Abstract
Recently, it has become clear that dendritic cells (DCs) are essential for the priming of T cell responses. However, their role in the maintenance of peripheral T cell tolerance remains largely undefined. Herein, an antigen-presenting cell (APC) transfer system was devised and applied to experimental allergic encephalomyelitis (EAE), to evaluate the contribution that DCs play in peripheral T cell tolerance. The CD8α−CD4+ subset, a minor population among splenic DCs, was found to mediate both tolerance and bystander suppression against diverse T cell specificities. Aggregated (agg) Ig-myelin oligodendrocyte glycoprotein (MOG), an Ig chimera carrying the MOG 35–55 peptide, binds and cross-links FcγR on APC leading to efficient peptide presentation and interleukin (IL)-10 production. Furthermore, administration of agg Ig-MOG into diseased mice induces relief from clinical EAE involving multiple epitopes. Such recovery could not occur in FcγR-deficient mice where both uptake of Ig-MOG and IL-10 production are compromised. However, reconstitution of these mice with DC populations incorporating the CD8α−CD4+ subset restored Ig-MOG–mediated reversal of EAE. Transfer of CD8α+ or even CD8α−CD4− DCs had no effect on the disease. These findings strongly implicate DCs in peripheral tolerance and emphasize their functional potency, as a small population of DCs was able to support effective suppression of autoimmunity.
Keywords: autoimmunity, antigen delivery, dendritic cells, peripheral T cell tolerance, Fcγ receptors
Introduction
Dendritic cells (DCs)* represent a phenotypically heterogeneous population endowed with an important biologic function, namely, the presentation of antigen to both B and T lymphocytes (1–4). To date, it is believed that priming of naive T cells and stimulation of primary T cell responses are mainly a function of DCs with minimal contribution from other professional APCs (2, 3). Furthermore, recent evidence has indicated that different DC subsets confer discrete developmental functions to T cells (5–8). Accordingly, human DC1 and mouse CD8α+ DC subsets were shown to promote differentiation into Th1 effectors, while human DC2 and mouse CD8α− subsets supported development of Th2 cells (6–8). More recently, DCs have been shown to internalize apoptotic bodies and necrotic debris (9, 10) and cross-present (11) antigens exogenously acquired from the dead cells by intersecting the endogenous pathway of antigen presentation. This phenomenon has proven quite potent in cross-priming cytolytic T lymphocytes (10–13) and may provide a valuable tool for therapeutic vaccination against tumors (14–15). Our current knowledge on the roles that DCs play in T cell priming has been growing steadily due to the availability of experimental models. However, little is known about the critical contribution of DCs to central tolerance (16–18), and our knowledge on their role in peripheral tolerance is still in its infancy (19). In this report, we have devised an APC transfer system that was applied to the well-characterized experimental allergic encephalomyelitis (EAE) model of autoimmunity and evaluated the contribution of splenic DCs to peripheral T cell tolerance.
In prior studies, we have demonstrated that Ig-myelin proteolipid protein (PLP)1, an Ig-chimera carrying the encephalitogenic PLP1 peptide corresponding to amino acid (aa) sequence 139–151 of proteolipid protein (PLP), is presented to T cells 100-fold better than free PLP1 (20). In addition, aggregation of Ig-PLP1 facilitates cross-linking of Fcγ receptors (FcγR) on APCs and induces production of IL-10 by both macrophages and DCs (21) without stimulating upregulation of costimulatory molecules (22). Consequently, aggregated (agg) Ig-PLP1 induced a dramatic reduction in paralytic severity and promoted full recovery from EAE when it was administered free of adjuvant into mice with ongoing disease (21, 22). The likely mechanism underlying the effective suppression of EAE by agg Ig-PLP1 maybe a synergy between efficient peptide presentation, lack of costimulation, and IL-10–mediated bystander suppression (22). In FcγR-deficient (FcγR−/−) mice, agg Ig-PLP1–mediated peptide presentation and IL-10 production by APC would be compromised. Thus, these mutant mice offer a suitable host into which to transfer wild-type DCs and evaluate their contribution to agg Ig-PLP1–mediated modulation of autoreactive T cells and maintenance of peripheral tolerance. However, due to the lack of FcγR−/− mice on the SJL/J (H-2s) background we opted to instead engineer the I-Ab-restricted myelin oligodendrocyte glycoprotein (MOG) aa 35–55 peptide (23) into the Ig backbone and use the resulting Ig-MOG chimera along with FcγR−/− C57Bl/6 mice (24, 25) to evaluate the contribution of DCs to peripheral tolerance. The results outlined in this report demonstrate that agg Ig-MOG reverses clinical EAE induced in C57Bl/6 mice by injection of central nervous system (CNS) homogenate, as did agg Ig-PLP1 in the SJL/J mice. This indicates that bystander suppression of diverse T cell specificities is also operative in the Ig-MOG/C57Bl/6 system. When a similar treatment regimen with agg Ig-MOG was applied to FcγR−/− C57Bl/6 mice recovery from disease did not occur. This result was anticipated as both agg Ig-MOG–driven peptide presentation and IL-10 production by APCs would be compromised in these FcγR−/− mice. However, transfer of wild-type C57Bl/6 DCs into the FcγR−/− mice before treatment with agg Ig-MOG restored Ig-MOG–mediated reversal of disease. To gain further insight into how DCs operate the modulation of autoreactive T cells, DCs were separated into subsets and tested both for cytokine production in vitro upon incubation with agg Ig chimeras and for restoration of agg Ig-chimera reversal of EAE in the FcγR−/− mice. The results indicated that upon cross-linking of FcγR by the Ig-chimeras the CD8α− DCs secreted IL-10 and reversed EAE in the mutant mice while the CD8α+ DCs were unable to reverse EAE and instead produced IL-12. More striking, further separation of the CD8α− DCs into CD8α−CD4+ and CD8α−CD4− populations indicated that IL-10 production was confined to the CD8α−CD4+ subset, which also supported suppression of autoreactive T cells and reversal of EAE in the FcγR-deficient mice.
Materials and Methods
Animals
sJL/J mice were purchased from Harlan-Sprague-Dawley and C57Bl/6 mice from The Jackson Laboratory. Fcerg1 (FcγRI and III−/−), Fcgr2b (FcγRII−/−), and Fcer1g/Fcgr2 (FcγRI, II, and III−/−) mice (24, 25) were purchased from Taconic. All mice were bred and maintained in our animal care facility for the duration of the experiments. All experimental procedures were performed according to the guidelines of the institutional animal care committee.
Antigens
Peptides.
The peptides used in this study were purchased from Research Genetics (Huntsville, AL) and were HPLC purified to >90% purity. PLP1 peptide (HSLGKWLGHPDKF) encompasses aa residues 139–151 of PLP and is encephalitogenic in SJL/J mice (26). MOG peptide (MEVGWYRSPFSRVVHLYRNGK), encompassing aa residues 35–55 of MOG, is encephalitogenic in C57Bl/6 mice (23).
CNS Homogenate.
50 frozen unstripped rat brains (Pelfreez Biologicals) were homogenized in PBS using a Waring blender and adjusted to 300 mg/ml with PBS.
Ig-Chimeras.
The Ig-PLP1 chimera harbors PLP1 peptide within the heavy chain CDR3 region and has been described previously (20–22). The Ig-MOG chimera harbors MOG 35–55 peptide within the heavy chain CDR3 region and, like Ig-PLP1, was constructed using the genes coding for the IgG2b, κ anti-arsonate antibody, 91A3 as described previously (20). In brief, the D segment was deleted from the CDR3 of the 91A3 heavy chain variable region and replaced with a nucleotide sequence that code for MOG35–55 peptide using mutagenesis procedures similar to those described for the generation of Ig-PLP1 (20). The resulting 91A3-MOG chimeric IgG2b heavy chain was cotransfected with the parental 91A3 κ chain into the non-Ig–producing SP2/0 myeloma B cell line, and the transfectoma cells producing complete Ig-MOG were selected with drugs as described previously (20). Transfection, cloning, sequencing, and purification procedures for Ig-MOG are similar to those used for Ig-PLP1 (20).
The chimeras were agg by precipitation with 50%-saturated (NH4)2SO4 as has been described previously (21, 22). Since both Ig-PLP1 and Ig-MOG derive from the same Ig backbone and thereby comprise identical IgG2b isotype, their Fc-associated functions including binding and cross-linking of FcγR will be similar. In this respect we may refer to them indistinguishably as Ig chimeras.
Isolation of DCs
Splenic DCs were purified according to the standard collagenase/differential adherence method (27). In brief, the spleen was disrupted in a collagenase solution, and isolated DCs floated on a dense BSA gradient. Subsequently, the cells were allowed to adhere to Petri dishes for 90 min at 37°C, washed, and incubated overnight. For isolation of DC subsets, the bulk DCs were incubated with anti-CD8α mAb coupled microbeads (Miltenyi Biotec) and separated into CD8α+ and CD8α− populations by MACS® (Miltenyi Biotec). The CD8α− fraction was repassed on magnetic columns after incubation with anti-CD8α+ mAb coupled microbeads to eliminate any residual CD8α+ cells. Subsequently, the CD8α− cells were further purified by positive selection using anti-CD11c mAb coupled microbeads (Miltenyi Biotec). For preparation of CD8α−CD4+ and CD8α−CD4− DC subsets, the CD8α− fraction was labeled with anti-CD4 mAb coupled microbeads (Miltenyi Biotec), and the subsets were separated as above. The CD4− fraction was further purified by positive selection using anti-CD11c mAb coupled microbeads (Miltenyi Biotec). Each fraction was assessed for purity and no population was used if contamination was >5%.
Induction of EAE
6–8-wk-old mice were induced for EAE by subcutaneous injection in the footpads and at the base of the limbs and tail with a 200 μl IFA/PBS (vol/vol) solution containing 6 mg CNS homogenate and 200 μg Mycobacterium tuberculosis H37Ra (Difco Laboratories). 6 h later the mice were given intravenous 200 ng of purified Bordetella pertussis toxin (List Biological Laboratories, Inc.). A second injection of B. pertussis toxin was given after 48 h. Subsequently, the mice were scored daily for clinical signs of EAE as follows: 0, no clinical score; 1, loss of tail tone; 2, hind limb weakness; 3, hind limb paralysis; 4, forelimb paralysis; and 5, moribund or death. EAE induced by CNS homogenate manifests as a monophasic disease in C57Bl/6 mice and as a relapsing/remitting disease in SJL/J mice. For induction of EAE with peptide-pulsed DCs, the following procedure was used: purified CD8α+ or CD8α− DCs (106 cells/ml) were pulsed overnight with 50 μg/ml PLP1 peptide, washed, resuspended in PBS, and injected subcutaneously into the hind limb footpads of mice at 3 × 105 cells per mouse. 6 and 24 h later, the mice were given intravenously 200 ng of B. pertussis toxin.
Treatment of EAE
Treatment with Peptide.
Mice induced for EAE with CNS homogenate began receiving treatment with 100 μg of PLP1 or MOG peptide after loss of tail tone, which occurs regularly between days 6 and 8 after disease induction. Treatment injections were given intraperitoneally in PBS on days 9, 13, and 17 as described previously (21).
Treatment with Ig-PLP1 or Ig-MOG.
Mice induced for EAE with CNS homogenate began receiving treatment with 300 μg of agg Ig-PLP1 or Ig-MOG after loss of tail tone. Treatment injections were given intraperitoneally in PBS on days 9, 13, and 17 after disease induction as described previously (21). Treatment of EAE in FcγR−/− mice reconstituted with wild-type DCs was performed as follows: on day 5 after disease induction, the mice were adoptively transferred intraperitoneal with either 0.3 or 0.6 × 106 purified DCs. Subsequently, the animals were treated with agg Ig-MOG on days 9, 13, and 17 as above.
Cytokine Detection
ELISA.
ELISA was performed according to BD PharMingen's standard protocol. The capture Abs were as follows: rat anti–mouse IFN-γ, R4–6A2; rat anti–mouse IL-10, JES5–2A5; rat anti–mouse IL-4, 11B11; and rat anti–mouse IL-12, 9A5. The biotinylated anti-cytokine Abs were as follows: rat anti–mouse IFN-γ, XMG1.2; rat anti–mouse IL-10, JES5–16E3; rat anti–mouse IL-4, BVD6–24G2; and rat anti–mouse IL-12, C17.8. All antibodies were purchased from BD PharMingen. Assays were read on a SPECTRAmax 190 counter (Molecular Devices). Graded amounts of recombinant mouse IL-4, IFN-γ (BD PharMingen), IL-10, or IL-12 (Peprotech) were included in all experiments for construction of standard curves. The cytokine concentration in culture supernatants was interpolated from the linear portion of the standard curve.
Intracellular Cytokine Staining.
CD8α+, CD8α−CD4+, or CD8α−CD4− DCs (106 cells/ml/well) were incubated with 0.6 μM agg Ig-chimera for 24 h to allow for FcγR cross-linking. During the last 8 h of incubation, brefeldin A (10 μg) (Epicentre Technologies) was added to the wells in order to block cytokine secretion. The cells were then harvested and stained with anti-CD11c-APC mAb, HL3, and FITC-labeled anti-CD8α mAb, 53–6.7, or anti-CD4 mAb, GK1.5. Subsequently, the cells were fixed, permeabilized with 0.5% saponin (Sigma-Aldrich), and stained for intracellular IL-10 using PE-labeled anti–IL-10 mAb, JES5–16E3. The FACS® data were analyzed on a FACSVantage™ Flow Cytometer (Becton Dickinson) using CELLQuest™ software (Becton Dickinson).
Stimulation of Cytokine Production by APCs
Splenocytes and subsets of DCs from normal or FcγR-deficient mice were plated with graded amounts of soluble (sol) or agg Ig-PLP1, and the culture was then incubated for 24 h. Detection and quantification of cytokines was then assessed by ELISA from 100 μl of culture supernatant as described above.
T Cells
TCC-PLP1–1B10.
The generation of the PLP1-specific Th0 cell clone, TCC-PLP1–1B10, has been described previously (21). In brief, SJL mice were immunized with 100 μg PLP1 peptide in CFA, and 10 d later the draining lymph nodes were removed and stimulated with PLP1. After three stimulation/resting cycles, the cells were cloned twice by limiting dilution, and positive clones were retested for reactivity with PLP1 peptide by both proliferation and cytokine production. Subsequently, one positive clone displaying a Th0 pattern was selected and designated TCC-PLP1–1B10.
MOG-specific T Cell Line.
A similar procedure was used to generate a MOG reactive T cell line and these cells were used for testing Ig-MOG for peptide presentation.
Analysis of Cytokine Responses Upon Presentation of agg Ig-PLP1 by Subsets of DCs
Purified bulk, CD8α+, or CD8α− DCs were plated at 5 × 104 cells/well/50 μl and incubated with graded amounts of sol or agg Ig-PLP1 (100 μl/well) for 1 h. Subsequently, TCC-PLP1–1B10 cells (5 × 104 cells per well per 50 μl) were added, and the culture was continued for 24 h. Detection and quantification of cytokines were assessed by ELISA from 100 μl of culture supernatant as described above.
Results
Broad Efficacy of agg Ig-myelin Chimeras in the Suppression of EAE.
We have previously demonstrated that agg Ig-PLP1 is effective in the induction of IL-10 by APC and modulation of EAE involving diverse T cell specificities (21, 22). Furthermore this study indicated that IL-10–mediated bystander suppression plays a critical role in T cell modulation and amelioration of EAE, as administration of anti–IL-10 mAb into mice during treatment of disease with agg Ig-PLP1 restored full severity of paralysis (21, 22). This experimental model of T cell tolerance was used in conjunction with FcγR-deficient mice to devise an adoptive APC transfer system suitable for evaluation of the role of DCs in peripheral tolerance. Since FcγR-deficient mice were not available in the H-2s (SJL/J) background we instead chose to use C57Bl/6 (H-2b) mice and therefore engineered an Ig-MOG chimera containing the I-Ab–restricted MOG35–55 peptide, an epitope that has been shown to induce EAE in C57Bl/6 mice (23). To ensure that the MOG peptide would indeed be processed from the chimera and presented to specific T cells, Ig-MOG was assayed for stimulation of a T cell line generated against MOG35–55 peptide. The results showed that the MOG-specific T cell line significantly proliferated when it was incubated with Ig-MOG in the presence of compatible APC, thus indicating that Ig-MOG was taken up by the APC and that a MOG peptide was generated and presented to the T cells (unpublished data).
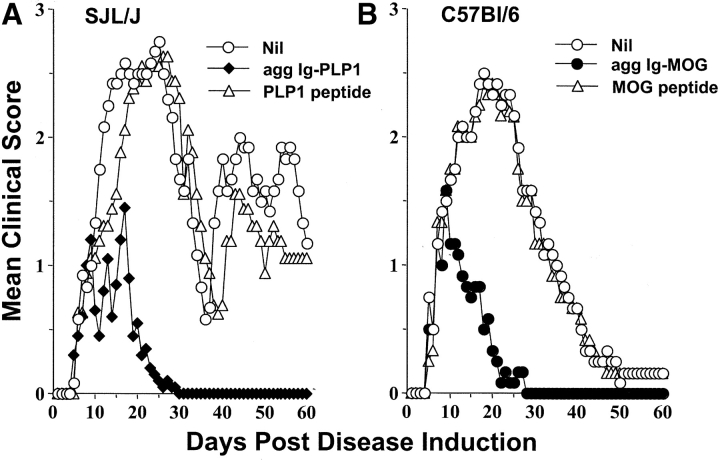
Subsequently, Ig-MOG was tested for amelioration of EAE in C57Bl/6 mice side by side with Ig-PLP1 in the SJL strain. The results indicate that agg Ig-MOG, like agg Ig-PLP1, was able to reverse CNS homogenate induced EAE and, most likely, involved downregulation of diverse T cell specificities (Fig. 1) . Indeed, while the untreated C57Bl/6 mice had a mean maximal score of 2.4 ± 0.2, those treated with agg Ig-MOG had a mean maximal score of 1.2 ± 0.3 (Fig. 1 B). In addition, while agg Ig-MOG treated animals fully recovered from the typical monophasic EAE by day 22 after disease induction, the recovery of the untreated mice was delayed and occurred on day 47. This concurs with the SJL/Ig-PLP1 system in which the untreated mice had a mean maximal disease score of 2.8 ± 0.3 and had not recovered by day 60 post disease induction, as relapses were still observed. Those treated with agg Ig-PLP1 had a mean maximal disease score of 1.4 ± 0.3 and fully recovered from this relapsing/remitting disease by day 26 after induction, and relapses were not observed (Fig. 1 A). Furthermore, free PLP1 and MOG peptides used at 17- and 19-fold excess, respectively, were unable to suppress the clinical signs of EAE in their respective mouse strains (Fig. 1). These results indicate that the agg Ig chimeras display a broad effectiveness in reversing disease involving multiple epitopes and, most likely, diverse T cell specificities.
Figure 1.
Agg Ig-MOG modulates disease involving diverse T cell specificities. Groups of SJL/J (A) or C57Bl/6 (B) mice were induced for EAE with 6 mg of CNS homogenate and were treated intraperitoneally with a saline solution containing 300 μg of agg Ig-PLP1 (agg Ig-PLP1), 300 μg agg Ig-MOG (agg Ig-MOG), 100 μg of free PLP1 peptide (PLP1 peptide), or 100 μg MOG peptide (MOG peptide) on days 9, 13, and 17 after disease induction. On the basis of the molecular weight of the Ig-chimeras and PLP1 and MOG peptide, we estimated that 300 μg of free peptide contains 17- and 19-fold higher copies of peptide, respectively, than the Ig-chimera. Groups of untreated mice (Nil) were included for comparison purposes. The clinical onset of disease was at day 5–7 after disease induction in these experimental groups. Each point represents the mean clinical score of six mice.
DCs Mediate the Reversal of EAE by agg Ig-myelin Chimeras.
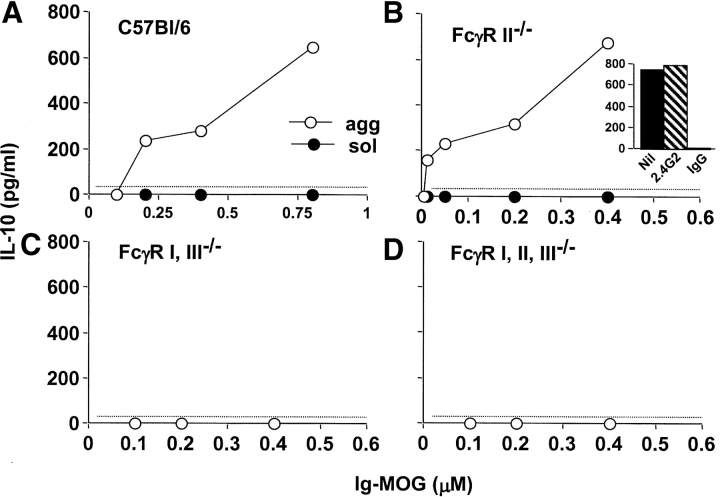
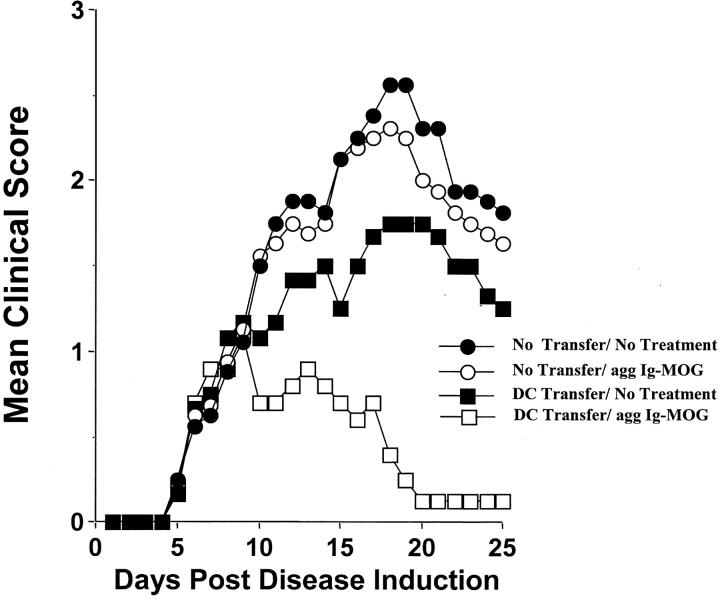
In prior studies, we demonstrated that DCs produce IL-10 upon cross-linking of their FcγR by agg Ig-PLP1 (21). Since neutralization of IL-10 during treatment of diseased mice with agg Ig-PLP1 restored clinical severity, it is possible that DCs play a critical role in the suppression of EAE (21). To evaluate the contribution of DCs to this form of peripheral T cell tolerance, an animal model where both antigen presentation and IL-10 secretion are compromised was needed. Since our delivery molecule belongs to the IgG2b subclass, an isotype that internalizes into APC via FcγR (20, 28) and induces IL-10 by the cross-linking of these receptors (21, 22), mice deficient in FcγR expression would provide such a model. The FcγR family is divided into three subfamilies that either employ an α-chain that mediates both Ig binding and signaling (FcγRII) or an α-chain that mediates Ig binding and a γ-chain responsible for signaling (FcγRI and III) (for a review, see reference 29). Mice in which the α-chain of FcγRII was knocked out generated animals that were deficient for FcγRII (FcγRII−/−) but displayed intact FcγRI and III (24). Also, gene targeted mutation of the common γ-chain generated mice that were deficient in FcγRI and III (FcγRI and III−/−), but not FcγRII expression (25). Genetic crossing of these two mutant strains generated mice deficient for all three types of FcγRs (FcγRI, II, and III−/−) (25). Using these mutant mice, we identified FcγRI as the major mediator of agg Ig-chimera induced IL-10 production by APCs (Fig. 2) . Indeed, splenocytes from C57Bl/6 or FcγRII−/− mice produced IL-10 upon incubation with agg Ig-MOG (Fig. 2, A and B), while those from FcγRI and III−/− or FcγRI, II, and III−/− mice did not, as IL-10 production remained at the background level seen with the negative control, sol Ig-MOG (Fig. 2, C and D). Similar results were obtained when DCs were used instead of splenocytes (unpublished data). These results indicated that FcγRI and/or FcγRIII was responsible for binding agg Ig-MOG and triggering the IL-10 production by the APC. However, blockade of FcγRIII with 2.4G2 antibody, which is specific for both FcγRII and III (30), on cells lacking FcγRII still allowed for equivalent IL-10 production indicating that FcγRI is most likely the major binder and, thus, mediator of agg Ig-MOG induced IL-10 production (Fig. 2, B insert). Therefore, FcγRI and III−/− mice provide an appropriate model for the adoptive transfer of wild-type C57Bl/6 APC in order to investigate the role of DCs in the suppression and reversal of EAE by agg Ig-chimeras. Accordingly, FcγRI and III−/− mice that had been induced for EAE with CNS homogenate were given C57Bl/6 DCs on day 5 after disease induction and were treated with agg Ig-MOG when EAE had became clinically apparent. As can be seen in Fig. 3 , the FcγRI and III−/− mice developed a severe disease (2.6 ± 0.2) in response to CNS homogenate (no transfer/no treatment group). In contrast to the wild-type C57Bl/6 mice, these FcγRI and III−/− mice could not reverse their disease upon similar treatment with the agg Ig-MOG (no transfer/agg Ig-MOG group). However, if these mice were reconstituted with wild-type DCs from C57Bl/6 mice before treatment with agg Ig-MOG, the severity of EAE was significantly reduced (mean maximal score of 1.1 ± 0.2), and the animals recovered by day 20 after disease induction (DC transfer/agg Ig-MOG group). Adoptive transfer of DCs without treatment with agg Ig-MOG had a marginal effect on disease manifestation (DC transfer/no treatment group). These results indicate that DCs play a critical role in this form of peripheral tolerance and suppression of autoimmunity.
Figure 2.
The induction of IL-10 production by agg Ig-chimeras is mediated primarily by FcγRI. Splenocytes from C57Bl/6 (A); FcγRII−/− (B); FcγRI, III−/− (C); and FcγRI, II, and III−/− (D) mice were incubated with graded amounts of agg (white circles) or sol (black circles) Ig-MOG for 24 h. Subsequently, the supernatant was used to measure IL-10 production by ELISA. To determine the contribution of FcγRI and FcγRIII in mediating IL-10 production, the following experiment was performed (insert to B): DCs from FcγRII−/− mice were incubated with 0.6 μM of agg Ig-chimera in the presence of 20 μg/ml 2.4G2 mAb (hatched bar) or 100 μg/ml mouse IgG (white bar), and IL-10 production was evaluated. Each point represents the mean of triplicate wells. The dotted line indicates the lower limit of cytokine detection in this ELISA.
Figure 3.
DCs mediate reversal of EAE by agg Ig-chimeras. Groups of FcγRI and III−/− mice (6–8 wk of age) were induced for EAE with 6 mg of CNS homogenate, and on day 5 after disease induction, adoptively transferred with 0.6 × 106 purified C57Bl/6 DCs (squares). Subsequently, the mice were treated intraperitoneally with 300 μg agg Ig-MOG on days 9, 13, and 17 after disease induction (white symbols). Groups of untreated mice that either received (black squares) or did not receive (black circles) DC transfer and mice that were treated with agg Ig-MOG but did not receive DC transfer (white circles) were included for comparison purposes. The clinical onset of disease was at day 5 in these experimental groups. Each point represents the mean clinical score of 6–8 mice.
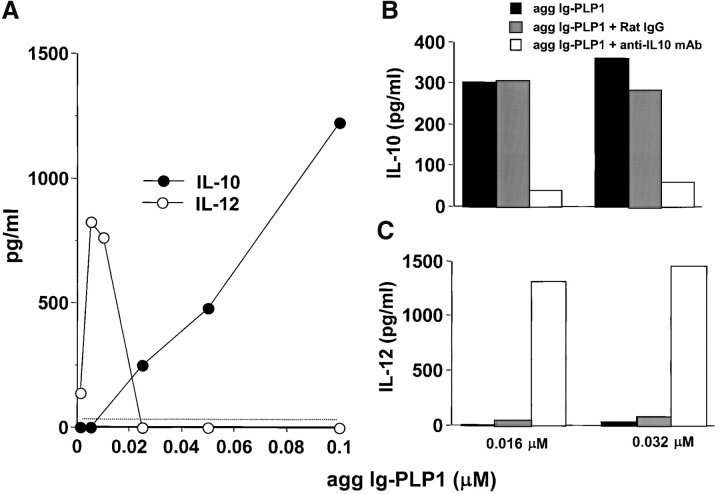
We have previously shown that modulation of disease by the Ig-chimeras is due to tolerization of myelin-specific T cells and suppression of their proliferation as well as IFN-γ production (21). In addition, the IL-10 produced by APC, upon binding of agg Ig-myelin chimeras was shown to play a critical role in T cell tolerization, as its neutralization by administration of anti–IL-10 antibodies restored disease severity (21). Suppression of EAE by agg Ig-myelin chimeras could operate through the direct binding of IL-10 to the autoreactive T cells leading to inhibition of their proliferation (31–33). Alternatively, IL-10 may downregulate IL-12 production by the APC and lead to defective T cell differentiation (31, 34, 35). To test this premise, we assayed DCs for production of IL-12 upon incubation with agg Ig-MOG and assessed whether any such IL-12 secretion would be subject to downregulation by IL-10. As can be seen in Fig. 4 , DCs produced both IL-10 and IL-12 upon incubation with agg Ig-MOG. However, the secretion of IL-12 decreased as IL-10 production increased suggesting a regulation of IL-12 by IL-10. In fact the addition of an anti–IL-10 mAb to the culture to neutralize IL-10 supported this view and allowed for IL-12 secretion at higher concentrations of agg Ig-chimera (Fig. 4, B and C).
Figure 4.
DCs produce IL-10 and downregulate IL-12 upon incubation with agg Ig-PLP1. 20,000 purified DCs (A) were incubated with graded amounts of agg Ig-PLP1 for 24 h, and the supernatant was then used to measure both IL-10 and IL-12 production by ELISA. In the right panel the stimulation with agg Ig-PLP1 was performed in the presence of 20 μg/ml anti–mouse IL-10 mAb antibody (2A5) or negative control rat IgG. Subsequently IL-10 (B) and IL-12 (C) were measured by ELISA. Each point or bar represents the mean of triplicate wells. The dotted line indicates the lower limit of cytokine detection in this ELISA.
The CD8α−, but not CD8α+, DC Population Is Responsible for IL-10 Production and Reversal of EAE upon Treatment with agg Ig-MOG.
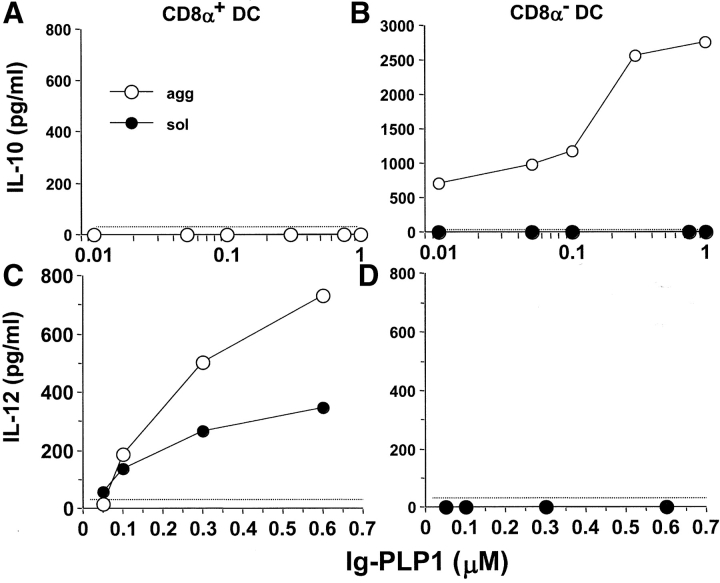
While all DCs defined to date function as APC, they are quite heterogeneous in their surface phenotypes (36, 37). Recently, it has become clear that the different DC subtypes display different trafficking patterns (38) and contribute distinct developmental functions in T cell priming (7, 8). Since DCs produce IL-10 and mediate reversal of EAE upon treatment with agg Ig-MOG, this model offers an opportunity to investigate the role of the DC subsets in peripheral T cell tolerance. Initially, DCs were separated into CD8α+ and CD8α− populations and tested for both IL-10 and IL-12 production upon incubation with agg Ig-MOG. As illustrated in Fig. 5 , CD8α− DCs produced IL-10, while CD8α+ DC secreted IL-12, upon cross-linking of their FcγRs by agg Ig-PLP1. The production of IL-12 by CD8α+ DCs was also inducible by sol Ig-PLP1, possibly indicating that binding of monomeric IgG to FcγRs is sufficient to trigger IL-12 secretion. Alternatively, some sol Ig-PLP1 might have agg during culture at 37°C. If this is the case, IL-12 induction requires only a small amount of agg Ig relative to that required for the induction of IL-10 (Fig. 4).
Figure 5.
CD8α− DCs produce IL-10, while CD8α+ DCs secrete IL-12 in response to agg Ig-PLP1. 50,000 purified CD8α+ (A and C) or CD8α− (B and D) DCs were incubated with graded amounts of agg (white circles) or sol (black circles) Ig-PLP1 for 24 h. Subsequently, the supernatant was used to measure both IL-10 (A and B) and IL-12 (C and D) production by ELISA. Each point represents the mean of triplicate wells. The results shown are representative of four experiments. The dotted line indicates the lower limit of cytokine detection in this ELISA.
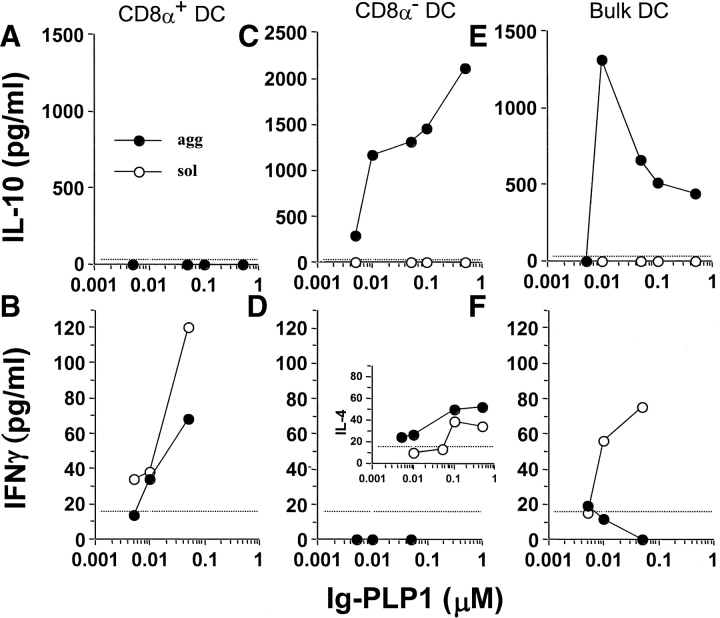
Since the CD8α− DCs produced IL-10 upon incubation with agg Ig-PLP1, T cells engaged by these APCs through peptide presentation should be tolerized rather than stimulated (31, 32). To test this premise, the PLP1-specific Th0 clone, TCC-PLP1–1B10 (21), was incubated with CD8α− DCs in the presence of sol or agg Ig-PLP1 and both IL-10 production by the APC and IFN-γ secretion by the T cells were measured. For comparison purposes, bulk and CD8α+ DCs were included in these experiments. As can be seen in Fig. 6, A and B , CD8α+ DCs, which do not produce IL-10 upon incubation with agg or sol Ig-PLP1, activated the Th0 clone to produce IFN-γ through presentation of PLP1 peptide. In contrast, CD8α− DCs, which produce IL-10 upon incubation with agg Ig-PLP1 (Fig. 6 C), were not able to drive production of IFN-γ by the Th0 clone (Fig. 6 D). Surprisingly, incubation with sol Ig-PLP1, which does not cross-link FcγR or trigger IL-10 production by the CD8α− DCs (Fig. 6 C), was unable to stimulate the Th0 clone for IFN-γ production (Fig. 6 D). The lack of IFN-γ was not due to defective presentation of sol Ig-PLP1 as the Th0 clone was able to produce IL-4 in response to such stimulation (Fig. 6, D insert). Nevertheless, the IL-10 produced by the CD8α− subset within the bulk DC population (Fig. 6 E) suppressed the capacity of the CD8α+ subset to drive IFN-γ production by the T cells (Fig. 6 F), a phenomenon that further argues for the tolerogenic function of CD8α− DCs. Overall, the results of this in vitro experiment with a Th0 clone demonstrate that CD8α− DCs do not support the polarization of T cells into IFN-γ–producing cells and can actively antagonize the activation of Th1 cells through IL-10 production.
Figure 6.
CD8α− DCs are unable to promote the production of IFN-γ by T cells. Purified CD8α+ (A and B), CD8α− (C and D), and unseparated CD11c+ (E and F) DC (5 × 104 cells per well) were incubated with graded amounts of agg (black circles) or sol (white circles) Ig-PLP1 for 1 h. Subsequently, TCC-PLP1–1B10 Th0 cells (5 × 104 cells per well) were added, and the incubation was continued for 24 h. IL-10 (A, C, and E) and IFN-γ (B, D, and F) production in the same culture well were then measured by ELISA from 100 μl of culture supernatant. The insert in D, measuring IL-4 secretion, indicates that the absence of IFN-γ production by the T cells upon stimulation with CD8α− DCs and Ig-PLP1 was not due to inadequate antigen presentation. Each point represents the mean of triplicate wells. The dotted line indicates the lower limit of cytokine detection in this ELISA.
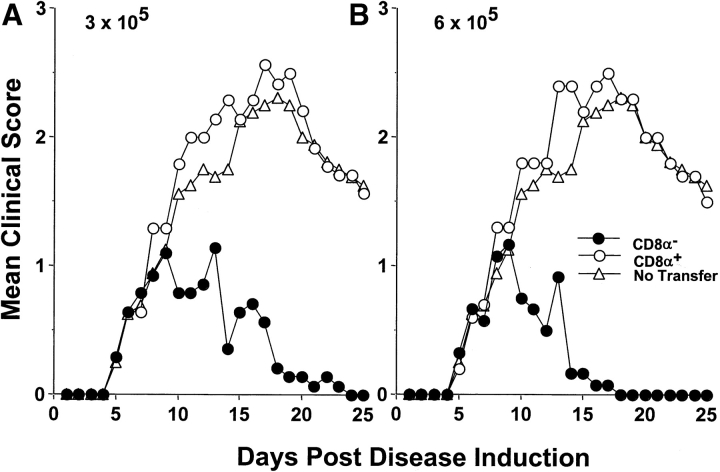
The ability of CD8α− DCs to mediate T cell tolerance in vivo was then analyzed. To this end, CD8α− DCs were adoptively transferred into FcγRI and III−/− mice and tested for reversal of EAE upon treatment with agg Ig-MOG. The results illustrated in Fig. 7 indicate that transfer of either 3 or 6 × 105 C57Bl/6 CD8α− DCs into FcγRI and III−/− mice with ongoing clinical EAE allowed for the reversal of paralysis when the mice were treated with agg Ig-MOG. However, mice adoptively transferred with CD8α+ DCs, instead of the CD8α− population, were unable to suppress the disease and had clinical scores similar to mice that did not receive any cell transfer. These results indicate that CD8α− DCs are responsible for tolerization of pathogenic myelin-specific Th1 T cells and, thus, play a critical role in this form of peripheral tolerance.
Figure 7.
CD8α− wild-type DCs restore agg Ig-MOG mediated reversal of EAE in FcγRI and III−/− mice. Groups of FcγRI and III−/− mice (6–8 wk of age) were induced for EAE with 6 mg of CNS homogenate. On day 5 after disease induction, the mice were given intraperitoneally 3 × 105 (A) or 6 × 105 (B) purified C57Bl/6 CD8α− (black circles) or CD8α+ DCs (white circles). Subsequently, the mice were treated intra-peritoneal with 300 μg agg Ig-MOG on days 9, 13, and 17 after disease induction. A group of mice that were treated with agg Ig-MOG but did not receive cell transfer (white triangles) was included for comparison purposes. The clinical onset of disease was at day 5 in these experimental groups. Each point represents the mean clinical score of 6–8 mice.
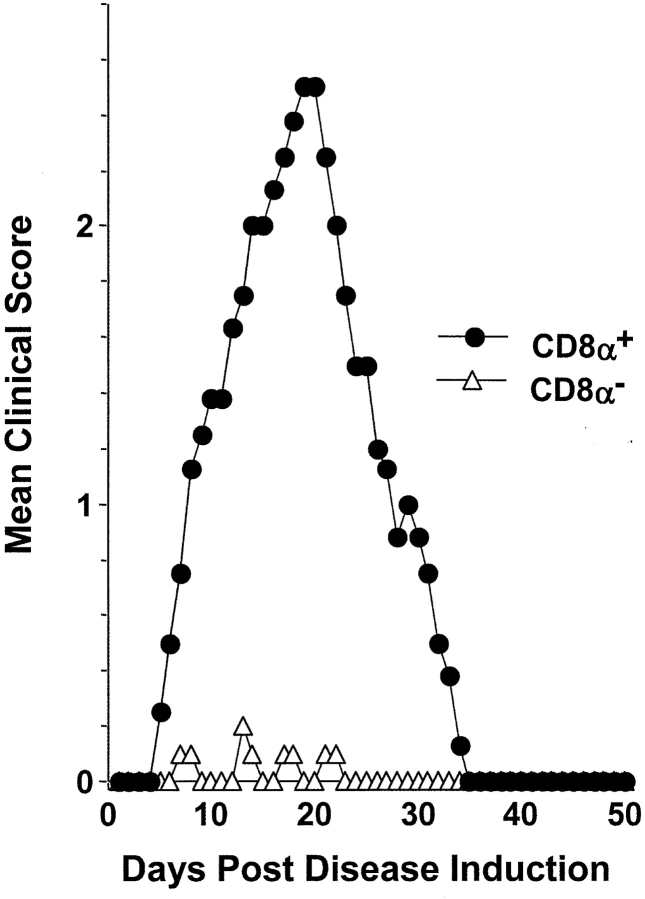
It has been previously shown that DCs loaded with antigenic peptide induce immune responses without the requirement for adjuvant (3). This regimen provided a useful approach to test the intrinsic tolerogenic function of the CD8α− DCs. Accordingly, CD8α− and CD8α+ DCs were loaded with an excess of PLP1 peptide in vitro and injected into mice according to a regimen defined to induce EAE (39). Daily analysis of paralytic scores indicated that CD8α+, but not CD8α− DCs, induced EAE (Fig. 8) . Again, these results argue that PLP1-reactive T cells were available but were unresponsive to PLP1 peptide when it was presented by these CD8α− DCs as activation and differentiation into Th1 effectors that cause disease did not occur (7, 8).
Figure 8.
CD8α+, but not CD8α−, DCs promote the development of EAE. Purified CD8α+ (black circles) or CD8α− (white triangles) DCs were pulsed overnight with 50 μg/ml PLP1 peptide, washed, resuspended in PBS, and injected (3 × 105 cells per mouse) into the hind limb footpads of SJL/J mice. 6 and 24 h later, the mice were given intravenous 200 ng of B. pertussis toxin. The clinical onset of disease was at day 6 in these experimental groups, and each point represents the mean clinical score of five mice.
CD8α−CD4+, but not CD8α−CD4−, DCs Produce IL-10 and Mediate Reversal of EAE upon Treatment with Ig-MOG.
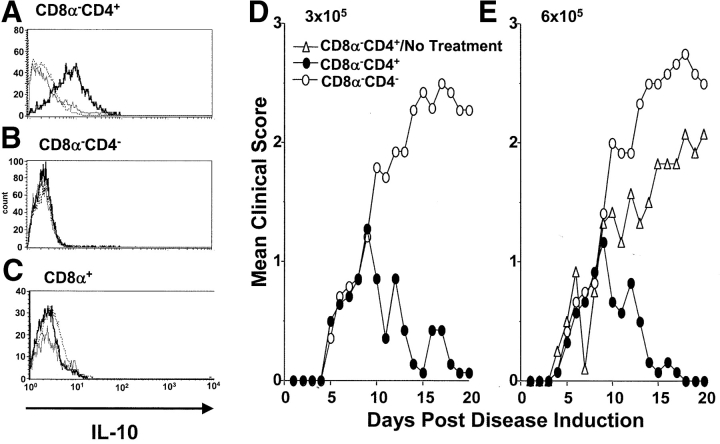
The CD8α− subset of splenic DCs is comprised of two phenotypically different populations based upon the expression of CD4 (37). However, whether these subsets, CD8α−CD4+ and CD8α−CD4−, display distinct biologic functions has not yet been clarified. Herein, the two subsets were separated from splenic DCs and tested for IL-10 production as well as reversal of EAE upon treatment with agg Ig-MOG. The results illustrated in Fig. 9 indicate that CD8α−CD4+, but not CD8α−CD4− DCs, produce IL-10 upon incubation with agg Ig-MOG as tested by intracellular cytokine staining (Fig. 9, A and B). IL-10 production was not observed with the CD8α+ subset that was used as a negative control (Fig. 9 C). Subsequently, the two subsets were adoptively transferred into FcγRI and III−/− mice with ongoing clinical EAE and tested for reversal of paralysis upon treatment with agg Ig-MOG. The results indicated that CD8α−CD4+, but not CD8α−CD4−, DCs were able to mediate tolerance and reverse the disease upon treatment with agg Ig-MOG (Fig. 9, D and E). Indeed, the mice transferred with 3 or 6 × 105 CD8α−CD4+ DCs had a mean maximal disease severity of 0.9 ± 0.3 and fully recovered by day 18 after disease induction. Those transferred with equivalent numbers of CD8α−CD4− DCs had a mean maximal disease severity of 2.6 ± 0.3 and did not recover from their paralysis during the 21-d period of clinical assessment. The reversal of EAE requires treatment with agg Ig-MOG because adoptive transfer of 6 × 105 CD8α−CD4+ DCs did not confer reduction of the severity of disease or recovery from paralysis when the animal were not given agg Ig-MOG. These results identify the CD8α− CD4+ DC subset as the tolerogenic population among mature splenic DCs.
Figure 9.
CD8α−CD4+ DCs produce IL-10 and mediate reversal of EAE. IL-10 production by purified (A) CD11c+CD8α−CD4+, (B) CD11c+CD8α−CD4−, and (C) CD11c+CD8α+ DCs was measured by intracellular cytokine staining. Accordingly, DCs (106 cells per well) were incubated with 0.6 μM agg Ig-PLP1 for 24 h and brefeldin A (10 μg) was added to the wells during the last 8 h of incubation, in order to block cytokine secretion. Subsequently, the cells were harvested and stained with anti–CD11c-APC and FITC-labeled anti-CD8α or anti-CD4 mAb. The cells were then fixed, permeabilized, and intracellular stained with PE-conjugated anti–IL-10 (thick lines) or isotype matched (dotted lines) mAb. Cells incubated with media without the addition of Ig-PLP1 (thin lines) and intracellular stained with anti–IL-10-PE mAb were included for control purposes. Shown are histogram plots representing the intensity of IL-10 expression by the indicated DC population. The results shown are representative of four experiments. In D and E, groups of FcγRI and III−/− mice (6–8 wk of age) were induced for EAE with 6 mg of CNS homogenate and on day 5 the mice were given intraperitoneally 3 × 105 (D) or 6 × 105 (E) purified C57Bl/6 CD8α−CD4+ (black circles) or CD8α−CD4− (white circles) DCs. Subsequently, the mice were treated intraperitoneal with 300 μg agg Ig-MOG on days 9, 13, and 17 after disease induction. A group of untreated mice that received 0.6 × 106 CD8α−CD4+ DC (white triangles) was included for comparison purposes. The clinical onset of disease was at day 5 in these experimental groups. Each point represents the mean clinical score of 6–8 mice.
Discussion
The results presented in this report show that C57Bl/6 mice recover from EAE when treated with agg Ig-MOG as did SJL/J mice after treatment with agg Ig-PLP1 (Fig. 1). However, C57Bl/6 mice deficient for FcγRI and III could not resolve EAE upon similar treatment with agg Ig-MOG unless reconstituted with DCs from wild-type C57Bl/6 mice before the treatment (Fig. 3). Previously, we have shown that APC internalize Ig-chimera of the same IgG2b backbone as Ig-MOG and Ig-PLP1 mainly via FcγRs leading to processing of the chimeras within endosomes and efficient loading of the incorporated peptide onto newly synthesized MHC molecules (40). Aggregation of the Ig-chimeras adds another feature to the Ig delivery system, namely the cross-linking of FcγR and production of IL-10 by the APC (21, 22). FcγRI was identified as the main target for Ig-MOG and mice lacking such a receptor could not resolve EAE (Fig. 3) most likely due to compromised presentation of Ig-MOG and lack of IL-10 production (Fig. 2). Furthermore, this report shows that DCs produce IL-12 upon cross-linking of their FcγR but that the simultaneous production of IL-10 negatively regulates such IL-12 secretion (Fig. 4). This observation prompted us to determine whether the two cytokines were produced by the same or by separate subsets of DCs and whether there was a correlation between a specific population of DCs and recovery from disease. The findings indicated that the IL-10 production was mediated by CD8α− DCs and IL-12 by the CD8α+ population (Fig. 5). While this result is in good agreement with data obtained recently with pansorbin (killed S. aureus) stimulated DCs (41), it is also supported by the observation that CD8α− but not CD8α+ DCs mediate recovery from EAE in the FcγRI and III−/− mice upon treatment with Ig-MOG (Fig. 7). Although different subsets of DCs may display different pattern of trafficking (38) and migration issues could be at play in this form of peripheral tolerance, a number of observations argue instead that these results represent intrinsic functions of the two populations. Fig. 6 shows that upon incubation with agg Ig-PLP1 the CD8α+ DCs produce IL-12, present the PLP1 peptide, and stimulate the PLP1-specific Th0 cells to produce IFN-γ. Therefore, these cells most likely would not support reversal of EAE upon encounter with pathogenic T cells when properly located. However, CD8α− DCs produced IL-10, did not stimulate IFN-γ production by the same Th0 cells (Fig. 6), and therefore not surprisingly supported the reversal of EAE (Fig. 7). Thus, in this in vitro T cell regulation system, where migration issues should not be at play, the DC subsets displayed functions that parallel the results obtained in vivo. Furthermore, when the two subpopulations were loaded with free peptide in vitro and then used to induce EAE, only the CD8α+ DCs produced paralysis. This observation suggests that both naive self-reactive T cells were available and that CD8α+ DCs were able to encounter these cells and drive their activation and induction of paralysis. However, the CD8α− DCs population seems to mediate unresponsiveness as they did not activate the T cells to produce EAE despite their ability to migrate through the circulation, encounter the T cells and mediate reversal of EAE as shown in Fig. 7. Thus, the intrinsic function of these cells most likely dictates the type of contribution that a DC subset displays in this model of peripheral tolerance. However, we cannot currently rule out that differential trafficking patterns between the two populations influence their functions.
In recent years reports have been made indicating that human immature DCs cross-presenting viral (42) or allogeneic (43) antigens induce IL-10–producing regulatory T cells that could potentially sustain peripheral tolerance. In addition, immature DCs were found to internalize apoptotic bodies generated from normal cells dying as a consequence of physiologic turnover, and transport the associated self-antigens to nearby lymph nodes populated with T cells (10, 44). Since T cell responses to those self-antigens were undetectable it was presumed that such transport served to maintain peripheral T cell tolerance (19, 44). The contribution of DCs to peripheral tolerance, however, remains largely undefined. Lately, it has been shown that repetitive injections of TNF-α matured peptide-loaded DCs can protect against EAE presumably via induction of IL-10–producing regulatory T cells (45). Moreover, peptide loading into the endosomal compartment of DCs in the absence of inflammation also supports T cell down-regulation and likely maintains peripheral tolerance (46). The Ig-chimeras drive both peptide loading into endosomes without causing inflammation, and induce IL-10 production by APC aligning powerful factors against the self-reactive T cells. Interestingly, among the mature splenic DCs, the CD8α− CD4+ subset seems to drive such tolerance as they produce IL-10 and support reversal of EAE in the FcγRI and III−/− mice (Fig. 9). Given the small size of the CD8α− CD4+ DCs population we suspect that such potency emanated from coordinated inhibitory functions. IL-10 produced by the CD8α− CD4+ DCs could inhibit the function of T cells (47) directly by alteration of CD28 expression (33), and/or indirectly by downregulation of IL-12 from the CD8α+ DCs (Figs. 4 and 6). Furthermore, we believe that the CD8α− DCs and more specifically the CD8α−CD4+ subpopulation display tolerizing effects on Th1 cells as they were unable to drive IFN-γ production by the Th0 T cell clone even upon stimulation with sol Ig-PLP1, a chimera which does not induce IL-10 production (Fig. 6). The observation that CD8α− DCs loaded with free PLP1 peptide (hence no IL-10 involvement) were unable to induce EAE, while CD8α+ DCs did, (Fig. 8) further supports the idea that CD8α− DCs are tolerogenic against Th1 cells.
CD8α− DCs, which proved tolerogenic against Th1 responses in this model, have previously been shown to promote the development of Th2 cells (7, 8). Thus, the question that arises is whether the same DCs display a functional plasticity and prime Th2 responses while tolerizing for Th1 immunity, or if each function is confined to a distinct subset within the CD8α− population. The latter was confirmed for the tolerizing function as the CD8α−CD4+, but not the CD8α−CD4−, subset was found to produce IL-10 and reverse EAE upon treatment with agg Ig-MOG (Fig. 9). Therefore, we believe the CD8α−CD4+ subset of mature splenic DCs displays intrinsic tolerizing properties complemented with the ability to produce IL-10 and play a critical role in peripheral tolerance against self-reactive T cells. Whether or not these cells represent the described previously (6, 19, 48) mature DC subset specialized in T cell tolerance is not clear at this time.
Our prior studies of disease reversal using agg Ig-PLP1 in the SJL system have not been able to detect regulatory T cells (21). Since regulatory T cells usually adopt a cycling pattern (49) and may be subject to self-limitation upon modulation of the pathogenic T cells, they may be difficult to detect subsequent to recovery from disease. If this is the case the possibility remains that CD8α−CD4+ DCs are able to induce regulatory T cells, particularly that they produce IL-10, a growth factor for regulatory T cells (34). Whatever the mechanism might be, these cells seem to be very potent, as tolerance has occurred in two different antigenic systems using two different mouse strains and the disease was reversed even in SJL mice where the autoreactive T cell repertoire encompasses an usual high frequency of self-reactive T cells (50).
Overall, these investigations have identified a DC subset that displays powerful modulatory effects on autoreactive T cells and provides a DC candidate that could sustain peripheral tolerance against self-antigens.
Acknowledgments
This work was supported by the grants RG2967A2/1 from the National Multiple Sclerosis Society, and RO1NS37406 from the National Institutes of Health and a contract from Astral, Inc., a subsidiary of Alliance Pharmaceutical Corp. (San Diego, CA).
K.L. Legge's present address is Beirne B. Carter Center for Immunology Research, University of Virginia, 400 Lane Rd., MR-4 Building, P.O. Box 801386, Charlottesville, VA 22908-1386.
Footnotes
Abbreviations used in this paper: aa, amino acid; agg, aggregated; CNS, central nervous system; DC, dendritic cell; EAE, experimental allergic encephalomyelitis; MOG, myelin oligodendrocyte glycoprotein; PLP, myelin proteolipid protein; sol, soluble.
References
- 1.Steinman, R.M. 1991. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 9:271–296. [DOI] [PubMed] [Google Scholar]
- 2.Macatonia, S.E., P.M. Taylor, S.C. Knight, and B.A. Askonas. 1989. Primary stimulation by dendritic cells induces antiviral proliferative and cytotoxic T cell response in vitro. J. Exp. Med. 169:1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inaba, K., J.P. Metlay, M.T. Crowley, and R.M. Steinman. 1990. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J. Exp. Med. 172:631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sornasse, T., V. Flamand, G. De Becker, H. Bazin, F. Tielemans, K. Thielemans, J. Urbain, O. Leo, and M. Moser. 1992. Antigen-pulsed dendritic cells can efficiently induce an antibody response in vivo. J. Exp. Med. 175:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kronin, V., K. Winkel, G. Shss, A. Kelso, W. Heath, J. Kirberg, H. von Boehmer, and K. Shortman. 1996. A subclass of dendritic cells regulates the response of naïve CD8 T cells by limiting their IL-12 production. J. Immunol. 157:3819–3827. [PubMed] [Google Scholar]
- 6.Rissoan, M.C., V. Soumelis, N. Kadowaki, G. Grouard, F. Briere, R. de Waal Malefyt, and Y.J. Liu. 1999. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 283:1183–1186. [DOI] [PubMed] [Google Scholar]
- 7.Maldonado-Lopez, R., T. De Smedt, P. Michel, J. Godfroid, B. Pajak, C. Heirman, K. Thielemans, O. Leo, J. Urbain, and M. Moser. 1999. CD8α+ and CD8α− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 189:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pulendran, B., J.L. Smith, G. Caspary, K. Brasel, D. Pettit, E. Maraskovsky, and C.R. Maliszewski. 1999. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. USA. 96:1036–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albert, M.L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 392:86–89. [DOI] [PubMed] [Google Scholar]
- 10.Sauter, B., M.L. Albert, L. Francisco, M. Larsson, S. Somersan, and N. Bhardwaj. 2000. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 191:423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bevan, M.J. 1976. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J. Exp. Med. 143:1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.den Haan, J.M., S.M. Lehar, and M.J. Bevan. 2000. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 192:1685–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sigal, J.L., S. Crotty, R. Anidino, and K.L. Rock. 1999. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature. 398:77–80. [DOI] [PubMed] [Google Scholar]
- 14.Berard, F., P. Blanco, J. Davoust, E.M. Neidhart-Berard, M. Nouri-Shirazi, N. Taquet, D. Rimoldi, J.C. Cerottini, J. Banchereau, and A.K. Paluka. 2000. Cross-priming of naive CD8 T cells against melanoma antigens using dendritic cells loaded with killed allogeneic melanoma cells. J. Exp. Med. 192:1535–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subklewe, M., C. Paludan, M.L. Tsang, K. Mahnke, R.M. Steinman, and C. Münz. 2001. Dendritic cells cross present latency gene products from Epstein-Barr virus-transformed B cells and expand tumor-reactive CD8+ killer T cells. J. Exp. Med. 193:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inaba, M., K. Inaba, M. Hosono, T. Kumamoto, T. Ishida, S. Muramatsu, T. Masuda, and S. Ikehara. 1991. Distinct mechanism of neonatal tolerance induced by dendritic cells and thymic B cells. J. Exp. Med. 173:549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khoury, S.J., L. Gallon, W. Chen, K. Betres, M.E. Russell, W.W. Hancock, C.B. Carpenter, M.H. Sayegh, and H.L. Weiner. 1995. Mechanisms of acquired thymic tolerance in experimental autoimmune encephalomyelitis: thymic dendritic-enriched cells induce specific peripheral T cell unresponsiveness in vivo. J. Exp. Med. 182:357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broker, T., M. Riedinger, and K. Karjalainen. 1997. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo. J. Exp. Med. 185:541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinman, R.M., S. Turley, I. Mellman, and K. Inaba. 2000. The induction of tolerance by dendritic cells that have captured apoptotic cells. J. Exp. Med. 191:411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Legge, K.L., B. Min, N.T. Potter, and H. Zaghouani. 1997. Presentation of a T cell receptor antagonist peptide by immunoglobulins ablates activation of T cells by a synthetic peptide or proteins requiring endocytic processing. J. Exp. Med. 185:1043–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Legge, K.L., B. Min, J.J. Bell, J.C. Caprio, L. Li, R.K. Gregg, and H. Zaghouani. 2000. Coupling of peripheral tolerance to endogenous interleukin 10 promotes effective modulation of myelin-activated T cells and ameliorates experimental allergic encephalomyelitis. J. Exp. Med. 191:2039–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Legge, K.L., J.J. Bell, L. Li, R. Gregg, J.C. Caprio, and H. Zaghouani. 2001. Multi-modal antigen specific therapy for autoimmunity. Intern. Rev. Immunol. 20:593–611. [DOI] [PubMed] [Google Scholar]
- 23.Bernard, C.C.A., T.G. Johns, A. Slavin, M. Ichikawa, C. Ewing, J. Lui, and J. Bettadapura. 1997. Myelin oligodendrocyte glycoprotein: a novel candidate autoantigen in multiple sclerosis. J. Mol. Med. 75:77–88. [DOI] [PubMed] [Google Scholar]
- 24.Takai, T., M. Ono, M. Hikida, H. Ohmori, and J.V. Ravetch. 1996. Augmented humoral and anaphylactic responses in Fcγ RII-deficient mice. Nature. 379:346–349. [DOI] [PubMed] [Google Scholar]
- 25.Takai, T., M. Li, D. Sylvestre, R. Clynes, and R.V. Ravetch. 1994. FcRγ chain deletion results in pleiotrophic effector cell defects. Cell. 76:519–529. [DOI] [PubMed] [Google Scholar]
- 26.Tuohy, V.K., Z. Lu, R.A. Sobel, R.A. Laursen, and M.B. Lees. 1989. Identification of an encephalitogenic determinant of myelin proteolipid protein for SJL mice. J. Immunol. 142:1523–1527. [PubMed] [Google Scholar]
- 27.Romani, N., N. Bhardwaj, M. Pope, F. Koch, W.J. Swiggard, U.O. Doherty, M.D. Witmer-Pack, L. Hoffman, G. Schuler, K. Inaba, and R.M. Steinman. 1996. Dendritic cells. Weirs Handbook of Experimental Immunology. L.A. Herzenberg, D. Weir, L.A. Herzenberg, and C. Blackwell, editors. Blackwell Science, Cambridge. 156.1–156.14.
- 28.Zaghouani, H., R.M. Steinman, R. Nonacs, H. Shah, W. Gerhard, and C. Bona. 1993. Presentation of a viral T cell epitope expressed in the CDR3 region of a self immunoglobulin molecule. Science. 259:224–227. [DOI] [PubMed] [Google Scholar]
- 29.Daeron, M. 1997. Fc receptor biology. Annu. Rev. Immunol. 15:203–234. [DOI] [PubMed] [Google Scholar]
- 30.Unkeless, J.C. 1979. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J. Exp. Med. 150:580–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore, K.W., R. de Waal Malefyt, R.L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683–765. [DOI] [PubMed] [Google Scholar]
- 32.Macatonia, S.E., T.M. Doherty, S.C. Knight, and A. O'Garra. 1993. Differential effect of interleukin 10 on dendritic cell-induced T cell proliferation and IFNγ production. J. Immunol. 150:3755–3765. [PubMed] [Google Scholar]
- 33.Joss, A., M. Akdis, A. Faith, K. Blaser, and C.A. Akdis. 2000. IL-10 directly acts on T cells by specifically altering the CD28 co-stimulation pathway. Eur. J. Immunol. 30:1683–1690. [DOI] [PubMed] [Google Scholar]
- 34.Groux, H., M. Bigler, J.E. de Vries, and M.G. Roncarolo. 1996. Interleukin-10 induces a long term antigen-specific anergic state in human CD4+ T cells. J. Exp. Med. 184:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinbrink, K., M. Wolfl, H. Jonuleit, J. Knop, and A.H. Enk. 1997. Induction of tolerance by IL-10 treated dendritic cells. J. Immunol. 159:4772–4780. [PubMed] [Google Scholar]
- 36.Vermec, D., M. Zorbas, R. Scollay, D.J. Saunders, C.F. Ardavin, L. Wu, and K. Shortman. 1992. The surface phenotype of dendritic cells purified from mouse thymus and spleen; investigation of the CD8 expression by a subpopulation of dendritic cells. J. Exp. Med. 176:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vermec, D., J. Pooley, H. Hochrein, L. Wu, and K. Shortman. 2000. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J. Immunol. 164:2978–2986. [DOI] [PubMed] [Google Scholar]
- 38.Pulendran, B., J. Lingappa, M.K. Kennedy, J. Smith, M. Teepe, A. Rudensky, C.R. Maliszewski, and E. Maraskovsky. 1997. Develpomental pathways of dendritic cells in vivo: distinct function, phenotype, and localization of dendritic cell subsets in FLT3 ligand-treated mice. J. Immunol. 159:2222–2231. [PubMed] [Google Scholar]
- 39.Dittel, B.N., I. Visintin, R.M. Merchant, and C.A. Janeway, Jr. 1999. Presentation of the self antigen myelin basic protein by dendritic cells leads to experimental autoimmune encephalomyelitis. J. Immunol. 163:32–39. [PubMed] [Google Scholar]
- 40.Brumeanu, T.D., W.J. Swiggard, R.M. Steinman, C.A. Bona, and H. Zaghouani. 1993. Efficient loading of identical viral peptide onto class II molecules by antigenized immunoglobin and influenza virus. J. Exp. Med. 178:1795–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maldonado-Lopez, R., C. Maliszewski, J. Urbain, and M. Moser. 2001. Cytokines regulate the capacity of CD8α1 and CD8α2 dendritic cells to prime Th1/Th2 cells in vivo. J. Immunol. 167:4345–4350. [DOI] [PubMed] [Google Scholar]
- 42.Dhodapkar, M.V., R.M. Steinman, J. Krasovsky, C. Münz, and N. Bhardwaj. 2001. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J. Exp. Med. 193:233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jonuleit, H., E. Schmitt, G. Schuler, J. Knop, and A.H. Enk. 2000. Induction of interleukin-10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J. Exp. Med. 192:1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang, F.P., N. Platt, M. Wykes, J.R. Major, T.J. Powell, C.D. Jenkins, and G.G. MacPherson. 2000. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 191:435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menges, M., S. Röflner, C. Voigtländer, H. Schindler, N.A. Kukutsch, C. Bogdan, K. Erb, G. Schuler, and M.B. Lutz. 2002. Repetitive injections of dendritic cells matured with tumor necrosis factor α induce antigen-specific protection of mice from autoimmunity. J. Exp. Med. 195:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hawiger, D., K. Inaba, Y. Dorsett, M. Guo, K. Mahnke, M. Rivera, J.V. Ravetch, R.M. Steinman, and M.C. Nussensweig. 2001. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 194:769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cua, D.J., B. Hutchins, D.M. LaFace, S.A. Stohlman, and R.L. Coffman. 2001. Central nervous system expression of IL-10 inhibits autoimmune encephalomyelitis. J. Immunol. 166:602–608. [DOI] [PubMed] [Google Scholar]
- 48.Suss, G., and K. Shortman. 1996. A subclass of dendritic cells kills CD4 T cells via Fas/Fas-ligand-induced apoptosis. J. Exp. Med. 183:1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jerne, N.K. 1974. Towards a network theory of the immune system. Annu. Immunol. 125C:373–389. [PubMed] [Google Scholar]
- 50.Anderson, A.C., L.B. Nicholson, K.L. Legge, V. Turchin, H. Zaghouani, and V.K. Kuchroo. 2000. High frequency of autoreactive myelin proteolipid protein-specific T cells in the periphery of naive mice: mechanisms of selection of the self-reactive repertoire. J. Exp. Med. 191:761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]