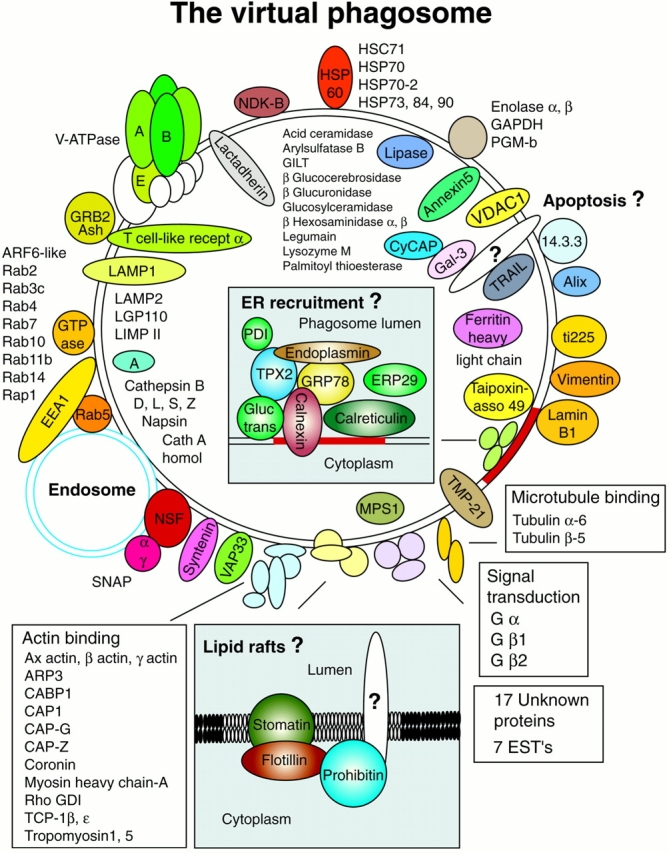
The virtual phagosome. The major proteins identified in the present study are presented in their potential interaction with phagosomes. In several cases, the localization to the lumen, the membrane, or the cytoplasmic aspect of the phagosome was indicated by the sensitivity to pronase proteolysis (Fig. 4). (Insets) New concepts proposed after the identification of novel phagosome proteins.
Acknowledgments
The authors thank C. Chatellard and B. Blot for help with making the antibody against Alix. We also thank Ruddy Wattiez, Geneviève Milon, and John Bergeron for their constructive comments and help and for the kind gift of anti–calnexin cytosolic antibody (J. Bergeron).
This work was supported by grants from the Medical Research Council (MRC) of Canada (GX-15526 and MT-12951) and from FCAR Equipe. J.-F. Dermine is the recipient of a studentship from Natural Sciences and Engineering Research Council of Canada, S. Duclos is the recipient of a studentship from the MRC, and M. Desjardins is a Scholar from Fonds de la recherche en santé du Quebec.
Footnotes
Drs. Garin and Diez contributed equally to this work and should be considered co-first authors.
Abbreviations used in this paper: 2-D, two-dimensional; ARF, ADP-ribosylation factor; EST, expression sequence tag; MHC, major histocompatibility complex; MS, mass spectrometry; PDI, protein disulfide isomerase; PMF, peptide mass fingerprinting.
References
- Alvarez-Dominguez C., Stahl P.D. Increased expression of Rab5a correlates directly with accelerated maturation of Listeria monocytogenes phagosomes. J. Biol. Chem. 1999;274:11459–11462. doi: 10.1074/jbc.274.17.11459. [DOI] [PubMed] [Google Scholar]
- Arunachalam B., Phan U.T., Geuze H.J., Cresswell P. Enzymatic reduction of disulfide bonds in lysosomescharacterization of a gamma-interferon-inducible lysosomal thiol reductase (GILT) Proc. Natl. Acad. Sci. USA. 2000;97:745–750. doi: 10.1073/pnas.97.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berón W., Colombo M.I., Mayorga L.S., Stahl P.D. In vitro reconstitution of phagosome-endosome fusionevidence for regulation by heterotrimeric GTPases. Arch. Biochem. Biophys. 1995;317:337–342. doi: 10.1006/abbi.1995.1172. [DOI] [PubMed] [Google Scholar]
- Bickel P.E., Scherer P.E., Schnitzer J.E., Oh P., Lisanti M.P., Lodish H.F. Flotillin and epidermal surface antigen define a new family of caveolae-associated integral membrane proteins. J. Biol. Chem. 1997;272:13793–13802. doi: 10.1074/jbc.272.21.13793. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 1981;256:1604–1607. [PubMed] [Google Scholar]
- Buettner R., Papoutsoglou G., Scemes E., Spray D.C., Dermietzel R. Evidence for secretory pathway localization of a voltage-dependent anion channel isoform. Proc. Natl. Acad. Sci. USA. 2000;97:3201–3206. doi: 10.1073/pnas.060242297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus V., Jahraus A., Tjelle T., Berg T., Kirschke H., Faulstich H., Griffiths G. Lysosomal enzyme trafficking between phagosomes, endosomes, and lysosomes in J774 macrophages. Enrichment of cathepsin H in early endosomes. J. Biol. Chem. 1998;273:9842–9851. doi: 10.1074/jbc.273.16.9842. [DOI] [PubMed] [Google Scholar]
- Cox D., Lee D.J., Dale B.M., Calafat J., Greenberg S. A Rab11-containing rapidly recycling compartment in macrophages that promotes phagocytosis. Proc. Natl. Acad. Sci. USA. 2000;97:680–685. doi: 10.1073/pnas.97.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defacque H., Egeberg M., Habermann A., Diakonova M., Roy C., Mangeat P., Voelter W., Marriott G., Pfannstiel J., Faulstich H., Griffiths G. Involvement of ezrin/moesin in de novo actin assembly on phagosomal membranes. EMBO (Eur. Mol. Biol. Organ.) J. 2000;19:199–212. doi: 10.1093/emboj/19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins M. Biogenesis of phagolysosomesthe ‘kiss and run’ hypothesis. Trends Cell Biol. 1995;5:183–186. doi: 10.1016/s0962-8924(00)88989-8. [DOI] [PubMed] [Google Scholar]
- Desjardins M., Celis J.E., van Meer G., Dieplinger H., Jahraus A., Griffiths G., Huber L.A. Molecular characterization of phagosomes J. Biol. Chem. 269 1994. 32194 32200b [PubMed] [Google Scholar]
- Desjardins M., Huber L.A., Parton R.G., Griffiths G. Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus J. Cell Biol. 124 1994. 677 688a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins M., Nzala N.N., Corsini R., Rondeau C. Maturation of phagosomes is accompanied by changes in their fusion properties and size-selective acquisition of solute materials from endosomes. J. Cell Sci. 1997;110:2303–2314. doi: 10.1242/jcs.110.18.2303. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C., van Donselaar E., Hsu V.W., Yang C., Stahl P.D., Peters P.J. ARF6 targets recycling vesicles to the plasma membraneinsights from an ultrastructural investigation. J. Cell Biol. 1998;140:603–616. doi: 10.1083/jcb.140.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclos S., Diez R., Garin J., Papadopoulou B., Descoteaux A., Stenmark H., Desjardins M. Rab5 regulates the kiss and run fusion between phagosomes and endosomes and the acquisition of phagosome leishmanicidal properties in RAW 264.7 macrophages. J. Cell Sci. 2000;113:3531–3541. doi: 10.1242/jcs.113.19.3531. [DOI] [PubMed] [Google Scholar]
- Dupuis M., Schaerer E., Krause K.H., Tschopp J. The calcium-binding protein calreticulin is a major constituent of lytic granules in cytolytic T lymphocytes. J. Exp. Med. 1993;177:1–7. doi: 10.1084/jem.177.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri K.F., Kroemer G. Control of apoptotic DNA degradation. Nat. Cell Biol. 2000;2:E63–E64. doi: 10.1038/35008692. [DOI] [PubMed] [Google Scholar]
- Forgac M. Structure and function of vacuolar class of ATP-driven proton pumps. Physiol. Rev. 1989;69:765–796. doi: 10.1152/physrev.1989.69.3.765. [DOI] [PubMed] [Google Scholar]
- Franco M., Peters P.J., Boretto J., van Donselaar E., Neri A., D'Souza-Schorey C., Chavrier P. EFA6, a sec7 domain-containing exchange factor for ARF6, coordinates membrane recycling and actin cytoskeleton organization. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:1480–1491. doi: 10.1093/emboj/18.6.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser S.A., Karimi R., Michalak M., Hudig D. Perforin lytic activity is controlled by calreticulin. J. Immunol. 2000;164:4150–4155. doi: 10.4049/jimmunol.164.8.4150. [DOI] [PubMed] [Google Scholar]
- Geppert M., Sudhof T.C. RAB3 and synaptotagminthe yin and yang of synaptic membrane fusion. Annu. Rev. Neurosci. 1998;21:75–95. doi: 10.1146/annurev.neuro.21.1.75. [DOI] [PubMed] [Google Scholar]
- Haas A. Reprogramming the phagocytic pathway—intracellular pathogens and their vacuoles. Mol. Membr. Biol. 1998;15:103–121. doi: 10.3109/09687689809074522. [DOI] [PubMed] [Google Scholar]
- Hackam D.J., Rotstein O.D., Bennett M.K., Klip A., Grinstein S., Manolson M.F. Characterization and subcellular localization of target membrane soluble NSF attachment protein receptors (t-SNAREs) in macrophages. Syntaxins 2, 3, and 4 are present on phagosomal membranes. J. Immunol. 1996;156:4377–4383. [PubMed] [Google Scholar]
- Ikonen E., Fiedler K., Parton R.G., Simons K. Prohibitin, an antiproliferative protein, is localized to mitochondria. FEBS Lett. 1995;358:273–277. doi: 10.1016/0014-5793(94)01444-6. [DOI] [PubMed] [Google Scholar]
- Jahraus A., Tjelle T.E., Berg T., Habermann A., Storrie B., Ullrich O., Griffiths G. In vitro fusion of phagosomes with different endocytic organelles from J774 macrophages. J. Biol. Chem. 1998;273:30379–30390. doi: 10.1074/jbc.273.46.30379. [DOI] [PubMed] [Google Scholar]
- Kopacek J., Sakaguchi S., Shigematsu K., Nishida N., Atarashi R., Nakaoke R., Moriuchi R., Niwa M., Katamine S. Upregulation of the genes encoding lysosomal hydrolases, a perforin-like protein, and peroxidases in the brains of mice affected with an experimental prion disease. J. Virol. 2000;74:411–417. doi: 10.1128/jvi.74.1.411-417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzchalia T.V., Parton R.G. Membrane microdomains and caveolae. Curr. Opin. Cell Biol. 1999;11:424–431. doi: 10.1016/s0955-0674(99)80061-1. [DOI] [PubMed] [Google Scholar]
- Lamond A.I., Mann M. Cell biology and the genome projects—a concerted strategy for characterizing multiprotein complexes by using mass spectrometry. Trends Cell Biol. 1997;7:139–142. doi: 10.1016/S0962-8924(97)01031-3. [DOI] [PubMed] [Google Scholar]
- Lang D.M., Lommel S., Jung M., Ankerhold R., Petrausch B., Laessing U., Wiechers M.F., Plattner H., Stuermer C.A. Identification of reggie-1 and reggie-2 as plasmamembrane-associated proteins which cocluster with activated GPI-anchored cell adhesion molecules in non-caveolar micropatches in neurons. J. Neurobiol. 1998;37:502–523. doi: 10.1002/(sici)1097-4695(199812)37:4<502::aid-neu2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Manoury B., Hewitt E.W., Morrice N., Dando P.M., Barrett A.J., Watts C. An asparaginyl endopeptidase processes a microbial antigen for class II MHC presentation. Nature. 1998;396:695–699. doi: 10.1038/25379. [DOI] [PubMed] [Google Scholar]
- Masters C. Interactions between glycolytic enzymes and components of the cytomatrix. J. Cell Biol. 1984;99:222s–225s. doi: 10.1083/jcb.99.1.222s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlroy D., Tanaka M., Sakahira H., Fukuyama H., Suzuki M., Yamamura K., Ohsawa Y., Uchiyama Y., Nagata S. An auxiliary mode of apoptotic DNA fragmentation provided by phagocytes. Genes Dev. 2000;14:549–558. [PMC free article] [PubMed] [Google Scholar]
- Méresse S., Steele-Mortimer O., Moreno E., Desjardins M., Finlay B.B., Gorvel J.P. Controlling the maturation of pathogen-containing vacuolesa matter of life or death. Nat. Cell Biol. 1999;1:E183–E188. doi: 10.1038/15620. [DOI] [PubMed] [Google Scholar]
- Missotten M., Nichols A., Rieger K., Sadoul R. Alix, a novel mouse protein undergoing calcium-dependent interaction with the apoptosis-linked-gene 2(ALG-2) protein. Cell Death Differ. 1999;6:124–129. doi: 10.1038/sj.cdd.4400456. [DOI] [PubMed] [Google Scholar]
- Morgan A., Burgoyne R.D. Exo1 and Exo2 proteins stimulate calcium-dependent exocytosis in permeabilized adrenal chromaffin cells. Nature. 1992;355:833–836. doi: 10.1038/355833a0. [DOI] [PubMed] [Google Scholar]
- Munier-Lehmann H., Mauxion F., Bauer U., Lobel P., Hoflack B. Re-expression of the mannose 6-phosphate receptors in receptor-deficient fibroblasts. Complementary function of the two mannose 6-phosphate receptors in lysosomal enzyme targeting. J. Biol. Chem. 1996;271:15166–15174. doi: 10.1074/jbc.271.25.15166. [DOI] [PubMed] [Google Scholar]
- Narahashi Y., Yanagita M. Studies on proteolytic enzymes (pronase) of Streptomyces griseus K-1. I. Nature and properties of the proteolytic enzyme system. J. Biochem. 1967;62:633–641. doi: 10.1093/oxfordjournals.jbchem.a128718. [DOI] [PubMed] [Google Scholar]
- Nguyen T.N., Wang H.J., Zalzal S., Nanci A., Nabi I.R. Purification and characterization of beta-actin-rich tumor cell pseudopodiarole of glycolysis. Exp. Cell Res. 2000;258:171–183. doi: 10.1006/excr.2000.4929. [DOI] [PubMed] [Google Scholar]
- Pasquali C., Fialka I., Huber L.A. Preparative two-dimensional gel electrophoresis of membrane proteins. Electrophoresis. 1997;18:2573–2581. doi: 10.1002/elps.1150181413. [DOI] [PubMed] [Google Scholar]
- Pitt A., Mayorga L.S., Stahl P.D., Schwartz A.L. Alterations in the protein composition of maturing phagosomes. J. Clin. Invest. 1992;90:1978–1983. doi: 10.1172/JCI116077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizon V., Desjardins M., Bucci C., Parton R.G., Zerial M. Association of Rap1a and Rap1b proteins with late endocytic/phagocytic compartments and Rap2a with the Golgi complex. J. Cell Sci. 1994;107:1661–1670. doi: 10.1242/jcs.107.6.1661. [DOI] [PubMed] [Google Scholar]
- Rabilloud T., Kieffer S., Procaccio V., Louwagie M., Courchesne P.L., Patterson S.D., Martinez P., Garin J., Lunardi J. Two-dimensional electrophoresis of human placental mitochondria and protein identification by mass spectrometrytoward a human mitochondrial proteome. Electrophoresis. 1998;19:1006–1014. doi: 10.1002/elps.1150190616. [DOI] [PubMed] [Google Scholar]
- Reymann S., Haase W., Krick W., Burckhardt G., Thinnes F.P. Endosomesanother extra-mitochondrial location of type-1 porin/voltage-dependent anion-selective channels. Pflügers Arch. 1998;436:478–480. doi: 10.1007/s004240050659. [DOI] [PubMed] [Google Scholar]
- Roberts R.L., Barbieri M.A., Pryse K.M., Chua M., Morisaki J.H., Stahl P.D. Endosome fusion in living cells overexpressing GFP-rab5. J. Cell Sci. 1999;112:3667–3675. doi: 10.1242/jcs.112.21.3667. [DOI] [PubMed] [Google Scholar]
- Roth D., Morgan A., Burgoyne R.D. Identification of a key domain in annexin and 14-3-3 proteins that stimulate calcium-dependent exocytosis in permeabilized adrenal chromaffin cells. FEBS Lett. 1993;320:207–210. doi: 10.1016/0014-5793(93)80587-k. [DOI] [PubMed] [Google Scholar]
- Santamaría I., Velasco G., Pendás A.M., Fueyo A., López-Otín C. Cathepsin Z, a novel human cysteine proteinase with a short propeptide domain and a unique chromosomal location. J. Biol. Chem. 1998;273:16816–16823. doi: 10.1074/jbc.273.27.16816. [DOI] [PubMed] [Google Scholar]
- Santoni V., Molloy M., Rabilloud T. Membrane proteins and proteomicsun amour impossible? Electrophoresis. 2000;21:1054–1070. doi: 10.1002/(SICI)1522-2683(20000401)21:6<1054::AID-ELPS1054>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Scianimanico S., Pasquali C., Lavoie J., Huber L.A., Gorvel J.P., Desjardins M. Two-dimensional gel electrophoresis analysis of endovacuolar organelles. Electrophoresis. 1997;18:2566–2572. doi: 10.1002/elps.1150181412. [DOI] [PubMed] [Google Scholar]
- Sheff D.R., Daro E.A., Hull M., Mellman I. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J. Cell Biol. 1999;145:123–139. doi: 10.1083/jcb.145.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A., Jensen O.N., Podtelejnikov A.V., Sagliocco F., Wilm M., Vorm O., Mortensen P., Shevchenko A., Boucherie H., Mann M. Linking genome and proteome by mass spectrometrylarge-scale identification of yeast proteins from two dimensional gels. Proc. Natl. Acad. Sci. USA. 1996;93:14440–14445. doi: 10.1073/pnas.93.25.14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Slembrouck D., Annaert W.G., Wang J.M., Potter W.P. Rab3 is present on endosomes from bovine chromaffin cells in primary culture. J. Cell Sci. 1999;112:641–649. doi: 10.1242/jcs.112.5.641. [DOI] [PubMed] [Google Scholar]
- Snyers L., Umlauf E., Prohaska R. Association of stomatin with lipid-protein complexes in the plasma membrane and the endocytic compartment. Eur. J. Cell Biol. 1999;78:802–812. doi: 10.1016/S0171-9335(99)80031-4. [DOI] [PubMed] [Google Scholar]
- Sonnichsen B., De Renzis S., Nielsen E., Rietdorf J., Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 2000;149:901–914. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilsbury K., O'Mara M.A., Wu W.M., Rowe P.B., Symonds G., Takayama Y. Isolation of a novel macrophage-specific gene by differential cDNA analysis. Blood. 1995;85:1620–1629. [PubMed] [Google Scholar]
- Steglich G., Neupert W., Langer T. Prohibitins regulate membrane protein degradation by the m-AAA protease in mitochondria. Mol. Cell. Biol. 1999;19:3435–3442. doi: 10.1128/mcb.19.5.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima M., Kim K.M., Adachi T., Nielsen P.J., Reth M., Kohler G., Lamers M.C. The IgM antigen receptor of B lymphocytes is associated with prohibitin and a prohibitin-related protein. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:3782–3792. doi: 10.1002/j.1460-2075.1994.tb06689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villadangos J.A., Ploegh H.L. Proteolysis in MHC class II antigen presentationwho's in charge? Immunity. 2000;12:233–239. doi: 10.1016/s1074-7613(00)80176-4. [DOI] [PubMed] [Google Scholar]
- Wu Y.C., Stanfield G.M., Horvitz H.R. NUC-1, a Caenorhabditis elegans DNase II homolog, functions in an intermediate step of DNA degradation during apoptosis. Genes Dev. 2000;14:536–548. [PMC free article] [PubMed] [Google Scholar]
- Xing H., Zhang S., Weinheimer C., Kovacs A., Muslin A.J. 14-3-3 proteins block apoptosis and differentially regulate MAPK cascades. EMBO (Eur. Mol. Biol. Organ.) J. 2000;19:349–958. doi: 10.1093/emboj/19.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Paszty C., Turetzky T., Tsai S., Kuypers F.A., Lee G., Cooper P., Gallagher P.G., Stevens M.E., Rubin E., Mohandas N., Mentzer W.C. Stomatocytosis is absent in “stomatin”-deficient murine red blood cells. Blood. 1999;93:2404–2410. [PubMed] [Google Scholar]




