Abstract
Highly conserved glycine residues within span I and span II of the phenylalanine and tyrosine transporter PheP were shown to be important for the function of the wild-type protein. Replacement by amino acids with increasing side chain volume led to progressive loss of transport activity. Second-site suppression studies performed with a number of the primary mutants revealed a tight packing arrangement between spans I and II that is important for function and an additional interaction between spans I and III. We also postulate that a third motif, GXXIG, present in span I and highly conserved within different members of the amino acid-polyamine-organocation family, may function as a dimerization motif. Surprisingly, other highly conserved residues, such as Y60 and L41, could be replaced by various residues with no apparent loss of activity.
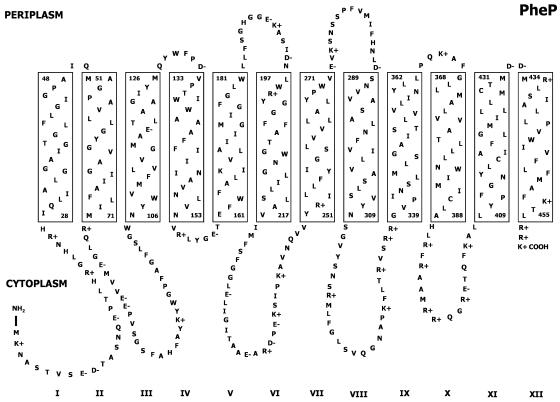
The PheP protein of Escherichia coli is a polytopic integral membrane protein which, when energized by the proton motive force, actively transports phenylalanine and tyrosine into the cell (1, 15, 20). The topology of the protein has been established from a combination of hydropathy plots and alkaline phosphatase sandwich fusions and has been shown to involve 12 membrane spans with both the N and C termini in the cytoplasm (17). The current topological model for the PheP protein is shown in Fig. 1. A number of residues in this protein that are of critical importance for the transport function have also been identified, as has one residue implicated in substrate recognition (1, 16, 18, 19). The PheP protein is part of the amino acid transporter family (AAT) [TC2.A.3.1], which in turn is a member of the amino acid-polyamine-organocation (APC) superfamily (9).
FIG. 1.
Topological model of the PheP permease.
Whereas the APC superfamily includes proteins from archaea, prokaryotes, and eukaryotes, members of the AAT family are all of bacterial origin. The AAT family is composed of 36 members, including seven characterized permeases which transport a variety of amino acids and 29 putative transporters whose functions are yet to be determined.
The proposed topological model of PheP shows that transmembrane spans I and II are closely associated with a tight periplasmic turn. Comparison of the amino acid sequences of members of the AAT family has shown that many residues found in span I and a smaller number of residues located in span II are very highly conserved in most members of the family. Spans I and II are also characterized by a relatively large number of highly conserved glycine residues. Glycine residues are commonly found in transmembrane spanning segments of membrane proteins (2, 11), and several studies have shown that these residues play a number of roles, including helix capping, modulation of helix flexibility, and mediation of close helix-helix association (22, 3, 10). The presence of glycine residues at helix-helix interfacial positions allows close approach of interacting helices because of the small size of the glycine side chain. Additional studies designed to identify helix packing motifs have shown that the sequence GXXXG can mediate high-affinity homo-oligomerization (7, 23). Evidence that glycine residues provide the basis for a close association between interacting helices also comes from studies which have shown that in general, glycine does not form packing voids in folded membrane proteins and has higher packing values than larger hydrophobic residues (3).
In this study, we investigated close helix packing by identifying the highly conserved small residues whose replacement by amino acids with larger side chains leads to a loss of transport activity and by showing that for some of these residues transport activity can be restored by complementary changes (large to small) in residues in other helices. In this way, we identified close helix packing between helices I and II and also between helices I and III. A possible role for some of the other highly conserved residues is also discussed below.
MATERIALS AND METHODS
Bacterial strains and media.
The following E. coli strains were used: JM101 [Δ(lac pro) supE thi (F′ traD36 proAB lacIqZΔM15)] for work involving bacteriophage M13mp18; XLI-Red [endA1 gyrA96 thi-1 hsdSR17 supE44 relA1 lac mutD5 mutS mutT Tn10 (Tetr); Stratagene], a random mutator strain which was used to introduce second-site suppressor mutations; JP7910 (aroP1000 pheP367 tyrP mtr-24 tnaAB2 tyrR366), which was used for transport assays and pulse-labeling of the PheP protein; JP4538 (pheA pheP aroP tyrP), which was used for selection of second-site revertants capable of transporting phenylalanine; and JP7912 (tyrA pheP aroP tyrP), which was used for selection of second-site revertants capable of transporting tyrosine. The minimal medium used was the half-strength medium 56 described by Monod et al. (14) supplemented with 0.2% glucose and required growth factors. Luria agar and Lennox broth were used as complete media. Kanamycin was added to nutrient and minimal media to a final concentration of 25 μg/ml.
Recombinant DNA techniques.
Standard recombinant DNA techniques were used essentially as described by Sambrook et al. (25).
Site-directed mutagenesis and sequence analysis.
Oligonucleotide-directed site-specific mutagenesis was used to introduce various amino acid substitutions into the pheP gene by the method described by Vandeyar et al. (26). mpMU3137 (17), a construct containing a modified pheP gene (with ATG instead of GTG as the start codon), was used as a template for mutagenesis. DNA sequencing of the entire pheP gene was performed to verify all the mutations. Automated DNA sequencing was performed with a model 377 DNA sequencer and ABI BigDye terminators (Perkin-Elmer Corporation).
Transport assays.
Active transport was measured as previously described (27). Briefly, wild-type and mutant pheP alleles carried on plasmid pLG339 were transformed into E. coli strain JP7910. Cultures were grown in half-strength buffer 56 containing 0.2% glucose, the appropriate growth factors, and kanamycin at 37°C to an optical density at 600 nm of 0.45. Cells were washed in half-strength buffer 56 containing 0.2% glucose and chloramphenicol (80 μg/ml). Samples were preincubated at 30°C for 5 min before l-[14C]phenylalanine was added to a final concentration of 10 μM. Aliquots (150 μl) were removed at the appropriate times and filtered through cellulose acetate filters, which were then washed twice with half-strength buffer 56. Intracellular activity was determined by liquid scintillation counting. Assays were done at least twice, and the variation in uptake values obtained in repeat tests was <10%.
Pulse-labeling and immunoprecipitation.
Cells harboring a plasmid expressing wild-type and mutant forms of PheP permease were cultured under the conditions described above for the transport assays and were grown to an optical density at 600 nm of 0.5. Aliquots (0.5 ml) of the cell culture were pulse-labeled with 50 μCi of [35S]methionine-cysteine for 1 min at 37°C. Cell extracts were prepared and immunoprecipitated by using the method specific for integral membrane proteins described by Ito and Akiyama (8). Specific proteins were precipitated with antiserum TTP7, which binds PheP permease. Samples were electrophoresed on sodium dodecyl sulfate-12% polyacrylamide gels. Each dried gel was exposed to X-ray film for at least 48 h. The densities of the radioactive bands were measured by scanning the autoradiographs with a Molecular Dynamics scanning densitometer, and the values were used to estimate the levels of mutant proteins relative to the levels of the wild-type proteins.
Pulse-chase labeling.
Cells expressing wild-type and mutant forms of the PheP protein were cultured by using the conditions described above for pulse-labeling and immunoprecipitation. Aliquots (2 ml) of the culture were labeled with 200 μCi of [35S]methionine-cysteine for 1 min at 37°C. Incorporation of radiolabeled amino acid was stopped after 1 min by addition of excess nonradioactive methionine-cysteine (200 μg/ml). Samples (0.5 ml) were taken 1, 5, 15 and 30 min after quenching for immunoprecipitation.
Random mutagenesis and screening for second-site suppressor mutations.
Random mutations were introduced into a pheP allele carrying a primary site null mutation by propagating a plasmid containing this gene in the mutator strain XLI-Red for three overnight cycles. Plasmids were harvested and then transformed into strains JP4538 and JP7912, which lacked the ability to synthesize phenylalanine and tyrosine, respectively. Transformed strain JP4538 was plated onto minimal medium containing 10−5M phenylalanine, while transformed strain JP7912 was plated onto minimal medium containing 10−5 or 10−6 M tyrosine. These media could only support the growth of cells capable of transporting phenylalanine and tyrosine, respectively (i.e., wild-type revertants or suppressed mutants). Plasmids from colonies showing the capacity to grow on these media were harvested and sequenced.
RESULTS
Importance of conserved small residues for transport activity.
Transmembrane spans I and II contain a total of 17 glycine and alanine residues. By using site-directed mutagenesis, various substitutions were introduced into each of these 17 positions, and the transport activities of each of the mutants are shown in Table 1. Table 1 also shows the degrees of conservation of these residues in the first four groups (32 members) of the AAT family. The four members of group V were not included in the analysis because they exhibited major differences in a number of highly conserved sequences shown elsewhere to be important for function (1, 16, 18, 19). Two conclusions can be drawn from the data in Table 1. First, in the case of these glycine and alanine residues there is a tight correlation between the degree of conservation and the importance of the residue for function. Second, for five of the glycines in span I and perhaps two of the glycines in span II, a progressive increase in the side chain volume of the amino acid occupying the position is accompanied by a progressive loss of transport activity. In general, the effects on phenylalanine and tyrosine transport are comparable. However, in some cases, complete loss of tyrosine transport compared with low levels of phenylalanine transport is invaluable for further analyses. Two alanine residues, A32 and A52, occupy positions sensitive to side chain volume.
TABLE 1.
Transport activities for phenylalanine of various PheP mutants, in which larger amino acids have been substituted for glycine or alanine
| Residuea | Frequencyb | Transport activities (% of wild-type activity) with the following substitutionsc:
|
||
|---|---|---|---|---|
| Alanine | Valine | Leucine | ||
| A32 | 22 | 24 (13) | 13 (7) | |
| G34 | 30 | 76 | 43 (2) | 19 (0) |
| G35 | 27 | 22 (5) | 10 (1) | 2 (0) |
| A36 | 20 | 75 (97) | 92 | |
| G38 | 32 | 30 | 13 (0) | 0 (5) |
| G40 | 31 | 12 (0) | 0 (0) | 0 (0) |
| G44 | 28 | 31 | 12 (0) | 5 (0) |
| G46 | 22 | 91 (205) | 77 | |
| A48 | 6 | 64 (39) | ||
| A52 | 28 | 78 (85) | 29 (25) | |
| G53 | 32 | 70 | 0 (0) | 0 (0) |
| A55 | 4 | 93 | ||
| G59 | 2 | 100 | 100 | |
| G61 | 4 | 102 (108) | 74 | |
| A63 | 8 | 75 (152) | 68 (93) | |
| G64 | 30 | 71 | 56 (22) | 0 (0) |
| A67 | 4 | 57 (22) | 175 (15) | |
Residues where a progressive increase in the side chain volume resulted in a progressive decrease in transport activity are indicated by boldface type.
Number of times that the residue occurs at the position in the 32 members of groups I to IV of the AAT family.
Steady-state phenylalanine uptake expressed as a percentage of the uptake by the wild-type permease. The values in parentheses are tyrosine uptake expressed as a percentage of the uptake by the wild-type permease.
Insertion of mutant proteins into the membrane.
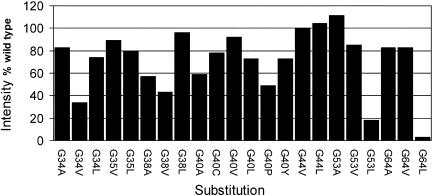
To test whether the lack of transport activity in the various mutants carrying replacements of glycine residues was a consequence of an inability of the PheP protein to insert into the membrane, cultures expressing wild-type and various mutant forms of PheP were pulse-labeled as described in Materials and Methods. Membrane fractions were prepared, and protein was immunoprecipitated by using the PheP-specific antiserum TTP7 according to the method of Ito and Akiyama (8). Samples were electrophoresed on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, and the intensities of the wild-type and mutant forms of the PheP protein were measured by densitometry and compared. The results of these experiments are shown in Fig. 2.
FIG. 2.
Densitometric analysis of amounts of mutant proteins expressed and inserted into the membrane relative to the wild-type level.
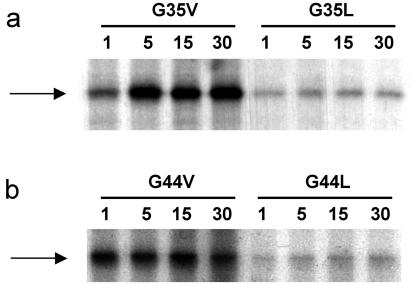
As the results show, the loss of transport activity in the majority of cases was not a consequence of a simple failure to insert into the membrane. However, in two cases replacement of glycine at positions 53 and 64 by leucine drastically reduced the amount of protein inserted into the membrane to less than 20% of wild-type level. Experiments involving pulse-chase labeling of cells were carried out to determine whether the loss of transport activity was due to instability of the protein in the membrane. Strains expressing wild-type and mutant forms of PheP were pulse-labeled with radioactive [35S]methionine-cysteine and then chased with an excess of cold methionine-cysteine. The PheP protein was then immunoprecipitated after the chase by using the PheP-specific antibody TTP7. The results of this study for four mutants which had lost most of the transport activity but retained their insertion into the membrane are shown in Fig. 3.
FIG. 3.
Stability of mutant forms of the PheP protein with G35V, G35L, G44V, and G44L substitutions. Strains expressing the PheP protein were pulse-labeled with [35S]methionine-cysteine for 1 min and then chased with excess cold methionine-cysteine. Protein was immunoprecipitated 1, 5, 15, and 30 min after the chase, and samples were electrophoresed on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. Bands representing the PheP protein were visualized by autoradiography.
As the results show, PheP proteins containing the substitutions G35V, G35L, G44V, and G44L were expressed and maintained in a stable form, and therefore, the loss of transport function was not due to degradation of the protein. Stability studies were also performed with strains expressing PheP proteins having substitutions at the other glycine residues that are shown in Fig. 2. Similarly, all these mutant forms of PheP appeared to be expressed stably (data not shown).
Replacement of other residues in spans I and II.
The remaining 27 residues that comprise spans I and II consist of amino acids with side chains larger than those of alanine or glycine. These residues were replaced by smaller amino acids (either alanine, glycine, or valine) to determine whether reducing the side chain volume adversely affected the transport function. Only changes in three residues (T39, F42, and I49) significantly affected transport, even though a number of residues (I37, L41, F42, I49, P54, Y60, and M71) were highly conserved within the family (data not shown). Replacement of T39 by alanine reduced the transport activity to 5% of the wild-type activity, replacement of F42 by valine reduced the transport activity to 14% of the wild-type activity, and replacement of I49 by alanine reduced the transport activity to 4% of the wild-type activity.
Selection of second-site suppressors.
Having identified a number of glycine residues in span I that are critical for activity, we decided to use second-site suppressor mutagenesis to identify any glycines in span I that might be involved in the close association between helices. In order to select second-site suppressors in primary mutants with mutations in the PheP protein, it was necessary to start with mutants that totally lacked transport activity. This is because the selection for suppressors was performed on minimal media supplemented with very low levels (10−5 to 10−6 M) of the amino acid being transported and experience has shown that even very low levels of transport activity in the original mutant are sufficient to allow growth to occur. The PheP transport protein transports both phenylalanine and tyrosine, but it has a 10-fold-higher affinity for phenylalanine (Km, 2 μM) than for tyrosine (Km, 30 μM) (1).
In a previous study in which second-site suppressors were selected (18), a transport-negative strain with a mutation (pheA) that prevented endogenous biosynthesis of phenylalanine was used. A number of the mutants in Table 1 exhibited low-level uptake of phenylalanine but fortunately were not able to transport tyrosine. Consequently, we introduced the mutant alleles into a transport-negative strain with a mutation (tyrA) that prevented tyrosine biosynthesis, and we were able to search for suppressed strains on minimal medium supplemented with very low levels of tyrosine.
Eleven primary mutants (G34V, G34L, G35V, G38V, G38L, G40C, G40V, G40L, G40Y, G44V and G44L) which exhibited no transport activity for either tyrosine or phenylalanine were chosen for this study. Suppressed strains having primary substitutions at G34, G35, G38, or G44 were selected on minimal medium containing 10−5 or 10−6 M tyrosine by using the tyrA host, while suppressed strains having primary substitutions at G40 were selected on minimal medium containing 10−5 M phenylalanine with the corresponding pheA host.
Plasmids carrying the pheP gene were isolated from 200 colonies which had grown on the selective medium, and these plasmids were then sequenced to confirm the presence of the primary mutation. Approximately 50% of the colonies that grew on selective medium were primary-site revertants and were not studied further. The pheP alleles that still retained the primary mutation were then fully sequenced in order to determine the type and position of any secondary mutations. Only five of the isolates were found to have a second-site mutation within pheP, and these isolates were used for further study, while the other isolates were discarded. The phenotypes of the five strains that were selected and the effects of the second-site mutations on transport activity are shown in Table 2. Second-site suppressor mutations were not isolated in strains carrying the primary substitutions G34V, G34L, G38L, G40V, G40L, and G44L, and it is possible that replacement of glycine by leucine perturbed the structure too much to allow any compensating changes to restore function.
TABLE 2.
Transport activities of strains expressing PheP protein carrying primary-site and second-site mutations
| Primary substitution | Transport activity (%)a
|
Second-site suppressor | Transport activity (%)a
|
Location of suppressor mutation | ||
|---|---|---|---|---|---|---|
| Phe uptake | Tyr uptake | Phe uptake | Tyr uptake | |||
| G40Y | 0 | 0 | S323F | 30 | 0 | Cytoplasmic loop between spans VIII and IX |
| G40C | 0 | 0 | Y60C | 15 | 15 | Span II |
| G35V | 10 | 1 | Y107C | 45 | 30 | Span III |
| G38V | 13 | 0 | G44A | 13 | 2 | Span I |
| G44V | 12 | 0 | G38A | 30 | 6 | Span I |
Phenylalanine and tyrosine uptake are expressed as percentages of the wild-type levels.
Second-site suppressors for mutations that changed glycine at position 40.
Following random mutagenesis and selection of suppressed strains on minimal medium containing a low level of phenylalanine (10−5 M), two mutants with independent second-site suppressor mutations were isolated (Table 2).
One suppressor mutation, a change at S323 to phenylalanine (S323F), significantly suppressed the defect in phenylalanine transport caused by the primary mutation G40Y, resulting in levels that were 30% of wild-type levels; at the same time the mutation did not have any effect on the inability of the mutant to transport tyrosine. According to the current topological model (17), the S323 residue is located within a cytoplasmic loop between spans VIII and IX, whereas G40 is located in the middle of span I. If the model is correct, it seems unlikely that there would be any direct interaction between these two residues, and the suppression effect of S323F in this case is most probably indirect. In a previous paper it was reported that the G40S change provides low-level suppression (4%) of the primary P341R null mutation (18), which is located at the C terminus of span IX.
The second suppressor mutation affected the primary substitution G40C and involved a change in a span II residue, changing Y60 to cysteine. This suppressor mutation restored both the level of tyrosine transport and the level of phenylalanine transport to 15% of the wild-type levels (Table 2). In contrast to the previous results, these two residues are located at about the same position in the membrane, since according to the topological model, G40 and Y60 are approximately in the middle of adjacent transmembrane spans, and this makes direct interaction a possibility. This possibility was investigated further, as described below.
Effects of side chain volume at position 40 on efficiency of suppression.
Starting with the primary mutant G40C, tyrosine 60 was changed to either alanine, serine, or methionine (Table 3). Each of these changes restored activity; Y60A restored the activity to levels that were about 80% of the wild-type level, Y60M restored the activity to levels that were more than 130% of the wild-type level, and Y60S restored the activity to levels that were 101% of the wild-type level for phenylalanine and 50% of the wild-type level for tyrosine. In other words, the requirement for a glycine residue at position 40 is determined by the nature of the residue occupying position 60 in span II.
TABLE 3.
Transport activities of strains expressing PheP protein carrying primary-site mutations at position 40 and second-site mutations at position 60
| Primary substitution | Secondary substitution | Phe uptake (% of wild type) | Tyr uptake (% of wild type) |
|---|---|---|---|
| G40C | 0 | 0 | |
| G40C | Y60A | 83 | 74 |
| G40C | Y60S | 101 | 50 |
| G40C | Y60M | 133 | 164 |
| G40A | 12 | 0 | |
| G40V | 0 | 0 | |
| G40L | 0 | 0 | |
| G40A | Y60M | 75 | 65 |
| G40V | Y60M | 0 | 0 |
| G40L | Y60M | 0 | 0 |
We next tested the ability of the suppressor Y60M to suppress the primary mutations G40A, G40V, and G40L. As Table 3 shows, although Y60M was an efficient suppressor of G40A, it was unable to suppress either of the two primary mutants with a larger primary substitution (G40V and G40L).
We constructed two more double mutants, G40V Y60A and G40L Y60A, but neither of these exhibited transport activity for either phenylalanine or tyrosine (data not shown).
Additional interactions between spans I and II.
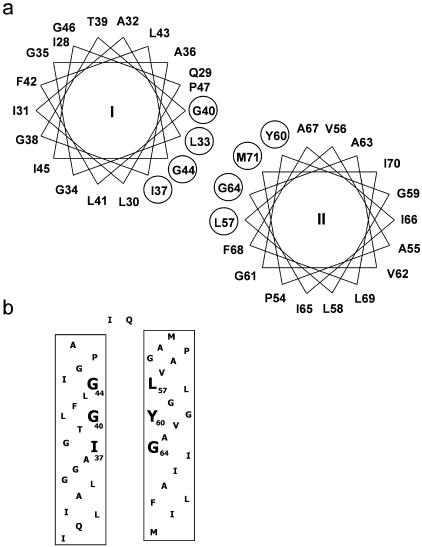
Figure 4a shows a helical wheel representation of residues in spans I and II, and Fig. 4b provides a possible lateral view of the helices within the membrane. In Fig. 4a the helical wheel plots demonstrate that residues I37, G44, L33, and G40 cluster on one side of span I, while residues L57, G64, M71, and Y60 are located on one face of span II. These residues are also shown in Fig. 4b lining one side of each transmembrane span. The residues indicated are located one helical turn apart along the length of each span. Since the data presented above provided evidence that there is a close interaction between residue G40 in span I and residue Y60 in span II, we investigated possible interactions between G44 and L57 and between I37 and G64. The results are shown in Table 4.
FIG. 4.
Diagrammatic representation of transmembrane spans I and II. (a) Helical wheel plots of spans I and II, assuming a periodicity of 3.4 residues per turn. Residues proposed to occupy interfacial positions are circled. (b) Possible lateral view of spans I and II in the membrane. Residues proposed to occupy interfacial positions are numbered and indicated by boldface type.
TABLE 4.
Suppressor effects of L57A and 137V on the primary-site mutations G44V and G64V, respectively
| Primary substitution | Secondary substitution | Phe uptake (% of wild type) | Tyr uptake (% of wild type) |
|---|---|---|---|
| G44V | 12 | 0 | |
| L57A | 120 | 113 | |
| G44V | L57A | 57 | 32 |
| G64L | 0 | 0 | |
| 137V | 127 | 114 | |
| G64V | 137V | 20 | 12 |
In the G44V mutant, replacement of L57 by the smaller amino acid alanine resulted in restoration of 57% of the phenylalanine transport activity and 32% of the tyrosine transport activity. The L57A substitution alone in the wild-type protein caused a slight increase in transport activity.
Similarly, the I37V change, when introduced into the primary mutant protein G64L, restored 20% of the phenylalanine transport activity and 12% of the tyrosine transport activity. In the wild-type protein, such a change alone caused a slight increase in transport activity.
Second-site suppressors of a mutation changing the glycine at position 35.
Strains containing the primary substitution G35V retained a low level of phenylalanine uptake (10% of the wild-type uptake), but the level of tyrosine transport was reduced to only 1% of the wild-type levels. Because of the relatively low affinity of the PheP protein for tyrosine, we endeavored to select suppressed strains on minimal medium containing 10−6M tyrosine. A mutant able to grow on this medium was isolated and subsequently shown to have a second mutation that caused a tyrosine-to-cysteine change at position 107 (Y107C). In the current topological model position 107 lies within transmembrane span III close to the cytoplasm, whereas position 35 is closer to the center of span I. In this double mutant, phenylalanine and tyrosine uptake increased by 4.5- and 30-fold, respectively, to 45 and 30% the wild-type levels (Table 2). In the wild-type protein the single Y107C change resulted in 2.6- and 2-fold increases in transport activity for phenylalanine and tyrosine, respectively (Table 5).
TABLE 5.
Transport activities of strains expressing PheP protein carrying mutations at positions 35 and 107
| Primary substitution | Secondary substitution | Phe uptake (% of wild type) | Tyr uptake (% of wild type) |
|---|---|---|---|
| G35V | 10 | 1 | |
| G35V | Y107C | 45 | 30 |
| Y107C | 258 | 197 | |
| Y107A | 316 | 224 | |
| Y107V | 115 | 100 | |
| Y107L | 100 | 54 | |
| G35A | 22 | NDa | |
| G35V | 10 | 1 | |
| G35L | 2 | 0 | |
| G35A | Y107A | 100 | 64 |
| G35V | Y107A | 55 | 27 |
| G35L | Y107A | 43 | 42 |
ND, not determined.
In the wild-type background changing Y107 to leucine did not alter phenylalanine transport, changing Y107 to valine resulted in a slight increase in phenylalanine transport, and changing Y107 to alanine resulted in a threefold increase in phenylalanine transport and a twofold increase in tyrosine transport (Table 5).
Having established that a reduction in the side chain volume at position 107 could suppress the effects of a primary-site mutation which involved an increase in the side chain volume at position 35, we also wanted to test whether the Y107A change could suppress the effects of a number of other substitutions at position 35 (namely, G35A, G35V, and G35L). Table 5 shows that the Y107A substitution significantly restored the transport activity for each of these primary changes. The suppression was most clearly seen with the suppressed G35L Y107A strain. In this case, the very low transport activities for phenylalanine and tyrosine (2 and 0%, respectively) observed in the single G35L mutant were increased to 43 and 42% of the wild-type levels in the G35L mutant with the Y107A suppressor.
Specificity of Y107C and Y60C suppressor mutations.
In the experiments described above, we showed that the Y107C change suppressed the effect of the primary-site G35V substitution. To further test the specificity of the Y107 suppressor, we tested the effect of the Y107C change on another primary-site mutant which produced a null phenotype, G40C. In this case there was no suppression of the original mutant phenotype (Table 6). We also wanted to test the specificity of the Y60C suppressor, which suppressed the effect of the primary-site substitution G40C. Table 6 shows that the Y60C substitution did not suppress the effects of the G35V primary-site substitution. These results confirm the specificity of the suppression caused by changes at Y60 and Y107.
TABLE 6.
Specificity of suppressor mutations Y107C and Y60C
| Primary substitution | Secondary substitution | Phe uptake (% of wild type) | Tyr uptake (% of wild type) |
|---|---|---|---|
| G40C | 0 | 0 | |
| G40C | Y107C | 0 | 0 |
| G35V | 10 | 1 | |
| G35V | Y60C | 14 | 0 |
Second-site suppressors of mutations that change the glycine at position 38 and the glycine at position 44.
Although the glycine at position 38 and the glycine at position 44 should be located on opposite sides of the helix in span I, they are considered together here because of the reciprocity of their suppressors. Replacement of the glycine at either position 38 or position 44 by valine resulted in a complete loss of tyrosine transport. In the case of G44V, a suppressed strain was found to have a second mutation, which changed the glycine at position 38 to alanine, and in the case of the primary mutant G38V, a suppressed strain was isolated with a second change, G44A (Table 2). In each case, the level of suppression, although low, was significant (6 and 2%). In each case, a change that caused an increase in the side chain volume on one side of the helix was partially suppressed by an additional change that also caused an increase in the side chain volume but occurred on the opposite face of the helix, six residues away. We do not have sufficient structural information to offer an explanation for this apparent reciprocity.
DISCUSSION
Transmembrane span I.
Span I is characterized by an unusually high prevalence of highly conserved small amino acids. The mutational studies confirmed the critical importance of side chain volume for most of these residues. Progressive replacement of these amino acids by other amino acids with larger side chain volumes led to a steady decrease in transport activity. The high level of conservation of span I residues in all members of the AAT family indicated that span I may play a central role in the overall structure of these proteins that is quite independent of the type of amino acid transported. The importance of small amino acids in helix packing could suggest that span I plays a central structural role in the association of various transmembrane spans within a tertiary structure. More than one side of the helix would be involved as the critical residues include the glycines at positions 34 and 38, the glycines at positions 40 and 44, and the glycine at position 35 (Fig. 5). One study, in which the authors investigated the structures of cytochrome c oxidase, bacteriorhodopsin, the photosynthetic reaction center of Rhodobacter sphaeroides, and the potassium channel of Streptomyces lividans, showed that in cases of close association between two helices, glycine residues occur predominantly along helix interfaces and also at helix crossing points and may serve as multiple notches for orienting multiple helices in a folded protein complex (10). The role of glycine residues in helix packing has also been well described for single-pass membrane proteins. For example, the homodimerization of glycophorin A, which consists of a single transmembrane domain, is mediated by a seven-residue motif (LIXXGVXXGVXXT) that includes two glycine residues located at helix interfacial positions. Replacement of either of these two glycine residues by residues with larger side chain volumes results in disruption of dimerization (12). Solution nuclear magnetic resonance studies have demonstrated that the glycine residues of one monomer form a groove into which the two valine residues of the opposing monomer pack, allowing favorable van der Waals interactions (13). The presence of β-branched residues adjacent to one of the glycines is also thought to play a role in these interactions. In span I there are two such motifs, 34GGAIG38 and 40GLFLG44. Changing any of the glycines to larger amino acids significantly reduces transport activity. Whereas G40 and G44 have been implicated in tight packing interactions between spans I and II, the failure to obtain second-site suppressors for either G34V or G38V that affect residues in other transmembrane domains may indicate that these residues are normally closely packed with other small residues and may constitute a dimerization domain.
FIG. 5.
Helical wheel representation of transmembrane span I, assuming a periodicity of 3.6 residues per turn. Critical residues are indicated by boldface type and are underlined.
The motif GXXIG is present in span I of five of the families of the APC superfamily, and a GXXXG motif is found in the sixth. The GXXIG motif is not present in four families (the ACT, APA, GGA, and SGP families), which cluster on one side of the phylogenetic tree. It is also found in span I of members of the related HAAP family TC2A.4.2 (24).
Proposed interaction between spans I and II.
The results clearly indicate that there is a close interaction between helices I and II, involving residues I37, G40, and G44 in span I and residues L57, Y60, and G64 in span II. Such a parallel association results in a tight hairpin-like structure, which might readily and spontaneously insert into the membrane in a manner suggested by Engelman and Steitz (4, 5, 21). Changes to G40 which destroy transport activity, however, are not associated with a failure of the protein to insert into the membrane. On the other hand, the topology of the mutant proteins within the membrane has not been examined and may differ from that of the wild type. The presence of the glycine-tyrosine pair, involving spans I and II, is highly conserved among members of the AAT family (30 of 32 members). Furthermore, in the case of PheP, replacement of the glycine at position 40 by either valine, leucine, cysteine, proline, or tyrosine completely destroys the transport function, and replacement by alanine reduces the level of phenylalanine transport to 12% of the wild-type level and eliminates transport of tyrosine. The strict requirements for the glycine at position 40 disappear when tyrosine 60 is changed. For example, when the tyrosine at position 60 is replaced by alanine, serine, or methionine, replacement of the glycine at position 40 by alanine or cysteine results in a protein with nearly wild-type levels of activity. Although it seems likely that the latter combinations can also provide a tight fit between helices I and II, the extraordinarily high levels of conservation of G40 and Y60 in the AAT family suggest that the interaction of these residues provides additional fitness to the protein, which is not measured in simple uptake experiments. Other examples of close packing between glycine and aromatic residues are found in the structure of cytochrome c (10) and bacteriorhodopsin (6).
The three glycine residues shown to be involved in the interaction between helices I and II (i.e., G40, G44 and G64) are all highly conserved and cannot be changed to other residues without dramatically affecting function. On the other hand, although I37 and Y60 are also highly conserved, replacement by smaller residues does not reduce the overall transport activity. One possibility that has not been tested is that the wild-type arrangement in all of the proteins may be important for the overall structure as it relates to the specificity of substrate selection and transport. Reducing the size of some of the residues, such as Y60, may increase the overall transport activity but decrease selectivity.
The fact that G40V is not complemented by Y60A indicates that there are some special requirements for the amino acid at position 40. Whether this is related to important constraints on helix packing or some other intrinsic feature of G40 has not been established yet. In light of these results, it is possible that the observed suppression of G40Y by S323F may involve major changes in the overall folding of the protein.
Suppression of mutants with substitutions at G35.
The finding that mutants such as the G35V and G35L mutants were suppressed by second-site changes affecting Y107 was unexpected because current topological models place Y107 much closer to the cytoplasmic border of the membrane than G35. These models are, however, based on a standard transmembrane span of 20 amino acids, and as discussed recently, it is possible to consider a span III which extends from position 96 to position 126 (1). Such an arrangement would place Y107 and G35 at about the same depth in the membrane. If the results that we obtained with the different substitutions reveal a close association between Y107 and G35, we would favor an interaction in which this represents a crossing point between the two helices rather than one region within a parallel association. The reason for this is that the extended face of span III involves F111, G115, E118, and A122. It has previously been shown that changing F111 to either tyrosine or isoleucine extends the substrate range of the protein to include tryptophan (1) and that E118 is a highly conserved and important residue that may play a part in a proton relay system (16). Consequently, we expect these residues to be exposed to some sort of aqueous channel rather than tightly packed against span I. Studies with AroP-PheP chimeras have shown that both G115 and A122 occur at side chain volume-sensitive positions (1). However, in both these cases the proposed helices with which they interact are postulated to be more distal rather than preceding span III (1).
Conclusions.
There are a number of conclusions that can be drawn from this work. The first is that second-site suppression combined with null mutants with conserved glycine residues has the potential to provide information on helix packing. The second is that the effects of increasing side chain volume in a residue in one helix can be partially compensated for by a decrease in the side chain volume of a specific residue in the interacting helix. The third is that the highly conserved spans I and II form a tight hairpin that appears to be essential for transport function. Fourth, span I also presents two other faces for interactions, with one face interacting with part of span III and the other face (GXXIG) interacting either with itself or with another span within the protein. Finally, we do not yet have a satisfactory explanation for the extremely high level of conservation of residues like Y60 and I37 in the AAT family and need to examine additional parameters in tests for phenotypic change.
Acknowledgments
We are grateful to T. Betteridge and J. H. An for technical assistance. We thank Ji Yang and Judy Praszkier for critical reading of the manuscript.
This work was supported by the Australian Research Council Large Grants Scheme.
REFERENCES
- 1.Cosgriff, A. J., G. Brasier, J. Pi, C. Dogovski, J. P. Sarsero, and A. J. Pittard. 2000. A study of AroP-PheP chimeric proteins and identification of a residue involved in tryptophan transport. J. Bacteriol. 182:2207-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deber, C. M., C. J. Brandl, R. B. Deber, L. C. Hsu, and X. K. Young. 1986. Amino acid composition of the membrane and aqueous domains of integral membrane proteins. Arch. Biochem. Biophys. 251:68-76. [DOI] [PubMed] [Google Scholar]
- 3.Eilers, M., S. C. Shekar, T. Shieh, S. O. Smith, and P. J. Fleming. 2000. Internal packing of helical membrane proteins. Proct. Natl. Acad. Sci. USA 97:5796-5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engelman, D. M., and T. A. Steitz. 1981. The spontaneous insertion of proteins into and across membranes: the helical hairpin hypothesis. Cell 23:411-422. [DOI] [PubMed] [Google Scholar]
- 5.Gallusser, A., and A. Kuhn. 1990. Initial steps in membrane protein insertion. Bacteriophage M13 procoat binds to the membrane surface by electrostatic interaction. EMBO J. 9:2723-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grigorieff, N., T. A. Ceska, K. H. Downing, J. M. Baldwin, and R. Henderson. 1996. Electron-crystallographic refinement of the structure of bacteriorhodopsin. J. Mol. Biol. 259:393-421. [DOI] [PubMed] [Google Scholar]
- 7.Haltia, T., and E. Freire. 1995. Forces and factors that contribute to the structural stability of membrane proteins. Biochim. Biophys. Acta 1228:1-27. [DOI] [PubMed] [Google Scholar]
- 8.Ito, K., and Y. Akiyama. 1991. In vivo analysis of integration of membrane proteins in Escherichia coli. Mol. Microbiol. 55:2243-2253. [DOI] [PubMed] [Google Scholar]
- 9.Jack, D. L., I. T. Paulsen, and M. H. Saier. 2000. The amino acid/polyamine/organocation (APC) superfamily of transporters specific for amino acids, polyamines and organocations. Microbiology 146:1797-1814. [DOI] [PubMed] [Google Scholar]
- 10.Javadpour, M. M., M Eilers, M. Groesbeek, and S. O. Smith. 1999. Helix packing in polytopic membrane proteins: role of glycine in transmembrane helix association. Biophys. J. 77:1609-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landolt-Marticorena, C., K. A. Williams, C. M. Deber, and R. A. Reithmeier. 1993. Non-random distribution of amino acids in the transmembrane segments of human type I single span membrane proteins. J. Mol. Biol. 229:602-608. [DOI] [PubMed] [Google Scholar]
- 12.Lemmon, M. A., J. M. Flanagan, H. R. Treutlein, J. Zhang, and D. M. Engelman. 1992. Sequence specificity in the dimerisation of transmembrane α-helices. Biochemistry 31:12719-12725. [DOI] [PubMed] [Google Scholar]
- 13.MacKenzie, K. R., J. H. Prestegard, and D. M. Engelman. 1997. A transmembrane helix dimer: structure and implications. Science 276:131-133. [DOI] [PubMed] [Google Scholar]
- 14.Monod, J., G. Cohen-Bazire, and M. Cohen. 1951. Sur la biosynthese de la β-galactosidase (lactase) chez Escherichia coli. La specificite de l'induction. Biochim. Biophys. Acta 7:585-599. [DOI] [PubMed] [Google Scholar]
- 15.Pi, J. 1991. Ph. D thesis. University of Melbourne, Melbourne, Australia.
- 16.Pi, J., and A. J. Pittard. 1993. Site-directed mutagenesis reveals the importance of conserved charged residues for the transport activity of the PheP permease of Escherichia coli. J. Bacteriol. 175:7500-7504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pi, J., and A. J. Pittard. 1996. Topology of the phenylalanine-specific permease of Escherichia coli. J. Bacteriol. 178:2650-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pi, J., H. Chow, and A. J. Pittard. 2002. Study of second-site suppression in the pheP gene for the phenylalanine transporter of Escherichia coli. J. Bacteriol. 184:5842-5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pi, J., C. Dogovski, and A. J. Pittard. 1998. Functional consequences of changing proline residues in the phenylalanine-specific permease of Escherichia coli. J. Bacteriol. 180:5515-5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pi, J., P. J. Wookey, and A. J. Pittard. 1991. Cloning and sequencing of the pheP gene, which encodes the phenylalanine-specific transport system of Escherichia coli. J. Bacteriol. 173:3622-3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popot, J. L., and D. M. Engelman. 1990. Membrane protein folding and oligomerisation: the two stage model. Biochemistry 29:4031-4037. [DOI] [PubMed] [Google Scholar]
- 22.Richardson, J. S., and D. C. Richardson. 1988. Amino acid preferences for specific locations at the ends of α-helices. Science 240:1648-1652. [DOI] [PubMed] [Google Scholar]
- 23.Russ, W. P., and D. M. Engelman. 2000. The GXXXG motif: a framework for transmembrane helix-helix association. J. Mol. Biol. 296:911-919. [DOI] [PubMed] [Google Scholar]
- 24.Saier, M. H. 2000. Families of transmembrane transporters selective for amino acids and their derivatives. Microbiology 146:1775-1795. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Vandeyar, M. A., M. P. Weiner, C. J. Hutton, and C. A. Batt. 1988. A simple and rapid method for the selection of oligodeoxynucleotide-directed mutants. Gene 65:129-133. [DOI] [PubMed] [Google Scholar]
- 27.Wookey, P. J., J. Pittard, S. M. Forrest, and B. E. Davidson. 1984. Cloning of the tyrP gene and further characterization of the tyrosine-specific transport protein in Escherichia coli K-12. J. Bacteriol. 160:169-174. [DOI] [PMC free article] [PubMed] [Google Scholar]