Abstract
We show that a combination of the immunosuppressive drugs, vitamin D3 and Dexamethasone, induced human and mouse naive CD4+ T cells to differentiate in vitro into regulatory T cells. In contrast to the previously described in vitro derived CD4+ T cells, these cells produced only interleukin (IL)-10, but no IL-5 and interferon (IFN)-γ, and furthermore retained strong proliferative capacity. The development of these IL-10–producing cells was enhanced by neutralization of the T helper type 1 (Th1)- and Th2–inducing cytokines IL-4, IL-12, and IFN-γ. These immunosuppressive drugs also induced the development of IL-10–producing T cells in the absence of antigen-presenting cells, with IL-10 acting as a positive autocrine factor for these T cells. Furthermore, nuclear factor (NF)-κB and activator protein (AP)-1 activities were inhibited in the IL-10–producing cells described here as well as key transcription factors involved in Th1 and Th2 subset differentiation. The regulatory function of these in vitro generated IL-10–producing T cells was demonstrated by their ability to prevent central nervous system inflammation, when targeted to the site of inflammation, and this function was shown to be IL-10 dependent. Generating homogeneous populations of IL-10–producing T cells in vitro will thus facilitate the use of regulatory T cells in immunotherapy.
Keywords: experimental autoimmune encephalomyelitis, vitamin D3, Dexamethasone, T regulatory lymphocyte, autoimmunity
Introduction
CD4+ T cell subsets include Th1 cells producing IFN-γ which are important for the clearance of intracellular pathogens but also mediate autoimmune pathologies (1, 2), and Th2 cells producing IL-4, IL-5, and IL-13 which play a role in the clearance of extracellular pathogens but are also implicated in allergic manifestations (1–3). IL-10 was originally isolated from Th2 cells (4) and it has been suggested that a Th1 to Th2 switch may result in the amelioration of organ-specific Th1-mediated autoimmune diseases (1, 5). However, observations showing that Th2 cells may also give rise to immunopathology (6) supported the theory for the existence of other regulatory T cell subsets (7–11). Furthermore, IL-10 can be produced by a number of different cells including Th1 cells and by affecting APC function and dendritic cell (DC)* maturation (12–14) can inhibit both Th1 and Th2 type responses, suggesting an important negative feedback role for this cytokine (15, 16). In addition, a number of studies suggest that IL-10–producing T cells may be induced in the absence of Th1 and Th2 responses and are involved in establishing non responsiveness (15, 17–19). These regulatory T cells could thus be of potential use in therapeutic intervention during inflammatory pathology. For example, Bacchetta et al. in 1994 isolated CD4+ T cell clones producing IL-10, IL-5, and little to no IL-2, from SCID patients in an HLA-mismatched situation after successful bone marrow transplantation, and these cells were implicated for the lack of GVHD (17). Also, repetitive stimulation of T cells using immature DCs as APCs resulted in T cells producing IL-10 but no other inflammatory cytokines (18). However, the low proliferative capacity of these populations of IL-10–producing regulatory cells provides a limitation for their use in clinical therapy. Various studies have suggested that the timing and possibly the localization of IL-10 production may critically affect its immunoregulatory function (20). Thus, an efficient strategy to avoid such complications would be the administration of antigen-specific T cells that produce IL-10 only upon stimulation through the TCR. However, such an alternate strategy has been hindered so far by the difficulty to reproducibly obtain a homogeneous population of IL-10–producing T cells in large numbers.
The generation in vitro of a regulatory T cell subset (named Tr1) by stimulating naive CD4+ T cells in the presence of IL-10, or a combination of IL-4 and IL-10, has previously been reported (21). However, Tr1 cells not only produce IL-10 but in addition inflammatory cytokines such as IFN-γ and IL-5 which can mediate inflammatory pathologies (100% of the IL-10–producing cells also make IL-5 [21]). Moreover, generating these cells using this protocol has proved difficult, as these culture conditions also invariably induce significant numbers of Th1 and Th2 cells (our results and reference 22) which suggest that IL-10 is probably not sufficient by itself to induce a homogeneous population of Tr1 cells. Indeed Levings et al. have recently attempted to obtain more homogeneous populations of IL-10–producing T cells by use of IFN-α as a possible cofactor. This led to a population of T cells producing high levels of IL-10 but in addition IFN-γ (23). Taken together, these findings question the use of these regulatory cells in adoptive immunotherapy, as they may simultaneously exacerbate alternate inflammatory pathologies.
We postulated that a combination of immunosuppressive drugs, 1,25(OH)2-vitamin D3 (VitD3) and Dexamethasone (Dex), known to inhibit key transcription factors involved in the regulation of a number of inflammatory cytokines genes, would allow the development of a homogeneous population of IL-10–producing cells. VitD3 inhibits Th1-mediated autoimmune diseases including experimental autoimmune encephalomyelitis (EAE), a model for multiple sclerosis (24, 25), and is currently used topically to treat psoriasis in humans (26). VitD3 affects APCs as well as lymphocytes and inhibits the production of a wide variety of pro-inflammatory and subsequent Th1 responses (26–28). Glucocorticoids (GCs), including Dex, are among the most potent immunosuppressive and antiinflammatory drugs currently available and are efficacious in the treatment of both Th1- and Th2-associated inflammatory diseases including rheumatoid arthritis and asthma (29, 30). GCs inhibit both T cells and APCs at the level of proliferation and cytokine production (31–33). Recently, it has been shown that Dex enhanced IL-10 and reduced IL-4, IL-5, and IL-13 production in human CD4+ and CD8+ T cells (34). In addition, myelin basic protein (MBP)-specific Th2 cell lines generated in vitro in the presence of Dex plus IL-4 were shown to protect rats from EAE, although these cells lines would have undoubtedly contained Th2 cells (35).
Most importantly, VitD3 and Dex are known to inhibit the activation and action of important transcription factors involved in cytokine gene regulation, such as nuclear factor of activated T cells (NFAT), activator protein (AP)-1, and nuclear factor (NF)-κB (30, 36–38). The repression of IL-2 gene transcription by VitD3 occurs via the vitamin D receptor (VDR)-dependent inhibition of NFAT and AP-1 complex formation (39). It has also been described that VitD3 mediates downregulation of NF-κB p50 and c-Rel protein expression in T cells (38). Glucocorticoids have been shown to interfere with the function of transcription factors such as AP-1 and NF-κB via protein–protein interactions (30, 36), and in addition to inhibit IL-4 and possibly IL-2 gene expression by interfering with NFAT-dependent transactivation (37).
Here, we describe a novel strategy to induce the development of IL-10–producing regulatory T cells, using a combination of the immunosuppressive drugs VitD3 and Dex, which are known to impair cytokine gene regulation. In addition, we define factors that positively and negatively regulate their development, and provide novel findings regarding the possible molecular mechanisms for the physiological development of these IL-10–producing regulatory T cells.
Materials and Methods
Mice and Reagents.
DO11.10 mice transgenic for an ovalbumin323–339 specific TCRαβ (40) and DO11.10 mice crossed with recombination activating gene (RAG)−/− mice were used as a source of antigen specific T cells. CSJLF1/J mice were obtained from The Jackson Laboratory. BALB/cAnN were obtained from Taconic Farms. All mice were housed under specific pathogen-free conditions and were used between 6–12 wk of age. Monoclonal anti-cytokine antibodies used in culture were anti–IL-4 (clone 11B11), anti–IL-12 (clone C17.8.20, a gift of G. Trinchieri, The Wistar Institute, Philadelphia, PA) and anti–IFN-γ (XMG 1.1; DNAX; as referenced in reference 41). Anti–mouse CD3 and CD28 mAbs used for T cell stimulation were purchased from BD PharMingen. mAbs used for T cell preparation were anti-B220, anti-CD8α, anti–Mac-1, anti-CD4-FITC, and anti-L-selectin-PE (all mouse specific; BD PharMingen). Anti–IL-10R mAb (1B1.3) was as described (42) and contained undetectable levels of endotoxin.
Culture Conditions with Mouse T Cells.
Naive CD4+ L-selectinhigh T cells were prepared as described previously (41). Cultures were set up with 2.5 × 105 sorted naive T cells, 5 × 106 splenic APCs (γ-irradiated, 3,000 rads), and 0.6 μM OVA323–339 peptide. Naive T cells were also stimulated using coated anti-CD3 (10 μg/ml) and anti-CD28 (1 μg/ml) and restimulated as described (43). When added, VitD3 at 4 × 10−8 M (BIOMOL Research Labs) and Dex at 10−8 M (Sigma-Aldrich) were present during the entire stimulation period. After each round of stimulation, cells were stimulated with PMA (50 ng/ml) and ionomycin (500 ng/ml), and after 2 h Brefeldin A (10 μg/ml) was added and 2 h later the cells were harvested and fixed with 2% paraformaldehyde (Sigma-Aldrich). Cells were then permeabilized with 0.5% saponin and stained with the cytokine antibodies as described (41). T cells were also restimulated using PMA/ionomycin at 5 × 105 per ml and supernatants were harvested at 48 h for assessment of cytokine production by ELISA.
Culture Conditions with Human T Cells.
Human peripheral blood–derived CD4+ T lymphocytes from healthy donors were positively selected using antibody-conjugated Dynabeads and then negatively depleted of CD45RO+ cells to give a >98% CD4+CD45RA+ population. The cells were cultured with plate-bound anti-CD3, 2 μg/ml soluble anti-CD28 and 50 U/ml IL-2. Some groups also received 50 U/ml IL-4 (NBS), 5 × 10−8 M Dex (Sigma-Aldrich), 10−7 M VitD3, and 5 μg/ml each of anti–IL-4 and anti–IFN-γ and anti–IL-12. The cultures underwent four rounds of stimulation and were then restimulated with anti-CD3, anti-CD28, and IL-2 alone for 18 h for intracellular cytokine staining, with addition of 2 μM monensin 2 h after the initiation of the culture. Alternatively culture supernatants were harvested at 48 h and IL-4, IL-5, IL-10, and IFN-γ content analyzed by ELISA.
Real-time Quantitative PCR (TaqMan) Analysis.
RNA from the different T cell subsets was extracted using RNeasy Kit (QIAGEN) according to the manufacturer's protocol and reverse transcribed with oligo dT14–18 (Life Technologies) and random hexamer primers (Promega) using standard protocols. cDNA was analyzed for the expression of IL-10, T-bet, erm, GATA-3, c-maf, and ubiquitin by PCR assay using a PerkinElmer ABI Prism 5700 Sequence Detection System (PerkinElmer). Quantification of target gene expression was calculated by correcting the values relative to the expression of ubiquitin.
Preparation of Nuclear Extracts and EMSA for AP-1 and NF-κB.
Prior to the preparation of nuclear extracts, 1–2 × 107 T cells were incubated in cRPMI with 2% FCS for 5 h and then activated with cross-linked anti-CD3 for 2 h. Nuclear extracts were prepared as described (44). In brief, cells were pelleted and cell membranes then disrupted by brief (1 min) incubation in PSB buffer (50 mM HEPES, 100 mM NaF, 10 mM NaPPi, 2 mM NaVO4, and 4 mM EDTA) with 0.2% Nonidet P-40 (NP-40) (Boehringer) and 10 mM MgCl2. Cells were then washed in 0.05% NP-40 with sucrose and 10 mM MgCl2 and nuclei lysed in 0.1% NP-40 in PSB. Protease inhibitors (leupeptin and aprotinin; Sigma-Aldrich), and Pefabloc (Boehringer) were added to all solutions used. EMSA were performed for AP-1 (c-fos) and NF-κB (p65) according to the manufacturer's protocol (Geneka Biotechnology).
EAE Protocol.
Mouse spinal cord homogenate (MSCH) was prepared from 8–12-wk-old BALB/cAnN mice as described previously (45). For active induction of EAE, mice were immunized intradermally over three right flank sites with a total of 150 μl of a PBS-oil emulsion containing 4 mg of MSCH and 75 μg of heat killed Mycobacterium tuberculosis (strain H37RA; Difco). Immunized mice were challenged over three left flank sites with the same MSCH preparation at day 7. Pertussis toxin (100 ng; List Bio Lab) was intravenously injected at day 1 and 8. OVA (10 μg) was precipitated using alum and injected intracranially 4 d before immunization and 1–3 × 106 cells were intravenously injected 3 d before immunization with MSCH. As controls, some mice were injected with alum alone to assess the influence of the intracranial injection by itself on the outcome of the response. Alternatively, mice were injected intraperitoneally with OVA (10 μg or 1 mg) precipitated using alum, or not injected with OVA (controls). Anti–IL-10R mAb or isotype control mAbs (1 mg per mouse) were administered, 1 h before injection of the cells as well as once a week during the EAE experiment. In BALB/c mice the incidence of disease is 30–70% while in CSJLF1/J mice, it is 90–100% with a greater than 50% mortality rate within 1 wk after the onset of the disease (45). Clinical signs of EAE were scored as described previously (45). For histopathology, mice were killed by CO2 asphyxiation and spinal cords removed, fixed in 10% formalin and embedded in paraffin blocks. Sections were stained with hematoxylin and eosin for light microscopy as described.
Results
Vit/Dex Enhances the Development of IL-10–producing T Cells.
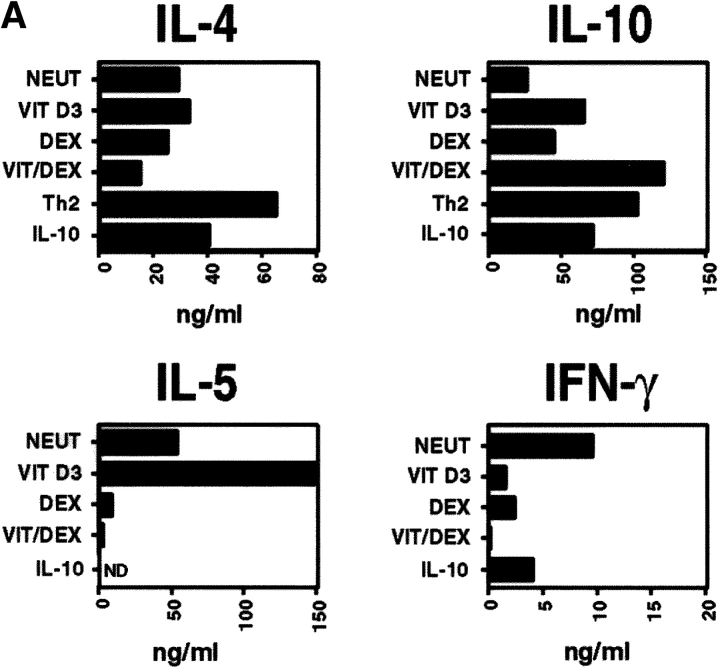
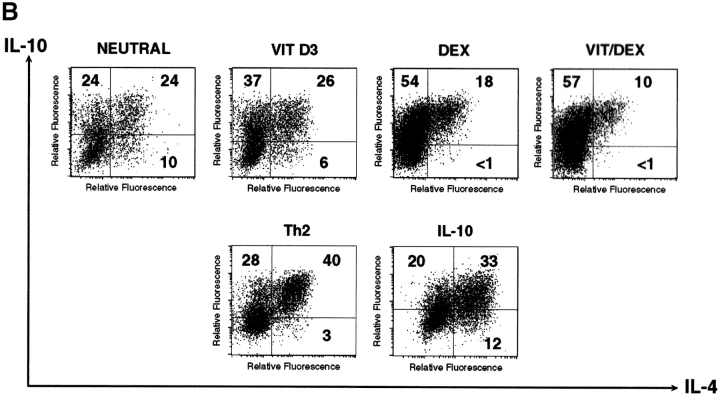
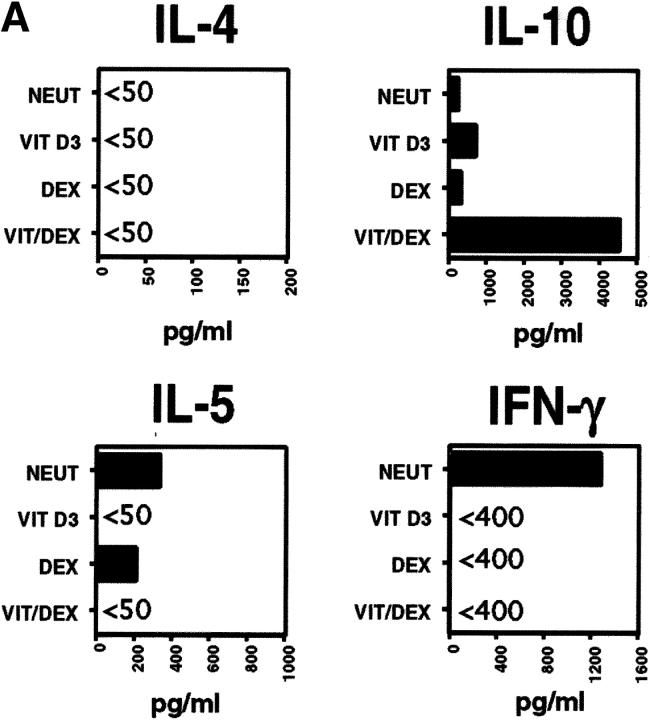
Naive CD4+ T cells were stimulated using APC and OVA in the presence of VitD3 and Dex in order to assess their role on T cell differentiation. VitD3 promoted the differentiation of naive OVA-specific TCR-transgenic CD4+ T cells, stimulated with antigen and APCs, into Th2 cells, enhancing IL-5 and IL-10 and reducing IFN-γ production relative to populations cultured with no additions (neutral condition; Fig. 1 A). Dex, in contrast, inhibited both IL-5 and IFN-γ production and slightly enhanced the production of IL-10 (Fig. 1 A). Neither of the drugs had a significant effect on IL-4 production. Using the two drugs in combination (Vit/Dex) led to the development of an increased number of IL-10–producing T cells and decreased numbers of IFN-γ, IL-4, and IL-5–producing cells (Fig. 1 B, and data not shown). This correlated with protein levels in the culture supernatants (Fig. 1 A). As already suggested in previous studies (22), addition of exogenous IL-10 (as originally described to generate Tr1 cells [21]) gave rise to heterogeneous populations of T cells containing IL-10–producing T cells as well as Th2 cells producing IL-10 and IL-4 (Fig. 1, A and B).
Figure 1.
VitD3/Dex enhances the development of IL-10–producing cells. Purified OVA-specific naive CD4+ T cells were activated using OVA323–339 peptide and APCs in the presence of VitD3 and/or Dex, IL-10 (10 ng/ml), or in neutral (10 ng/ml of IL-2) or Th2 conditions (10 ng/ml of IL-4 and 10 μg/ml of anti–IL-12 mAb). After three rounds of stimulation, cells were characterized for cytokine production by immunoassay (A) and by intracellular flow cytometric analysis (B). Identical results were obtained when T cells were isolated from DO11.10 RAG−/−. Representative results of more than five experiments are shown.
Inhibition of Th1 and Th2 Type Cytokines Enhances the Development of Vit/Dex-induced IL-10–producing T Cells.
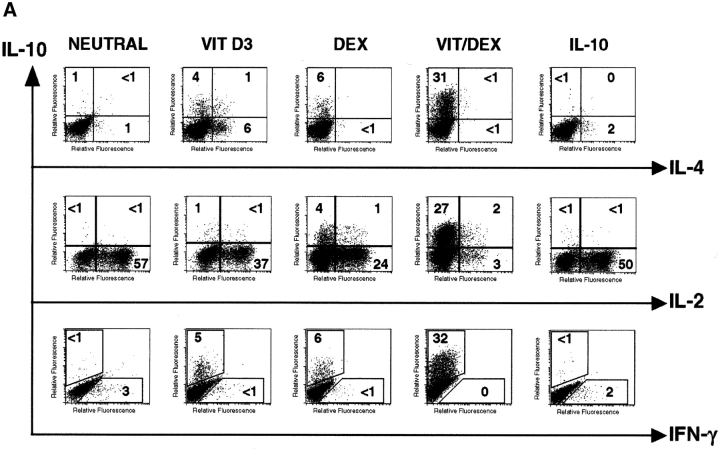
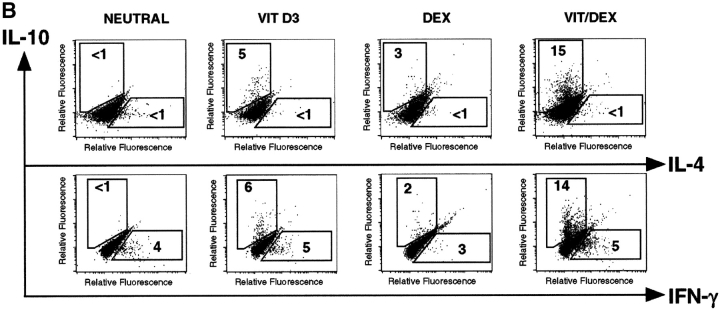
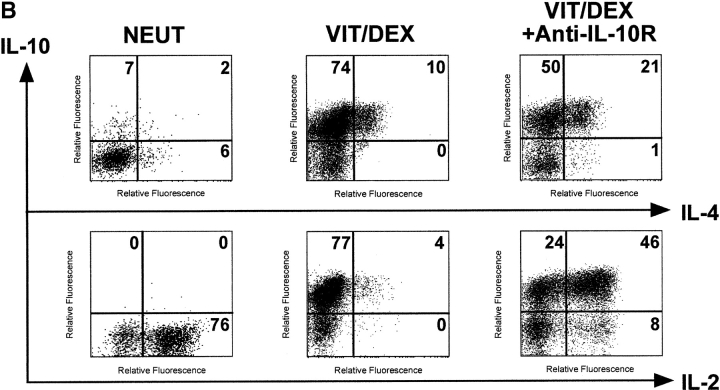
To determine whether Vit/Dex-derived T cells can develop independently of both Th1 and Th2 polarizing cytokines, we performed similar stimulations in the presence of neutralizing mAbs to IL-12, IFN-γ, and IL-4. Under these conditions, VitD3 and Dex synergized to give rise to a population of T cells producing IL-10 but no IL-4, IL-5, or IFN-γ, and low amounts of IL-2 (Fig. 2 A, and data not shown). The inhibition of both Th1 and Th2 polarizing cytokines was essential for this effect (data not shown). Absolute percentages of IL-10-producing cells as well as absolute amounts of IL-10 protein were decreased compared with those obtained in the absence of neutralizing mAbs (Fig. 1) which can be explained by the removal of IL-4–dependent IL-10–producing cells (including Th2 cells). Identical results were obtained when T cells were isolated from DO11.10 RAG−/−, which do not contain any CD4+CD25+ T cells. These results using the combination Vit/Dex plus neutralizing mAbs were unique and in contrast to cells cultured similarly but with VitD3 or Dex alone, where only very low numbers of cells produced IL-10. Strikingly, cells cultured with IL-10 as described previously (21), but here in the absence of IL-4, IL-12, and IFN-γ, lost their capacity to make IL-10 and resembled undifferentiated cells in that they mainly produced IL-2 (Fig. 2 A). Remarkably, inhibition of Th1- and Th2-polarizing cytokines enhanced the growth of IL-10–producing T cells generated with Vit/Dex. The expansion of the cells increased from 2–4-fold (Vit/Dex alone) to 15–25-fold (Vit/Dex plus anti–IL-12, IFN-γ, and IL-4 mAbs) after three rounds of stimulation. This suggests that IL-10–producing T cells can be controlled by Th1 and Th2 cells, and has enormous implications for the physiological interplay between these cells during an immune response.
Figure 2.
VitD3/Dex induces the development of T cells producing IL-10 only and no inflammatory cytokines upon neutralization of Th1 and Th2 polarizing cytokines. (A) Purified OVA-specific naive CD4+ T cells were activated under the same conditions as in Fig. 1; except that neutralizing anti–IL-12, anti–IFN-γ, and anti–IL-4 mAbs were also added. After three rounds of stimulation, cells were characterized for cytokine production by intracellular flow cytometric analysis. Identical results were obtained when T cells were isolated from DO11.10 RAG−/−. Representative results of more than five experiments are shown. (B) Purified naive CD4+ T cells from DO11.10 RAG−/− mice were activated using anti-CD3 and anti-CD28 stimulation under neutral or VitD3/Dex conditions. After three rounds of stimulation, cells were characterized for cytokine production by intracellular flow cytometric analysis. Identical results were obtained using DO11.10 or BALB/c mice. Representative results of three experiments are shown.
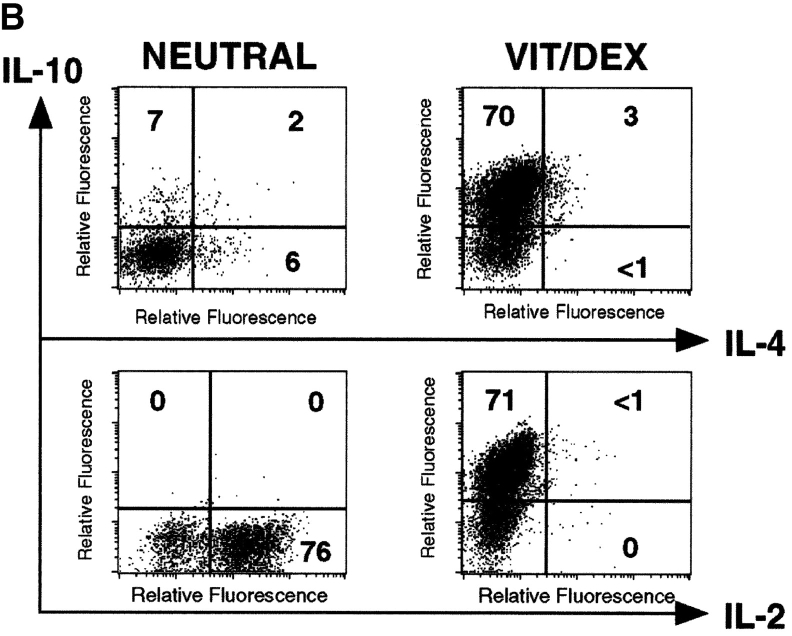
Vit/Dex Can Mediate Its Effect on T Cells in the Absence of APCs.
VitD3 and Dex have been reported to have a strong inhibitory effect on APCs (26–28, 30). Both drugs can indeed affect the maturation of DCs (28, 46), which might be decisive for the development of IL-10–producing T cells (18, 19). However, we show that Vit/Dex also induced the development of IL-10–producing T cells when naive CD4+ T cells were stimulated with anti-CD3 and anti-CD28 only (Fig. 2 B), in contrast to cells activated in the presence of Vit or Dex alone (data not shown). In fact, culture of T cells in the complete absence of APCs allowed the development of much higher numbers of IL-10–producing cells in response to Vit/Dex (increased from 33% with APCs, to 70% without APCs), and very low numbers of IL-4–producing cells (Fig. 2 B) or IFN-γ–producing cells (data not shown). This was a reproducible increase as compared with cultures containing APCs and was seen in all of four experiments. Again, identical results were obtained using either DO11.10 RAG−/−, DO11.10, or BALB/c mice
Vit/Dex Enhances the Development of Human IL-10–producing T Cells.
In view of the potential implications of our results regarding immunotherapy, conditions for generating IL-10–producing T cells in the mouse, using Vit/Dex and anti-CD3 and anti-CD28 stimulation in the complete absence of IL-4 and IFN-γ, were reproduced with human T cells. Naive human CD4+CD45RA+ T cells cultured under these conditions resulted in T cells producing high levels of IL-10 and no IL-4, IL-5, or IFN-γ (Fig. 3 A). This was also achieved in the presence of APC (data not shown). Similarly to our results using mouse T cells (Fig. 2 B), the combination of Vit/Dex led to an enhanced percentage of human IL-10–producing T cells, compared with the effects of Vit and Dex alone (Fig. 3 B). These cells did not produce any IL-4 or IFN-γ. The ability to generate human T cells producing IL-10 in the absence of Th1 or Th2-type cytokines, is a significant step toward their potential application in adoptive immunotherapy.
Figure 3.
VitD3/Dex induces the development of human T cells producing IL-10 and no IL-4, IL-5, or IFN-γ. Purified human CD4+CD45RA+ were stimulated with plate-bound anti-CD3, soluble anti-CD28, and IL-2 in the presence of neutralizing anti–IL-4, anti–IFN-γ, and anti–IL-12 mAbs. After four rounds of stimulation, cells were characterized for cytokine production by immunoassay (A) as well as by intracellular flow cytometric analysis (B). Representative results of four experiments are shown.
IL-10–producing T Cells Induced by Vit/Dex Can Prevent EAE.
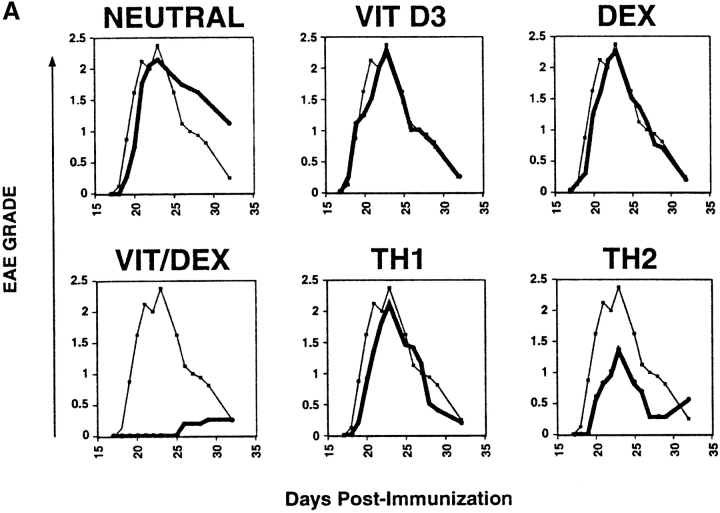
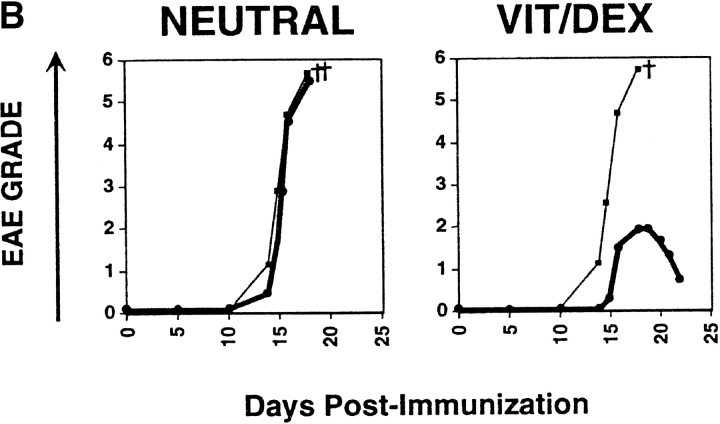
CD4+ T cells producing primarily IL-10 would be predictably potent inhibitors of inflammatory responses. However, before they could be considered regulatory T cells, it was necessary to prove this directly in vivo. To demonstrate the regulatory capacity of the Vit/Dex induced IL-10–producing T cells to inhibit inflammatory pathologies, OVA-specific DO11.10 T cells were transferred into mice 3 d before the induction of EAE with MSCH. These cells included Th1 and Th2 cells as well as cells derived under neutral conditions, in the presence of VitD3 alone, or Dex alone as controls, or T cells derived using the combination Vit/Dex, all in the presence of neutralizing mAbs to IL-12, IFN-γ, and IL-4. 1 d before the cell transfer, OVA adsorbed to Alum was injected intracranially to provide a reservoir of antigen for activating the regulatory T cell population. Only cells induced with the combination of Vit/Dex were able to prevent EAE with absolute abrogation of disease onset and absence of clinical signs, which is consistent to what was observed in vitro regarding cytokine production (Fig. 4 A). The EAE incidence was 30–70% in the BALB/c mice where disease was induced in the presence of, no cells, or T cells driven under neutral, Th1, Vit alone, or Dex alone, while in the mice treated with the Vit/Dex T cell group the incidence of EAE was <15% (with only 7% of the mice reaching over grade 1 of the disease; Table I). Th2 cells conferred incomplete and marginally significant protection (Fig. 4 A and Table I). Identical results were obtained when IL-10–producing regulatory T cells were generated with Vit/Dex obtained from DO11.10 RAG−/− mice, and furthermore when derived in the absence of APCs (data not shown). In a more severe model of EAE using a genetically susceptible strain derived from BALB/c × SJL/J (CSJLF1/J mice [45]), the IL-10–producing T regulatory cells induced by Vit/Dex completely prevented death and delayed the onset of the disease (Fig. 4 B). Furthermore, although in the CSJLF1/J mice, the incidence of EAE in the mice injected with Vit/Dex-derived IL-10–producing cells was 64% compared with >90% in the no cells group, importantly, only 21% of the mice reached over grade 3 with all the mice recovering from the disease, compared with control groups where >90% of the mice reached over grade 3 with accompanying mortality (Table I). In contrast, Th2 cells that gave minor protection using the BALB/c model did not confer any protection in the CSJLF1/J mice model (data not shown). In untreated CSJLF1/J mice, intense mononuclear infiltrates in the white matter of the spinal cord correlated with the clinical symptoms of EAE. In contrast, recipients of IL-10–producing T regulatory cells derived in the combination of Vit/Dex were EAE resistant and spinal cords showed reduced inflammatory infiltrates that were restricted to the perivascular spaces of the white matter (Fig. 4 C).
Figure 4.
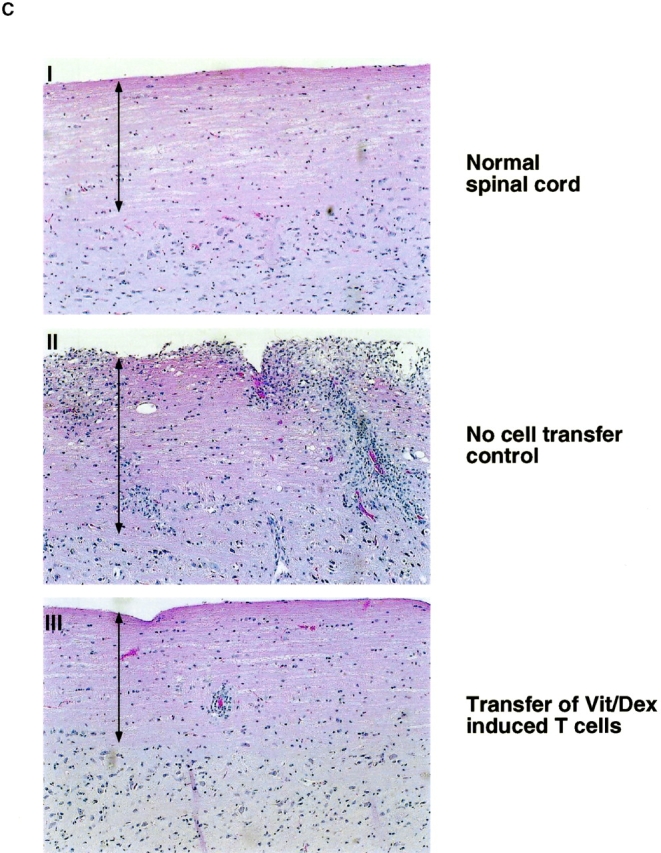
IL-10–producing T cells derived in VitD3/Dex prevent the induction of EAE: cells (1–3 × 106 cells per mouse) activated in the presence of neutralizing anti–IL-12, anti–IFN-γ, and anti–IL-4 mAbs as described in Fig. 2 with a combination of VitD3/Dex, in the presence of VitD3 or Dex alone, or as controls under neutral, Th1, or Th2 conditions were injected into BALB/c (A) or CSJLF1/J mice (B and C) as indicated on the plots, 24 h after intracranial injection of OVA/Alum. EAE was then induced as described (thin line, no cells: thick line, cells developed in vitro as described). The experiments each were performed using 5–10 mice per group and all mice included in the groups were used in the analysis. One representative experiment of five is shown (so 40–50 mice per group in total were analyzed in the BALB/c, 25–35 in the CSJLF1/J mice) although the total number of experiments is presented in Table I. (C) Sections of spinal cord were stained with hematoxylin and eosin for light microscopy as described (reference 45). Arrows indicate the white matter of the spinal cord. Original magnification was 100×.
Table I.
Incidence of EAE in both BALB/c and CSJLF1/J Mice
| BALB/c mice
|
CSJLF1/J mice
|
|||
|---|---|---|---|---|
| Disease incidence |
No. of mice over grade 1 |
Disease incidence |
No. of mice over grade 3 |
|
| No cells | 31/50 (62%) | 28/50 (56%) | 30/32 (94%) | 29/32 (91%) |
| Neut | 24/40 (60%) | 22/40 (55%) | 28/30 (93%) | 28/30 (93%) |
| VitD3 | 24/45 (53%) | 22/45 (49%) | ||
| Dex | 27/45 (60%) | 25/45 (56%) | ||
| Vit/Dex | 6/45a (13%) | 3/45a (7%) | 18/28b (64%) | 6/28a (21%) |
| Th1 | 27/40 (68%) | 26/40 (65%) | ||
| Th2 | 22/40 (55%) | 15/40c (38%) | ||
Statistical analyses were performed using the χ2 method.
Difference with no cells group is statistically significant (P < 0.0001).
Difference with no cells group is statistically significant (P < 0.01).
Difference with no cells group is statistically marginally significant (P = 0.0932).
IL-10 Is a Positive Autocrine Factor that Acts Directly on the T Cell in the Absence of APCs to Enhance the Development of IL-10–producing T Cells.
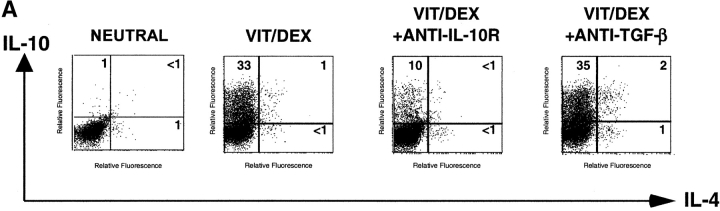
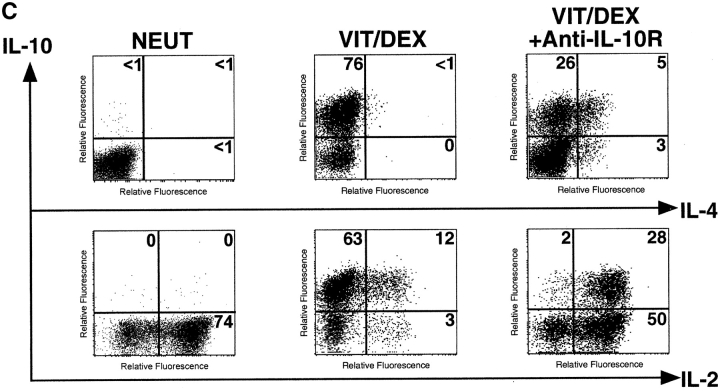
To understand the mechanisms underlying the Vit/Dex-induced development of these IL-10–producing T cells, we assessed the role of IL-10 and TGF-β in this process. Using the antigen and APC stimulation cultures of DO11.10 T cells as described earlier in Fig. 2 A, we demonstrated that the differentiation of IL-10–producing T cells was significantly abrogated when a neutralizing anti–IL-10R mAb, but not an anti–TGF-β mAb, was simultaneously added to the culture (Fig. 5 A). No extra additive effect was observed when both neutralizing anti–IL-10R and anti–TGF-β mAbs were present in the culture (data not shown). Furthermore, the addition of anti–IL-10R did not have any effect on the cells driven in neutral conditions in the presence of the neutralizing anti–IL-4, IL-12, and IFN-γ mAbs (data not shown). As IL-10 has a strong effect on APCs, we wished to determine whether its effect was solely APC mediated. Similar experiments using the APC-free system, where T cells were stimulated with anti-CD3 plus anti-CD28, showed that neutralization of IL-10 significantly inhibited the development of the IL-10–producing cells in response to Vit/Dex from 74 to 50% (anti-CD3/anti-CD28 only; Fig. 5 B) and from 76 to 26% (anti-CD3/anti-CD28 plus neutralizing anti–IFN-γ and anti–IL-4 mAbs) of single IL-10–producing T cells (Fig. 5 C). Furthermore, the addition of anti–IL-10R in the culture increased the number of IL-4–producing cells (from 10 to 22%, Fig. 5 B; and from 0 to 8%, Fig. 5 C) and of IL-2 producing cells (from 4 to 54%, Fig. 5 B; and from 15 to 78%, Fig. 5 C). These results demonstrate for the first time that IL-10 is a positive autocrine factor that acts directly on the T cell in the absence of APCs to enhance the development of IL-10–producing T cells, which is in contrast to its negative feedback role in the development of both Th1 and Th2 responses (20). However, as IL-10–producing T cells only developed when stimulated in the presence of Vit/Dex, this shows clearly that IL-10 is required but not sufficient for this process.
Figure 5.
IL-10 is a positive autocrine factor to enhance the development of IL-10–producing T cells induced by Vit/Dex. (A) Cells were activated as in Fig. 2 in the combination of Vit/Dex, plus neutralizing anti–IL-12, anti–IFN-γ, and anti–IL-4 mAbs in the absence or presence of either anti–IL-10R (10 μg/ml) or anti–TGF-β (10 μg/ml). Additionally, T cells were stimulated using anti-CD3 and anti-CD28 with Vit/Dex in the absence (B) or presence of neutralizing anti–IFN-γ and anti–IL-4 mAbs (C), in the presence or absence of anti–IL-10R mAbs. After three rounds of stimulation, cells were characterized for cytokine production by intracellular flow cytometric analysis. Representative results of five experiments are shown.
Prevention of EAE by IL-10–producing T Cells Is Abrogated by Administration of Anti–IL-10R mAbs In Vivo and Requires Antigenic Stimulation in the Central Nervous System.
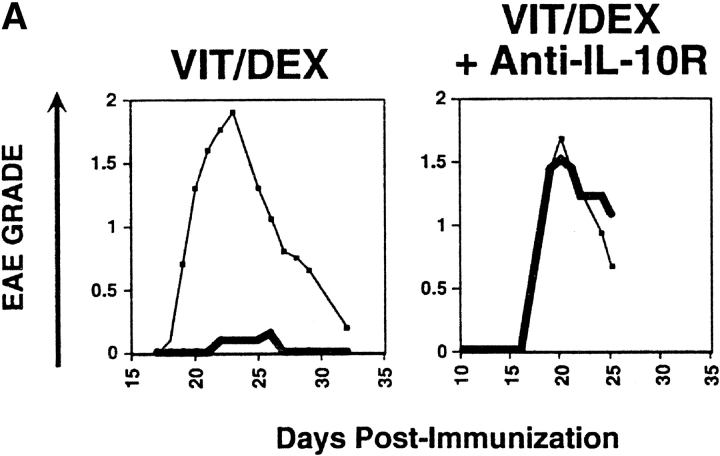
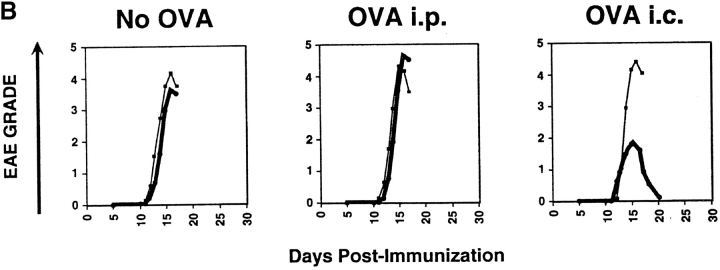
The regulatory function of these IL-10–producing cells was completely abrogated when a neutralizing anti-IL10R mAb (1 mg per mouse) was simultaneously administered in vivo at the time of T cell transfer (Fig. 6 A), demonstrating that IL-10 plays a crucial role for the function as well as the development of these regulatory T cells. Moreover, the prevention from EAE by the Vit/Dex induced IL-10–producing T cells did not occur in mice that received alum intracranially in the absence of OVA, demonstrating that these cells require antigenic stimulation in order to function (Fig. 6 B). Furthermore, if OVA (10 μg per mouse) was delivered intraperitoneally this was not sufficient to induce the action of the IL-10–producing regulatory T cells. This dose of OVA was chosen since it is sufficient to prime T cells in the periphery but on the other hand would be at too low a dose to penetrate the central nervous system (CNS). Under these conditions the IL-10–producing cells failed to inhibit EAE, in contrast to when OVA was delivered intracranially where full protection was observed (Fig. 6 B). However, experiments were also performed with a higher dose of OVA (1 mg per mouse) delivered intraperitoneally and under these conditions, the IL-10–producing regulatory T cells were still unable to prevent the disease (data not shown). Taken altogether, this suggests that these cells have to be activated to produce IL-10 at the site of inflammation in order to manifest their regulatory function.
Figure 6.
Prevention of EAE by IL-10–producing T cells derived in VitD3/Dex is abrogated by administration of anti-IL-10R mAbs in vivo and requires antigenic stimulation in the CNS. (A) Cells activated in the presence of neutralizing anti–IL-12, anti–IFN-γ, and anti–IL-4 mAbs as described in Fig. 2 with a combination of VitD3/Dex were injected into BALB/c mice 24 h after intracranial injection of OVA (thin line, no cells: thick line, cells). Anti–IL-10R mAbs or isotype control (1 mg per mouse) was delivered to the mice 1 h before administration of regulatory T cells as well as once a week during the course of the experiment. (B) Alternatively, cells activated with a combination of VitD3/Dex also were injected into CSJLF1/J mice, after intracranial injection of Alum alone (No OVA, left panel); after injection of OVA, intraperitoneally (i.p.; 10 μg in Alum, middle panel) or after intracranial (i.c.) injection of OVA (right panel). As control, OVA was also injected intraperitoneally at a higher dose (1 mg in Alum) but the experiments gave similar results. The experiments each were performed using 5–10 mice per group and all mice included in the groups were used in the analysis. One representative experiment of three is shown.
Transcription Factors Involved in Th1 and Th2 Differentiation Are Downregulated in IL-10–producing T Cells.
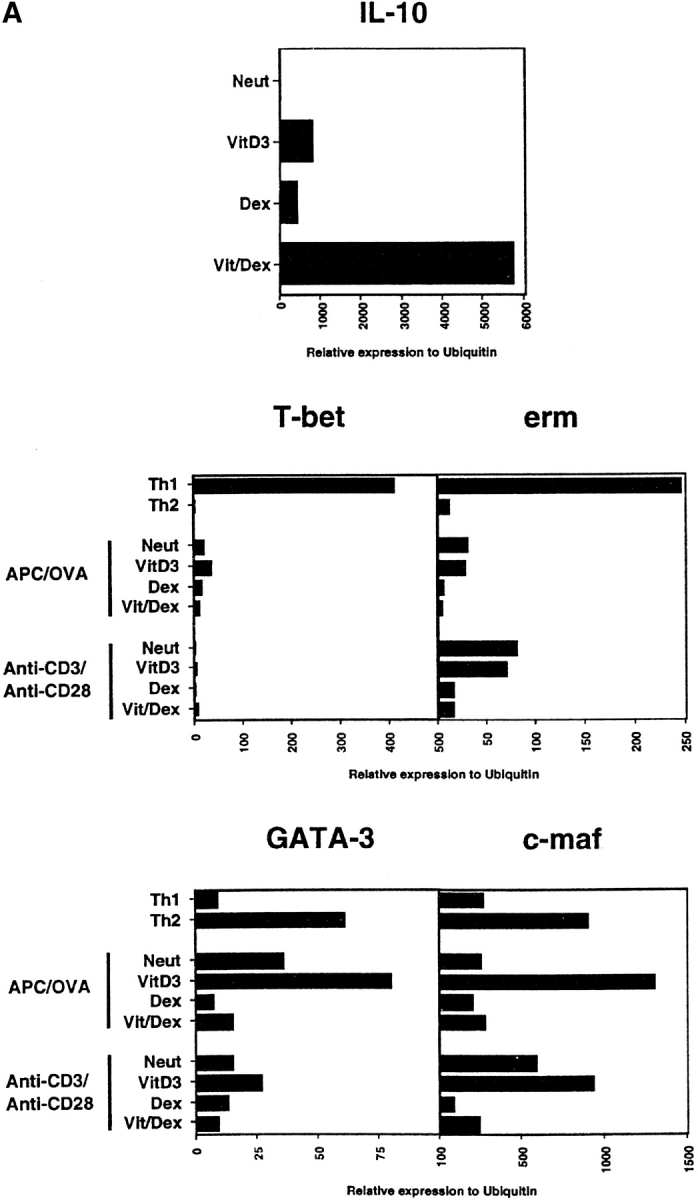
Key transcription factors have been associated with the differentiation of Th1 and Th2 cell subsets (47, 48). Indeed, it has been shown that erm was expressed only in Th1 cells and is induced by IL-12 in a Stat4-dependent manner, but its function is as yet unknown (49). Also, T-bet was shown to be restricted to Th1 cells and it has been shown that ectopic expression of T-bet both transactivates the IFN-γ gene and induces endogenous IFN-γ production (43). Moreover, introducing T-bet into polarized Th2 cells could redirect them toward a Th1 phenotype producing IFN-γ and low amounts of IL-4 (43). Similarly, c-maf and GATA-3 are selectively expressed in Th2 cells. C-maf can direct the expression IL-4 and plays an important role in Th2 differentiation (50), while GATA-3 has been shown to induce Th2 cytokines when introduced into developing Th1 conditions demonstrating its predominant role in Th2 differentiation (41, 51–53). We thus assessed the transcription level of T-bet, erm, GATA-3, and c-maf in the population of cells driven either using OVA and APCs (as in Fig. 2 A) or using anti-CD3 and anti-CD28 (as in Fig. 5 B), both in the presence of neutralizing anti–IFN-γ, anti–IL-12, and anti–IL-4 mAbs. In accordance with the previous published findings (41–43, 50–53), the controls in our system showed identical profiles of reciprocal expression of erm and T-bet versus c-maf and GATA-3 in Th1 and Th2 cells, respectively (Fig. 7 A). In contrast, these transcription factors were all downregulated in the IL-10–producing Vit/Dex-induced T cells showing that the exclusive production of IL-10 in this population is not resulting from a specific upregulation of one of these transcription factors (Fig. 7 A).
Figure 7.
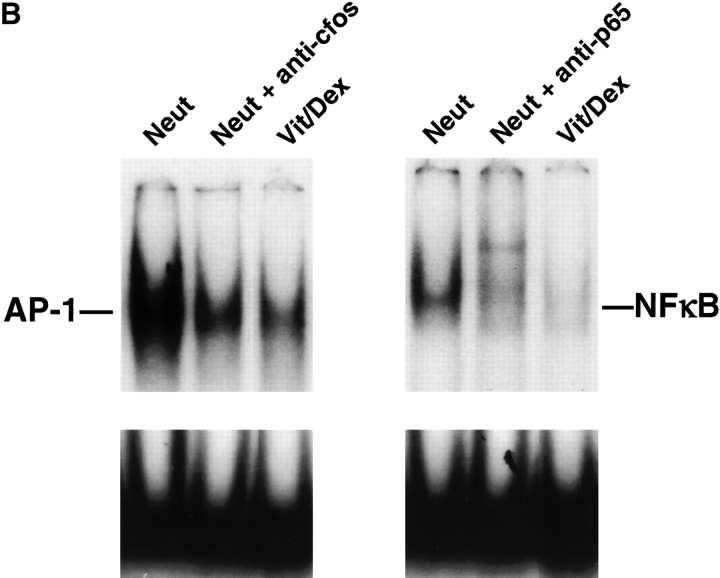
Transcription factors involved in Th1 and Th2 differentiation are downregulated in IL-10–producing T cells. (A) Expression level of T-bet, erm, GATA-3, and c-maf was assessed by Taqman in T cells developed under neutral (Neut), VitD3 alone, Dex alone, and Vit/Dex in the presence of neutralizing antibodies for IL-4, IL-12, and IFN-γ using APC/OVA stimulation as described in Fig. 2 A or anti-CD3/anti-CD28 as described in Fig. 5 C. (B) NF-κB and AP-1 activities were evaluated using 1–2 μg of nuclear extracts from cells developed under neutral conditions or Vit/Dex as described in Fig. 2 A. One representative experiment of three is shown.
As the combination of Vit/Dex led to enhanced IL-10 gene expression and inhibition of the Th1 and Th2 specific transcription factors (Fig. 7 A), we then determined the effect of the drug combination on NF-κB and AP-1 activity, as both VitD3 and Dex has been individually shown to affect these transcription factors. We demonstrated that NF-κB and AP-1 activities are both inhibited in Vit/Dex induced regulatory cells as shown by EMSA when compared with cells driven under neutral conditions (Fig. 7 B). Further studies will of course be necessary to assess which transcription factors are crucial for the generation of regulatory cells. However, our study provides for the first time the concept that IL-10–producing regulatory T cells must arise in vivo under conditions where the engagement of the signaling pathways required for Th1 and Th2 development is minimal.
Discussion
IL-10–producing T cells distinct from Th1 and Th2 cells have been described that undoubtedly play a role in regulating inflammatory pathologies, but factors determining their development are unknown. We report in this study the isolation of such a population of CD4+ T cells, that produce IL-10 and no IL-4, IL-5, or IFN-γ, which was able to prevent autoimmune disease when adoptively transferred in two mouse models of central nervous inflammation. This population was only induced when the immunosuppressive drugs VitD3 and Dex were used in combination, together with stimulation of the cells through their TCR, while using these drugs individually did not lead to the generation of such regulatory cells. We also showed that these IL-10–producing cells can develop independently of Th1 and Th2 polarizing cytokines which conversely were shown to inhibit the development of these regulatory T cells in vitro. Although not sufficient for the development of regulatory T cells, endogenous IL-10 delivered a positive signal to these IL-10–producing T cells during their generation with Vit/Dex. Furthermore, our data suggests that the development of such regulatory T cells occurs under conditions where the expression and/or activation of key transcription factors involved in Th1 and Th2 differentiation as well as NF-κB and AP-1 is minimal.
A likely in vivo counterpart of these regulatory T cells are the allospecific IL-10–producing T cells which have been suggested to account for tolerance in patients with SCID transplanted with HLA-mismatched hematopoietic stem cells (17). Other studies have shown that chronic antigenic stimulation in vivo can lead to the generation of IL-10–producing T cells and suggest how these cells may play a physiological role in regulating undesirable immune responses (54, 55). Thus, this type of IL-10–producing regulatory T cell population may arise from chronic encounter of self-antigens on subsets of specialized APCs e.g., DCs, resident in particular organs or particular stages of maturity (18, 19), and in the absence or low activation of the innate immune response normally required for the generation of inflammatory responses required to eradicate pathogens (56). In addition, on a functional basis, the mouse CD4+ CD45RBlow T cells have been shown to act via IL-10 to inhibit inflammatory bowel disease (IBD) and this heterogeneous population is likely to contain such IL-10–producing cells but in addition also Th2 cells (5, 9, 57). Although much evidence supports the existence of regulatory T cells in vivo, the signals required for their development are still largely unknown. It was recently reported by Jonuleit et al. that immature DCs can stimulate T cells toward a phenotype of IL-10 producers with low proliferative capacity (18). Interestingly, both VitD3 and Dex have been shown to affect DC maturation (28, 46) which may account for the generation of regulatory T cells in our study. It is possible that endogenous levels of both VitD3 and glucocorticoids play a role in dampening immune responses to self-antigens during development thus avoiding the development of pathology inducing autoreactive T cells. Macrophages have been shown to synthesize VitD3 upon activation with LPS and IFN-γ (58), suggesting that upon triggering of APCs by microbial factors VitD3 is synthesized by these cells and thus may regulate the production of inflammatory mediators. Furthermore, glucocorticoids released from the adrenal glands in response to stress can have profound effects on the immune system (59). In addition, it has been demonstrated that genetic variation in the magnitude of such stress responses and the release of glucocorticoids can determine susceptibility to an experimental autoimmune disease (59, 60). Other immunosuppressive drugs may also favor regulatory cells development in this in vitro–driven situation (61).
The Tr1 cells that were derived in vitro in the presence of exogenous IL-10 or in the presence of IL-4 with IL-10 (21) present similarities but also have numerous differences with the single IL-10–producing cells derived in our study with VitD3 and Dex. First of all, Tr1 cells not only produce IL-10 but in addition a large amount of inflammatory cytokines such as IL-5 and IFN-γ (21). To support this, Levings et al. recently defined human Tr1 cells as IL-10 and IFN-γ positive cells, and their use of IFN-α as a possible cofactor to enhance the development of these cells, suggested that IL-10 is not sufficient to generate human IL-10–producing T cells (23). This is not the case with cells derived with VitD3/Dex described herein, which produce only IL-10 and no IFN-γ, IL-4, or IL-5 when cultured in the presence of anti–IL-4, anti–IL-12, and anti–IFN-γ (Fig. 2 A). Second, as shown in Fig. 1 and as already suggested in other studies (22), addition of exogenous IL-10 during antigenic stimulation led to heterogeneous populations of T cells producing IL-4 and thus resembling Th2 cells. Furthermore, culture of T cells in the presence of IL-10 but in the absence of IL-4 generates cells which produce no IL-10 (Fig. 2 A, and data not shown using IL-4 KO mice). This is totally in contrast with the IL-10–producing T cells driven in our study with Vit/Dex which produce IL-10 and no IL-4, IL-5, or IFN-γ, and furthermore whose development does not require, but can be inhibited by IL-4 (Fig. 2). Most importantly, the cells derived in our study with Vit/Dex in the absence of Th1 and Th2 polarizing cytokines proliferate, in contrast to the Tr1 cells which do not. The fact that Th1 and Th2 polarizing cytokines downregulated the number and purity of IL-10–producing regulatory T cells resulting from culture in Vit/Dex provides a novel concept regarding the control of regulatory T cells by Th1 and Th2 cells, and has significant implications for the physiological interplay between these cells during an immune response.
We have obtained IL-10–producing cells using a combination of two drugs, Vitamin D3 and Dexamethasone (Vit/Dex). Interestingly, despite their well documented role in inducing cytokine genes in both Th1 and Th2 subsets, none of the T-bet, erm, GATA-3, or c-maf genes appeared responsible for the specific activation of the IL-10 gene in the Vit/Dex-induced cells, as their expression was inhibited in T cells induced with Vit/Dex while IL-10 mRNA expression is dramatically induced in these cells. These results question whether one or few specific transcription factors are specific for the induction in vivo of such a population of regulatory cells. The use of the IL-10–producing cells derived in our study will of course be of advantage in order to answer this question. We also show herein that signaling pathways known to be affected by both these drugs including AP-1 and NF-κB activities are inhibited in these cells as compared with cells driven under neutral conditions. Taken together, this suggests that IL-10–producing regulatory T cells arise in vivo under conditions where the engagement of the signaling pathways previously described to be important for the regulation of cytokine genes produced by Th1 and Th2 cells is minimal. These observations that transcription factors and signaling pathways shown to be involved in the activation of Th1 and Th2-type cytokine genes and thus leading to inflammation, are reduced in the IL-10–producing regulatory T cells developed in our study support the concept that the effect of the immunosuppressive drugs on the development of regulatory T cells producing IL-10 is likely to mimic events that occur during development as autoreactive T cells encounter antigen in the periphery under tolerogenic conditions. Furthermore, our data suggest that in contrast to other cytokine genes, IL-10 gene regulation is not only refractory to the inhibitory action of VitD3 and Dex, but conversely is induced by the synergistic action of the signaling pathways induced by these drugs (Fig. 2). A reciprocal synergistic action of these drugs was also apparent with respect to the downregulation of IL-2 (Fig. 2 A, middle panel).
The use of regulatory T cells in adoptive immunotherapy has been hindered first by the contamination of such populations with Th1- or Th2-derived cytokines that may exacerbate inflammatory pathologies, and second by the inability to expand them in large numbers. The IL-10–producing regulatory T cells derived by the strategy presented here do not suffer these limitations. They could indeed be produced as a homogeneous population after stimulation of T cells with the antigen of interest, and for example adoptively transferred into patients suffering from GVHD, organ-specific autoimmune disorders, or other inflammatory diseases. Alternatively, such populations could be generated to respond either to a well-defined self-antigen or to an unrelated protein antigen provided that the T cells are appropriately triggered and thus could function as regulators via a bystander mechanism. Other studies describing T cells mediating bystander suppression of pathology-inducing T cells (EAE, diabetes, IBD) support the notion that regulatory T cells when located at the site of inflammation could effectively downregulate an immune response (21, 62, 63). Generating regulatory T cells specific for a distinct antigen to that which the pathogenic T cells react to could be a strategy to inhibit inflammation and autoimmune manifestations where the antigen is unknown. However, this still needs to be addressed in clinical situations. Initially IL-10–producing T cells generated with VitD3/Dex are more likely to be used in situations where the antigen is well defined such as GVHD or organ transplantation.
There is a clear paradox of how regulatory T cells preferentially inhibit T cell responses to distinct antigens, while simultaneously allowing responses to pathogens to occur. Clearly there are various thresholds imposed to regulate the activation of T cells, including positive and negative signals delivered to T cells from the APCs that may differ according to the previous activations encountered by the T cell (64, 65). In addition, signals from the innate immune response required to overcome negative signals delivered to the T cell or the APC (66), or the distribution of antigen and the recirculation patterns of T cells, may determine whether a T cell is activated or suppressed (65, 67, 68). Finally, the ratio of regulatory T cells to effector T cells will undoubtedly also contribute to the outcome of whether an immune response or tolerance ensues (65).
The relationship between IL-10–producing regulatory T cells and other described populations of regulatory T cells (9, 11, 17, 69–73) is still unknown. Our ability to generate homogeneous populations of regulatory cells in vitro with Vit/Dex will certainly be key in order to address this issue. Indeed, we have generated IL-10–producing T cells using CD4+ T cells from DO11.10 RAG−/− mice, which are known not to contain CD4+CD25+ cells (74). CD45RBlow CD4+ T cells can inhibit CD45RBhighCD4+ T cell–mediated colitis via the action of both TGF-β and IL-10 (9, 57), suggesting a role for both cytokines in regulation of mucosal inflammation. A role for TGF-β has also been demonstrated for a number of T regulatory populations, including Th3 and Tr1 cells, in inhibition of autoimmune pathologies, gut inflammation, and/or proliferation of antigen-specific T cells (8, 21). In addition to its broad antiinflammatory properties, TGF-β has been shown to inhibit the development of both Th1 and Th2 responses (75–77). As we now show that Th1 and Th2 cytokines can downregulate the development of IL-10–producing regulatory T cells, this may explain some of the effects of TGF-β to inhibit inflammatory pathologies, as well as its relationship with regulatory T cells (8).
Our studies now demonstrate that IL-10–producing regulatory T cells require antigenic stimulation to function to regulate EAE. As protection was only observed when the antigen was delivered intracranially but not intraperitoneally, this strongly suggests that the regulatory cells require activation at the site of inflammation. Furthermore, the inhibition of EAE by these OVA-specific T cells was via a bystander fashion, as they are inhibiting autoreactive T cells responding to CNS-specific antigens and require IL-10 for their function.
In conclusion, our present findings describing the generation of homogeneous populations of IL-10–producing regulatory T cells has allowed us to determine some of the regulatory mechanisms underlying their development and function. Our study will facilitate their potential use in clinical intervention and will be key to further understand the molecular events required for the differentiation and function of these cells.
Acknowledgments
We would like to thank Drs. Anne Rascle and Naoko Arai for advice to perform EMSA, Drs. Stephen Hurst and Jonathon Sedgwick and Drs. Jean Langhorne and Brigitta Sockinger, NIMR, for their helpful comments during the project and on the manuscript, as well as Drs. Yong-Jun Liu, Vassilli Soumelis, Paulo Vieira, and Lewis Lanier for their critical reading of the manuscript.
C. Hawrylowicz is supported by the National Asthma Campaign of Great Britain. DNAX Research Institute of Molecular and Cellular Biology is supported by Schering-Plough Corporation.
D.J. Cua and A. Boonstra contributed equally to this work.
C.M. Hawrylowicz and R.L. Coffman contributed equally to this work.
R.L. Coffman's present address is Dynavax Technologies, 717 Potter St., Suite 100, Berkeley, CA 94710.
Footnotes
Abbreviations used in this paper: AP, activator protein; CNS, central nervous system; DC, dendritic cell; Dex, Dexamethasone; EAE, experimental autoimmune encephalomyelitis; MSCH, mouse spinal cord homogenate; NF, nuclear factor; NFAT, nuclear factor of activated T cells; RAG, recombination activating gene; VitD3, 1,25(OH)2-vitamin D3.
References
- 1.O'Garra, A. 1998. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 8:275–283. [DOI] [PubMed] [Google Scholar]
- 2.Sher, A., and R.L. Coffman. 1992. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu. Rev. Immunol. 10:385–409. [DOI] [PubMed] [Google Scholar]
- 3.Romagnani, S. 1994. Lymphokine production by human T cells in disease states. Annu. Rev. Immunol. 12:227–257. [DOI] [PubMed] [Google Scholar]
- 4.Fiorentino, D.F., M.W. Bond, and T.R. Mosmann. 1989. Two types of mouse helper T cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 170:2081–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mason, D., and F. Powrie. 1998. Control of immune pathology by regulatory T cells. Curr. Opin. Immunol. 10:649–655. [DOI] [PubMed] [Google Scholar]
- 6.Lafaille, J.J., F. Van de Keere, A.L. Hsu, J.L. Baron, W. Haas, C.S. Raine, and S. Tonegawa. 1997. Myelin basic protein-specific T helper 2 (Th2) cells cause experimental autoimmune encephalomyelitis in immunodeficient hosts rather than protect them from disease. J. Exp. Med. 186:307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakaguchi, S. 2000. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 101:455–458. [DOI] [PubMed] [Google Scholar]
- 8.Weiner, H.L. 1997. Oral tolerance for the treatment of autoimmune diseases. Annu. Rev. Med. 48:341–351. [DOI] [PubMed] [Google Scholar]
- 9.Powrie, F., J. Carlino, M.W. Leach, S. Mauze, and R.L. Coffman. 1996. A critical role for transforming growth factor-β but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RBlow CD4+ T cells. J. Exp. Med. 183:2669–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seddon, B., and D. Mason. 2000. The third function of the thymus. Immunol. Today. 21:95–99. [DOI] [PubMed] [Google Scholar]
- 11.Thornton, A.M., and E.M. Shevach. 1998. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koppelman, B., J.J. Neefjes, J.E. de Vries, and R. de Waal Malefyt. 1997. Interleukin-10 down-regulates MHC class II ab peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity. 7:861–871. [DOI] [PubMed] [Google Scholar]
- 13.Ding, L., P.S. Linsley, L.Y. Huang, R.N. Germain, and E.M. Shevach. 1993. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the upregulation of B7 expression. J. Immunol. 151:1224–1234. [PubMed] [Google Scholar]
- 14.Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. [DOI] [PubMed] [Google Scholar]
- 15.Akdis, C.A., T. Blesken, M. Akdis, B. Wuthrich, and K. Blaser. 1998. Role of interleukin 10 in specific immunotherapy. J. Clin. Invest. 102:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grunig, G., D.B. Corry, M.W. Leach, B.W.P. Seymour, V.P. Kurup, and D.R. Rennick. 1997. Interleukin-10 is a natural suppressor of cytokine production and inflammation in a murine model of allergic bronchopulmonary aspergillosis. J. Exp. Med. 185:1089–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bacchetta, R., M. Bigler, J.L. Touraine, R. Parkman, P.A. Tovo, J. Abrams, R. de Waal Malefyt, J.E. de Vries, and M.G. Roncarolo. 1994. High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. J. Exp. Med. 179:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonuleit, H., E. Schmitt, G. Schuler, J. Knop, and A.H. Enk. 2000. Induction of Interleukin 10–producing, Nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J. Exp. Med. 192:1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhodapkar, M.V., R.M. Steinman, J. Krasovsky, C. Munz, and N. Bhardwaj. 2001. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J. Exp. Med. 193:233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore, K.W., R. de Waal Malefyt, R.L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683–765. [DOI] [PubMed] [Google Scholar]
- 21.Groux, H., A. O'Garra, M. Bigler, M. Rouleau, J. de Vries, and M.-G. Roncarolo. 1997. Generation of a novel regulatory CD4+ T-cell population, which inhibits antigen-specific T-cell responses. Nature. 389:737–742. [DOI] [PubMed] [Google Scholar]
- 22.Olivares-Villagomez, D., A.K. Wensky, Y. Wang, and J.J. Lafaille. 2000. Repertoire requirements of CD4+ T cells that prevent spontaneous autoimmune encephalomyelitis. J. Immunol. 164:5499–5507. [DOI] [PubMed] [Google Scholar]
- 23.Levings, M.K., R. Sangregorio, F. Galbiati, S. Squadrone, R. de Waal Malefyt, and M.G. Roncarolo. 2001. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J. Immunol. 166:5530–5539. [DOI] [PubMed] [Google Scholar]
- 24.Cantorna, M.T., C.E. Hayes, and H.F. DeLuca. 1996. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc. Natl. Acad. Sci. USA. 93:7861–7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattner, F., S. Smiroldo, F. Galbiati, M. Muller, P. Di Lucia, P.L. Poliani, G. Martino, P. Panina-Bordignon, and L. Adorini. 2000. Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1,25-dihydroxyvitamin D(3). Eur. J. Immunol. 30:498–508. [DOI] [PubMed] [Google Scholar]
- 26.Lemire, J.M., D.C. Archer, L. Beck, and H.L. Spiegelberg. 1995. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J. Nutr. 125:1704S–1708S. [DOI] [PubMed] [Google Scholar]
- 27.D'Ambrosio, D., M. Cippitelli, M.G. Cocciolo, D. Mazzeo, P. Di Lucia, R. Lang, F. Sinigaglia, and P. Panina-Bordignon. 1998. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J. Clin. Invest. 101:252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piemonti, L., P. Monti, M. Sironi, P. Fraticelli, B.E. Leone, E. Dal Cin, P. Allavena, and V. Di Carlo. 2000. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J. Immunol. 164:4443–4451. [DOI] [PubMed] [Google Scholar]
- 29.Wilckens, T., and R. De Rijk. 1997. Glucocorticoids and immune function: unknown dimensions and new frontiers. Immunol. Today. 18:418–424. [DOI] [PubMed] [Google Scholar]
- 30.Karin, M. 1998. New twists in gene regulation by glucocorticoid receptor: is DNA binding dispensable? Cell. 93:487–490. [DOI] [PubMed] [Google Scholar]
- 31.Blotta, M.H., R.H. DeKruyff, and D.T. Umetsu. 1997. Corticosteroids inhibit IL-12 production in human monocytes and enhance their capacity to induce IL-4 synthesis in CD4+ lymphocytes. J. Immunol. 158:5589–5595. [PubMed] [Google Scholar]
- 32.Vieira, P.L., P. Kalinski, E.A. Wierenga, M.L. Kapsenberg, and E.C. de Jong. 1998. Glucocorticoids inhibit bioactive IL-12p70 production by in vitro-generated human dendritic cells without affecting their T cell stimulatory potential. J. Immunol. 161:5245–5251. [PubMed] [Google Scholar]
- 33.Visser, J., A. van Boxel-Dezaire, D. Methorst, T. Brunt, E.R. de Kloet, and L. Nagelkerken. 1998. Differential regulation of interleukin-10 (IL-10) and IL-12 by glucocorticoids in vitro. Blood. 91:4255–4264. [PubMed] [Google Scholar]
- 34.Richards, D.F., M. Fernandez, J. Caulfield, and C.M. Hawrylowicz. 2000. Glucocorticoids drive human CD8(+) T cell differentiation towards a phenotype with high IL-10 and reduced IL-4, IL-5 and IL-13 production. Eur. J. Immunol. 30:2344–2354. [DOI] [PubMed] [Google Scholar]
- 35.Ramirez, F., and D. Mason. 2000. Induction of resistance to active experimental allergic encephalomyelitis by myelin basic protein-specific Th2 cell lines generated in the presence of glucocorticoids and IL-4. Eur. J. Immunol. 30:747–758. [DOI] [PubMed] [Google Scholar]
- 36.De Bosscher, K., M.L. Schmitz, W. Vanden Berghe, S. Plaisance, W. Fiers, and G. Haegeman. 1997. Glucocorticoid-mediated repression of nuclear factor-kappaB-dependent transcription involves direct interference with transactivation. Proc. Natl. Acad. Sci. USA. 94:13504–13509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen, R., T.F. Burke, J.E. Cumberland, M. Brummet, L.A. Beck, V. Casolaro, and S.N. Georas. 2000. Glucocorticoids inhibit calcium- and calcineurin-dependent activation of the human IL-4 promoter. J. Immunol. 164:825–832. [DOI] [PubMed] [Google Scholar]
- 38.Yu, X.P., T. Bellido, and S.C. Manolagas. 1995. Down-regulation of NF-kappa B protein levels in activated human lymphocytes by 1,25-dihydroxyvitamin D3. Proc. Natl. Acad. Sci. USA. 92:10990–10994. [published erratum at 93:524]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alroy, I., T.L. Towers, and L.P. Freedman. 1995. Transcriptional repression of the interleukin-2 gene by vitamin D3: direct inhibition of NFATp/AP-1 complex formation by a nuclear hormone receptor. Mol. Cell. Biol. 15:5789–5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy, K.M., A.B. Heimberger, and D.Y. Loh. 1990. Induction by antigen of interthymic apoptosis of CD4+ CD8+ TCR-lo thymocytes in vivo. Science. 250:1720–1722. [DOI] [PubMed] [Google Scholar]
- 41.Ferber, I.A., H.-J. Lee, F. Zonin, V. Heath, A. Mui, N. Arai, and A. O'Garra. 1999. GATA-3 significantly down-regulates IFN-γ production from developing Th1 cells in addition to inducing IL-4 and IL-5 levels. Clin. Immunol. 91:134–144. [DOI] [PubMed] [Google Scholar]
- 42.O'Farrell, A.-M., Y. Liu, K.W. Moore, and A.L. Mui. 1998. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and independent pathways. EMBO J. 17:1006–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szabo, S.J., S.T. Kim, G.L. Costa, X. Zhang, C.G. Fathamn, and L.H. Glimcher. 2000. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 100:655–669. [DOI] [PubMed] [Google Scholar]
- 44.Mui, A.L., A. Miyajima, N. Harada, A.M. O'Farrell, and H. Wakao. 1995. Interleukin 3, granulocyte-macrophage colony stimulating factor and interleukin 5, transduce signals through two Stat5 homologs. EMBO J. 14:1166–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cua, D.J., H. Groux, D.R. Hinton, S.A. Stohlman, and R.L. Coffman. 1999. Transgenic interleukin 10 prevents induction of experimental autoimmune encephalomyelitis. J. Exp. Med. 189:1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rea, D., C. van Kooten, K.E. van Meijgaarden, T.H. Ottenhoff, C.J. Melief, and R. Offringa. 2000. Glucocorticoids transform CD40-triggering of dendritic cells into an alternative activation pathway resulting in antigen-presenting cells that secrete IL-10. Blood. 95:3162–3167. [PubMed] [Google Scholar]
- 47.Glimcher, L.H., and K.M. Murphy. 2000. Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev. 14:1693–1711. [PubMed] [Google Scholar]
- 48.O'Garra, A., and N. Arai. 2000. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends Cell Biol. 10:542–550. [DOI] [PubMed] [Google Scholar]
- 49.Ouyang, W., N.G. Jacobson, D. Bhattacharya, J.D. Gorham, D. Fenoglio, W.C. Sha, T.L. Murphy, and K.M. Murphy. 1999. The Ets transcription factor ERM is Th1-specific and induced by IL-12 through a Stat4-dependent pathway. Proc. Natl. Acad. Sci. USA. 96:3888–3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim, J., I.C. Ho, M. Grusby, and L.H. Glimcher. 1999. The transcription factor c-maf controls the production of IL-4 but not other Th2 cytokines. Immunity. 10:745–751. [DOI] [PubMed] [Google Scholar]
- 51.Zheng, W.-P., and R.A. Flavell. 1997. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4T cells. Cell. 89:587–596. [DOI] [PubMed] [Google Scholar]
- 52.Ouyang, W., S.H. Ranganath, K. Weindel, D. Bhattacharya, T.L. Murphy, W.C. Sha, and K.M. Murphy. 1998. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 9:745–755. [DOI] [PubMed] [Google Scholar]
- 53.Lee, H.J., N. Takemoto, H. Kurata, Y. Kamogawa, S. Miyatake, A. O'Garra, and N. Arai. 2000. GATA-3 induces Th2 cytokine expression and chromatin remodeling in committed Th1 cells. J. Exp. Med. 192:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buer, J., A. Lanoue, A. Franzke, C. Garcia, H. von Boehmer, and A. Sarukhan. 1998. Interleukin 10 secretion and impaired effector function of major histocompatibility complex class II-restricted T cells anergized in vivo. J. Exp. Med. 187:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller, C., J.A. Ragheb, and R.H. Schwartz. 1999. Anergy and cytokine-mediated suppression as distinct superantigen-induced tolerance mechanisms in vivo. J. Exp. Med. 190:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Medzhitov, R., and C.J. Janeway. 2000. Innate immune recognition: mechanisms and pathways. Immunol. Rev. 173:89–97. [DOI] [PubMed] [Google Scholar]
- 57.Asseman, C., S. Mauze, M.W. Leach, R.L. Coffman, and F. Powrie. 1999. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 190:995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hewison, M. 1992. Vitamin D and the immune system. J. Endocrinol. 132:173–176. [DOI] [PubMed] [Google Scholar]
- 59.Mason, D. 1991. Genetic variation in the stress response: susceptibility to experimental allergic encephalmyelitis and implications for human inflammatory disease. Immunol. Today. 12:57–60. [DOI] [PubMed] [Google Scholar]
- 60.MacPhee, I.A., F.A. Antoni, and D.W. Mason. 1989. Spontaneous recovery of rats from experimental allergic encephalomyelitis is dependent on regulation of the immune system by endogenous adrenal corticosteroids. J. Exp. Med. 169:431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gregori, S., M. Casorati, S. Amuchastegui, S. Smiroldo, A.M. Davalli, and L. Adorini. 2001. Regulatory T cells induced by 1alpha,25-dihydroxyvitamin d(3) and mycophenolate mofetil treatment mediate transplantation tolerance. J. Immunol. 167:1945–1953. [DOI] [PubMed] [Google Scholar]
- 62.Homann, D., A. Holz, A. Bot, B. Coon, T. Wolfe, J. Petersen, T.P. Dyrberg, M.J. Grusby, and M.G. von Herrath. 1999. Autoreactive CD4+ T cells protect from autoimmune diabetes via bystander suppression using the IL-4/Stat6 pathway. Immunity. 11:463–472. [DOI] [PubMed] [Google Scholar]
- 63.Nicholson, L.B., A. Murtaza, B.P. Hafler, A. Sette, and V.K. Kuchroo. 1997. A T cell receptor antagonist peptide induces T cells that mediate bystander suppression and prevent autoimmune encephalomyelitis induced with multiple myelin antigens. Proc. Natl. Acad. Sci. USA. 94:9279–9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matzinger, P. 1994. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12:991–1045. [DOI] [PubMed] [Google Scholar]
- 65.Maloy, K.J., and F. Powrie. 2001. Regulatory T cells in the control of immune pathology. Nat. Immunol. 2:816–822. [DOI] [PubMed] [Google Scholar]
- 66.Medzhitov, R., and C.A.J. Janeway. 1997. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 91:295–298. [DOI] [PubMed] [Google Scholar]
- 67.Mackay, C.R. 1993. Homing of naive, memory and effector lymphocytes. Curr. Opin. Immunol. 5:423–427. [DOI] [PubMed] [Google Scholar]
- 68.Zinkernagel, R.M., S. Ehl, P. Aichele, S. Oehen, T. Kundig, and H. Hengartner. 1997. Antigen localisation regulates immune responses in a dose- and time-dependent fashion: a geographical view of immune reactivity. Immunol. Rev. 156:199–209. [DOI] [PubMed] [Google Scholar]
- 69.Chen, Y., V.K. Kuchroo, J.-I. Inobe, D.A. Hafler, and H.L. Weiner. 1994. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 265:1237–1240. [DOI] [PubMed] [Google Scholar]
- 70.Bridoux, F., A. Badou, A. Saoudi, I. Bernard, E. Druet, R. Pasquier, P. Druet, and L. Pelletier. 1997. Transforming growth factor β (TGF-β)-dependent inhibition of T helper 2 (Th2)-induced autoimmunity by self-major histocompatibility complex (MHC) class II-specific, regulatory CD4+ T cell lines. J. Exp. Med. 185:1769–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han, H.S., H.S. Jun, T. Utsugi, and J.W. Yoon. 1996. A new type of CD4+ suppressor T cell completely prevents spontaneous autoimmune diabetes and recurrent diabetes in syngeneic islet-transplanted NOD mice. J. Autoimmun. 9:331–339. [DOI] [PubMed] [Google Scholar]
- 72.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151–1164. [PubMed] [Google Scholar]
- 73.Olivares-Villagomez, D., Y. Wang, and J.J. Lafaille. 1998. Regulatory CD4+ T cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. J. Exp. Med. 188:1883–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Itoh, M., T. Takahashi, N. Sakaguchi, Y. Kuniyasu, J. Shimizu, F. Otsuka, and S. Sakaguchi. 1999. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 162:5317–5326. [PubMed] [Google Scholar]
- 75.Swain, S.L., G. Huston, S. Tonkonogy, and A.D. Weinberg. 1991. Transforming growth factor-beta and IL-4 cause helper T cell precursors to develop into distinct effector helper cells that differ in lymphokine secretion pattern and cell surface phenotype. J. Immunol. 147:2991–3000. [PubMed] [Google Scholar]
- 76.Sad, S., and T.R. Mosmann. 1994. A single IL-2-secreting precursor CD4 T cell can develop into either a Th1 or Th2 secreting phenotype. J. Immunol. 153:3514–3522. [PubMed] [Google Scholar]
- 77.Heath, V., E. Murphy, C. Crain, M. Tomlinson, and A. O'Garra. 2000. TGF-beta1 downregulates Th2 development and results in decreased IL-4-induced Stat6 activation and GATA-3 expression. Eur. J. Immunol. 30:2639–2649. [DOI] [PubMed] [Google Scholar]