Abstract
The major histocompatibility complex (MHC) restriction element for a human Ni2+ reactive T cell, ANi-2.3, was identified as DR52c. A series of experiments established that the functional ligand for this T cell was a preformed complex of Ni2+ bound to the combination of DR52c and a specific peptide that was generated in human and mouse B cells, but not in fibroblasts nor other antigen processing–deficient cells. In addition, ANi-2.3 recognition of this complex was dependent on His81 of the MHC β chain, suggesting a role for this amino acid in Ni2+ binding to MHC. We propose a general model for Ni2+ recognition in which βHis81 and two amino acids from the NH2-terminal part of the MHC bound peptide coordinate Ni2+ which then interacts with some portion of the Vα CDR1 or CDR2 region.
Keywords: hypersensitivity, T cell receptor, antigen presentation, hapten, nickel
Introduction
Although much of what we know about αβTCR recognition comes from the study of peptide antigens, αβTCRs can recognize ligands that contain moieties other than, or in addition to, peptides. For example, the class Ib MHC molecule CD1 presents lipids and glycolipids to T cells (1). Haptens are another group of chemicals that have been shown to be capable of forming part of the αβTCR receptor ligand. Small organic haptens such as dinitrophenol (DNP), trinitrophenol (TNP), and fluorescein (FL) have long been used experimentally to study T cell responses and hypersensitivity. Also, metals, such as nickel, gold, or beryllium, form another group of chemicals for which T cell hypersensitivity has been demonstrated. Contact sensitivity to nickel in jewelry is quite common (2). Patients undergoing colloidal gold therapy can develop hypersensitivity (3). Sensitivity to beryllium is a serious problem in industries that handle this element (4).
In the case of CD1, the MHC binding groove is very hydrophobic and appears to bind the lipid portion of the antigen, presenting the most hydrophilic portion of the antigen on the MHC surface (5). Studies with TNP have suggested that a hapten-modified MHC bound peptide is most often the relevant antigen (6, 7). However, there are a few cases in which haptens appear to dominate the interaction with the αβTCR, such that the interaction can be measured even in the absence of peptide and MHC (8, 9).
In the case of hypersensitivity to metals, it is most likely that αβTCRs recognize metal ions complexed with the MHC/peptide surface in a manner analogous to the organic haptens, but to date there is no formal demonstration of this complex. In the present study, we have defined the ligand for the αβTCR of a Ni2+ reactive T cell clone (ANi-2.3) isolated from a patient with Ni2+ hypersensitivity (10, 11). We show that recognition by this αβTCR requires the combination Ni2+, a particular MHC molecule, DR52c (DRA*0101, DRB3*0301), and an unknown specific MHC bound peptide produced in B cells, but not fibroblasts nor other nonprofessional APC. These results are consistent with the recognition of Ni2+ bound to the MHC surface by amino acid side chains from the MHC and/or the MHC bound peptide.
Materials and Methods
Oligonucleotides, Peptides, and Superantigen.
Oligonucleotides used in DNA constructions, mutagenesis and sequencing were produced in the Molecular Resources Center at National Jewish Medical and Research Center. The following peptides were also produced at this facility: pTT, amino acids 830–840 of tetanus toxin (12); pTu, amino acids 342–359 of the elongation factor Tu; pDRA, amino acids 110–128 of HLA DR α chain; pλ, amino acids 11–29 of human Ig lambda chain (13). The superantigen, staphylococcal enterotoxin B (SEB),* was purchased from Sigma-Aldrich.
Cell Lines.
Production and characterization of the Ni2+ reactive T cell transfectoma bearing the αβTCR of ANi2.3, has been described previously (14). The T cell transfectoma, AL8.1, was a gift from Dr. U. Blank (Pasteur Institute, Paris, France; reference 12). It is specific for pTT presented by an allele of DR13 (DRB1*1302, DRA). The EBV transformed B cell line, HO301, was a gift from Dr. J. Hansen (University of Washington, Seattle, WA). The MHC class II alleles expressed in HO301 are DRA*01012, DRB1*1302, DRB3*0301, DQA*101021, DQB1*0604, DPA1*01, and DPB1*1601 (15). The mouse B cell lymphoma line, M12.C3, was obtained from Dr. Laurie Glimcher (Harvard University, Cambridge, MA). It is a variant of M12 that fails to express MHC class II due to mutations in both the IAd and IEd β chain genes (16). The mouse fibroblast cell line DAP was a gift from Dr. J. Bill (University of Colorado Health Sciences Center, Denver, CO). The human cell line T2 is a B cell/T cell hybrid (17) that carries a large genomic deletion (18) including the structural genes for all MHC class II molecules and for DM. The MHC class II− mouse mastocytoma line, P815 and the DR1+ (DRB1*0101,DRA) EBV-transformed human B cell line, LG2, were obtained from the American Type Culture Collection.
Monoclonal Antibodies.
B cell hybridoma, FK7.3.19.1, which produced a mAb specific for DR52c (19), was a gift from Dr. F. Koning (Leiden University Medical Center, Netherlands). L243 (anti-DRB1) and L227 (anti-DRα) B cell hybridomas were obtained from the American Type Culture Collection.
Cell Fixation.
EBV B cells were fixed with paraformaldehyde. 2 × 106 cells were incubated with 1 ml of 0.25% paraformaldehyde at 37°C for 40 min and then washed thoroughly with balanced salt solution (BSS).
IL-2 Production.
IL-2 production by stimulated transfectoma T cells was assayed as previously described (20, 21) using the IL-2–dependent T cell line, HT-2. Briefly, cultures were prepared containing 105 transfectoma T cells and 105 antigen-presenting cells. Unless otherwise stated, stimuli were either nothing, 100 μM Ni2+ (as NiCl2), or 50 μg/ml SEB.
Preparation of Soluble DR52c.
DR52c was isolated from lysates of HO301 by an adaptation of published methods (22, 23). Briefly, 1–5 × 109 HO301 cells were harvested and washed three times with cold BSS. Cells were suspended in lysis buffer (20 mM octanoyl-N-methylglucamide (MEGA-8), 20 mM nonanoyl-N-methylglucamide (MEGA-9), 1 mM PMSF, 50 mM iodoacetamide, 10 μg/ml leupeptin, 0.2 mg/ml EDTA, and 0.7 μg/ml pepstatin A in PBS, pH 7.4) and incubated on ice for 30 min. The lysate was centrifuged at 100,000 g for 1 h at 4°C and passed through a prewashed FK7.3.19.1 coupled Sepharose column. Class II molecules were eluted with pH 11.4, 50 mM 3-[cyclohexylamino]-1-propanesulfonic acid, 150 mM NaCl, 20 mM MEGA-8, and 20 mM MEGA-9. The eluate was collected into siliconized glass tubes and neutralized with 2 M Tris (pH 6.8). All reagents were purchased from Sigma-Aldrich. To remove the transmembrane domain from natural DR52c, 8 vol of 1.5 mg/ml DR52c were incubated with 3 vol of 0.1 mM dithiothreitol, 0.1 mM EDTA, 1 mM Tris, and 0.1 mg/ml papain solution for 1 h at 37°C. The reaction was stopped with 1 vol of 20 mM iodoacetamide and 100 mM Tris solution, pH 8, incubated on ice for 30 min. This was stored in PBS.
Extraction of MHC Bound Peptides.
DR52c molecules in 10 mM Tris buffer, pH 7.5, were incubated 2× with 2.5 M acetic acid for 30 min at 37°C. This solution was passed twice through Centricon C-10 filters. The pass-through was collected and lyophilized to dryness. The residue was redissolved in water and lyophilized to dryness three more times.
Vectors, Constructs, and Transduction of Cell Lines.
The genes for the α and β chains of DR52c were transduced into various cells using an MSCV retroviral system in which green fluorescent protein (GFP) or thy-1.1 served as surrogate markers (24, 25). Bacteria stock carrying the plasmid pBEX WT46 BIII that encoded the DRB3–0301 β chain of DR52c, was a gift from Dr. J. Gorski (Milwaukee Blood Center, Milwaukee, WI). cDNA encoding the full length DR52C β chain was cloned into MSCV-GFP between the BglII and NotI restriction sites of the polylinker. cDNA encoding the full length DRα chain gene was cloned into MSCV-thy1.1 between the EcoRI and NotI restriction sites of the polylinker. The plasmids were transfected into a retroviral packaging cell line as described (25). 4 ml of the resultant viral stock was then used to transduce 5 × 105 target cells using a spinfection protocol. Transductants were then cloned at limiting dilution. A variant of the DR52c β chain/MSCV-GFP construct was made in which the PCR was used to change the codon for His (CAC) to that of Gln (CAG) at the position encoding amino acid 81 of the β chain.
Results
DRβ3–0301 Is the Restriction Element for ANi-2.3.
The ANi-2.3 T cell clone was originally isolated from a patient with nickel hypersensitivity (11). The clone and a T cell hybridoma transfectant (14) expressing an αβTCR containing the ANi-2.3 Vα and Vβ linked to mouse Cα and Cβ respond to autologous antigen-presenting cells pulsed with Ni2+. Based on the reactivity of the clone to Ni2+ presented by a series of APCs of different HLA genotypes and the inhibition of its reactivity with a specific anti-DRα mAb, the restriction element of this clone was thought to be DR13 (DRB1*1302, DRA*0101; references 11 and 14). However, in preliminary experiments in which we transfected the DRB1*1302 β chain gene into a number of cells types that contained the DRα gene, we were unable to transfer Ni2+ presenting ability (data not shown). Therefore, we considered that some other class II MHC molecule in this patient was the Ni2+ presenting element. As DRB1*1302 is in very tight linkage disequilibrium with the DR52c β chain gene (26, 27), we turned our attention to this molecule.
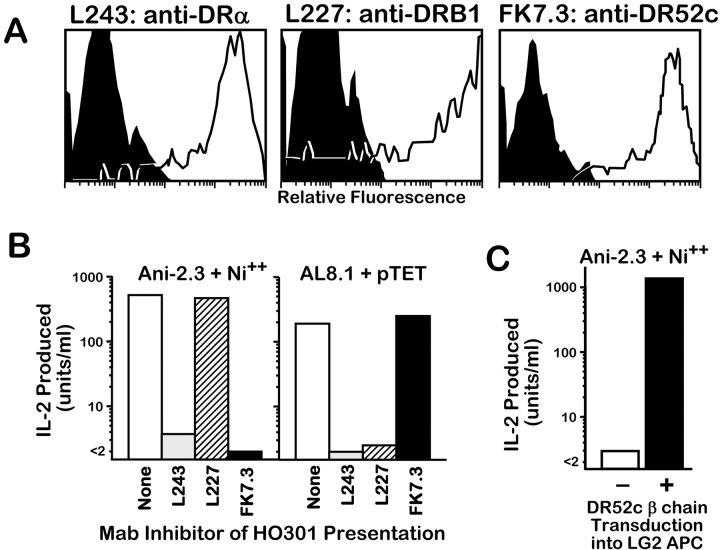
Two types of experiments convincingly demonstrated that DR52c is in fact the MHC restriction element for Ni2+ presentation to ANi-2.3. In the first, we used the EBV transformed cell line, HO301, which is homozygous for both DRB1*1302 and DR52c, as an APC for Ni2+ presentation. Fig. 1 A shows the expression of DR13 and DR52c on HO301 using the β specific mAbs L227 (anti-DRB1) and FK-7.3 (anti-DR52c). Both β chains are well expressed as is the common DRα chain detected with the mAb, L243. Fig. 1 B shows the reactivity of ANi-2.3 to Ni2+ presented by HO301. As a control we used another T cell transfectoma, AL8.1, which is specific for a tetanus peptide presented by DRB1*1302 (12). ANi-2.3 responded to Ni2+ presented by HO301 and AL8.1 responded to the tetanus peptide. The response of ANi-2.3 to Ni2+ was nearly completely blocked by the DR52c and DRα specific mAbs, but not the DRB1 specific mAb. As expected, the AL8.1 response to the tetanus peptide was inhibited by the DRB1 and DRα specific mAbs, but not by the DR52c specific mAb. These results strongly implicated DR52c as the Ni2+ presenting MHC restriction element for ANi-2.3.
Figure 1.
Identification of DR52c as the Ni2+ presenting element for ANi-2.3. (A) Fluorescent staining with mAb's was used to assess the surface expression of DR13 and DR52c on the surface of HO301 cells. Three mAbs were used: L243, specific for the common DRα chain; L227, specific for all DRB1 β chains (in this case DRB1*1302); and FK7.3, specific for DR52c β chain. HO301 cells were incubated with biotinylated versions of the antibodies, which were then detected with phycoerythrin streptavidin (unfilled histograms). The negative controls (filled histograms) were HO301 cells incubated with only phycoerythrin streptavidin. (B) The same set of anti-DR mAb's was used in an attempt to inhibit IL-2 production by either ANi-2.3 stimulated by Ni2+ or AL-8.1 stimulated by pTT presented by HO301. The antibodies (10 μg/ml) were added at the initiation of the IL-2 production cultures. (C) The DRB1*0101 homozygous EBV transformed B cell line, LG2, was tested before and after transduction with the gene for DR52c β chain for its ability to present Ni2+ to ANi-2.3.
In a second experiment, we used a retroviral vector to transduce the gene for the DR52c β chain into the EBV transformed cell line, LG2. LG2 is homozygous for DRB1*0101 and does not express DR52c. We then compared LG2 to its DR52c transductant for the ability to present Ni2+ to ANi-2.3 (Fig. 1 C). The nontransduced LG2 cells did not present Ni2+ to ANi-2.3, but the transductant presented Ni2+ very well. Taken together these results confirmed that DR52c was the required MHC restriction element for Ni2+ presentation to ANi-2.3.
Ni2+ Presentation Occurs via a Preformed Peptide/MHC Complex.
Previous experiments had shown that fixed APCs could present Ni2+ to ANi-2.3 cells (10), indicating that antigen processing was not required and suggesting that the Ni2+ was presented by a preformed MHC/peptide complex. We performed several experiments to confirm this suggestion. In the first experiment, we showed that Ni2+ interaction with fixed APC was reversible under conditions consistent with a protein/Ni2+ complex. Fixed H0301 cells were preincubated with Ni2+, extensively washed and then exposed to various pHs in an attempt to remove any bound Ni2+. The treated cells were tested for stimulation of ANi-2.3 cells with or without adding back Ni2+ to the IL-2 production culture. The results are shown in Fig. 2 . Ni2+ pulsed, fixed H0301 cells exposed to neutral pH presented Ni2+ to ANi-2.3 cells equally well, whether or not additional Ni2+ was added to the culture, showing that the preincubation with Ni2+ stably saturated the presenting ability of the fixed cells. As the pH of the treatment was lowered below pH 5.5, there was a precipitous drop of presenting ability that could be restored by adding Ni2+ back to the culture medium. Treatment below pH3.5 resulted in irreversible loss of Ni2+ presenting ability.
Figure 2.
pH-dependent, reversible binding of Ni2+ to fixed HO301 cells. HO301 cells were fixed with paraformaldehyde and then incubated with 250 μM Ni2+ overnight at 37°C. The cells were washed to remove unbound Ni2+ and then incubated at various pHs for 40 min at 37°C. The cells were then centrifuged and washed three times with BSS. The treated cells were used as antigen presenting cells for ANi-2.3, alone (squares), plus 100 μM Ni2+ (circles), or plus 50 ng/ml SEB (triangles).
These results suggest that Ni2+ is reversibly bound to the fixed APC surface via a pH sensitive (pH 3.5 to 5.0) interaction consistent with coordination by amino acid side chains from histidine (pK ∼6) and/or aspartic/glutamic acids (pK ∼3.5). These amino acids and cysteine are those most commonly found coordinating transition metal ions in proteins. The irreversible loss of Ni2+ presenting ability at pH 3 and below suggested loss of DR52c integrity, perhaps by loss of peptide from its binding groove, a possibility we explored further as described below in the following section.
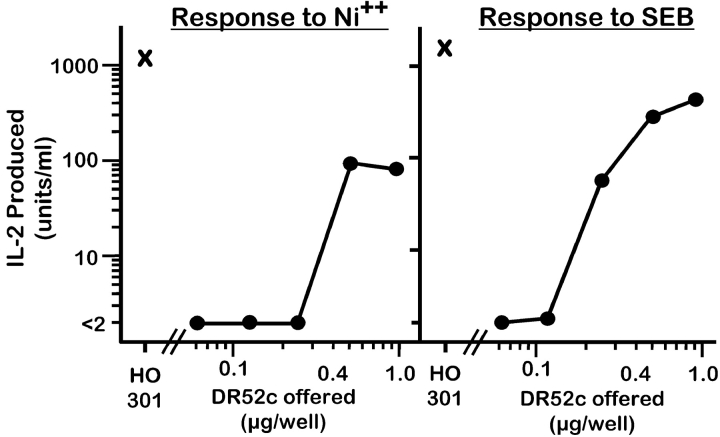
We then tested directly whether or not the MHC/peptide complex for Ni2+ presentation preexisted on the surface of H0301. In tissue culture wells, we immobilized DR52c that had been immunoaffinity purified from lysates of H0301. This immobilized MHCII was able to present Ni2+ to ANi-2.3 (Fig. 3) . This result indicated that Ni2+, DR52c, and a bound peptide were sufficient for engaging the αβTCR of ANi-2.3.
Figure 3.
Natural DR52c purified from HO301 cells can present Ni2+. Various amounts of papain treated DR52c, immunopurified from HO301 cells were immobilized by absorption to plastic tissue culture wells. The immobilized MHCII was then used to present either Ni2+ (100 μM, left panel) or SEB (50 ng/ml, right panel) to ANi-2.3. As a control, HO301 cells (X) were used for antigen presentation.
Ni2+ Presentation to ANi-2.3 Requires a Specific B Cell–derived Peptide.
While our results thus far established that a preformed DR52c/peptide complex was required for Ni2+ presentation to ANi-2.3, they did not tell us the function of the peptide in the recognition. It was possible that this peptide functioned only to stabilize the DR52c molecule and that its specific sequence was not important for Ni2+ presentation. Several experiments have led us to conclude that this is not the case and that a particular bound peptide is required for DR52c presentation of Ni2+ to ANi-2.3.
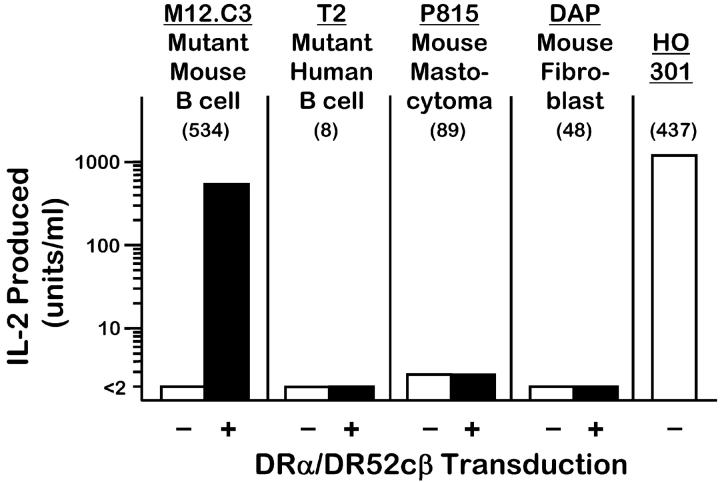
First, in transduction experiments, we expressed the DR52c molecule on the surface of a number of different mouse and human cell types, by cotransducing the DR52c and the DRα gene. The transductants were compared for their ability to present Ni2+ to ANi-2.3. The results are shown in Fig. 4 . As was the case with the human EBV B cell line, LG2, good Ni2+ presentation was seen with the transduced mouse IAd β –/IEd β – BALB/c B lymphoma variant cell line, M12.C3 (16). However, three transduced cell types failed to present Ni2+: P815, a MHCII−/H2-DM− mouse mastocytoma cell line; T2, a human mutant B/T cell hybrid line that lacks the genes for MHCII and HLA-DM; and DAP, a mouse fibroblast line. While these latter three transductants expressed less surface DR52c than did the M12.C3 transductant, the results suggest that the correct Ni2+ presenting DR52c/peptide complex is generated only in a professional, functional antigen-presenting cell and may only occur in B cells. Two possibilities are that this peptide is derived from a B cell–specific protein or is generated from a ubiquitous protein but for loading into DR52c requires a fully functioning CII vesicle compartment that is lacking in P815, T2, and DAP.
Figure 4.
Ni2+ presentation requires DR52c expression in a professional APC. A number of human and murine cell lines lacking DR expression were transduced with the genes for the common DR α chain and the DR52c β chain. Surface expression of DR52c on the transductants was monitored by staining with FK-7.3 (mean channel fluorescence shown in parentheses). Cells were used before (white bar) and after (black bar) transduction to present Ni2+ (100 μM) to ANi-2.3. Untransduced HO301 cells were used as a control APC.
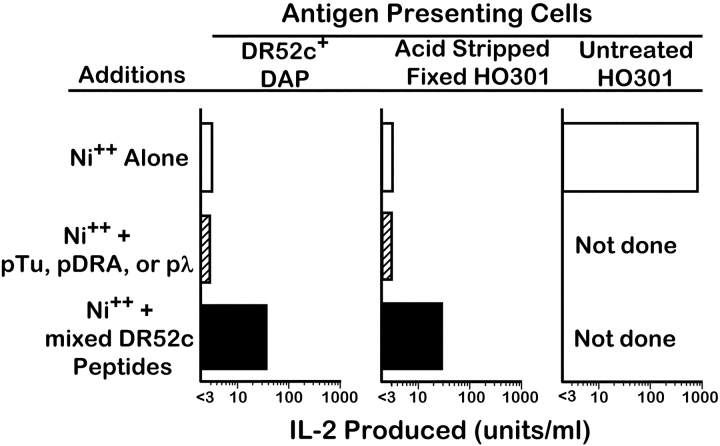
To test this idea of a specific peptide further, we extracted and purified bulk peptides from DR52c immunopurified from H0301 lysates. We preincubated these peptides with two types of APCs that expressed DR52c, but failed to present Ni2+ to ANi-2.3: DR52c transduced DAP fibroblasts and fixed, pH 3 treated H0301 cells. The peptide preincubated cells were then tested for Ni2+ presentation to ANi-2.3 (Fig. 5) . As above, neither the transduced DAP cells nor the fixed and acid stripped H0301 cells could present Ni2+ to ANi-2.3. However, after preincubation with H0301-derived DR52c bound peptides, both APCs presented Ni2+. Several peptides dominantly bound to DR52c on an EBV transformed cell line have been identified (13). To see if any peptide strongly bound to DR52c would restore Ni2+ presentation we tested three of these peptides, pTu, pDRA, and pλ, for their ability to confer Ni2+ presenting activity to these APC. A high concentration of any of these peptides was not able to restore Ni2+ presentation when added to the DR52c bearing cells. We concluded from these experiments that ANi-2.3 requires Ni2+, DR52c, and a particular peptide that is generated in human and mouse B cells (and perhaps other professional APCs), but not in other cell types.
Figure 5.
Peptides isolated from HO301 produced DR52c can transfer Ni2+ presenting ability to other DR52c bearing cells. Peptides were acid stripped and purified from DR52c immunopurified from HO301 cells. An aliquot of the peptides (3 × 108 cell equivalents) were added to either DR52c expressing DAP cells or pH3-treated fixed HO301 cells. The cells were used either before (white bar) or after (black bar) peptide exposure to present Ni2+ (100μM) to ANi-2.3 cells. As negative controls (hatched bar), peptides (pTu, pDRA, pλ) known to bind well to DR52c were used (100 μg/ml). Presentation by untreated HO301 cells served as the positive control.
Possible Role of DR52c β81His in Ni++ Presentation.
Our results suggested that surface amino acids of the DR52c and/or its bound peptide coordinate Ni2+ in a way that presents the cation for recognition by the αβTCR. We had no information about sequence of the functional peptide, but we examined the predicted surface amino acids of the MHC α or β chains for possible candidates for Ni2+ coordination. As our experiments suggested the involvement of histidine, we looked for a predicted pair of histidines, such as is often found in transition metal ion binding sites. In fact, only one MHC histidine (β81) is predicted to lie on the surface of DR52c within what has been the αβTCR interaction footprint on MHC seen in structures of αβTCR/MHC complexes (for reviews, see references 28 and 29). This histidine lies on the top of the β chain α helix near the NH2-terminal end of the bound peptide. It is conserved in nearly all MHCII alleles and isotypes of all species. In all crystal structures of MHCII thus far, the side chain of this histidine points toward the peptide making a important H-bond with the peptide backbone. This histidine has in fact been shown be capable of coordinating Zn2+ as part of the interaction site between MHCII and Zn2+ containing bacterial superantigens (30, 31).
Therefore, we tested the importance of this histidine in Ni2+ presentation to ANi-2.3 by mutating it in the DRB3-*0301 β chain to glutamine. The geometry of the glutamine side chain should allow it to H-bond to the peptide backbone similarly to histidine, but glutamine would not normally be predicted to coordinate Ni2+. The mutated β chain gene was transduced into LG2. The mutated β chain was well expressed on the surface of the transduced LG2 cells, but had lost its ability to present Ni2+ to ANi-2.3 (Fig. 6) . This result is consistent with a role for β histidine 81 in coordinating Ni2+, but, of course, does not eliminate the alternate interpretation that this amino acid is a contact residue for αβTCR/MHC, rather than Ni2+/MHC, interaction.
Figure 6.
DR52c βHis81 is important for ANi-2.3 recognition of DR52c presented Ni2+. LG2 cells were transduced with the gene for the DR52c β chain in which the codon for βHis81 was changed to that for Gln. Presentation of Ni2+ to ANi-2.3 cells by the LG2 cells transduced with the mutant β chain (hatched bar) was compared with that seen with untransduced LG2 cells (white bar) or LG2 cells transduced with the wild type β chain (black bar).
Discussion
A number of laboratories have isolated Ni2+ reactive T cell clones from patients with Ni2+ hypersensitivity (10, 32, 33). The properties of these clones have varied dramatically. There has been no particular MHCII allele or isotype associated with Ni2+ sensitivity. Some clones, such as the ANi-2.3 clone studied here are self-MHC–restricted in their specificity requiring one of the patient's allelic forms of an MHC molecule as the Ni2+ presenting element. Others have proven to be much more promiscuous, in that they can respond to Ni2+ presented by foreign allelic forms of the MHC molecule. These results contrast to those seen in berylliosis in which there is a very high correlation between Be sensitivity and the presence MHCII DP2 allele (34). Furthermore, in vitro, T cells from berylliosis patients require DP2 bearing antigen presenting cells to respond to Be (35).
The specific role of the MHC bound peptide in Ni2+ presentation has not been extensively examined. The ability of Ni2+ to bind to an MHC bound peptide was demonstrated in one series of experiments, by showing that peptide dependent binding of Ni2+ to an MHC/peptide complex could interfere with conventional T cell recognition of the complex (36). However, there is very little direct data on the role of the MHC bound peptide in Ni2+ presentation.
Our experiments with ANi-2.3 suggest an essential role for a particular MHC bound peptide in Ni2+ presentation whose function is more than simply stabilizing the MHCII molecule. Among the cells we examined, only B cells with an intact antigen processing pathway were able to generate this peptide. However, we did not examine other professional antigen presenting cells such as macrophages and dendritic cells. Furthermore, other peptides with a wider cellular distribution and less stringent processing requirements may be adequate for Ni2+ presentation to other T cell clones (37). Since in our studies we used a costimulation independent T cell transfectoma to detect Ni2+ presentation, we cannot say whether in vivo a requirement for costimulation might also limit the type and state of antigen presenting cells driving the response to Ni2+.
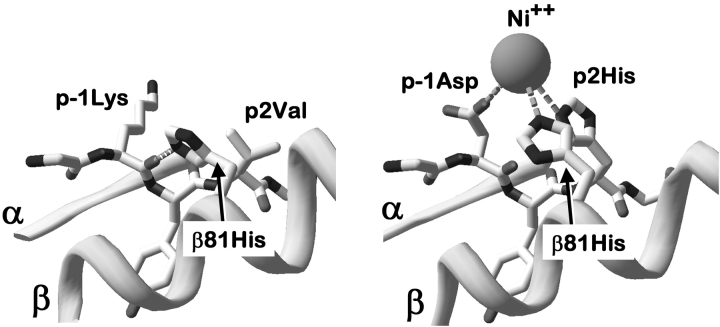
The variety of MHCII alleles capable of Ni2+ presentation and the MHC promiscuity of some Ni2+ reactive clones might be taken as evidence that Ni2+ can be presented to T cells in many different ways. However, our findings that the ANi-2.3 clone requires a specific peptide for Ni2+ presentation and is dependent on His81 of the MHC β chain suggest the possibility of a common theme in Ni2+ presentation. As mentioned above, βHis81 is conserved in nearly all MHCII molecules regardless of allele or isotype. In crystal structures reported thus far this His forms a hydrogen bond to the peptide backbone. For example, Fig. 7 A shows this area of the DR1 molecule bound to an influenza hemagglutinin peptide (38). In this case βHis81 is hydrogen bonded to the backbone carbonyl of a Lys at the p-1 position of the peptide. It is reasonable to assume that the βHis81 of DR52c is in a similar configuration.
Figure 7.
Possible model for Ni2+ coordination by MHCII βHis81 and amino acids at positions p-1 and p2 of the peptide. In the left panel a portion of the structure of the DR1 (DRA*0101, DRB1*0101) bound to a peptide from influenza hemagglutinin (reference 38) is shown in the vicinity of βHis81. The conserved hydrogen bond between βHis81 and the peptide backbone is shown as a dashed line. In the right panel, using Swiss PDB Viewer, peptide amino acids at position p-1 and p2 were changed to Asp and His, respectively. These amino acids and βHis81 were given rotamers that create the correct geometry for three ligands for potential tetragonal coordination (dashed lines) of a Ni2+ ion (sphere).
If the loss of Ni2+ recognition by ANi-2.3 upon our mutation of DR52c βHis81 to Gln, indicates a role for βHis81 in Ni2+ coordination, than we can predict several additional properties of the Ni2+ presenting MHC/peptide complex. First, there must be a new rotamer for the side chain of βHis81. Second, as there are no other suitable amino acids on the MHC surface in the vicinity to provide additional Ni2+ coordination, these must be supplied by the peptide. The peptide amino acids in positions to perform this function are located at p-1 and p2. Therefore, in the DR1 structure we modeled an Asp and a His for the influenza peptide amino acids at positions p-1 and p2 respectively (Fig. 7 B). Allowing only preferred rotamers for these two substituted amino acids and for βHis81, we could easily place these side chains in positions nearly ideal for tetragonal coordination of Ni2+. No accommodations were required of the MHC or peptide backbone or of the rotamers of any other amino acids. Square planar or tetragonal coordination involving aspartic acid and histidine is very common in Ni2+ ion complexes to proteins (39–41) and would be consistent with our findings of the low pH sensitivity of Ni2+ binding to fixed antigen presenting cells. While the order of the Asp and His in the peptide theoretically could be reversed, this alternate configuration did not yield ideal tetragonal geometry with only preferred rotamers.
The attraction of this model is that it suggests a conserved peptide dependent mode of Ni2+ presentation among many different MHCII alleles and isotypes. This hypothesis is also consistent with the finding that T cells reactive to Ni2+ often cross react with other metal ions. For example, ANi-2.3 also responds to copper and gold cations. These could be expected to be coordinated similarly to Ni2+. Furthermore, if sometimes the coordinated Ni2+ dominates other peptide contributions to the interface with the αβTCR, then one could also explain the promiscuity of some T cell clones in recognizing Ni2+ bound to a variety of MHC alleles. As long as the bound peptide provided the appropriate coordination groups, the combination of the bound Ni2+ and other conserved amino acids on the MHC surface common to many alleles could satisfy the T cell receptor. These ideas can be tested in structural and functional studies.
One consequence of placing Ni2+ at a particular location on the MHC/peptide surface is a prediction about the portion of the αβTCR that is likely to be in contact with the Ni2+. There are now numerous crystal structures of αβTCRs bound to MHC/peptide complexes involving both MHCI and MHCII molecules. In all cases, the receptor has a somewhat diagonal orientation on the MHC/peptide surface with the Vα portion toward the peptide NH2 terminus, the Vβ region toward the peptide COOH terminus and the CDR3 regions focused on the center of the peptide. Within these constraints there is quite a bit of rotational variation among different αβTCRs. Therefore, while it is not possible to predict precisely the orientation of the ANi-2.3 αβTCR on a Ni2+/peptide/DR52c complex, the CDR1 or CDR2 of Vα is most likely to be oriented over a Ni2+ coordinated by βHis81 and the p-1 and p2 amino acids of the peptide. The Vα element used by ANi-2.3 is AV01s4, whose CDR1 sequence is SYGATPY and CDR2 sequence is KYFSGDTLV. The most obvious candidate to provide a fourth group to complete the tetragonal coordination of Ni2+ is the Asp in the CDR2 region. Interestingly, an Asp or Glu is present in the COOH-terminal half of the CDR2 regions of nearly all of the Vα elements of the αβTCRs of Ni2+ reactive T cell previously reported (11, 14, 33). In fact, a T cell with a highly MHC promiscuous response to Ni2+ bears a Vα element with a pair of Asp in this region (33).
Although direct coordination of Ni2+ by the side chain of an αβTCR His or acidic amino acid is the most obvious choice, there are other possibilities. For example, in the various solved structures of Ni2+ ions bound to proteins, one of the ligands for the metal can be an oxygen from water, the protein backbone or a side chain from Ser or Thr (39, 41). Furthermore, we cannot rule out the possibility that the αβTCR recognizes the MHC and peptide amino acid side chains that have changed their configuration in order to accommodate the Ni2+ rather than the Ni2+ itself. The definitive answer to these questions will require crystal structures of these complexes. However, in the mean time the model we propose here offers a good starting point for designing structure/function studies.
Acknowledgments
The authors would like to acknowledge Jerry Bill, Janice White, Francis Crawford, and David Krum for assistance and support.
This work was supported in part by U.S. Public Health Service grants AI-17134, AI-18785 and AI-22295.
Jörg Vollmer's present address is Coley Pharmaceutical GmbH, Elisabeth-Selbert-Str. 9, D-40764 Langenfeld, Germany.
Corinne Moulon's present address is Dictagene, Chemin de la Vuiliette 4, 1000 Lausanne 25, Switzerland.
Footnotes
Abbreviations used in this paper: BSS, balanced salt solution; GFP, green fluorescent protein; SEB, staphylococcal enterotoxin B.
References
- 1.Melian, A., E.M. Beckman, S.A. Porcelli, and M.B. Brenner. 1996. Antigen presentation by CD1 and MHC-encoded class I-like molecules. Curr. Opin. Immunol. 8:82–88. [DOI] [PubMed] [Google Scholar]
- 2.Budinger, L., and M. Hertl. 2000. Immunologic mechanisms in hypersensitivity reactions to metal ions: an overview. Allergy. 55:108–115. [DOI] [PubMed] [Google Scholar]
- 3.Hostynek, J.J. 1997. Gold: an allergen of growing significance. Food Chem. Toxicol. 35:839–844. [DOI] [PubMed] [Google Scholar]
- 4.Newman, L.S., J. Lloyd, and E. Daniloff. 1996. The natural history of beryllium sensitization and chronic beryllium disease. Environ. Health Perspect. 104S:937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng, Z., A.R. Castano, B.W. Segelke, E.A. Stura, P.A. Peterson, and I.A. Wilson. 1997. Crystal structure of mouse CD1: An MHC-like fold with a large hydrophobic binding groove. Science. 277:339–345. [DOI] [PubMed] [Google Scholar]
- 6.von Bonin, A., B. Ortmann, S. Martin, and H.U. Weltzien. 1992. Peptide-conjugated hapten groups are the major antigenic determinants for trinitrophenyl-specific cytotoxic T cells. Int. Immunol. 4:869–874. [DOI] [PubMed] [Google Scholar]
- 7.Franco, A., T. Yokoyama, D. Huynh, C. Thomson, S.G. Nathenson, and H.M. Grey. 1999. Fine specificity and MHC restriction of trinitrophenyl-specific CTL. J. Immunol. 162:3388–3394. [PubMed] [Google Scholar]
- 8.Rao, A., W.W. Ko, S.J. Faas, and H. Cantor. 1984. Binding of antigen in the absence of histocompatibility proteins by arsonate-reactive T-cell clones. Cell. 36:879–888. [DOI] [PubMed] [Google Scholar]
- 9.Diamond, D.J., P. Szalay, D. Symer, P. Hao, H.S. Shin, R.Z. Dintzis, H.M. Dintzis, E.L. Reinherz, and R.F. Siliciano. 1991. Major histocompatibility complex independent T cell receptor-antigen interaction: functional analysis using fluorescein derivatives. J. Exp. Med. 174:229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moulon, C., J. Vollmer, and H.U. Weltzien. 1995. Characterization of processing requirements and metal cross-reactivities in T cell clones from patients with allergic contact dermatitis to nickel. Eur. J. Immunol. 25:3308–3315. [DOI] [PubMed] [Google Scholar]
- 11.Vollmer, J., M. Fritz, A. Dormoy, H.U. Weltzien, and C. Moulon. 1997. Dominance of the BV17 element in nickel-specific human T cell receptors relates to severity of contact sensitivity. Eur. J. Immunol. 27:1865–1874. [DOI] [PubMed] [Google Scholar]
- 12.Blank, U., B. Boitel, D. Mege, M. Ermonval, and O. Acuto. 1993. Analysis of tetanus toxin peptide/DR recognition by human T cell receptors reconstituted into a murine T cell hybridoma. Eur. J. Immunol. 23:3057–3065. [DOI] [PubMed] [Google Scholar]
- 13.Verreck, F.A., A. van de Poel, J.W. Drijfhout, R. Amons, J.E. Coligan, and F. Koning. 1996. Natural peptides isolated from Gly86/Val86-containing variants of HLA-DR1, -DR11, -DR13, and -DR52. Immunogenetics. 43:392–397. [DOI] [PubMed] [Google Scholar]
- 14.Vollmer, J., H.U. Weltzien, and C. Moulon. 1999. TCR reactivity in human nickel allergy indicates contacts with complementarity-determining region 3 but excludes superantigen-like recognition. J. Immunol. 163:2723–2731. [PubMed] [Google Scholar]
- 15.Gorski, J., C. Irle, E.M. Mickelson, M.J. Sheehy, A. Termijtelen, C. Ucla, and B. Mach. 1989. Correlation of structure with T cell responses of the three members of the HLA-DRw52 allelic series. J. Exp. Med. 170:1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glimcher, L.H., D.J. McKean, E. Choi, and J.G. Seidman. 1985. Complex regulation of class II gene expression: analysis with class II mutant cell lines. J. Immunol. 135:3542–3550. [PubMed] [Google Scholar]
- 17.Salter, R.D., and P. Cresswell. 1986. Impaired assembly and transport of HLA-A and -B antigens in a mutant TxB cell hybrid. EMBO J. 5:943–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erlich, H., J.S. Lee, J.W. Petersen, T. Bugawan, and R. DeMars. 1986. Molecular analysis of HLA class I and class II antigen loss mutants reveals a homozygous deletion of the DR, DQ, and part of the DP region: implications for class II gene order. Hum. Immunol. 16:205–219. [DOI] [PubMed] [Google Scholar]
- 19.Koning, F., I. Schreuder, M. Giphart, and H. Bruning. 1984. A mouse monoclonal antibody detecting a DR-related MT2-like specificity: serology and biochemistry. Hum. Immunol. 9:221–230. [DOI] [PubMed] [Google Scholar]
- 20.Walker, E., N.L. Warner, R. Chesnut, J. Kappler, and P. Marrack. 1982. Antigen-specific. I region-restricted interactions in vitro between tumor cell lines and T cell hybridomas. J. Immunol. 128:2164–2169. [PubMed] [Google Scholar]
- 21.Mosmann, T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 65:55–63. [DOI] [PubMed] [Google Scholar]
- 22.Nelson, C.A., R.W. Roof, D.W. McCourt, and E.R. Unanue. 1992. Identification of the naturally processed form of hen egg white lysozyme bound to the murine major histocompatibility complex class II molecule I-Ak. Proc. Natl. Acad. Sci. USA. 89:7380–7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gugasyan, R., I. Vidavsky, C.A. Nelson, M.L. Gross, and E.R. Unanue. 1998. Isolation and quantitation of a minor determinant of hen egg white lysozyme bound to I-Ak by using peptide-specific immunoaffinity. J. Immunol. 161:6074–6083. [PubMed] [Google Scholar]
- 24.Mitchell, T.C., D. Hildeman, R.M. Kedl, T.K. Teague, B.C. Schaefer, J. White, Y. Zhu, J. Kappler, and P. Marrack. 2001. Immunological adjuvants promote activated T cell survival via induction of Bcl-3. Nat. Immunol. 2:397–402. [DOI] [PubMed] [Google Scholar]
- 25.Schaefer, B.C., T.C. Mitchell, J.W. Kappler, and P. Marrack. 2001. A novel family of retroviral vectors for the rapid production of complex stable cell lines. Anal. Biochem. 297:86–93. [DOI] [PubMed] [Google Scholar]
- 26.Tiercy, J.M., J. Gorski, H. Betuel, A.C. Freidel, L. Gebuhrer, M. Jeannet, and B. Mach. 1989. DNA typing of DRw6 subtypes: correlation with DRB1 and DRB3 allelic sequences by hybridization with oligonucleotide probes. Hum. Immunol. 24:1–14. [DOI] [PubMed] [Google Scholar]
- 27.Sintasath, D.M., T. Tang, R. Slack, E.E. Tilley, J. Ng, R.J. Hartzman, and C.K. Hurley. 1999. Relative HLA-DRB1*13 allele frequencies and DRB3 associations of unrelated individuals from five US populations. Hum. Immunol. 60:1001–1010. [DOI] [PubMed] [Google Scholar]
- 28.Hennecke, J., and D.C. Wiley. 2001. T cell receptor-MHC interactions up close. Cell. 104:1–4. [DOI] [PubMed] [Google Scholar]
- 29.Rudolph, M.G., and I.A. Wilson. 2002. The specificity of TCR/pMHC interaction. Curr. Opin. Immunol. 14:52–65. [DOI] [PubMed] [Google Scholar]
- 30.Li, Y., H. Li, N. Dimasi, J.K. McCormick, R. Martin, P. Schuck, P.M. Schlievert, and R.A. Mariuzza. 2001. Crystal structure of a superantigen bound to the high-affinity, zinc-dependent site on MHC class II. Immunity. 14:93–104. [DOI] [PubMed] [Google Scholar]
- 31.Petersson, K., M. Hakansson, H. Nilsson, G. Forsberg, L.A. Svensson, A. Liljas, and B. Walse. 2001. Crystal structure of a superantigen bound to MHC class II displays zinc and peptide dependence. EMBO J. 20:3306–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinigaglia, F., D. Scheidegger, G. Garotta, R. Scheper, M. Pletscher, and A. Lanzavecchia. 1985. Isolation and characterization of Ni-specific T cell clones from patients with Ni-contact dermatitis. J. Immunol. 135:3929–3932. [PubMed] [Google Scholar]
- 33.Vollmer, J., H.U. Weltzien, K. Gamerdinger, S. Lang, Y. Choleva, and C. Moulon. 2000. Antigen contacts by Ni-reactive TCR: typical ab chain cooperation versus a chain-dominated specificity. Int. Immunol. 12:1723–1731. [DOI] [PubMed] [Google Scholar]
- 34.Fontenot, A.P., M. Torres, W.H. Marshall, L.S. Newman, and B.L. Kotzin. 2000. Beryllium presentation to CD4+ T cells underlies disease-susceptibility HLA-DP alleles in chronic beryllium disease. Proc. Natl. Acad. Sci. USA. 97:12717–12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fontenot, A.P., L.S. Newman, and B.L. Kotzin. 2001. Chronic beryllium disease: T cell recognition of a metal presented by HLA-DP. Clin. Immunol. 100:4–14. [DOI] [PubMed] [Google Scholar]
- 36.Romagnoli, P., A.M. Labhardt, and F. Sinigaglia. 1991. Selective interaction of Ni with an MHC-bound peptide. EMBO J. 10:1303–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nasorri, F., S. Sebastiani, V. Mariani, O. De Pita, P. Puddu, G. Girolomoni, and A. Cavani. 2002. Activation of nickel-specific CD4+ T lymphocytes in the absence of professional antigen-presenting cells. J. Invest. Dermatol. 118:172–179. [DOI] [PubMed] [Google Scholar]
- 38.Hennecke, J., A. Carfi, and D.C. Wiley. 2000. Structure of a covalently stabilized complex of a human alphabeta T-cell receptor, influenza HA peptide and MHC class II molecule, HLA-DR1. EMBO J. 19:5611–5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White, A., X. Ding, J.C. vanderSpek, J.R. Murphy, and D. Ringe. 1998. Structure of the metal-ion-activated diphtheria toxin repressor/tox operator complex. Nature. 394:502–506. [DOI] [PubMed] [Google Scholar]
- 40.Benning, M.M., T. Haller, J.A. Gerlt, and H.M. Holden. 2000. New reactions in the crotonase superfamily: structure of methylmalonyl CoA decarboxylase from Escherichia coli. Biochemistry. 39:4630–4639. [DOI] [PubMed] [Google Scholar]
- 41.Hall, D.R., D.G. Gourley, G.A. Leonard, E.M. Duke, L.A. Anderson, D.H. Boxer, and W.N. Hunter. 1999. The high-resolution crystal structure of the molybdate-dependent transcriptional regulator (ModE) from Escherichia coli: a novel combination of domain folds. EMBO J. 18:1435–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]