Abstract
Natural killer T (NKT) cells have been implicated in diverse immune responses ranging from suppression of autoimmunity to tumor rejection. Thymus-dependent NKT cells are positively selected by the major histocompatibility complex class I–like molecule CD1d, but the molecular events downstream of CD1d are still poorly understood. Here, we show that distinct members of the Rel/nuclear factor (NF)-κB family of transcription factors were required in both hematopoietic and nonhematopoietic cells for normal development of thymic NKT cells. Activation of NF-κB via the classical IκBα-regulated pathway was required in a cell autonomous manner for the transition of NK-1.1–negative precursors that express the TCR Vα14-Jα18 chain to mature NK-1.1–positive NKT cells. The Rel/NF-κB family member RelB, on the other hand, had to be expressed in radiation resistant thymic stromal cells for the generation of early NK-1.1–negative NKT precursors. Moreover, NF-κB–inducing kinase (NIK) was required for both constitutive thymic DNA binding of RelB and the specific induction of RelB complexes in vitro. Thus, distinct Rel/NF-κB family members in hematopoietic and nonhematopoietic cells regulate NKT cell development with a unique requirement for NIK-mediated activation of RelB in thymic stroma.
Keywords: RelB, NKT cells, thymus, aly, NIK
Introduction
NKT cells represent a subset of mature T lymphocytes, which are characterized by the coexpression of NK cell surface markers and the TCR as well as high levels of cytokine production, in particular IL-4 and IFN-γ. NKT cells are most frequent in thymus, bone marrow (BM),* spleen, and liver and represent a smaller proportion of T cells in lymph nodes, blood, and lung. In mouse thymus, NKT cells are either CD4−CD8− or CD4+CD8− and represent 0.3–0.8% of total thymocytes and up to 20% of the mature heat stable antigen (HSA)neg-low thymocyte fraction (for reviews, see references 1–3). Thymus-dependent NKT cells are positively selected by CD1d, a nonclassical MHC class I–like, β2-microglobulin-associated molecule, and they predominantly express the invariant Vα14-Jα18 TCR (4–7). These so-called Vα14i NKT cells respond to the marine sponge-derived glycolipid α-galactosyl ceramide (α-GalCer) and can be identified by staining with α-GalCer–loaded CD1d tetramers (8–10). Other populations of NKT cells, defined by NK-1.1 and TCR expression, do not express the Vα14-Jα18 TCR and thus are unresponsive to α-GalCer (11, 12).
Several independent observations indicate that the requirements for NKT cell and conventional TCRαβ T lymphocyte development are fundamentally different. First, whereas thymic epithelial cells are the predominant cell type for positive selection of conventional T cells, BM-derived immature CD4+CD8+ double positive (DP) thymocytes are required for the positive selection of NKT cells (6, 13). Second, many mutations/gene deletions that impair development of conventional T cells do not have a severe effect on the development of NKT cells and in a few cases the opposite is true (for a review, see reference 14). These observations suggest that mouse NKT cells belong to a special lineage of T lymphocytes and that NKT precursors follow the conventional developmental pathway to the CD4+CD8+ DP thymocyte to branch off into the NKT lineage as a result of CD1d mediated instructions given to the Vα14-Jα18 TCR (1). The recent finding that NKT cells are indeed derived from DP thymocytes (15) supports this model, but the molecular events downstream of CD1d selection are still poorly understood.
Gene knockout studies demonstrate that lymphotoxin (LT) plays an important role in organogenesis and maintenance of secondary lymphoid tissue microarchitecture (for a review, see reference 16). Membrane LT signaling is defective in the absence of functional nuclear factor (NF)-κB–inducing kinase (NIK) and NIK-deficient as well as alymphoplasia (aly/aly) mice, which carry an amino acid substitution in the COOH-terminal domain of NIK, have similar defects in lymphoid organ development (16–19). Interestingly, both LT signaling and NIK are required for normal mouse NKT cell development, but dispensable for the development of conventional T cells (20–22).
The Rel/NF-κB transcription factors, consisting of the family members NF-κB1 (encoding p50 and its precursor p105), NF-κB2 (encoding p52 and its precursor p100), RelA (p65), RelB, and c-Rel, play an important role in immune responses, inflammation, cell survival, and cancer (23–25). In resting cells, NF-κB proteins are retained in the cytoplasm as inactive forms via the association with inhibitory IκB molecules. The classical induction of p50-RelA NF-κB heterodimers involves activation of the IκB kinase (IKK) complex by a wide range of stimuli, resulting in the phosphorylation and ubiquitin-dependent degradation of IκBα. As a consequence, RelA NF-κB complexes translocate to the nucleus and regulate the expression of κB target genes (26, 27). Recently, an alternative pathway has been described that involves NIK/IKKα-mediated phosphorylation of the inhibitory p100 precursor, its processing to p52, and nuclear translocation of p52-RelB heterodimers (28).
Rel/NF-κB proteins have essential and distinct roles in development and function of the immune system. Similar to LT signaling, Rel/NF-κB family members are required for proper organogenesis and maintenance of secondary lymphoid tissues (29–31). Importantly, while NF-κB regulates specific T cell functions, such as antigen/mitogen-induced proliferation and cytokine production, thymic development of conventional T cells is not affected by the targeted deletion of individual Rel/NF-κB family members or the blockage of the classical NF-κB pathway with a mutant nondegradable mIκBα super-inhibitor (32).
We show here that, similar to aly/aly mice, RelB-deficient animals lacked thymus-dependent Vα14i NKT cells. Whereas RelB had to be expressed in radiation-resistant thymic stromal cells, classical NF-κB was required in NKT precursors for their maturation and expansion. We further show that NIK regulates RelB DNA binding in vitro and in vivo, suggesting a unique requirement for NIK-mediated activation of RelB in NKT cell development.
Materials and Methods
Mice.
Generation of nfkb1 − / − (33), nfkb2 − / − (34), relB − / − (35), ltbr − / − (36), tnfr1 − / − (37), and mIκBαtg mice (38) has been described previously. Alymphoplasia (aly/aly) (17, 19) mice were kindly provided by Dr. Thomas Böhm, Max-Planck-Institute of Immunobiology, Freiburg, Germany. C57BL/6-Ly-5.1 mice were obtained from Charles River Laboratories. Adoptive BM transfers were performed as previously described (30) and chimeric mice were analyzed 12–14 wk after reconstitution. All animals were housed and bred under standardized conditions with water and food ad libitum in the SPF mouse facility of the Forschungszentrum Karlsruhe.
Flow Cytometric Analyses.
Flow cytometry was performed using a Becton Dickinson LSR flow cytometer. Single cell suspensions were washed twice with staining buffer (PBS, 2% FCS) and incubated with Fc Block™ (clone 2.4G2). The following conjugated mAbs were used: anti-NK-1.1-PE (clone PK136, diluted 1:200), anti-TCRβ-FITC (clone H57–597, diluted 1:100), anti-CD24/HSA-Biotin (clone M1/69, diluted 1:100), anti-CD4-FITC/Biotin (clone RM4–5, diluted 1:100), anti-CD8α-PE/Biotin (clone 53–6.7, diluted 1:200), anti-CD1d-FITC (clone 1B1, diluted 1:100), anti-CD44-Biotin (clone IM7, diluted 1:100), and anti-Ly-5.2-Biotin (clone 104, diluted 1:100). All mAbs were from BD Biosciences. Biotinylated mAbs were detected with Streptavidin-PerCP (BD Biosciences, diluted 1:200). Apoptotic cells were detected by Annexin V-FITC staining according to the manufacturer's recommendations (Roche). Incubations were for 30 min on ice followed by two washes with staining buffer. Staining with α-GalCer–loaded CD1d tetramers was performed as described previously (39). Data were analyzed using CELLQuest Pro™ software (BD Biosciences).
Cell Culture.
Primary mouse embryonic fibroblasts (MEFs) were prepared from E15.5 embryos according to standard procedures (40), cultured in DMEM supplemented with 10% heat-inactivated FCS, penicillin (100 U/ml), streptomycin (100 μg/ml), l-glutamine (2 mM), and treated with agonistic anti-LTβR mAb (1 μg/ml clone AC.H6; gift from Dr. Jeffrey Browning and Dr. Paul Rennert, Biogen, Cambridge, MA).
RNA Analysis.
RNA extraction and quantitative RT-PCR were performed as reported previously (30). Briefly, amplification conditions using an MJ Research PTC-225 thermal cycler were 94°C for 1 min, 55°C for 1 min, 72°C for 1 min in the presence of 1 μCi [α-32P]dCTP. The linear range of amplification was determined for each primer pair: Vα14/Jα18, 26 cycles; Cβ, 18 cycles; IL-15, 29 cycles. Amplified products were separated in 6% polyacrylamide gels. Quantifications were done with a Fujix BAS 1000 Phosphorimager. PCR primers were: Vα14 (GTT GTC CGT CAG GGA GAG AA) and Jα18 (CAA TCA GCT GAG TCC CAG CT), IL-15 (TCA GCA GAT AAC CAG CCT AC and TTT CTC CTC CAG CTC CTC AC), and Cβ (CAC TGA TGT TCT GTG TGA CA and GAG GAT CTG AGA AAT GTG ACT CCA C).
Electrophoretic Mobility Shift Assays.
Whole thymus tissue extracts and nuclear extracts from fibroblasts were prepared and electrophoretic mobility shift assays (EMSAs) were performed as described previously (38, 41). Integrity of extracts was checked using an Oct oligodeoxynucleotide.
Online Supplemental Material.
Fig. S1 shows reduced numbers of Vα14i/NKT cells in relB − / − and mIκBαtg mice. Thymocytes from 3-mo-old mice were analyzed for tetramer binding and TCRβ expression by flow cytometry (lymphocyte gate). Numbers indicate mean values ± SD from three mice. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20022234/DC1.
Results
NF-κB Is Essential for Normal NKT Cell Development.
To examine the role of individual Rel/NF-κB subunits in NKT cell development we analyzed mice with targeted disruptions of RelB (relB − / −), p50/NF-κB1 (nfkb1 − / −), and p52/NF-κB2 (nfkb2 − / −) as well as transgenic mice, in which NF-κB function is inhibited in T cells by the expression of a nondegradable mutant form of the IκBα molecule under the control of the T cell–specific lck proximal promoter (mIκBαtg). The percentage of NK-1.1+TCRβ+ cells in thymus of relB − / − mice was dramatically reduced (∼20-fold) compared with wild-type mice (Fig. 1 A). Thymic NK-1.1+TCRβ+ cells were also markedly reduced in nfkb1 − / − mice (∼4-fold), whereas nfkb2 − / − mice showed a milder phenotype with two- to threefold reduced NK-1.1+TCRβ+ cell numbers (Fig. 1 A). The observed defects in NKT cell development in relB − / −, nfkb1 − / −, and nfkb2 − / − mice could be due to the lack of these proteins in all cells of the thymus (including stroma) or to impaired NF-κB function in hematopoietic thymocytes that develop into mature T cells. We therefore analyzed mIκBαtg mice, in which NF-κB function is blocked in Lck-expressing thymocytes (38). Interestingly, the specific inhibition of NF-κB in these cells also resulted in a ∼5-fold reduced NKT cell population compared with nontransgenic littermates (Fig. 1 B).
Figure 1.
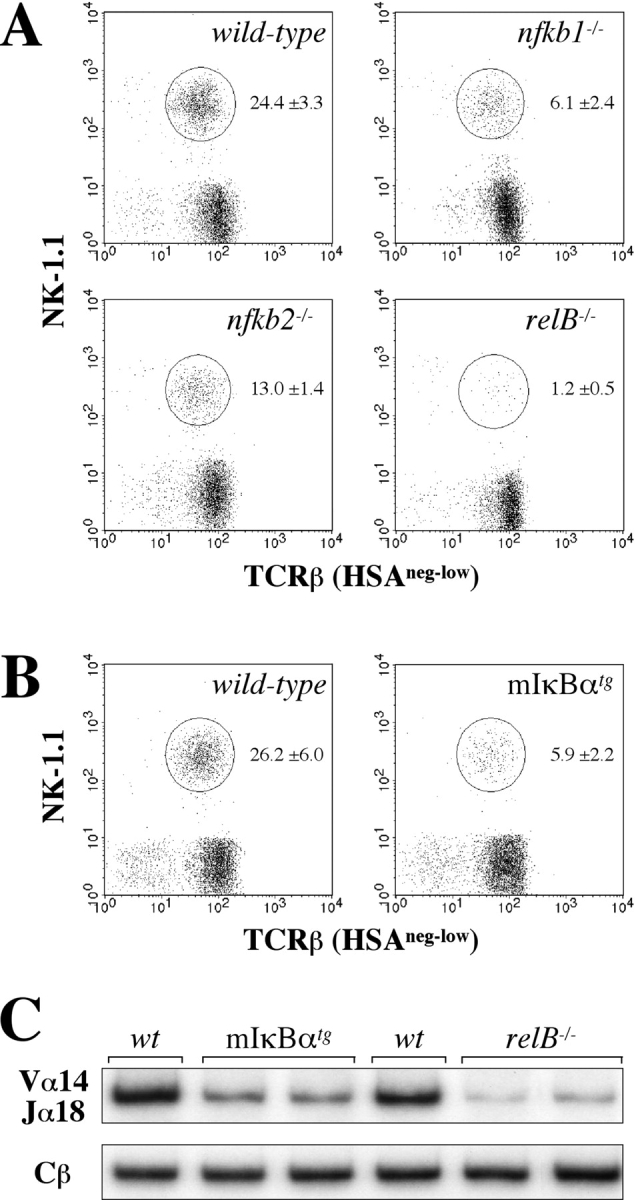
Reduced numbers of thymic NKT cells in NF-κB–deficient mice. Thymocytes from adult wild-type, nfkb1 − / −, nfkb2 − / − , and relB − / − (A) as well as from control and mIκBαtg mice (B) were analyzed for NK-1.1 and TCRβ expression by flow cytometry. Percentages of NKT cells (circles) were increased by gating on HSAneg-low cells. Numbers indicate mean values ± SD from 5–7 mice. (C) RT-PCR analysis of Vα14-Jα18 expression in wild-type, mIκBαtg, and relB − / − mice. Expression of the Cβ chain is shown as an amplification control.
Since NKT cell development can still occur in the absence of the NK-1.1 marker (42), the observed reduction of NK-1.1+TCRβ+ cells in relB − / −, nfkb1 − / −, nfkb2 − / −, and mIκBαtg mice could be due to the lack of NK-1.1 expression. Expression of the Vα14-Jα18 chain is considered a more stringent measure for the major population of NKT cells in thymus (43). Semiquantitative RT-PCR analysis revealed reduced Vα14-Jα18 mRNA levels in relB − / −, nfkb1 − / −, nfkb2 − / −, and mIκBαtg thymocytes (Fig. 1 C, and unpublished data). Staining with α-GalCer/CD1d tetramers confirmed the reduced Vα14i NKT cell numbers, indicating that these mice have a true defect in NKT cell development (online supplemental Fig. S1).
Selective Loss of CD4+ NKT Cells in Spleen and Bone Marrow of relB−/− and nfkb1−/− Mice.
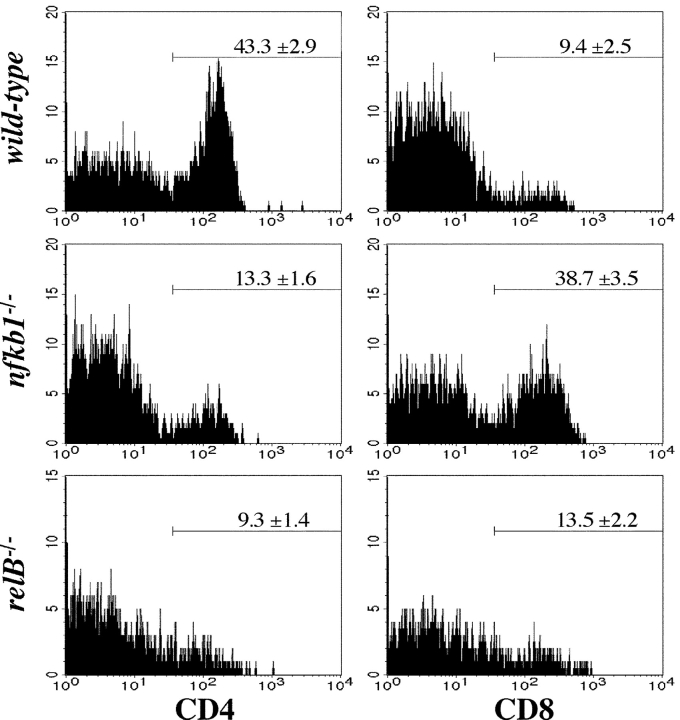
Spleen and BM are other major lymphoid organs where NKT cells are found. Despite the significant reduction in thymic NKT cells, only a moderate reduction in total NKT cell numbers was observed in spleen and BM of relB − / −, nfkb1 − / −, and mIκBαtg mice (unpublished data). When different peripheral NKT cell subpopulations were analyzed thymus-dependent CD4+ NKT cells were clearly reduced in spleens from relB − / − (4.5-fold) and nfkb1 − / − mice (threefold). Interestingly, nfkb1 − / − spleens showed a selective fourfold increase in the percentage of CD8+ NK-1.1+ T cells, whereas this subpopulation was only minimally increased in relB − / − mice (Fig. 2) . As the CD8+ NK-1.1+ subset is unrelated to thymus-dependent CD4+ NKT cells (12), this result indicates a specific requirement of NF-κB for the development of Vα14i NKT cells rather than for the total NK-1.1+ T cell population.
Figure 2.
Selective loss of peripheral CD4+ NKT cells in relB − / − and nfkb1 − / − mice. NKT cells from adult wild-type, nfkb1 − / −, and relB − / − spleens were analyzed for CD4 and CD8 expression by flow cytometry. Histograms show CD4 (left) and CD8 (right) expression levels on NK-1.1+TCRβ+ cells. Note the reduction of CD4+ NKT cells in nfkb1 − / − and relB − / − mice and the increase of CD8+ NK-1.1+ T cells in nfkb1 − / − but not in relB − / − animals. Numbers indicate mean values ± SD from 3–7 mice.
Differential Requirement of Distinct NF-κB Complexes in Hematopoietic and Radiation-resistant Stromal Cells for NKT Cell Development.
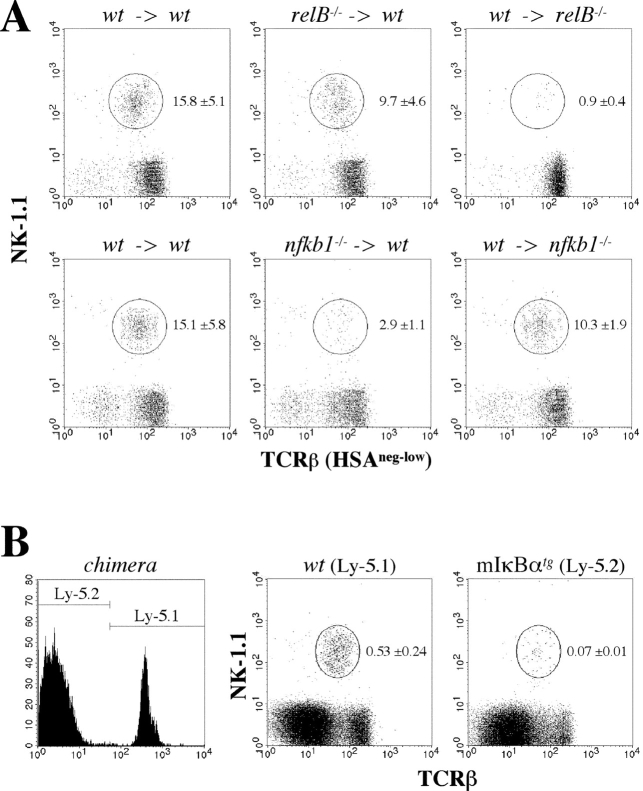
Thymic NKT cells are positively selected via the interaction of their Vα14-Jα18 TCR with CD1d on DP cortical thymocytes (6) but stromal components are also required for normal NKT cell development (22). Flow cytometric analysis revealed normal CD1d levels on thymocytes from relB − / −, nfkb1 − / −, nfkb2 − / −, and mIκBαtg mice, indicating that NF-κB is not required for the expression of CD1d (unpublished data). Next, we examined the requirement of individual NF-κB members in both hematopoietic and nonhematopoietic cells by radiation chimera analysis. Reciprocal BM transplantations between wild-type and relB − / − mice revealed that RelB in radiation resistant stromal components was required for NKT cell development, as wild-type BM transferred into lethally irradiated relB − / − recipients resulted in severely impaired development of NKT cells (Fig. 3 A). RelB appeared to play a minor role in hematopoietic cells, as adoptive transfers of relB − / − BM into wild-type recipients resulted only in a 1.5-fold reduction of NKT cell numbers compared with controls. Interestingly, reciprocal BM transfers between wild-type and nfkb1 − / − mice revealed that p50/NF-κB1 was required in hematopoietic cells rather than in the stromal compartment (Fig. 3 A). In contrast to p50/NF-κB1 but similar to RelB, p52/NF-κB2 was required in radiation-resistant nonhematopoietic cells for proper NKT cell development (unpublished data).
Figure 3.
Analysis of NKT cell development in BM chimeras. (A) Thymocytes were isolated from lethally irradiated mice that were reconstituted with BM as indicated (donor BM → irradiated recipient). HSAneg-low cells were analyzed for NK-1.1 and TCRβ expression by flow cytometry. Percentages of NKT cells (mean values from 3–7 mice ± SD) are indicated. (B) Competitive BM chimeras were generated by mixing wild-type Ly-5.1 with mIκBαtg Ly-5.2 BM to reconstitute lethally irradiated wild-type Ly-5.2 mice. Chimerism was checked by flow cytometry (left). Thymocytes derived from wild-type (Ly-5.1+) and from mIκBαtg (Ly-5.2+) BM were stained for NK-1.1 and TCRβ expression and analyzed by flow cytometry (right). Percentages of NKT cells among total thymocytes (mean values from 2–3 mice ± SD) are indicated.
To determine whether the reduced numbers of NKT cells in mIκBαtg mice was a direct effect of blocking the NF-κB pathway in NKT precursors or due to the lack of NF-κB in accessory thymocytes, mixed chimeric mice were generated. BM cells from Ly-5.1 wild-type and Ly-5.2 mIκBαtg mice were mixed and injected into lethally irradiated Ly-5.2 wild-type recipients. Flow cytometric analysis showed that ∼70% of the thymocytes were derived from Ly-5.2 mIκBαtg BM whereas the Ly-5.1 wild-type BM contributed the remaining 30% (Fig. 3 B). While only Ly-5.1 wild-type BM resulted in the generation of NKT cells, BM from Ly-5.2 mIκBαtg mice was unable to efficiently generate NKT cells despite the coexistence of wild-type thymocytes (Fig. 3 B). Together, these data indicate that thymic NKT cell development is dependent on both RelB in the nonhematopoietic compartment and, in a cell-autonomous manner, on classical NF-κB activity in NKT precursors.
RelB Is Required for Normal Thymic IL-15 mRNA Expression.
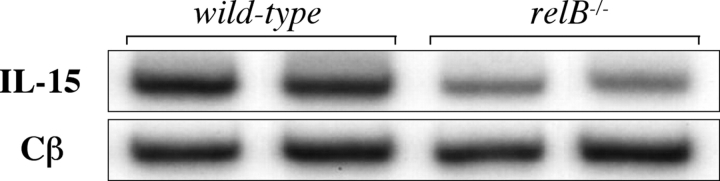
As NF-κB regulates gene expression we analyzed total thymus steady-state mRNA levels of several genes that have been shown to be crucial for NKT cell development (14). Focusing on relB − / − and mIκBαtg mice, we did not observe significant defects in the expression of cathepsin S, cathepsin L, Ets-1, Fyn, GM-CSFRβ, IL-15Rα, IRF-1, LTα, LTβ, and Vav-1 compared with wild-type controls (unpublished data). In contrast, steady-state levels of IL-15 mRNA were reduced ∼3-fold in relB − / − but not in mIκBαtg thymus (Fig. 4) . IL-15 expression was not detected in thymocyte single cell suspensions (unpublished data), indicating that RelB in stromal cells is required for the maintenance of normal IL-15 levels.
Figure 4.
Analysis of IL-15 mRNA expression in thymus from NF-κB–deficient mice. RT-PCR analysis of whole thymus RNA revealed reduced IL-15 steady-state mRNA levels in relB − / − mice. Expression of IL-15 in thymus from mIκBαtg mice was similar to controls (unpublished data). Expression of the Cβ chain is shown as an amplification control.
DNA Binding of RelB Complexes Is Defective in Thymus of aly/aly Mice.
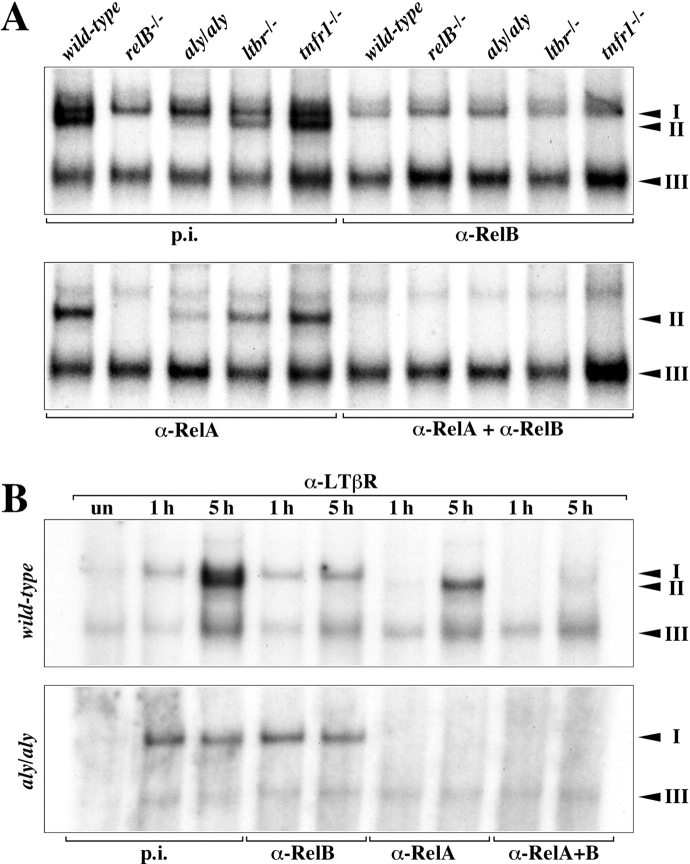
NIK is a key player in LT signaling and it has previously been shown that NIK is required in nonhematopoietic cells for normal NKT cell development (22). As thymic NKT cell development was also dependent on stromal RelB expression we analyzed whether the lack of functional NIK affects constitutive DNA-binding of RelB complexes in thymus. When whole thymus extracts were analyzed in EMSAs a marked reduction of RelB κB-binding (complex II) was observed in aly/aly extracts and to a lesser extent also in ltbr − / − mice, whereas binding of RelA (complex I) was not affected (Fig. 5 A). Extracts from relB − / − and tnfr1 − / − mice were included as a specificity control. To address whether NIK is also required for the induced binding of RelB complexes we stimulated fibroblasts with agonistic anti-LTβR mAb. LTβR activation in wild-type MEFs resulted in the rapid induction of RelA (complex I) and at later time points also RelB (complex II), while no binding of RelB complexes could be detected in aly/aly MEFs despite normal induction of RelA (Fig. 5 B). Thus, both constitutive and surface receptor-induced RelB κB-binding requires functional NIK, whereas binding of classical RelA NF-κB complexes is independent of this kinase.
Figure 5.
LTβR and NIK regulate RelB DNA-binding in thymus. (A) Whole thymus extracts from wild-type, relB − / −, aly/aly, ltbr − / −, and tnfr1 − / − mice were prepared and analyzed for κB-binding activity in EMSAs. Complexes are indicated by arrowheads and their identity was determined with Abs specific for individual Rel/NF-κB family members. p.i., preimmune serum; complex I, RelA heterodimers; complex II, RelB heterodimers. Complex III consists of p50 homodimers (unpublished data). (B) LTβR-mediated induction of RelB is dependent on NIK. Primary MEFs from wild-type and aly/aly mice were either left untreated (un) or treated for 1 and 5 h with anti-LTβR mAb and nuclear extracts were analyzed for κB-binding. Complexes were identified with specific Abs.
Lack of RelB and NF-κB Blocks Early NKT Cell Development at Different Steps.
Recent evidence indicates that NK-1.1− NKT precursors that bind to CD1d tetramers give rise to mature NK-1.1+ tetramer+ cells in both thymus and peripheral lymphoid tissues (15, 44, 45). As the frequency of NK-1.1− precursors is low in adult compared with young animals, we analyzed 2-wk-old mice by flow cytometry. Staining of wild-type thymocytes with α-GalCer–loaded CD1d tetramer and anti-NK-1.1 mAb revealed that tetramer+ cells at this age consisted predominantly of NK-1.1− precursors and a smaller portion of mature NK-1.1+ NKT cells. In contrast, the NK-1.1− precursor population was markedly reduced in relB − / − mice whereas mature NKT cells were less severely affected. On the other hand, mIκBαtg mice had a normal NK-1.1− precursor frequency but a ninefold reduction in the percentage of mature NK-1.1+ tetramer+ NKT cells (Fig. 6 A). Annexin V staining of thymocytes from 2-wk-old mice did not reveal increased apoptosis of tetramer+ cells in relB − / − or mIκBαtg mice (Fig. 6 B).
Figure 6.
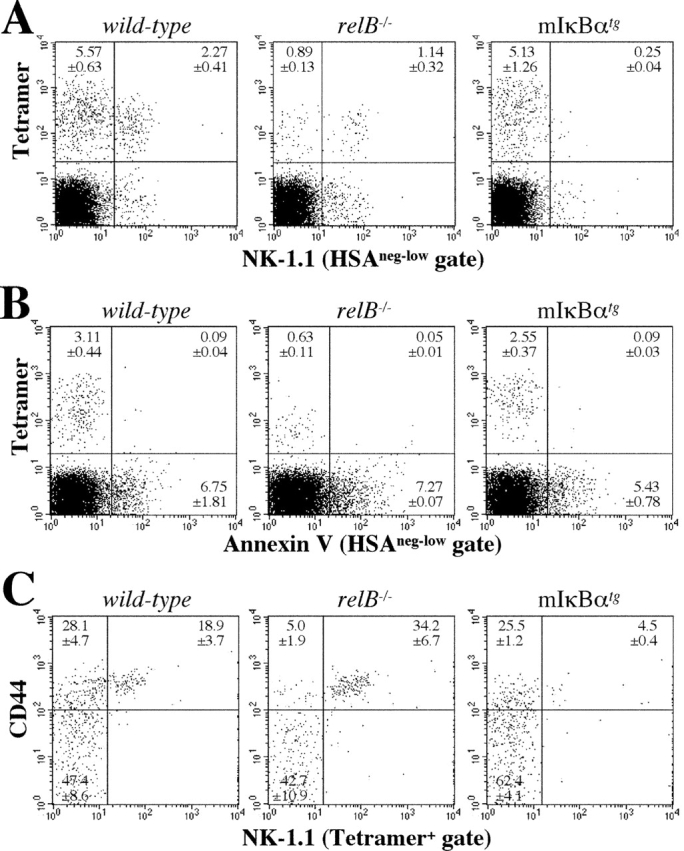
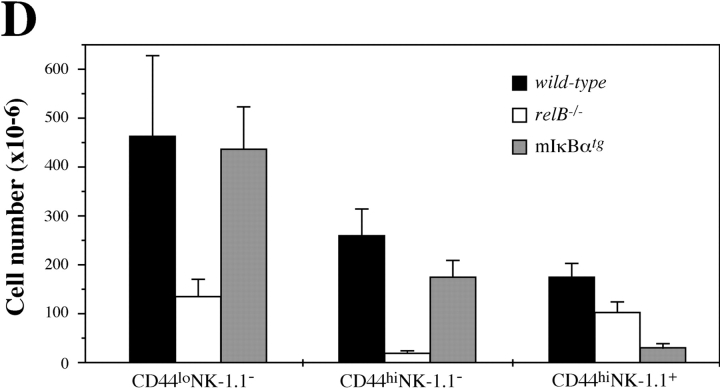
Analysis of developmental intermediates of Vα14i NKT cells in NF-κB-deficient mice. (A) Staining of thymocytes from 2-wk-old C57BL/6 wild-type, relB − / −, and mIκBαtg mice with anti-NK-1.1 and α-GalCer CD1d tetramer. (B) Analysis of apoptotic cells in thymus from 2-wk-old C57BL/6 wild-type, relB − / −, and mIκBαtg mice. Thymocytes were stained with Annexin V and α-GalCer CD1d tetramer. HSAneg-low cells in panels A and B were gated as in Fig. 1. (C) Expression of CD44 and NK-1.1 on tetramer+ thymocytes from 2-wk-old wild-type, relB − / −, and mIκBαtg mice was determined by flow cytometric analysis. Numbers in quadrants indicate mean values of percentages ± SD from three mice. (D) Numbers of tetramer+ cells in 106 thymocytes from 2-wk-old wild-type, relB − / −, and mIκBαtg mice based on their CD44 and NK-1.1 expression patterns.
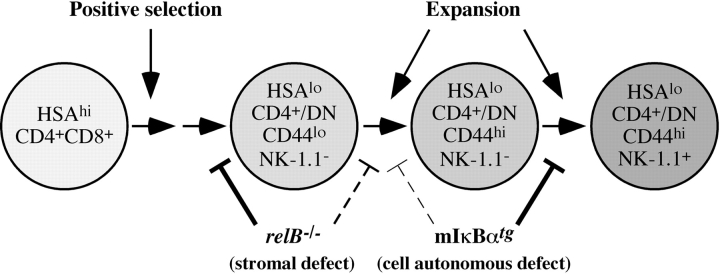
After positive selection, NKT precursors progress through CD44lo and CD44hi stages before they become positive for the NK-1.1 marker (for a review, see reference 14). We therefore compared the CD44/NK-1.1 profiles of tetramer+ thymocytes in 2-wk-old wild-type, relB − / −, and mIκBαtg mice. Whereas the percentage of mature CD44hiNK-1.1+ NKT cells was not reduced, the frequency of the intermediate CD44hiNK-1.1− NKT population was strongly reduced in relB − / − mice. In contrast, intermediate CD44hiNK-1.1− NKT precursors were only mildly affected in mIκBαtg mice, while further differentiation into mature CD44hiNK-1.1+ NKT cells was clearly impaired (Fig. 6 C). This difference between wild-type, relB − / −, and mIκBαtg mice became even more obvious when numbers of the different subpopulations were calculated (Fig. 6 D). Fig. 6 D also shows that RelB is required for the generation of normal numbers of early CD44loNK-1.1− NKT precursors, whereas this population is not reduced in mIκBαtg mice. Our data indicate that these early but distinct blocks caused by the lack of RelB in stromal and classical NF-κB in hematopoietic cells, respectively, result in severely reduced numbers of mature Vα14i NKT cells in adult mice.
Discussion
This study reveals distinct nonredundant roles for the NF-κB family members p50/NF-κB1, p52/NF-κB2, and RelB in both hematopoietic and nonhematopoietic cells for the development of thymic NKT cells. While p50/NF-κB1 is required in hematopoietic cells, RelB is required in the nonhematopoietic compartment. Interestingly, the lack of RelB in radiation-resistant stromal cells results in a more severe decrease in NKT cell numbers than blocking total NF-κB function via the nondegradable mIκBα inhibitor in NKT precursors. CD4+ NKT cells are thymus-dependent (11, 12) and because this subpopulation is also reduced in nfkb1 − / −, relB − / −, and mIκBαtg spleen it is unlikely that the reduced numbers of thymic NKT cells is due to increased emigration from thymus. The selective loss of these cells is accompanied by an increase in CD8+ NK-1.1+ T cells in the spleen of nfkb1 − / − but not in relB − / − mice. One possible explanation for this observation is that p50/NF-κB1 prevents the expansion of CD8+ NK-1.1+ T cells. Alternatively, the lack of p50/NF-κB1 may result in the up-regulation of the NK-1.1 marker on conventional CD8+ T cells (46). Due to the very low frequency, it is difficult to analyze CD8+ NKT cells and the specific increase of this subpopulation in nfkb1 − / − mice may help to study the function and origin of CD8+ NKT cells in more detail.
NKT cells are derived from a CD4+CD8+ DP thymic subset (15), suggesting that NKT cell development is similar to conventional T cells until the DP stage and that at this stage the interaction of CD1d with the Vα14-Jα18 TCR triggers both positive selection and NKT lineage commitment. As a CD1d/Vα14-Jα18 TCR-specific signal regulating positive selection and/or commitment to the NKT lineage has not been identified yet (14) it is important to identify key factors within signaling pathways, which regulate the development of NKT independent from conventional T cells.
Functional NIK in nonhematopoietic cells is essential for NKT cell development (22), which is consistent with the lack of constitutive RelB DNA binding in thymus of aly/aly mice and the stromal requirement of RelB for NKT cell development. Both relB − / − and aly/aly mice show similar structural disorganization of the thymus (17, 35, 47) and the marked defect in RelB DNA binding in aly/aly thymus offers an explanation for the similarity of this phenotype. In contrast to aly/aly mice, thymic DNA binding of RelB is only mildly affected in ltbr − / − and normal in tnfr1 − / − mice, indicating that another receptor associated with NIK may be involved in RelB activation, NKT cell development, and the establishment of proper thymic architecture. NIK is required for the processing of the NF-κB2 p100 precursor to p52 (48, 49) and since the COOH-terminal domain of p100 specifically inhibits RelB function (50–52), lack of RelB DNA binding in the absence of functional NIK most likely is due to defective processing of the inhibitory p100 precursor. Similar to RelB, p52/NF-κB2 is also required in stromal cells for normal NKT cell development. This observation correlates with a stromal requirement of both RelB and p52/NF-κB2 for proper spleen (29, 30) and Peyer's patch development (52, 53), emphasizing that p52-RelB rather than p50-RelB heterodimers are involved in the regulation of NKT cell development, although we cannot rule out that p50-RelB complexes also contribute.
Thymic steady-state mRNA levels of many genes that have been demonstrated in knockout mouse models to be important for NKT cell development are not affected in relB − / − and mIκBαtg mice. However, we observed a reproducible reduction of IL-15 mRNA levels in thymus from relB − / − mice. This finding is consistent with a stromal function of RelB since IL-15 produced by thymic stromal cells has been shown to be essential for early NKT cell development and homeostasis (54, 55). An NF-κB binding site has been reported in the IL-15 promoter (56) but it remains to be shown whether RelB directly or indirectly regulates expression of IL-15.
Recent reports indicate that committed NK-1.1− NKT precursors are derived from a CD4+CD8+ DP thymocyte subpopulation. They proliferate and differentiate through CD44loNK-1.1− and CD44hiNK-1.1− stages into mature CD44hiCD4+/DN NK-1.1+ NKT cells (15, 44, 45). We did not observe a significant reduction in the frequency of tetramer+ DPhi NKT precursors in 12-d-old relB − / − and mIκBαtg mice (unpublished data), indicating that NF-κB is not required for the rearrangement of the Vα14-Jα18 TCR. As expression of CD1d is normal in relB − / − and mIκBαtg mice, it is unlikely that initial positive selection is impaired in these mice. The analysis of 2-wk-old mice revealed, however, that RelB function is required for the generation of normal numbers of early CD44loNK-1.1− NKT precursors, indicating that RelB in stromal cells regulates maturation/expansion of positively selected Vα14i NKT precursors. We also observed markedly reduced percentages and numbers of CD44hiNK-1.1− NKT precursors in thymus from 2-wk-old relB − / − mice. This can be explained by an impaired transition from the CD44lo to the CD44hi stage, which is supported by the analysis of 6-wk-old mice (unpublished data), but normal progression from the CD44hiNK-1.1− to the mature CD44hiNK-1.1+ stage (see also Fig. 7) . Interestingly, IL-15–deficient mice also show an early block in NKT cell development (55) and the reduced IL-15 expression in relB − / − thymus may contribute to the reduced NKT cell numbers in RelB-deficient mice. Analysis of mIκBαtg mice, on the other hand, revealed that classical NF-κB function within NKT precursors is dispensable for the early generation of tetramer+ NK-1.1− cells, whereas it is required at a later step of NKT cell development. In particular, the progression from CD44hiNK-1.1− to the mature CD44hiNK-1.1+ stage is impaired with a milder block at the CD44lo to CD44hi transition (Fig. 7). It is unlikely that the blocks in NKT cell development are due to increased apoptosis since we did not observe increased Annexin V staining of tetramer+ cells in relB − / − and mIκBαtg mice compared with wild-type controls.
Figure 7.
Model of NF-κB-regulated Vα14i NKT cell development in thymus. Lack of RelB in stromal cells results in impaired generation of CD44loNK-1.1− precursors. Blocking NF-κB in precursors results in impaired expansion of NKT cells predominantly at the NK-1.1− to NK-1.1+ transition. For more details see Discussion.
Our results demonstrate a distinct involvement of different NF-κB subunits in the development of NKT cells with an important role of the classical NF-κB pathway within NKT precursor cells, while the alternative NIK/NF-κB2/RelB pathway operates in the thymic stroma. The identification of stromal cell–derived factor(s) regulated by RelB and hematopoietic cell–derived factor(s) regulated by classical NF-κB would help to better define the role of NF-κB in the regulation of different stages of NKT cell development.
Acknowledgments
We gratefully acknowledge Tanja Herrmann, Miriam Koch, Michal Malewicz, Heike Mondrzak, Debra Weih, and Buket Yilmaz for excellent technical assistance and suggestions as well as Harald Krug and Silvia Diabaté for help with the flow cytometer. We are indebted to Jeffrey Browning and Paul Rennert for providing agonistic anti-LTβR mAb. We also thank Mitchell Kronenberg and Peter Herrlich for suggestions and support as well as Selma Huber and all the staff in the animal facility at the Institute of Toxicology and Genetics.
This work was supported by the Deutsche Forschungsgemeinschaft (grants We2224/1, We2224/3, and Pf 259/2-5/6).
The online version of this article contains supplemental material.
Footnotes
Abbreviations used in this paper: α-GalCer, α-galactosylceramide; BM, bone marrow; DP, double positive; EMSA, electrophoretic mobility shift assay; HSA, heat-stable Ag; LT, lymphotoxin; MEF, mouse embryonic fibroblast; NF, nuclear factor; NIK, NF-κB–inducing kinase.
References
- 1.Bendelac, A., M.N. Rivera, S.H. Park, and J.H. Roark. 1997. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 15:535–562. [DOI] [PubMed] [Google Scholar]
- 2.Godfrey, D.I., K.J. Hammond, L.D. Poulton, M.J. Smyth, and A.G. Baxter. 2000. NKT cells: facts, functions and fallacies. Immunol. Today. 21:573–583. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald, H.R. 2002. Development and selection of NKT cells. Curr. Opin. Immunol. 14:250–254. [DOI] [PubMed] [Google Scholar]
- 4.Lantz, O., and A. Bendelac. 1994. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I–specific CD4+ and CD4−8− T cells in mice and humans. J. Exp. Med. 180:1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bendelac, A., O. Lantz, M.E. Quimby, J.W. Yewdell, J.R. Bennink, and R.R. Brutkiewicz. 1995. CD1 recognition by mouse NK1+ T lymphocytes. Science. 268:863–865. [DOI] [PubMed] [Google Scholar]
- 6.Bendelac, A. 1995. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J. Exp. Med. 182:2091–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendiratta, S.K., W.D. Martin, S. Hong, A. Boesteanu, S. Joyce, and L. Van Kaer. 1997. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 6:469–477. [DOI] [PubMed] [Google Scholar]
- 8.Kawano, T., J. Cui, Y. Koezuka, I. Toura, Y. Kaneko, K. Motoki, H. Ueno, R. Nakagawa, H. Sato, E. Kondo, et al. 1997. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 278:1626–1629. [DOI] [PubMed] [Google Scholar]
- 9.Burdin, N., L. Brossay, Y. Koezuka, S.T. Smiley, M.J. Grusby, M. Gui, M. Taniguchi, K. Hayakawa, and M. Kronenberg. 1998. Selective ability of mouse CD1 to present glycolipids: α-galactosylceramide specifically stimulates Vα14+ NK T lymphocytes. J. Immunol. 161:3271–3281. [PubMed] [Google Scholar]
- 10.MacDonald, H.R. 2000. CD1d-glycolipid tetramers: a new tool to monitor natural killer T cells in health and disease. J. Exp. Med. 192:F15–F20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eberl, G., R. Lees, S.T. Smiley, M. Taniguchi, M.J. Grusby, and H.R. MacDonald. 1999. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J. Immunol. 162:6410–6419. [PubMed] [Google Scholar]
- 12.Hammond, K.J., S.B. Pelikan, N.Y. Crowe, E. Randle-Barrett, T. Nakayama, M. Taniguchi, M.J. Smyth, I.R. van Driel, R. Scollay, A.G. Baxter, and D.I. Godfrey. 1999. NKT cells are phenotypically and functionally diverse. Eur. J. Immunol. 29:3768–3781. [DOI] [PubMed] [Google Scholar]
- 13.Robey, E., and B.J. Fowlkes. 1994. Selective events in T cell development. Annu. Rev. Immunol. 12:675–705. [DOI] [PubMed] [Google Scholar]
- 14.Kronenberg, M., and L. Gapin. 2002. The unconventional lifestyle of NKT cells. Nat. Rev. Immunol. 2:557–568. [DOI] [PubMed] [Google Scholar]
- 15.Gapin, L., J.L. Matsuda, C.D. Surh, and M. Kronenberg. 2001. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat. Immunol. 2:971–978. [DOI] [PubMed] [Google Scholar]
- 16.Fu, Y.-X., and D.D. Chaplin. 1999. Development and maturation of secondary lymphoid tissues. Annu. Rev. Immunol. 17:399–433. [DOI] [PubMed] [Google Scholar]
- 17.Miyawaki, S., Y. Nakamura, H. Suzuka, M. Koba, R. Yasumizu, S. Ikehara, and Y. Shibata. 1994. A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur. J. Immunol. 24:429–434. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto, M., K. Iwamasa, P.D. Rennert, T. Yamada, R. Suzuki, A. Matsushima, M. Okabe, S. Fujita, and M. Yokoyama. 1999. Involvement of distinct cellular compartments in the abnormal lymphoid organogenesis in lymphotoxin-α-deficient mice and alymphoplasia (aly) mice defined by the chimeric analysis. J. Immunol. 163:1584–1591. [PubMed] [Google Scholar]
- 19.Shinkura, R., K. Kitada, F. Matsuda, K. Tashiro, K. Ikuta, M. Suzuki, K. Kogishi, T. Serikawa, and T. Honjo. 1999. Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-κb-inducing kinase. Nat. Genet. 22:74–77. [DOI] [PubMed] [Google Scholar]
- 20.Iizuka, K., D.D. Chaplin, Y. Wang, Q. Wu, L.E. Pegg, W.M. Yokoyama, and Y.-X. Fu. 1999. Requirement for membrane lymphotoxin in natural killer cell development. Proc. Natl. Acad. Sci. USA. 96:6336–6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elewaut, D., L. Brossay, S.M. Santee, O.V. Naidenko, N. Burdin, H. De Winter, J. Matsuda, C.F. Ware, H. Cheroutre, and M. Kronenberg. 2000. Membrane lymphotoxin is required for the development of different subpopulations of NK T cells. J. Immunol. 165:671–679. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa, K., K. Iwabuchi, K. Ogasawara, M. Ato, M. Kajiwara, H. Nishihori, C. Iwabuchi, H. Ishikura, R.A. Good, and K. Onoe. 1997. Generation of NK1.1+ T cell antigen receptor α/β+ thymocytes associated with intact thymic structure. Proc. Natl. Acad. Sci. USA. 94:2472–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karin, M., and A. Lin. 2002. NF-κB at the crossroads of life and death. Nat. Immunol. 3:221–227. [DOI] [PubMed] [Google Scholar]
- 24.Karin, M., Y. Cao, F.R. Greten, and Z.W. Li. 2002. NF-κB in cancer: from innocent bystander to major culprit. Nat. Rev. Cancer. 2:301–310. [DOI] [PubMed] [Google Scholar]
- 25.Li, Q., and I.M. Verma. 2002. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2:725–734. [DOI] [PubMed] [Google Scholar]
- 26.Pahl, H.L. 1999. Activators and target genes of Rel/NF-κB transcription factors. Oncogene. 18:6853–6866. [DOI] [PubMed] [Google Scholar]
- 27.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621–663. [DOI] [PubMed] [Google Scholar]
- 28.Pomerantz, J.L., and D. Baltimore. 2002. Two pathways to NF-κB. Mol. Cell. 10:693–695. [DOI] [PubMed] [Google Scholar]
- 29.Poljak, L., L. Carlson, K. Cunningham, M.H. Kosco-Vilbois, and U. Siebenlist. 1999. Distinct activities of p52/NF-κB required for proper secondary lymphoid organ microarchitecture: functions enhanced by Bcl-3. J. Immunol. 163:6581–6588. [PubMed] [Google Scholar]
- 30.Weih, D.S., Z.B. Yilmaz, and F. Weih. 2001. Essential role of RelB in germinal center and marginal zone formation and proper expression of homing chemokines. J. Immunol. 167:1909–1919. [DOI] [PubMed] [Google Scholar]
- 31.Alcamo, E., N. Hacohen, L.C. Schulte, P.D. Rennert, R.O. Hynes, and D. Baltimore. 2002. Requirement for the NF-κB family member RelA in the development of secondary lymphoid organs. J. Exp. Med. 195:233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerondakis, S., M. Grossmann, Y. Nakamura, T. Pohl, and R. Grumont. 1999. Genetic approaches in mice to understand Rel/NF-κB and IκB function: transgenics and knockouts. Oncogene. 18:6888–6895. [DOI] [PubMed] [Google Scholar]
- 33.Sha, W.C., H.-C. Liou, E.I. Tuomanen, and D. Baltimore. 1995. Targeted disruption of the p50 subunit of NF-κB leads to multifocal defects in immune responses. Cell. 80:321–330. [DOI] [PubMed] [Google Scholar]
- 34.Caamaño, J.H., C.A. Rizzo, S.K. Durham, D.S. Barton, C. Raventos-Suarez, C.M. Snapper, and R. Bravo. 1998. Nuclear factor (NF)-κB2 (p100/p52) is required for normal splenic microarchitecture and B cell-mediated immune responses. J. Exp. Med. 187:185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weih, F., D. Carrasco, S.K. Durham, D.S. Barton, C.A. Rizzo, R.-P. Ryseck, S.A. Lira, and R. Bravo. 1995. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-κB/Rel family. Cell. 80:331–340. [DOI] [PubMed] [Google Scholar]
- 36.Fütterer, A., K. Mink, A. Luz, M.H. Kosco-Vilbois, and K. Pfeffer. 1998. The lymphotoxin β receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 9:59–70. [DOI] [PubMed] [Google Scholar]
- 37.Pfeffer, K., T. Matsuyama, T.M. Kündig, A. Wakeham, K. Kishihara, A. Shahinian, K. Wiegmann, P.S. Ohashi, M. Krönke, and T.W. Mak. 1993. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 73:457–467. [DOI] [PubMed] [Google Scholar]
- 38.Vallabhapurapu, S., R.-P. Ryseck, M. Malewicz, D.S. Weih, and F. Weih. 2001. Inhibition of NF-κB in T cells blocks lymphoproliferation and partially rescues autoimmune disease in gld/gld mice. Eur. J. Immunol. 31:2612–2622. [DOI] [PubMed] [Google Scholar]
- 39.Matsuda, J.L., O.V. Naidenko, L. Gapin, T. Nakayama, M. Taniguchi, C.R. Wang, Y. Koezuka, and M. Kronenberg. 2000. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 192:741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hogan, B., R. Beddington, F. Costantini, and E. Lacy. 1994. Preparing mouse embryo fibroblasts. In Manipulating the Mouse Embryo. Cold Spring Harbor Laboratory Press, New York. 260–261.
- 41.Weih, F., D. Carrasco, and R. Bravo. 1994. Constitutive and inducible Rel/NF-κB activities in mouse thymus and spleen. Oncogene. 9:3289–3297. [PubMed] [Google Scholar]
- 42.Lantz, O., L.I. Sharara, F. Tilloy, A. Andersson, and J.P. DiSanto. 1997. Lineage relationships and differentiation of natural killer (NK) T cells: intrathymic selection and interleukin (IL)-4 production in the absence of NKR-P1 and Ly49 molecules. J. Exp. Med. 185:1395–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tilloy, F., J.P. Di Santo, A. Bendelac, and O. Lantz. 1999. Thymic dependence of invariant Vα14+ natural killer-T cell development. Eur. J. Immunol. 29:3313–3318. [DOI] [PubMed] [Google Scholar]
- 44.Benlagha, K., T. Kyin, A. Beavis, L. Teyton, and A. Bendelac. 2002. A thymic precursor to the NK T cell lineage. Science. 296:553–555. [DOI] [PubMed] [Google Scholar]
- 45.Pellicci, D.G., K.J. Hammond, A.P. Uldrich, A.G. Baxter, M.J. Smyth, and D.I. Godfrey. 2002. A natural killer T (NKT) cell developmental pathway involving a thymus-dependent NK1.1-CD4+ CD1d-dependent precursor stage. J. Exp. Med. 195:835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slifka, M.K., R.R. Pagarigan, and J.L. Whitton. 2000. NK markers are expressed on a high percentage of virus-specific CD8+ and CD4+ T cells. J. Immunol. 164:2009–2015. [DOI] [PubMed] [Google Scholar]
- 47.Burkly, L., C. Hession, L. Ogata, C. Reilly, L.A. Marconi, D. Olson, R. Tizard, R. Cate, and D. Lo. 1995. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 373:531–536. [DOI] [PubMed] [Google Scholar]
- 48.Xiao, G., E.W. Harhaj, and S.-C. Sun. 2001. NF-κB-inducing kinase regulates the processing of NF-κB2 p100. Mol. Cell. 7:401–409. [DOI] [PubMed] [Google Scholar]
- 49.Yamada, T., T. Mitani, K. Yorita, D. Uchida, A. Matsushima, K. Iwamasa, S. Fujita, and M. Matsumoto. 2000. Abnormal immune function of hemopoietic cells from alymphoplasia (aly) mice, a natural strain with mutant NF-κB-inducing kinase. J. Immunol. 165:804–812. [DOI] [PubMed] [Google Scholar]
- 50.Dobrzanski, P., R.-P. Ryseck, and R. Bravo. 1995. Specific inhibition of RelB/p52 transcriptional activity by the C-terminal domain of p100. Oncogene. 10:1003–1007. [PubMed] [Google Scholar]
- 51.Solan, N.J., H. Miyoshi, E.M. Carmona, G.D. Bren, and C.V. Paya. 2002. RelB cellular regulation and transcriptional activity are regulated by p100. J. Biol. Chem. 277:1405–1418. [DOI] [PubMed] [Google Scholar]
- 52.Yilmaz, Z.B., D.S. Weih, V. Sivakumar, and F. Weih. 2003. RelB is required for Peyer's patch development: differential regulation of p52-RelB heterodimers by lymphotoxin and TNF. EMBO J. 22:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paxian, S., H. Merkle, M. Riemann, M. Wilda, G. Adler, H. Hameister, S. Liptay, K. Pfeffer, and R.M. Schmid. 2002. Abnormal organogenesis of Peyer's patches in mice deficient for NF-κB1, NF-κB2, and Bcl-3. Gastroenterology. 122:1853–1868. [DOI] [PubMed] [Google Scholar]
- 54.Ohteki, T., S. Ho, H. Suzuki, T.W. Mak, and P.S. Ohashi. 1997. Role for IL-15/IL-15 receptor β-chain in natural killer 1.1+ T cell receptor-αβ+ cell development. J. Immunol. 159:5931–5935. [PubMed] [Google Scholar]
- 55.Matsuda, J.L., L. Gapin, S. Sidobre, W.C. Kieper, J.T. Tan, R. Ceredig, C.D. Surh, and M. Kronenberg. 2002. Homeostasis of Vα14i NKT cells. Nat. Immunol. 3:966–974. [DOI] [PubMed] [Google Scholar]
- 56.Azimi, N., K. Brown, R.N. Bamford, Y. Tagaya, U. Siebenlist, and T.A. Waldmann. 1998. Human T cell lymphotropic virus type I Tax protein trans-activates interleukin 15 gene transcription through an NF-κB site. Proc. Natl. Acad. Sci. USA. 95:2452–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]