Abstract
Cryptococcus neoformans is an opportunistic fungal pathogen that causes life-threatening meningoencephalitis in immunocompromised patients. The Ca2+-calmodulin-activated protein phosphatase calcineurin is necessary for virulence of C. neoformans. Mutants lacking the calcineurin catalytic (Cna1) or regulatory (Cnb1) subunit fail to grow at elevated temperature and are defective in virulence and hyphal elongation. Here we isolated a multicopy suppressor gene, CTS1, which restores growth of a calcineurin mutant strain at 37°C. The CTS1 gene (for calcineurin temperature suppressor 1) encodes a protein containing a C2 domain and a leucine zipper motif that may function as an effector of calcineurin. The CTS1 gene was disrupted by homologous recombination, and cts1 mutants were viable but exhibited defects in cell separation, growth, mating, and haploid fruiting. In addition, cts1 mutants were inviable when calcineurin was mutated or inhibited. Taken together, these findings suggest that calcineurin and Cts1 function in parallel pathways that regulate growth, cell separation, and hyphal elongation.
Signal transduction cascades are utilized by all organisms to sense signals at the cell surface and to transmit this information to effectors within the cell (17, 50). The primary components of signal transduction cascades are protein kinases and phosphatases, which act on protein targets via a mechanism of reversible phosphorylation. Many pathogenic fungi utilize signal transduction pathways to sense the host and rapidly adapt to changing environmental conditions (24). Our studies address the signaling cascades that enable pathogenic fungi to sense and interact with the host.
The human fungal pathogen Cryptococcus neoformans is a leading cause of disease among immunosuppressed individuals, resulting in an often-fatal form of meningoencephalitis. However, even healthy individuals are at increased risk due to the ubiquitous environmental presence of this fungus. A recent outbreak of infection with Cryptococcus neoformans var. gattii among residents and visitors to Vancouver Island has resulted in more than 66 cases of cryptococcal disease, including several deaths, since 1999 (44). In addition to the incidence of cryptococcal infections among apparently healthy persons, isolates have been reported that are resistant to currently available antifungal drugs (36). Thus, further research into the pathogenic mechanisms of this medically important fungus and the discovery of novel drug targets are needed.
Calcineurin is a highly conserved Ca2+-calmodulin-activated serine/threonine protein phosphatase that is necessary for the pathogenesis of C. neoformans and other medically important fungi (reviewed in reference 14). In other organisms, calcineurin regulates many physiological processes necessary for life, including morphogenesis, cell wall biosynthesis, septation, and cytokinesis (16, 26, 31, 33, 51, 52, 54). Calcineurin is the target of the immunosuppressive drugs FK506 and cyclosporin, which inhibit calcineurin activity (25). In C. neoformans, calcineurin is essential for growth at 37°C and for virulence in animal models of cryptococcosis (9, 13, 35). In addition to a role in growth at 37°C, calcineurin is also required for morphogenic events involving hyphal elongation in C. neoformans, a process central to the development of infectious spores (7).
In this study we examined the hypothesis that calcineurin-dependent protein effectors are present in C. neoformans and that their overexpression will suppress the temperature-sensitive growth defect conferred by a calcineurin mutation. To identify and characterize components of the calcineurin signaling pathway in C. neoformans, we isolated multicopy suppressors of the temperature-sensitive defect of a calcineurin-deficient strain. Plasmid-dependent transformants were isolated which restored growth at high temperature, revealing a novel gene, named CTS1 for calcineurin temperature suppressor. CTS1 encodes a protein containing a phospholipid-binding C2 domain and a leucine zipper motif. Overexpression of Cts1 conferred resistance to FK506 and cyclosporin in wild-type organisms. Disruption of the CTS1 gene by homologous recombination resulted in temperature sensitivity, a cell separation and septal positioning defect, a reduction in growth, inhibition of hyphal elongation and virulence, synthetic lethality with calcineurin mutations, and enhanced sensitivity to FK506 and cyclosporin. In addition, domain analysis revealed that, while the C-terminal leucine zipper motif is important for full Cts1 function, the C2 domain is absolutely required for high-temperature growth, cell separation, phospholipid binding, and suppression of calcineurin mutation. Our findings suggest that calcineurin and Cts1 function in parallel pathways that control growth, cell separation, and hyphal elongation.
MATERIALS AND METHODS
Strains, media, and reagents.
All C. neoformans strains used in this study are derived from the congenic serotype D (Cryptococcus neoformans var. neoformans) strains JEC21 (MATα) and JEC20 (MATa). The genotype of strain MCC3 is MATa cna1::ADE2 ura5. Strain JEC43 (MATα ura5) is a 5-fluoroorotic acid-resistant derivative of JEC21. The genotypes of the cts1 deletion or truncation strains derived from JEC20, JEC43, or JEC21 are as follows: DSF22 (MATα cts1-570::NAT), DSF20 (MATa cts1-570::NAT), DSF42 (MATa cts1Δ::NAT), DSF45 (MATα cts1Δ::NAT), DSF50 (MATα cts1Δ::NAT ura5), and DSF11 (MATα cnb1::NAT). C. neoformans strains were grown on standard yeast medium except where otherwise indicated.
Oligonucleotide primers and sequencing.
Oligonucleotide primers for PCR and sequencing were synthesized by Integrated DNA Technologies, Inc. Sequencing was performed by the Duke University DNA analysis facility with the ABI 377 sequencer, version 3.3 (PE Applied Biosystems). Sequence data were analyzed with MacVector, version 7.0, and the DNASTAR software suite, version 4.0. Primers used include JOHE6289 (5′-CATACAACGCACTGCAAGTGCCC), JOHE6297 (5′-TACATTACTCTTCTCATCTCC), JOHE8912 (5′-GCACCCCTATAGATTATAAGGATGATGATGATAAGGAACCCAAAGAGC), JOHE8913 (5′-CTCTTTGGGTTCCTTATCATCATCATCCTTATAATCTATAGGGGTGC), JOHE8914 (5′-GGCACGTTGATCGATTATAAGGATGATGATGATAAGGTCGTTCAACGG), JOHE8915 (5′-CCGTTGAACGACCTTATCATCATCATCCTTATAATCGATCAACGTGCC), JOHE8916 (5′-GCGCAACATGGCGATTATAAGGATGATGATGATAAGCCGATGTCGTCG), and JOHE8917 (5′-CGACGACATGCCCTTATCATCATCATCCTTATAATCGCCATGTTGCGC).
Multicopy suppressor library.
The multicopy suppressor library was constructed as previously described (7). Briefly, genomic DNA from the serotype D MATα strain C21F2 was partially digested with Sau3AI, size selected to yield fragments from 6 to 12 kb, and ligated into the BamHI site of the C. neoformans shuttle vector pPm8 as described previously (32). The resulting genomic library was linearized with I-SceI and introduced by electroporation into MCC3. Transformants were grown on medium lacking uracil to maintain the pPm8 plasmid.
Disruption of the C. neoformans CTS1 gene.
The CTS1 gene was disrupted by homologous recombination with a cassette containing the nourseothricin dominant drug resistance gene from Streptomyces noursei, nat1, fused to the C. neoformans ACT1 promoter and TRP1 terminator and flanked by CTS1 gene sequence (29). The cassette was introduced as a 1.7-kb EcoRV fragment into the blunted NdeI sites of the CTS1 gene. Disruption was confirmed by Southern blot analysis of HindIII-digested genomic DNA from wild type and cts1-570::nat and cts1Δ::nat disruptants with a 300-bp probe corresponding to the CTS1 gene. The 1.7-kb EcoRV fragment containing the gene for resistance to the aminoglycoside antibiotic nourseothricin, nat1, fused to the C. neoformans actin promoter, was generated as previously described (13, 29).
Virulence assay.
The pathogenicity of the cts1Δ and cts1-570 mutant strains was determined in the murine inhalation model of cryptococcosis. Strains to be analyzed were grown to mid-log phase in yeast extract-peptone-dextrose (YPD) at 25°C for 24 h, washed, and resuspended in phosphate-buffered saline (PBS) to a concentration of 5 × 104 organisms per 0.1 ml. Organisms (wild type or mutant) were introduced by intranasal inoculation of complement (C5)-deficient DBA/1 mice with 5 × 104 organisms introduced per mouse with 10 mice per strain tested. Mice were monitored for survival over a period of 105 days. Surviving mice were analyzed for the presence of viable C. neoformans organisms as previously described (13).
Mating and haploid fruiting assays.
Mating assays were performed by growing strains of opposite mating types on YPD solid medium for 48 to 72 h at 25°C prior to coculturing them on V8 medium at 25°C for 7 days in the dark. Haploid fruiting assays were performed similarly, with strains being grown on YPD solid medium prior to being spotted onto filamentation agar followed by incubation at 25°C for 14 days in the dark. Filamentation for both mating and haploid fruiting assays was scored microscopically, as previously described (7).
FLAG tagging and site-directed mutagenesis of Cts1.
The FLAG epitope (DYKDDDDK) was introduced into the CTS1 coding region by using a PCR overlap approach to generate an N-terminal FLAG-Cts1 fusion. Briefly, PCR products for the left end of the CTS1 coding region (JOHE6289 and JOHE8913) and the right end (JOHE6297 and JOHE8912) were generated, purified, and combined to generate the full-length 3-kb overlap product with the flanking primer set (JOHE6289 and JOHE6297). Domain deletions of Cts1 were generated by replacement of the predicted phospholipid-binding C2 domain or a region bearing limited homology to a calmodulin binding domain (CMD) with the FLAG epitope. Briefly, the C2 domain (amino acids 16 to 161) and putative CMD (amino acids 284 to 305) of Cts1 were replaced by an in-frame substitution with the FLAG epitope (DYKDDDDK) by the use of a PCR overlap method as described above with primers JOHE8914 and JOHE8915 being paired with the flanking primer set to generate the C2 domain deletion product and primers JOHE9816 and JOHE8917 being paired with the flanking primer set to generate the CMD deletion product. All overlap products were then ligated into the TA cloning vector pCR2.1 (Invitrogen) and introduced into Escherichia coli TOP10 cells (Invitrogen).
Expression and purification of Cts1 fusion proteins.
To generate the FLAG-Cts1, Cts1ΔC2-FLAG, and Cts1ΔCMD-FLAG protein fusions, the FLAG::CTS1, CTS1ΔC2::FLAG, and CTS1ΔCMD::FLAG coding regions were isolated as BamHI-XbaI fragments from the pCR2.1 constructs described above and ligated into the BamHI and XbaI sites of the pPm8 shuttle vector. The 1.7-kb CTS1 promoter region was then introduced as a BglII fragment into the BamHI-BglII sites of each pPm8 construct in both forward and reverse orientations for FLAG::CTS1 and in the sense direction for CTS1ΔC2::FLAG and CTS1ΔCMD::FLAG. The resulting constructs were linearized and introduced by electroporation into DSF50. Transformants were grown on medium lacking uracil to maintain the pPm8 plasmid. Total protein was isolated from transformants grown at 25°C with complete yeast extraction buffer (Calbiochem), purified with anti-FLAG M2 affinity agarose (Sigma), eluted with 0.1 M glycine-HCl (pH 3.5), precipitated with acetone, and resuspended in PBS with protease inhibitors.
Phospholipid binding assays.
The binding of FLAG-Cts1 or the Cts1ΔC2-FLAG fusion proteins was evaluated by a dot blot assay utilizing phosphoinositide (PIP) strips (Molecular Probes). Purified fusion protein in PBS containing 0.1% Tween 20 (PBS-T) with 5% bovine serum albumin was used to probe the PIP membrane strips overnight at 4°C. Bound protein was visualized by Western blot analysis with a primary mouse anti-FLAG M2 antibody (Sigma) and a secondary sheep anti-mouse antibody conjugated to horseradish peroxidase (Amersham Biosciences) followed by detection with SuperSignal West Femto maximum-sensitivity substrate (Pierce). All antibody incubations were performed in PBS-T with 3% bovine serum albumin at room temperature, and washes were performed in PBS-T at room temperature.
Chitinase assays.
C. neoformans strains were grown to log phase in liquid YPD medium at 25°C, washed with PBS, and treated with chitinase (Sigma, 2.5 U) or PBS alone for 2 h at 25°C. Cells were then examined by differential interference contrast (DIC) microscopy with a Zeiss AxioPhot 2 fluorescence microscope with a Zeiss AxioCam MR digital camera and image capture software.
Fluorescence microscopy.
For fluorescence microscopic analysis C. neoformans strains were grown to log phase in liquid YPD medium at 25°C prior to fixation and permeabilization. Cells were fixed by incubation in PBS containing 7.7% formaldehyde for 1 h at room temperature, washed in PBS, and fixed in PBS containing 1% Triton X-100. Fixed and permeabilized cells were stained with Alexa Fluor 488-conjugated wheat germ agglutinin (WGA; Molecular Probes) to visualize chitin and Alexa Fluor 594-conjugated phalloidin (Molecular Probes) to visualize F-actin. Fluorescence and DIC microscopy were performed on a Zeiss AxioPhot 2 fluorescence microscope equipped with a Zeiss AxioCam MR digital camera and software for image capture and analysis.
Electron microscopy.
Conventional electron microscopy was performed as previously described with the following modifications (19). Cells were grown to mid-log phase in YPD at 25°C, rinsed in 0.1 M cacodylate buffer (pH 7.4), and fixed in 1% osmium tetroxide in 0.1 M cacodylate buffer containing 0.15% ruthenium red for 1 h at 25°C. Following three washes in cacodylate buffer, cells were dehydrated through a graded series of ethanol from 30 to 100% and subjected to an extended infiltration series in propylene oxide-Spurr resin prior to being embedded in 100% Spurr resin. Embedded samples were cut on an RMC MT-7 ultramicrotome, and sections of 50 to 80 nm were collected on Formvar-coated grids and stained with uranyl acetate and Reynold's lead citrate. All sections were viewed on a JEOL 1200EX transmission electron microscope.
Nucleotide sequence accession numbers.
The cDNA and genomic sequences for CTS1 have been deposited in GenBank under the accession numbers AY163383 (cDNA) and AY163382 (genomic).
RESULTS
Identification of a suppressor of calcineurin mutant temperature sensitivity.
To identify genes whose products function downstream of calcineurin, or in parallel pathways, a multicopy suppressor screen was conducted to discover genes that restore growth at 37°C of a calcineurin mutant strain. The multicopy genomic library, containing genomic fragments of 6 to 12 kb of the serotype D strain C21F2 cloned in the shuttle vector pPm8, was linearized with I-SceI and introduced into the calcineurin A (cna1) mutant strain MCC3 by electroporation (7). Transformants were selected on medium lacking uracil at 25°C and screened for growth at 35°C by replica plating. Four suppressors were isolated from a screen of 2,000 transformants. We verified that growth at 35°C was plasmid dependent by plasmid loss on 5-fluoroorotic acid medium to counterselect the URA5 marker. Plasmids were rescued from suppressor strains, and the inserts were sequenced. One of the four suppressors was found to contain the CNA1 gene, which encodes the calcineurin catalytic subunit. The three remaining suppressors contained genes of unknown function.
Sequence analysis of the open reading frame (ORF) of one of the three remaining suppressors revealed a predicted protein of 834 amino acid residues that possessed homology within the amino terminus to a putative C2 domain-containing protein of unknown function in Neurospora crassa encoded by the NCU09847.1 locus. In addition to the phospholipid-binding (C2) domain at the amino terminus, two additional functional domains were identified, including a predicted transmembrane domain adjacent to the C2 domain and a putative leucine zipper region at the carboxy terminus. Because phospholipid-associated proteins, such as Its3 in Schizosaccharomyces pombe, have been shown to function coordinately with calcineurin, we chose to examine this novel gene in greater detail, designating the gene CTS1 for calcineurin temperature suppressor 1 (54).
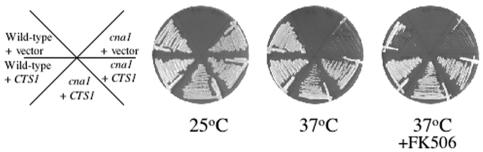
To confirm that the overexpression of CTS1 sufficed to restore growth at 37°C in calcineurin mutants, the rescued plasmid containing the CTS1 gene under the control of its own promoter (pCTS1) or the plasmid pPm8 was introduced into isogenic wild-type and cna1 mutant strains (JEC34 and MCC3, respectively). Overexpression of CTS1 restored growth at 37°C in calcineurin mutants (Fig. 1). However, overexpression of CTS1 did not restore hyphal elongation during mating or haploid fruiting in calcineurin mutant strains (data not shown) (7). Taken together, these results suggest that Cts1 and calcineurin share an essential function in the regulation of growth at elevated temperature. As this functional overlap is specific to the regulation of growth at 37°C, Cts1 is probably not involved in all aspects of calcineurin function. However, the possibility exists that Cts1 could have the potential to suppress the additional calcineurin mutant phenotypes if expressed at higher levels.
FIG. 1.
Overexpression of the CTS1 gene restores growth of calcineurin mutant strains at 37°C. Isogenic wild-type JEC34 (ura5) and calcineurin MCC3 (cna1 ura5) mutant strains were transformed with the rescued suppressor plasmid pCTS1 or the control plasmid pPm8 and grown on synthetic dextrose medium lacking uracil for 3 days at 25 or 37°C in the presence or absence of 1 μg of FK506/ml.
Disruption of the CTS1 gene by homologous recombination.
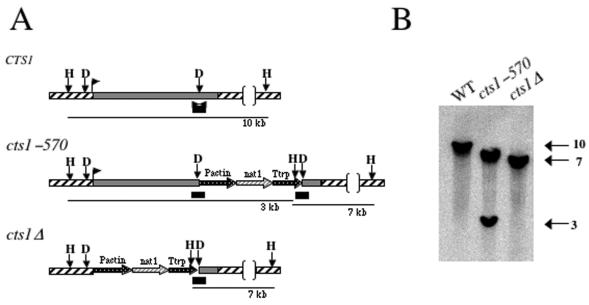
The CTS1 gene was disrupted by homologous recombination with a cassette containing the nourseothricin resistance gene nat1. The nat1 selectable marker cassette was either introduced into a single NdeI site at position 5146 (corresponding to amino acid 570) of CTS1 to generate the C-terminal truncation insertion allele cts1-570::nat1 or introduced between the NdeI sites at positions 2685 and 5146 within the coding region of CTS1 to generate the cts1Δ::nat1 deletion allele (Fig. 2A). The resulting truncation and deletion cassettes were introduced into the isogenic serotype D wild-type strains JEC20 (MATa) and JEC21 (MATα). Nourseothricin-resistant transformants were screened by colony PCR for the absence of the wild-type CTS1 gene, and 8 MATα cts1Δ (8 of 16, 50%), 11 MATa cts1Δ (11 of 22, 50%), 8 MATα cts1-570 (8 of 20, 40%), and four MATa cts1-570 (4 of 20, 20%) mutants were identified. Mutation of the CTS1 gene was verified by Southern blotting (Fig. 2B). In addition, each confirmed transformant was subjected to Northern analysis to verify that the CTS1 transcript was absent in cts1Δ mutant strains or truncated in the cts1-570 mutant strain (data not shown and Fig. 10).
FIG. 2.
Disruption of the CTS1 gene by homologous recombination. (A) The CTS1 gene was disrupted by the insertion of the dominant selectable marker nat1 (nourseothricin resistance) between two NdeI sites in the CTS1 gene, generating the cts1Δ::nat allele, while the cts1-570 truncation mutation was generated by introducing the nat cassette into the 3′ NdeI site corresponding to amino acid 570 of Cts1. The ORF of CTS1 is a solid box, the 5′ and 3′ untranslated regions are indicated by diagonal lines, and the transcription start site is marked by a bent arrow. The 300-bp bar denotes the region used as a probe in panel B. Restriction sites are HindIII (H) and NdeI (D). (B) Southern blot analysis of HindIII-digested genomic DNA from wild-type (WT; JEC21), cts1-570 (DSF22), and cts1Δ (DSF45) strains. Sizes in kilobases are indicated at right.
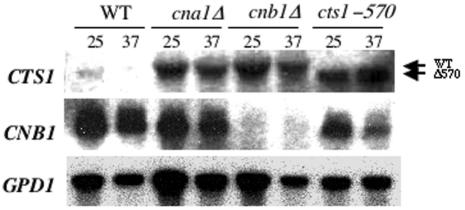
FIG. 10.
CTS1 mRNA expression is upregulated in calcineurin and cts1Δ570 mutants. Shown are results of Northern blot analysis of total RNA isolated from isogenic wild-type (JEC21) and cna1 (MCC3), cnb1 (DSF11), and cts1-570 (DSF22) mutant strains grown at 25 or 37°C for 4 h. Following hybridization with the CTS1 300-bp probe, the blot was stripped and reprobed with CNB1, followed by GPD1 as a control for loading and hybridization conditions. WT, wild type.
CTS1 is required for growth at elevated temperature.
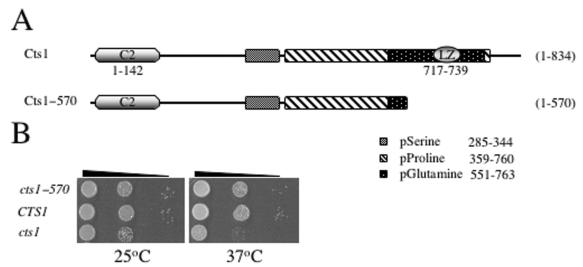
Both the catalytic (Cna1) and the regulatory (Cnb1) subunits of calcineurin are necessary for the growth of C. neoformans at 37°C (9, 13, 35). Because the multicopy expression of CTS1 was sufficient to suppress the temperature-sensitive growth defect of a calcineurin mutant, we reasoned that Cts1 might also be required for growth at 37°C. In addition, as leucine zipper motifs have been shown to provide essential functions in many membrane-associated proteins, we also sought to examine the importance of the leucine zipper region for Cts1 protein function (Fig. 3A). To address these questions, we examined the influence of the complete absence of functional Cts1, as well as the loss of the leucine zipper motif of Cts1, by testing the viability of both the cts1Δ and cts1-570 mutant strains at 25 and 37°C. The viability of the cts1Δ mutant was significantly reduced compared to that of the wild type at 37°C and moderately reduced for viability at 25°C (Fig. 3B). However, the cts1-570 truncation mutant lacking the C-terminal leucine zipper region was indistinguishable from wild type at either temperature (Fig. 3B). The inability of the cts1Δ mutant to grow at 37°C is the result of the loss of functional Cts1 because transformation of the cts1Δ strain with an episomal shuttle plasmid containing the CTS1 gene under the control of its endogenous promoter restored growth at 37°C (Fig. 8B and 9A). Thus, full-length Cts1, but not the leucine zipper region, is required for growth at 37°C.
FIG. 3.
Growth of cts1Δ mutants is temperature sensitive. (A) Diagram of the domain structure of Cts1 showing the amino-terminal C2 domain (C2); the serine, proline, or glutamine repeat regions (hatched); and the carboxy-terminal leucine zipper region (LZ). The amino acid positions for each repeat or domain are indicated. The cts1Δ allele completely disrupts the ORF of CTS1, resulting in the absence of Cts1 protein production, while the cts1-570::nat allele truncates 264 amino acids from the C terminus. (B) cts1Δ confers a growth defect. The isogenic wild-type (JEC21) and cts1Δ (DSF45) and cts1-570 (DSF22) mutant strains were serially diluted and grown on YPD medium for 3 days at 25 or 37°C.
FIG. 8.
The C2 domain of Cts1 is necessary for proper septation. (A) cts1Δ mutant strains overexpressing FLAG-Cts1 under the control of the CTS1 promoter in either sense or antisense orientation, Cts1ΔC2-FLAG, or Cts1ΔCMD-FLAG were grown to log phase at 25°C in YPD and visualized by DIC optics. (B) cts1Δ mutant strains overexpressing FLAG-CTS1 under the control of the CTS1 promoter in either sense or antisense orientation, Cts1ΔC2-FLAG, or Cts1ΔCMD-FLAG were serially diluted and grown on YPD medium for 3 days at 37°C in the presence of 1 μg of FK506/ml. (C) Domain structure of Cts1 showing the amino-terminal C2 domain, the putative CMD (hatched box), and the carboxy-terminal leucine zipper region. The position of the FLAG epitope is indicated by a filled octagon.
FIG. 9.
The C2 domain of Cts1 is necessary for growth at 37°C and binds phosphatidylinositol-derived phospholipids. (A) Isogenic wild-type (JEC21) and cts1Δ (DSF45) and cnb1Δ (DSF11) mutant strains overexpressing FLAG-Cts1 under the control of the CTS1 promoter in either sense or antisense orientation or Cts1ΔC2-FLAG were serially diluted and grown on YPD medium for 3 days at 37°C in the presence or absence of 1 μg of FK506/ml. (B) Purified FLAG-Cts1 and Cts1ΔC2-FLAG fusion proteins and the extract control were overlaid on the PIP array membrane. Bound protein was detected with a monoclonal anti-FLAG antibody. The input material for each binding assay was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride, and detected with anti-FLAG antibody. At the bottom of panel B is a table showing the relative density of input sample binding to either PI(4)P or PI(5)P expressed as the percent density of each sample relative to the FLAG-Cts1 sample.
CTS1 is necessary for virulence.
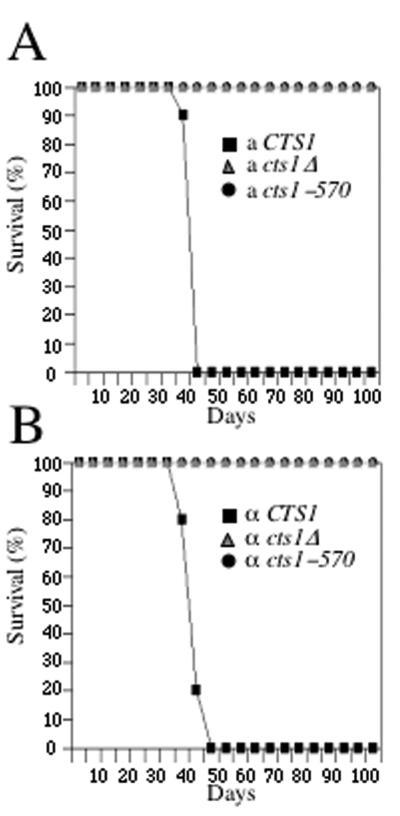
The ability to grow at elevated temperatures is essential for virulence of C. neoformans (13, 22, 35). Because the cts1Δ mutant is unable to grow at 37°C, we anticipated that Cts1 would be required for virulence. In contrast, because the cts1-570 mutant is viable at 37°C, this allele allowed us to test whether Cts1 plays a role in virulence beyond growth at 37°C. Wild-type, cts1Δ, and cts1-570 mutant strains were introduced by intranasal inoculation into mice lacking the C5 component of complement (DBA), and survival was monitored over a period of 105 days. Survival of mice infected with the wild-type strain was significantly reduced compared with that of mice inoculated with either the cts1Δ or cts1-570 mutant strain (Fig. 4). The a and α wild-type strains resulted in similar mortality values, with 50% mortality by day 40 and 100% mortality by day 42 to 46 (Fig. 4). In contrast, 100% of mice infected with either the cts1Δ mutant or the cts1-570 truncation mutant strain survived to day 105 irrespective of mating type, indicating that Cts1 and its leucine zipper region are required for virulence (Fig. 4). The ability to produce both melanin and a polysaccharide capsule is an importance virulence determinant for C. neoformans (15, 22, 23, 37). To determine whether the unanticipated loss of virulence in the cts1-570 mutant strain was due to a defect in either melanin or capsule production, the cts1Δ deletion and cts1-570 truncation mutant strains were examined. Interestingly, both strains were able to produce melanin and capsule at levels comparable to those of wild-type cells, suggesting that, in addition to calcineurin, Cts1 also plays an essential role in virulence that is independent of the ability to synthesize melanin or capsule components.
FIG. 4.
Cts1 is required for virulence of C. neoformans. Complement (C5)-deficient DBA mice were infected with 5 × 104 cells of the prototrophic wild-type a strain JEC20 (▪) or the congenic a cts1Δ (▴) or a cts1-570 (•) mutant (DSF42 or DSF20, respectively) (A) and the prototrophic wild-type α strain JEC21 (▪) or the congenic α cts1Δ (▴) or α cts1-570 (•) mutant (DSF45 or DSF22, respectively) (B). Mice were monitored daily for survival over 105 days, and the percentage of surviving mice was plotted versus time.
Cts1 is required for hyphal elongation.
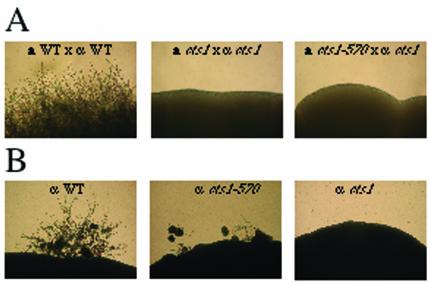
In addition to growth at 37°C, calcineurin is also required for hyphal elongation during mating and haploid fruiting in C. neoformans (7). To examine whether Cts1 also plays a role in mating, cts1Δ and cts1-570 mutant strains were analyzed for the ability to produce filaments. There were no defects in mating when MATα cts1Δ or MATa cts1Δ mutant strains were mixed with wild-type tester strains in a unilateral cross on mating (V8) medium, as numerous filaments and basidiospores were produced, typical of a wild-type mating reaction (data not shown). In contrast, when MATα cts1Δ and MATa cts1Δ mutant strains were cocultured in a bilateral cross, no filamentation was observed and no basidia or basidiospores were produced (Fig. 5A). These findings indicate that, in addition to calcineurin, Cts1 is also required for mating, as loss of Cts1 confers a bilateral mating defect. However, the carboxyl-terminal portion of Cts1, which contains the leucine zipper region, is not absolutely essential for mating, as filamentation and basidiospore production arise from a bilateral cross of cts1-570 mutants, although the filaments generated from this cross are abnormal (data not shown). We note that the leucine zipper region does become necessary for mating when the opposite mating partner lacks Cts1 entirely (Fig. 5A).
FIG. 5.
Cts1 is required for hyphal elongation. (A) Deletion of the CTS1 gene results in bilateral sterility. Isogenic a and α cts1Δ (DSF42 and DSF45), cts1-570 (DSF20 and DSF22), and wild-type (JEC20 and JEC21) strains were coincubated on nitrogen-limiting agar (V8) and photographed at 100× after incubation at 25°C for 7 days in the dark. (B) Cts1 is required for haploid fruiting. Isogenic wild-type (JEC21), cts1-570 (DSF22), and cts1Δ (DSF45) α strains were incubated on filamentation agar at 25°C for 14 days in the dark. WT, wild type.
In addition to a defect in mating, α cts1Δ mutant strains were also unable to undergo haploid fruiting, a differentiation process that occurs in α cells in response to nitrogen limitation and desiccation (Fig. 5B) (49). Haploid fruiting of the cts1-570 mutant strains lacking the leucine zipper region was reduced compared to that of wild type, but not abolished (Fig. 5B). These results demonstrate that Cts1 is involved in hyphal elongation, a process required for both mating and haploid fruiting. Thus, the carboxyl-terminal portion of Cts1 containing the leucine zipper region is important but not essential for hyphal elongation during mating and haploid fruiting.
Cts1 mutation confers a septation defect.
For the fission yeast S. pombe, calcineurin has been shown to play an important role in morphogenic processes including cytokinesis, septation, polarity acquisition, and mating (52). As mutations in either CTS1 or calcineurin confer defects in growth, virulence, and hyphal elongation in C. neoformans, we sought to examine whether Cts1 plays a role in morphogenesis. The wild-type, cts1Δ mutant, calcineurin (cnb1Δ) mutant, and cts1-570 mutant strains were grown to mid-log phase in YPD at 25°C and fixed and permeabilized. Fixed cells were then stained with phalloidin or WGA to visualize actin or chitin, respectively.
Wild-type cells treated with phalloidin revealed a pattern typical for C. neoformans, with punctate staining of cortical actin in the cytoplasm, as well as staining at the mother-bud neck and at the growing end of the emerging bud (data not shown) (18). Although there were minor differences between the actin staining pattern for the cnb1Δ, cts1Δ, or cts1-570 mutant cells and that for the wild type, the most striking difference was the increased frequency among the cts1Δ and cts1-570 mutant strains of cells that failed to complete separation, resulting in long chains of cells with some branching (data not shown).
Analysis of chitin staining with WGA revealed that, in contrast to wild-type cells or calcineurin-deficient cells, the cts1Δ and cts1-570 mutant cells fail to complete cell separation. Septal material that stained with WGA could be found at the mother-bud junction and at bud scars in wild-type cells (Fig. 6A). However, compared to wild-type cells, the accumulation of chitin at the mother-bud neck in both cts1Δ and cts1-570 mutant cells was quite striking (Fig. 6A). To determine the role of chitin accumulation in the failure of cts1 mutant cells to separate, we exposed cells to chitinase and examined the effects of chitin digestion on the morphology of cts1Δ mutant cells and found that exposure of cts1Δ mutant cells to chitinase resulted in the separation of cells at the mother-bud neck (Fig. 6B).
FIG. 6.
cts1Δ mutation confers a separation defect. (A) Isogenic wild-type (JEC21), cts1Δ mutant (DSF45), and cts1-570 mutant (DSF22) strains were grown to logarithmic phase at 25°C in YPD, fixed and permeabilized, stained with Alexa Fluor-conjugated WGA, and visualized by fluorescence and DIC optics. (B) Isogenic wild-type (JEC21) and cts1Δ mutant (DSF45) strains were grown to logarithmic phase at 25°C in YPD, washed, and incubated in PBS in the presence or absence of chitinase for 2 h prior to visualization by DIC optics. Magnification, ×1,000.
To examine the cell separation defect of the cts1Δ and cts1-570 mutants in greater detail, transmission electron microscopy was performed. Ultrastructural studies revealed that both the cts1Δ and cts1-570 mutant cells had thickened septa with frequent defects in septal formation and positioning (Fig. 7B to D). A more detailed examination of early septal formation in both wild-type and cts1Δ cells revealed that, while completed primary septa were observed in wild-type cells (Fig. 7E), the cts1Δ mutant cells did not form typical primary septa and instead appeared to close the neck by an abnormal advancement of septal material and gradual thickening (Fig. 7F). Neither the calcineurin-deficient cells nor wild-type cells exhibited any septal defects (Fig. 7A and data not shown). These observations indicate that Cts1, but not calcineurin, is required for proper septal positioning and formation.
FIG. 7.

Defective septal positioning and formation in cts1Δ and cts1-570 mutants. Transmission electron micrograph images of isogenic wild-type (JEC21) (A and E), cts1-570 (DSF22) (B and C), and cts1Δ (DSF45) (D and F) cells grown to logarithmic phase at 25°C in YPD and fixed in osmium tetroxide. Arrows indicate abnormally positioned and aberrant septa in the cts1Δ and cts1-570 mutant strains. Bars, 2 (A to D) and 0.1 (E and F) μm.
The C2 domain is essential for Cts1 function in vivo.
We next sought to examine the function of the C2 domain of the Cts1 protein, a candidate calcineurin effector. FLAG-tagged versions of the wild-type allele (FLAG::CTS1) and alleles in which the C2 domain (CTS1ΔC2::FLAG) or the putative CMD (CTS1ΔCMD::FLAG) was replaced with the FLAG epitope-encoding sequence were expressed under the control of the CTS1 promoter in the pPm8 shuttle plasmid in the temperature-sensitive cts1Δ and cnb1Δ strains (Fig. 8C). While the wild-type CTS1 and CTS1ΔCMD alleles restored growth at 37°C in the presence of the calcineurin inhibitor FK506 in both cts1Δ and cnb1Δ strains, neither the CTS1ΔC2 allele nor the antisense CTS1 promoter control construct restored growth in either cts1Δ or cnb1Δ strains (Fig. 8B and 9A). In addition, cts1Δ cells expressing the wild-type CTS1 and CTS1ΔCMD alleles were restored for normal septation, whereas cts1Δ cells expressing the CTS1ΔC2 allele or the antisense CTS1 promoter control were septation defective (Fig. 8A).
C2 domains have been shown to confer phospholipid-, inositol polyphosphate-, and protein-binding functions on many proteins involved in diverse cellular processes, including cytoskeletal organization, cell growth and gene expression, protein transport, signal transduction, and vesicular trafficking. To address the role of Cts1 in phospholipid binding, we characterized the PIP-binding capacity of the C2 domain of Cts1 by a protein-lipid overlay assay. FLAG-tagged Cts1 and Cts1ΔC2 fusion proteins were expressed in C. neoformans, purified by immunoaffinity anti-FLAG resin from C. neoformans protein extracts, and eluted from the resin. The FLAG-Cts1 and Cts1ΔC2-FLAG fusion proteins were each applied to a membrane-fixed PIP array in a protein-lipid overlay assay. The FLAG-Cts1 fusion protein bound phosphatidylinositol-5-phosphate [PI(5)P] and PI(4)P, whereas the Cts1ΔC2-FLAG fusion did not show significant binding, demonstrating that the C2 domain is essential for the phospholipid-binding activity of Cts1 (Fig. 9B). The binding of Cts1 was specific to PI(5)P and PI(4)P, and no binding to other phospholipids, including PI(3)P, PI(4,5)P2, and PI(3,4)P2 was detected (data not shown).
Taken together, these data provide compelling evidence that the C2 domain is important for the in vivo function of Cts1, as the C2 domain of Cts1 is required for growth at high temperature, septation, and phospholipid binding. In addition, deletion of the putative CMD of Cts1 had no effect on growth or septation.
Cts1 and calcineurin function in parallel pathways.
We next examined whether Cts1 and calcineurin function in the same or parallel pathways by genetic epistasis tests. The MATα cts1-570 mutant strain was crossed with a MATa cna1 mutant strain, and basidiospores were isolated. Although 13 meiotic recombinants were obtained from this cross, no viable cts1-570 cna1 double mutants were obtained, indicating that the cts1 mutation is synthetically lethal in combination with a calcineurin mutation (data not shown). Thus, calcineurin becomes essential at all growth temperatures in a strain lacking full-length Cts1, providing evidence that the two function in either parallel pathways or a branched pathway. In support of this conclusion, overexpression of Cts1 conferred resistance to FK506 in wild-type cells (Fig. 1 and 8B). Together, these results demonstrate that Cts1 can compensate for the loss of calcineurin function to permit growth at 37°C and that Cts1 and calcineurin interact genetically to maintain viability upon exposure to elevated temperatures. Additionally, these data indicate that the carboxyl terminus of Cts1, which contains the leucine zipper region, is not normally required for growth at elevated temperature but becomes necessary for growth in the absence of functional calcineurin.
To further study the functional relationship between calcineurin and Cts1, we examined the influence of calcineurin on the transcriptional regulation of the CTS1 gene. By Northern analysis, the CTS1 gene was transcribed at elevated levels in both cna1 and cnb1 mutant strains and in the cts1-570 truncation mutant at both 25 and 37°C but was transcribed at barely detectable levels in the wild type (Fig. 10). These results indicate that in the absence of calcineurin function, or in the absence of fully functional Cts1, the expression of the CTS1 mRNA is elevated.
We further addressed the functional relationship between calcineurin and Cts1 by in vitro protein binding assay analysis. Resin-immobilized FLAG-Cts1 was incubated with purified bovine calcineurin. No binding of Cts1 to calcineurin was detected in the presence or absence of Ca2+-calmodulin or Ca2+ (data not shown), suggesting that Cts1 and calcineurin do not physically interact. In addition, no interaction between Cts1 and either the catalytic (Cna1) or the regulatory (Cnb1) subunit of calcineurin from C. neoformans could be detected by yeast two-hybrid analysis or FLAG-Cts1 pull-down experiments with C. neoformans protein extracts (data not shown). Although the methods employed to detect possible interactions between Cts1 and calcineurin are suitable for the detection of a majority of protein-protein interactions, it is possible that these methods would not detect an interaction involving a bridging protein or protein complex. Therefore, we cannot definitively rule out the possibility that Cts1 and calcineurin physically interact.
DISCUSSION
The goal of this study was to identify calcineurin-dependent protein effectors through a multicopy suppressor approach. As a result of this screen, we isolated the calcineurin temperature suppressor gene, CTS1, which encodes a novel protein containing both a phospholipid-binding C2 domain and a leucine zipper motif. Overexpression of the CTS1 gene restored growth at elevated temperatures in a calcineurin-deficient strain and conferred resistance to the calcineurin inhibitor FK506 when the gene was overexpressed in wild-type cells. Disruption of the CTS1 gene conferred temperature-sensitive growth and inhibited virulence in the murine model of cryptococcosis, similar to the calcineurin mutant strains. In addition, the cts1Δ mutation conferred hypersensitivity to FK506, synthetic lethality with a calcineurin mutation, and a septation defect. Taken together, these data demonstrate that the novel C2 domain-containing protein Cts1 is essential for virulence of C. neoformans and performs essential functions that overlap with those of the Ca2+-calmodulin-activated serine/threonine phosphatase calcineurin in vivo.
Interestingly, we found that CTS1 expression is responsive to calcineurin activity, as the expression of CTS1 is induced in calcineurin-deficient strains. Although this result is suggestive of a potential derepression of CTS1 in the absence of functional calcineurin, a recent study involving a genome-wide analysis of calcineurin-dependent gene expression has found that calcineurin primarily directs the activation of gene expression rather than repression in Saccharomyces cerevisiae (53). However, this does not preclude the possibility that calcineurin could direct gene repression in C. neoformans. Therefore, although the induction of CTS1 expression could be the result of derepression due to a lack of functional calcineurin, a more plausible explanation fitting with our observations is that CTS1 expression is induced as part of a compensation mechanism in response to the lack of functional calcineurin.
Calcineurin plays a central role in many physiological processes in fungi and has been shown to be necessary for virulence in both C. neoformans and Candida albicans (reviewed in references 3, 4, 8, and 14). The functions of calcineurin have been extensively studied in two model fungi, the budding yeast, S. cerevisiae, and the fission yeast, S. pombe (1, 45-47). In S. cerevisiae, calcineurin controls gene expression necessary for cell wall biosynthesis, cation homeostasis, and morphogenesis via the regulation of the activity and localization of the calcineurin-responsive transcription factor Crz1 (5, 28, 30, 42, 43, 53). However, no Crz1 homolog is apparent in the C. neoformans 10× genome coverage of the serotype A or D C. neoformans strains.
In addition, several proteins that function coordinately with calcineurin to regulate cytokinesis, ion homeostasis, and septation in S. pombe have been identified (16, 26, 51, 54). Among these are two proteins, Its3 and Its10, which function in the regulation of cytokinesis and septation. Its3 is a homolog of the S. cerevisiae PI(4)P 5-kinase Mss4, which is localized to the plasma membrane and concentrated at the septum of dividing cells (10, 54). The its10 gene encodes a putative novel allele of the cdc7 gene, which encodes a serine/threonine protein kinase involved in the initiation of septum formation, and Its10 has been shown to function in a calcineurin-dependent manner (11, 26). Taken together, these findings support a central role for calcineurin in the regulation of cytokinesis and suggest that calcineurin may act to regulate multiple steps in cytokinesis, including formation of the actin ring at the mother-bud neck, septum formation, and cell separation.
We have identified Cts1, a suppressor of calcineurin temperature sensitivity that functions in septal positioning and septation in C. neoformans. By functional domain analysis, we have shown that the carboxyl-terminal leucine zipper region is not necessary for growth at 37°C but is required for virulence in the murine model of cryptococcosis, proper hyphal elongation, and viability in the absence of calcineurin function. Loss of this region also results in a defect in septal positioning and cell separation. Thus, the leucine zipper region is essential for several functions of Cts1. Although no obvious homologs of Cts1 have been identified, analysis of functional domains among proteins with similar functions identified a leucine-zipper-containing protein in S. pombe, Cdc14, which is also required for septum formation, likely via the mediation of protein-protein interactions with other septation proteins, including Cdc7 (12).
In addition to the leucine zipper motif, Cts1 also possesses an amino-terminal C2 domain that binds PI(4)P and PI(5)P. The Cts1 C2 domain shares homology with nonclassical Ca2+-independent protein kinase C isoforms that contain amino-terminal C2 domains (34, 38). C2 domains have been identified in many proteins involved in signal transduction, gene expression, and cytoskeletal organization and function to bind phospholipids in either a Ca2+-dependent or an independent manner. C2 domains facilitate protein-protein interactions and signal transduction membrane association (6, 21, 40).
We have shown that Cts1 and calcineurin function coordinately to regulate morphogenic events necessary for growth, cytokinesis, and filamentation. Cts1 and calcineurin are components of separate pathways with shared functions essential for growth at 37°C, virulence, and hyphal elongation. In addition, Cts1 is required for proper septal positioning and formation, and chains of cts1Δ cells can be separated by chitinase treatment, suggesting that Cts1 may promote the localization or assembly of protein complexes that carry out polarized chitin deposition or dissolution. Polarized chitin deposition is essential for proper septum biogenesis and septation in yeast and involves the actions of several proteins, including chitin synthases, chitinases, endoglucanases, and components of the contractile ring (39, 41, 48). Although Cts1 does not share homology with chitinases or endoglucanases known to be necessary for septum dissolution, recent studies have identified a role for lipid signaling in chitin deposition during septum biogenesis in S. cerevisiae (2, 27, 48). In these studies, the PIP phosphatase Sac1, an integral membrane protein that acts on PI(4)P, was found to be necessary for the proper sorting of the Chs3 chitin synthase from the septum to the trans-Golgi network (48). Therefore, Cts1 may regulate the localization of chitinases or endoglucanases to the septum to promote septal dissolution via interactions with PIPs and other proteins. Alternatively, Cts1 may be necessary for the proper localization of chitin synthesis machinery, wherein the lack of Cts1 leads to excessive accumulation of chitin at the septum. The ability of Cts1 to suppress the calcineurin defect when overexpressed supports a model in which Cts1 promotes the proper regulation of chitin synthase localization, as calcineurin mutants have severe cell wall and membrane integrity defects that could be suppressed by excess chitin deposition at the cell periphery (20). Future studies will address the role of Cts1 in the regulation of chitin biogenesis at the septum and cell periphery, as well as the role of Cts1 in chitin dissolution at the septum. In conclusion, the identification of the novel calcineurin temperature suppressor Cts1 has provided insights into the functions of the calcineurin signal transduction pathway in C. neoformans and may further our understanding of the roles of calcineurin in virulence and differentiation.
Acknowledgments
We thank Wandy Beatty at Washington University (St. Louis, Mo.) for performing the transmission electron microscopy experiments, James Fraser and Connie Nichols for comments and discussions, Fujisawa, Inc., for providing FK506, and members of the C. neoformans genome project at the Stanford Genome Technology Center and Nagasaki University for sequence data supported by the NIH (U01 AI47087).
This work was supported by NIAID (AIDS training grant) postdoctoral fellowship AI07392-10 (to D. Fox); NIAID R01 grants AI39115, AI42159, and AI50438 (to J. Heitman); and P01 award AI44975 from NIAID to the Duke University Mycology Research Unit. Gary Cox was supported by a Burroughs Wellcome New Investigator Award in Molecular Pathogenic Mycology. Joseph Heitman is a Burroughs Wellcome Scholar in Molecular Pathogenic Mycology and an associate investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Aramburu, J., A. Rao, and C. B. Klee. 2000. Calcineurin: from structure to function. Curr. Top. Cell Regul. 36:237-295. [DOI] [PubMed] [Google Scholar]
- 2.Baladron, V., S. Ufano, E. Duenas, A. B. Martin-Cuadrado, F. del Rey, and C. R. Vazquez de Aldana. 2002. Eng1p, an endo-1,3-β-glucanase localized at the daughter side of the septum, is involved in cell separation in Saccharomyces cerevisiae. Eukaryot. Cell 1:774-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blankenship, J. R., W. J. Steinbach, J. R. Perfect, and J. Heitman. 2003. Teaching old drugs new tricks: reincarnating immunosuppressants as antifungal drugs. Curr. Opin. Investig. Drugs 4:192-199. [PubMed] [Google Scholar]
- 4.Blankenship, J. R., F. L. Wormley, M. K. Boyce, W. A. Schell, S. G. Filler, J. R. Perfect, and J. Heitman. 2003. Calcineurin is essential for Candida albicans survival in serum and virulence. Eukaryot. Cell 2:422-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boustany, L. M., and M. S. Cyert. 2002. Calcineurin-dependent regulation of Crz1p nuclear export requires Msn5p and a conserved calcineurin docking site. Genes Dev. 16:608-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catz, S. D., J. L. Johnson, and B. M. Babior. 2002. The C2A domain of JFC1 binds to 3′-phosphorylated phosphoinositides and directs plasma membrane association in living cells. Proc. Natl. Acad. Sci. USA 99:11652-11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz, M. C., D. S. Fox, and J. Heitman. 2001. Calcineurin is required for hyphal elongation during mating and haploid fruiting in Cryptococcus neoformans. EMBO J. 20:1020-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz, M. C., A. L. Goldstein, J. R. Blankenship, M. Del Poeta, D. Davis, M. E. Cardenas, J. R. Perfect, J. H. McCusker, and J. Heitman. 2002. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 21:546-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz, M. C., R. A. Sia, M. Olson, G. M. Cox, and J. Heitman. 2000. Comparison of the roles of calcineurin in physiology and virulence in serotype D and serotype A strains of Cryptococcus neoformans. Infect Immun. 68:982-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desrivieres, S., F. T. Cooke, P. J. Parker, and M. N. Hall. 1998. MSS4, a phosphatidylinositol-4-phosphate 5-kinase required for organization of the actin cytoskeleton in Saccharomyces cerevisiae. J. Biol. Chem. 273:15787-15793. [DOI] [PubMed] [Google Scholar]
- 11.Fankhauser, C., and V. Simanis. 1994. The cdc7 protein kinase is a dosage dependent regulator of septum formation in fission yeast. EMBO J. 13:3011-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fankhauser, C., and V. Simanis. 1993. The Schizosaccharomyces pombe cdc14 gene is required for septum formation and can also inhibit nuclear division. Mol. Biol. Cell 4:531-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox, D. S., M. C. Cruz, R. A. Sia, H. Ke, G. M. Cox, M. E. Cardenas, and J. Heitman. 2001. Calcineurin regulatory subunit is essential for virulence and mediates interactions with FKBP12-FK506 in Cryptococcus neoformans. Mol. Microbiol. 39:835-849. [DOI] [PubMed] [Google Scholar]
- 14.Fox, D. S., and J. Heitman. 2002. Good fungi gone bad: the corruption of calcineurin. Bioessays 24:894-903. [DOI] [PubMed] [Google Scholar]
- 15.Fromtling, R. A., H. J. Shadomy, and E. S. Jacobson. 1982. Decreased virulence in stable, acapsular mutants of Cryptococcus neoformans. Mycopathologia 79:23-29. [DOI] [PubMed] [Google Scholar]
- 16.Fujita, M., R. Sugiura, Y. Lu, L. Xu, Y. Xia, H. Shuntoh, and T. Kuno. 2002. Genetic interaction between calcineurin and type 2 myosin and their involvement in the regulation of cytokinesis and chloride ion homeostasis in fission yeast. Genetics 161:971-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gustin, M. C., J. Albertyn, M. Alexander, and K. Davenport. 1998. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 62:1264-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopecka, M., M. Gabriel, K. Takeo, M. Yamaguchi, A. Svoboda, M. Ohkusu, K. Hata, and S. Yoshida. 2001. Microtubules and actin cytoskeleton in Cryptococcus neoformans compared with ascomycetous budding and fission yeasts. Eur. J. Cell Biol. 80:303-311. [DOI] [PubMed] [Google Scholar]
- 19.Kopecka, M., M. Yamaguchi, M. Gabriel, K. Takeo, and A. Svoboda. 2000. Morphological transitions during the cell division cycle of Cryptococcus neoformans as revealed by transmission electron microscopy of ultrathin sections and freeze substitution. Scr. Med. 73:369-380. [Google Scholar]
- 20.Kraus, P. R., D. S. Fox, G. M. Cox, and J. Heitman. 2003. The Cryptococcus neoformans MAP kinase Mpk1 regulates cell integrity in response to antifungal drugs and loss of calcineurin function. Mol. Microbiol. 48:1377-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulkarni, S., S. Das, C. D. Funk, D. Murray, and W. Cho. 2002. Molecular basis of the specific subcellular localization of the C2-like domain of 5-lipoxygenase. J. Biol. Chem. 277:13167-13174. [DOI] [PubMed] [Google Scholar]
- 22.Kwon-Chung, K. J., I. Polacheck, and T. J. Popkin. 1982. Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J. Bacteriol. 150:1414-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon-Chung, K. J., and J. C. Rhodes. 1986. Encapsulation and melanin formation as indicators of virulence in Cryptococcus neoformans. Infect. Immun. 51:218-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lengeler, K. B., R. C. Davidson, C. D'Souza, T. Harashima, W. C. Shen, P. Wang, X. Pan, M. Waugh, and J. Heitman. 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64:746-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, J., J. D. Farmer, Jr., W. S. Lane, J. Friedman, I. Weissman, and S. L. Schreiber. 1991. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66:807-815. [DOI] [PubMed] [Google Scholar]
- 26.Lu, Y., R. Sugiura, T. Yada, H. Cheng, S. O. Sio, H. Shuntoh, and T. Kuno. 2002. Calcineurin is implicated in the regulation of the septation initiation network in fission yeast. Genes Cells 7:1009-1019. [DOI] [PubMed] [Google Scholar]
- 27.Martin-Cuadrado, A. B., E. Duenas, M. Sipiczki, C. R. De Aldana, and F. Del Rey. 2003. The endo-beta-1,3-glucanase eng1p is required for dissolution of the primary septum during cell separation in Schizosaccharomyces pombe. J. Cell Sci. 116:1689-1698. [DOI] [PubMed] [Google Scholar]
- 28.Matheos, D. P., T. J. Kingsbury, U. S. Ahsan, and K. W. Cunningham. 1997. Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev. 11:3445-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDade, H. C., and G. M. Cox. 2001. A new dominant selectable marker for use in Cryptococcus neoformans. Med. Mycol. 39:151-154. [DOI] [PubMed] [Google Scholar]
- 30.Mendizabal, I., A. Pascual-Ahuir, R. Serrano, and I. F. de Larrinoa. 2001. Promoter sequences regulated by the calcineurin-activated transcription factor Crz1 in the yeast ENA1 gene. Mol. Genet. Genomics 265:801-811. [DOI] [PubMed] [Google Scholar]
- 31.Mendoza, I., F. J. Quintero, R. A. Bressan, P. M. Hasegawa, and J. M. Pardo. 1996. Activated calcineurin confers high tolerance to ion stress and alters the budding pattern and cell morphology of yeast cells. J. Biol. Chem. 271:23061-23067. [DOI] [PubMed] [Google Scholar]
- 32.Mondon, P., Y. C. Chang, A. Varma, and K. J. Kwon-Chung. 2000. A novel episomal shuttle vector for transformation of Cryptococcus neoformans with the ccdB gene as a positive selection marker in bacteria. FEMS Microbiol. Lett. 187:41-45. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura, T., T. Ohmoto, D. Hirata, E. Tsuchiya, and T. Miyakawa. 1996. Genetic evidence for the functional redundancy of the calcineurin- and Mpk1-mediated pathways in the regulation of cellular events important for growth in Saccharomyces cerevisiae. Mol. Gen. Genet. 251:211-219. [DOI] [PubMed] [Google Scholar]
- 34.Nalefski, E. A., and J. J. Falke. 1996. The C2 domain calcium-binding motif: structural and functional diversity. Protein Sci. 5:2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Odom, A., S. Muir, E. Lim, D. L. Toffaletti, J. Perfect, and J. Heitman. 1997. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 16:2576-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perfect, J. R., and G. M. Cox. 1999. Drug resistance in Cryptococcus neoformans. Drug Resist. Updates 2:259-269. [DOI] [PubMed] [Google Scholar]
- 37.Rhodes, J. C., I. Polacheck, and K. J. Kwon-Chung. 1982. Phenoloxidase activity and virulence in isogenic strains of Cryptococcus neoformans. Infect. Immun. 36:1175-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rizo, J., and T. C. Sudhof. 1998. C2-domains, structure and function of a universal Ca2+-binding domain. J. Biol. Chem. 273:15879-15882. [DOI] [PubMed] [Google Scholar]
- 39.Roncero, C. 2002. The genetic complexity of chitin synthesis in fungi. Curr. Genet. 41:367-378. [DOI] [PubMed] [Google Scholar]
- 40.Scheid, M. P., M. Huber, J. E. Damen, M. Hughes, V. Kang, P. Neilsen, G. D. Prestwich, G. Krystal, and V. Duronio. 2002. Phosphatidylinositol (3,4,5)P3 is essential but not sufficient for protein kinase B (PKB) activation; phosphatidylinositol (3,4)P2 is required for PKB phosphorylation at Ser-473: studies using cells from SH2-containing inositol-5-phosphatase knockout mice. J. Biol. Chem. 277:9027-9035. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt, M., B. Bowers, A. Varma, D. H. Roh, and E. Cabib. 2002. In budding yeast, contraction of the actomyosin ring and formation of the primary septum at cytokinesis depend on each other. J. Cell Sci. 115:293-302. [DOI] [PubMed] [Google Scholar]
- 42.Stathopoulos, A. M., and M. S. Cyert. 1997. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 11:3432-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stathopoulos-Gerontides, A., J. J. Guo, and M. S. Cyert. 1999. Yeast calcineurin regulates nuclear localization of the Crz1p transcription factor through dephosphorylation. Genes Dev. 13:798-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stephen, C., S. Lester, W. Black, M. Fyfe, and S. Raverty. 2002. Multispecies outbreak of cryptococcosis on southern Vancouver Island, British Columbia. Can. Vet. J. 43:792-794. [PMC free article] [PubMed] [Google Scholar]
- 45.Sugiura, R. 2002. Functional analysis of calcineurin-mediated signalling pathway using fission yeast as a model system. Nippon Yakurigaku Zasshi 119:155-161. [DOI] [PubMed] [Google Scholar]
- 46.Sugiura, R., T. Kuno, and H. Shuntoh. 1998. Calcineurin-mediated signal transduction pathways in yeast. Tanpakushitsu Kakusan Koso 43:1021-1028. [PubMed] [Google Scholar]
- 47.Sugiura, R., S. O. Sio, H. Shuntoh, and T. Kuno. 2002. Calcineurin phosphatase in signal transduction: lessons from fission yeast. Genes Cells 7:619-627. [DOI] [PubMed] [Google Scholar]
- 48.Tahirovic, S., M. Schorr, A. Then, J. Berger, H. Schwarz, and P. Mayinger. 2003. Role for lipid signaling and the cell integrity MAP kinase cascade in yeast septum biogenesis. Curr. Genet. 43:71-78. [DOI] [PubMed] [Google Scholar]
- 49.Wickes, B. L., M. E. Mayorga, U. Edman, and J. C. Edman. 1996. Dimorphism and haploid fruiting in Cryptococcus neoformans: association with the alpha-mating type. Proc. Natl. Acad. Sci. USA 93:7327-7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Widmann, C., S. Gibson, M. B. Jarpe, and G. L. Johnson. 1999. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol. Rev. 79:143-180. [DOI] [PubMed] [Google Scholar]
- 51.Yada, T., R. Sugiura, A. Kita, Y. Itoh, Y. Lu, Y. Hong, T. Kinoshita, H. Shuntoh, and T. Kuno. 2001. Its8, a fission yeast homolog of Mcd4 and Pig-n, is involved in GPI anchor synthesis and shares an essential function with calcineurin in cytokinesis. J. Biol. Chem. 276:13579-13586. [DOI] [PubMed] [Google Scholar]
- 52.Yoshida, T., T. Toda, and M. Yanagida. 1994. A calcineurin-like gene ppb1+ in fission yeast: mutant defects in cytokinesis, cell polarity, mating and spindle pole body positioning. J. Cell Sci. 107:1725-1735. [DOI] [PubMed] [Google Scholar]
- 53.Yoshimoto, H., K. Saltsman, A. P. Gasch, H. X. Li, N. Ogawa, D. Botstein, P. O. Brown, and M. S. Cyert. 2002. Genome-wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae. J. Biol. Chem. 277:31079-31088. [DOI] [PubMed] [Google Scholar]
- 54.Zhang, Y., R. Sugiura, Y. Lu, M. Asami, T. Maeda, T. Itoh, T. Takenawa, H. Shuntoh, and T. Kuno. 2000. Phosphatidylinositol 4-phosphate 5-kinase Its3 and calcineurin Ppb1 coordinately regulate cytokinesis in fission yeast. J. Biol. Chem. 275:35600-35606. [DOI] [PubMed] [Google Scholar]