Abstract
Deficiency of serum immunoglobulin (Ig)M is associated with the development of a lupus-like disease in mice. Recent studies suggest that classical complement components facilitate the clearance of apoptotic cells and that failure to do so predisposes mice to lupus. Since IgM is a potent activator of the classical complement pathway, we examined IgM binding to dying cells. IgM, but not IgG, bound to apoptotic T cells through the Fab′ portion of the antibody. Exposure of apoptotic cell membranes to phospholipase (PL) A2 increased, whereas PLD reduced, IgM binding and complement activation. Absorption studies combined with direct plate binding assays, revealed that IgM antibodies failed to bind to phosphatidyl lipids, but did recognize lysophosphatidylcholine and the phosphorylcholine head group. Both iPLA2 and cPLA2 are activated during apoptosis. Since inhibition of iPLA2, but not cPLA2, attenuated IgM binding to apoptotic cells, these results strongly suggest that the endogenous calcium independent PLA2, iPLA2, is involved in the hydrolysis of plasma membrane phospholipids and exposure of the epitope(s) recognized by IgM. We propose that recognition of dying cells by natural IgM antibodies is, in part, responsible for complement activation on dying cells leading to their safe clearance.
Keywords: IgM, apoptosis, complement, macrophages, autoimmunity
Introduction
Many perturbations of the immune system predispose to systemic autoimmune diseases such as systemic lupus erythematosus (for a review, reference 1). Amongst the possible causes of autoimmunity, recent attention has focused on defective clearance of dying cells as a potential source of immunogens. The strongest evidence supporting this mechanism is the expression of a lupus-like disease in mice deficient in the tyrosine kinase, mer (2). These mice demonstrate increased numbers of apoptotic cells in lymphoid organs associated with a failure to phagocytose apoptotic cells in vitro. The observations that mice with disruption of genes encoding Dnase 1 (3), C1q (4), or acute phase proteins (5) also develop lupus and that C1q and CRP opsonize dying cells for phagocytosis by macrophages in vitro (6, 7), support the argument that defective clearance of dying cells predisposes to lupus-like diseases.
Two independent studies recently reported that mice deficient in the secreted form of IgM develop lupus-like autoimmunity (8, 9). This association could be explained by increased infections, alterations in the composition of immune complexes, modulation of T or B cell function, or deficient clearance of self-antigens in serum IgM-deficient mice. Since we (6, 7) and others (10) have observed that complement components can be detected on the surface of apoptotic cells and facilitate their clearance by macro-phages, we tested the idea that IgM is responsible for complement activation on apoptotic cells. Here, we report that IgM activates complement on dying cells and that these antibodies bind to lysophospholipids, particularly lysophosphatidylcholine.
Materials and Methods
Serologic Reagents and Antibodies.
Human serum was obtained from normal individuals and from patients (provided by M. Sweetser, University of Washington, Seattle, WA and M. Cooper, University of Alabama, Birmingham, AL) with agammaglobulinemia (serum 1) or common variable immune deficiency (serum 2 and 3) and was stored in aliquots at −70°C. The serum concentrations (μg/ml) of Igs were: serum 1, IgG = 575; IgM < 58; IgA < 7; serum 2, IgG < 42, IgM < 7, IgA = 18; serum 3, IgG = 51; IgM = 9; IgA < 7 (normal ranges IgG, 6,300–13,000; IgM, 400–1,000; IgA, 400–1,350). Human sera with IgM anticardiolipin autoantibodies (range 63–300 MPL units) were obtained as described previously (11). Secretory IgM–deficient mice on a C57BL/6 and 129 backgrounds (8) were provided by J. Chen (MIT, Cambridge, MA).
Serum depleted of C1q (C1q-D), serum depleted of C8 (C8-D), mAb to C3b/bi, C1q, and a neoantigen created by the formation of the membrane attack complex (MAC; C5b-9), anti–Sc5–9, polyclonal goat anti–human C1q were purchased from Advanced Research Technologies. mAbs against PARP and β2-microglobulin were purchased from BD PharMingen, and polyclonal antibodies against the following proteins (and their commercial sources) were as follows: FITC-conjugated anti–human IgG, IgM (heavy-chain specific), sheep anti–mouse IgG, HRP-conjugated goat anti–mouse IgG (Jackson ImmunoResearch Laboratories); FITC-conjugated goat anti–mouse C3 (ICN). Purified human IgM, trypsin, trypsin inhibitor, hydrogen peroxide, and myeloperoxidase were purchased from Sigma-Aldrich. The mAbs, T15 (anti-phosphorylcholine[PC]*), SUV (anti-phosphatidyl choline), EO6 (anti-oxidized phospholipid) were provided by M. Scharff, Albert Einstein College of Medicine, Bronx, NY, R. Hardy, Fox Chase Cancer Center, Philadelphia, PA, and J. Witztum, UCSD, San Diego, CA, respectively.
Trypsin Digestion of IgM.
IgM was digested with trypsin at a ratio of 200:1 (w/w) at 65°C for 20 min and the reaction terminated by the addition of soybean trypsin inhibitor. Fc and Fab fragments were resolved by Fast Performance Liquid Chromatography (FPLC) using a HR16/60 S-300 column (Amersham Pharmacia Biotech). Purity of each fragment was determined by SDS-PAGE and Coomassie blue staining.
Cells and Induction of Apoptosis.
Jurkat T cells or peripheral blood derived T cells from normal donors were cultured in RPMI 1640 medium supplemented with 10% FBS, l-glutamine, and penicillin-streptomycin as described previously (12). Normal T cells were activated by PHA (5 μg/ml) and 100 U/ml of IL-2. Apoptosis of Jurkat T cells or activated peripheral blood T cells was induced by incubation with 0.2 μg/ml staurosporine (Sigma-Aldrich) for 6–8 h. 7-h incubation with dexamethasone (10−6 M) was used to induce cell death of mouse thymocytes. Necrosis was induced by heat treatment of cells at 65°C for 1 h (13). The percentage of early and late apoptotic cells was quantified by flow cytometry analysis using Annexin V and propidium iodide according to the manufacturer's instructions (Travigen, Inc.). Distinction between apoptotic and necrotic cells was based on exclusion of trypan blue as determined by light microscopy. Intracellular caspase-3 activity was quantified by cleavage of the PhiPhiLux-G1D2 substrate (OncoImmune, Inc.) per the manufacturer's instructions. Nuclear morphology was evaluated by staining cells with bisBENZIMIDE (2′-[4-Ethoxyphenyl]-5-[4-methyl-1-piperazinyl]-2,5′-bi-1H-benzimidazole, Hoechst No.33342) and immunofluorescence microscopy.
Complement Activation and Flow Cytometry.
Complement binding and activation studies were performed by incubation of 106 cells with 100 μl of medium containing 20% normal human serum (NHS) or a hypogammaglobulinemia serum (HGS) in TC buffer (140 mM NaCl, 2 mM CaCl2, 10 mM Tris, pH 7.4, supplemented with 1 mM Mg2+ and 1% BSA) for 30 min at 37°C. Cell-bound antibody or complement were detected by staining cells with FITC-conjugated antibodies for 30 min at 4°C. Flow cytometry was performed on a FACScan™ instrument operating with CELLQuest™ software (Becton Dickinson) as described previously (12). Debris was excluded by FCS/SSC gating. Data were displayed on a logarithmic scale of increasing fluorescence intensity.
Western Blot Analysis and Immunoprecipitation.
To detect protein antigens on apoptotic cells, cell membranes were labeled with D-Biotinyl-E-amidocaproic acid N-hydroxysuccinimide ester (Pierce Chemical Co.) according to the manufacturer's instructions. Western blot analysis was performed as described previously (12) and developed using an ECL kit (Amersham Biosciences) according to the manufacturer's instructions. The cells were incubated with 20% NHS or HGS, washed, and immunoprecipitated with rabbit IgG anti–human IgM adsorbed to protein G beads. Immunoprecipitated proteins were resolved by SDS-PAGE and detected by immunoblotting with streptavidin as described previously (12). Detection of ribosomal protein P0 (14) was used as a loading control.
Oxidation and Enzymatic Hydrolysis of the Plasma Membrane.
Purified human oxidized LDL was obtained from Fred deBeer (University of Kentucky, Lexington, KY). Live or early apoptotic PBLs were incubated with 1 μg/ml of myeloperoxidase with or without 0.5 mM of H202 for 30 and 60 min at room temperature with gentle shaking. The reaction was terminated by adding ice cold-PBS and extensive washing.
Type I (snake venom) and type III (bee venom) sPLA2, PLD and propranolol were purchased from Sigma. Enzymatic hydrolysis of intact apoptotic cells with PLs was performed by incubation of cells with varying concentrations of enzymes for 1 h either at 0° or 37°C. The iPLA2 inhibitor, bromoeonol lactone (BEL) was purchased from Cayman and the selective sPLA2 inhibitor, Shionogi-1 (15), was provided by M. Gelb, University of Washington, Seattle, WA.
Adsorption of Serum IgM by PC or Phospholipid Vesicles Absortion of Serum by Phospholipid Vesicles.
PC chloride (PC-Cl), O-Phosphorylethanolamine (PE), and O-Phospho-l-Serine were purchased from Sigma-Aldrich, and PC-KLH from BioResearch Technologies, Inc. The synthetic phospholipids,1,2-diacyl-sn-glycero-3-phosphocholine (PtC), 1,2-diacyl-sn-glycero-3-[phospho-l-serine (PtS), and 1,2-diacyl-sn-3-phosphoethanolamine (PtE), 1-acyl-2-hydroxy-sn-glycero-3-phosphocholine (lysoPtC), 1-acyl-2-hydroxy-sn-glycero-3-phosphoethanolamine (lysoPtE), and 1-acyl-2-hydroxy-sn-glycero-3-phospho-L-serine (lysoPtS) were purchased from Avanti Polar Lipids, Inc. Phospholipids were dissolved in chloroform and liposomes prepared as described previously (16). In brief, the phospholipid solutions were evaporated and dried under a nitrogen stream for 60 min and the lipid film resuspended in PBS, pH 7.4. The suspension was sonicated using a Misonix sonicator (1 min at 30% power, for 5–10 cycles on ice). The final applied lipid concentration was 10 mM.
Antibody absorptions with phospholipid liposomes were performed by incubating 20% serum with varying concentrations of liposome for 60 min at 37°C. After incubation, serum and phospholipids were separated by low speed centrifugation (13,000 rpm × 10 min). Serum absorption by lysophospholipids was achieved by first coating 96-well plates with lysophospholipids in methanol at 50 μg/ml for 18 h at 4°C. The following day, 20% of NHS, HGS, or purified IgM diluted in 1% BSA/PBS was applied to the plates for 2.5 h and the supernatant harvested.
Results
Serum IgM Binds to Apoptotic Cells and Recruits Complement.
We have previously shown that apoptotic lymphocytes activate complement resulting in the deposition of C3b/bi on the surface of the cells (7). Depending on the concentration of other serum opsonins such as CRP as well as the stage of cell death, both early (C1q) and late (the MAC) complement components may also be observed on dying cells (7). To examine whether natural antibodies could be responsible for complement activation on dying cells, we examined IgM and IgG binding to apoptotic cells. As shown in Fig. 1 A, IgM, but not IgG, bound to the cell surface. Furthermore, ∼2/3 of C3b/bi-positive apoptotic cells costained for IgM (Fig. 1 B). Human (Fig. 1, B and C) and mouse (Fig. 1 D) sera that were deficient in gammaglobulins (HGS) or secretory IgM (sIgM−/−) respectively, showed a striking reduction (66–75% in human and 50–60% in mouse) in complement deposition on the apoptotic cell surface. These findings could not be explained by abnormalities in the HGS or the IgM-deficient sera since these sera permitted complement recruitment to the surface of cells sensitized with control IgM antibodies and the reconstitution of HGS with IgM, but not IgG, restored C3b/bi deposition on the apoptotic cells (Fig. 1 E).
Figure 1.
IgM activates complement on apoptotic cells. (A) Normal peripheral blood–derived T cells (PBT) were either incubated in medium or induced to undergo apoptosis by staurosporine (STS) as described in Materials and Methods. The cells were then incubated with either purified human IgG (10 mg/ml) or IgM (1 mg/ml) for 20 min at 37°C and examined for Ig binding by flow cytometry (thin line, live cells; thick line, apoptotic cells; dotted line, apoptotic cells incubated with second antibody alone). The change in mean channel fluorescence (Δ) between the cells incubated with second antibody alone versus IgG or IgM followed by second antibody is shown. Representative of five experiments. (B) Apoptotic PBT cells were incubated with either HGS or autologous NHS for 30 min followed by two-color flow cytometric analysis of IgM and C3b/bi binding. Representative of three experiments. (C) Apoptotic PBT cells were incubated with either 20% autologous NHS or HGS no. 2 and 3 for 30 min at 37°C. Deposition of cell surface C3b/bi and the MAC were quantified by flow cytometry as described in Materials and Methods. As a control for complement activity in the HGS sera, C3b/bi, and MAC deposition on T cells were quantified after incubation with an IgM monoclonal anti-β2 microglobulin antibody followed by either NHS or HGS as a source of complement. The results are expressed as complement component binding in HGS/complement component binding in NHS ×100 (mean ± SD of 4 experiments). (D) C57BL/6 (B6) thymocytes were incubated with 1 μM dexamethasone for 6 h to induce apoptosis. Apoptotic thymocytes were incubated with 20% of autologous wild-type serum or serum obtained from sIgM−/− deficient mice on a B6 or 129 background for 30 min at 37°C. C3 binding to the cells was quantified by flow cytometry. As a control, C3 deposition was also quantified following preincubation of the apoptotic cells with IgM anti-Thy.1 antibodies. The results are expressed as percentage of complement activation. Two experiments gave virtually identical results. (E) Apoptotic PBT cells prepared as in C, were incubated with NHS, HGS, or HGS reconstituted with purified human 10 mg/ml IgG or 1 mg/ml IgM. C3b/bi deposition was detected by flow cytometry and are expressed as the percentage of cells positive for staining (mean ± SD of 3 experiments).
IgM Antibodies Recruit C1q to the Surface of Apoptotic Lymphocytes and Promote the Activation of C3.
It has previously been reported that C1q binds strongly to permeabilized apoptotic keratinocytes (10) and we have observed weaker C1q binding to the surface of nonpermeabilized apoptotic T cells (6). Since IgM activates complement through the classical pathway and we observed that maximal C3b/bi deposition on apoptotic cells required classical pathway activation (7), we evaluated C1q binding to apoptotic cells by Western blot analysis. As shown in Fig. 2 A, whereas little C1q binding was detected on live cells incubated with NHS (lane 3) or apoptotic cells incubated with HGS (lane 5), strong C1q binding was detected on apoptotic cells incubated with NHS (lane 7) or HGS reconstituted with normal IgM (lane 6). Furthermore, when isolated components were used, the addition of IgM to apoptotic cells markedly enhanced C1q recruitment to the cell surface (Fig. 2 B). Together, these findings indicate that IgM is, in large part, responsible for the activation of the classical complement pathway on apoptotic cells.
Figure 2.
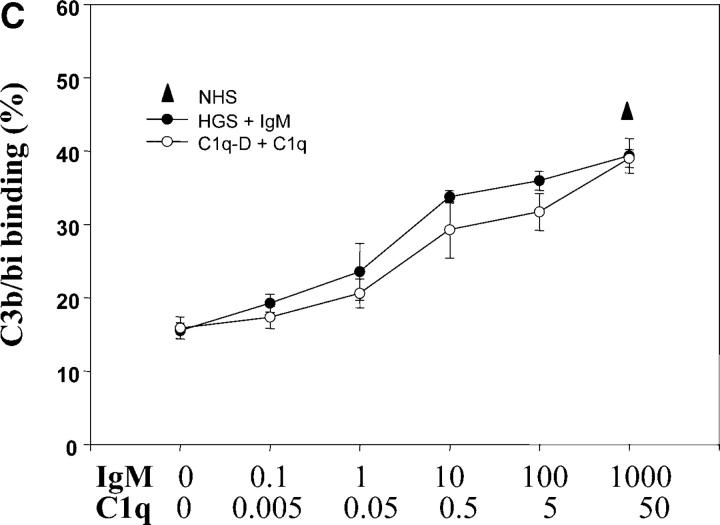
IgM recruits C1q to apoptotic cells leading to C3 activation. (A) Live or apoptotic PBT cells were incubated with medium alone (−), NHS, HGS, or HGS reconstituted with IgM (1 mg/ml) as indicated for 30 min at 37°C. The cells were washed and C1q binding detected by Western blot analysis as described in Materials and Methods. For Western blot analysis, protein loading was compared by probing the same membrane with anti-ribosomal P antiserum (38 kD). Representative of three experiments. (B) Apoptotic PBT cells were incubated with purified human IgM (1 mg/ml) as above, washed and then incubated with purified human C1q (10 μg/ml) for 20 min at 37°C. C1q binding on the surface of apoptotic cells was detected by flow cytometry using a monoclonal anti–human C1q antibody. The results are expressed as the mean ± SD of three experiments. (C) Apoptotic PBT cells were incubated as in A. with either NHS, HGS, or HGS to which IgM was added to the final concentrations (μg/ml) indicated. These concentrations correspond to serial 10-fold dilutions of the normal serum concentration (1 mg/ml). Parallel experiments were performed with C1q depleted serum (C1q-D) to which serial 10-fold dilutions of C1q were added (normal serum concentration is 50 μg/ ml). C3b/bi binding was detected by flow cytometry and expressed as the percentage of cells positive for staining as in Fig. 1 E (mean ± SD of 3 experiments).
To determine the relative roles of IgM catalyzed versus IgM-independent activation of C3 on apoptotic cells, we compared C3b/bi binding on apoptotic cells in serum deficient in C1q or HGS and then reconstituted these sera with the relevant missing protein. As shown in Fig. 2 C, sera deficient in either C1q or IgM promoted a low, but equivalent, “baseline” C3b/bi binding. The fact that in HGS, reconstitution with IgM was required to restore C3b/bi deposition to levels equivalent to normal serum (Fig. 2 C), strongly reinforces the interpretation that IgM is the limiting factor for C3b/bi deposition on apoptotic cells. The strikingly parallel curves also suggests that most C3 activation on apoptotic cells occurs by the same mechanism, namely IgM recruitment of C1q.
IgM antibodies recognize the potential universe of foreign antigens and binding is usually of low affinity (17). The Fc portion of IgM is heavily glycosylated and may interact with lectins and other structures. To determine whether IgM binding to apoptotic cells was due to recognition of antigens by Fab or could be explained by Fcμ interactions with molecules on the surface of the apoptotic cell, IgM was digested with trypsin, and the FPLC-purified Fab and Fcμ fragments tested for binding to apoptotic cells. As shown in Fig. 3 , when highly purified Fab and Fcμ fragments were tested for binding to apoptotic cells the Fab, but not the Fcμ, fragment bound to the cells. These results cannot be explained by tryptic digestion of the Fcμ epitope since the antibody recognized the Fcμ fragment on a Western blot (unpublished data). We conclude that IgM binding to apoptotic cells is due to antigen recognition by Fab.
Figure 3.
IgM binds to apoptotic cells by its Fab domain. Fc and Fab fragments of IgM were prepared by tryptic digestion and were isolated by FPLC as described in Materials and Methods. (A) Purity of the fragments (20 μg per lane) was assessed by SDS -10% PAGE under reducing conditions and proteins detected by Commassie-Blue staining. Lane 1, molecular mass standards; lane 2, IgM before digestion; lane 3, Fcμ; lane 4, Fab′. (B) Isolated IgM, IgM heated to 65°C for 10 min, IgM Fcμ or Fab′ fragments (all tested at a concentration of 1 pM) were examined for binding to apoptotic PBT as in Fig. 1 except that IgM Fc or Fab′ binding were detected with polyclonal antibodies specific for the fragment. The results are expressed as the mean ± SD of four experiments.
Natural IgM Antibodies Recognize Surface Structures Exposed on Late Apoptotic Cells.
To begin to address the specificity of the natural antibodies that bound to apoptotic cells, we first examined the kinetics of IgM binding in relation to cell surface membrane changes. Whereas almost half of the apoptotic cells bound annexin V6 h after apoptosis induction, the kinetics of IgM binding was slower and more closely approximated entry of PI into the cell (Fig. 4 A). IgM stained only PI-positive, trypan blue-negative, cells. Taken together, these findings indicate that IgM antibodies recognized antigen(s) exposed on late apoptotic cells, but before substantial disruption of cell membrane integrity. IgM also bound to heat-induced necrotic cells (unpublished data).
Figure 4.
Kinetics and specificity of IgM binding to apoptotic cells. (A) Apoptosis of PBT cells was induced as in Fig. 1 and at time 0, 2, 4 and 6 h, the cells were incubated in medium containing 20% NHS or HGS. Cells were analyzed by flow cytometry for Annexin V or IgM binding as well as for permeability to PI and trypan blue as indicated in the Figure. The results are expressed as the percentage of cells positive. The results are expressed as the mean ± SD of three experiments. (B) Purified IgM was incubated with liposomes containing either 50 or 500 ug/ml PtS or PtC for 30 min at 37°C. Samples were centrifuged and the supernates tested for binding to apoptotic cells. Annexin V and SUV (an anti-PtC specific mAb), were used as positive controls for binding to PtS and PtC, respectively. The results are expressed as percentage of inhibition of binding, calculated from (binding in medium − binding after preadsorbtion with liposome/binding in medium) × 100. The results are expressed as the mean ± SD of three experiments.
Although purified IgM bound to apoptotic cells, indicating that complement-mediated damage was not necessary for antigen exposure, we examined whether complement activity in NHS influenced IgM binding through MAC induced lysis of the membrane. In three consecutive experiments, the percentage of cells positive for PI (mean ± SE) was 53 ± 1.3 (NHS) and 52 ± 2.7 (C8-D) and little change in IgM binding (NHS = 26 ± 4.8 versus C8-D = 25 ± 2.8) was observed. These results indicate that complement did not induce significant membrane lysis under these conditions.
Consistent with the different kinetics of annexin V and IgM antibody binding to apoptotic cells, PtS-containing liposomes minimally inhibited IgM binding (16.28% and <40% at 50 μg/ml and at 500 μg/ml, respectively; Fig. 4 B) and there was a poor correlation between the binding of IgM to apoptotic cells and the anticardiolipin antibody activity as quantified by ELISA (unpublished data). Similarly, although PtC liposomes absorbed binding of a monoclonal anti-PtC antibody, the PtC-liposomes failed to absorb natural antibodies that bound to apoptotic cells (Fig. 4 B). Attempts to immunoprecipitate biotinylated cell surface proteins with IgM or Western blot analysis of apoptotic cell extracts failed to reveal protein or glycoprotein antigens (unpublished data).
Natural IgM Antibodies Bind to Lysophospholipids on Apoptotic Cells and Have Specificity for PC.
In addition to translocation of phosphorylserine (PS), alterations such as oxidation (18, 19) or action of PLs (20) could effect exposure of antigens to the outer cell surface membrane of apoptotic cells. Wiztum and colleagues (21) reported that certain murine natural antibodies, including the classic T15 mAb (22), bind to oxidized LDL and to the surface of apoptotic endothelial cells (23, 21). When we preincubated IgM with intentionally oxidized apoptotic cells or oxidized LDL, IgM binding was reduced by 15 ± 2% (unpublished data). This indicates that only a small subset of IgM antibodies had a specificity identical to the mAbs described above. In contrast, exposure of apoptotic cells to sPLA2, that catalyzes the hydrolysis of the sn-2 fatty acyl bond to form lysophosphopholipids, markedly increased IgM binding to human apoptotic cells (Fig. 5 A). sPLA2 exerted a similar effect on C3 binding to murine apoptotic cells which was considerably greater in wild-type compared with IgM deficient sera. (Fig. 5 B). Since some C3 was deposited on sPLA2 treated apoptotic cells incubated in IgM deficient serum, the altered cell surface must also activate the alternative or/and MBL complement pathways, albeit less efficiently than the classical pathway. By comparing C3b/bi deposition on apoptotic cells in the presence or absence of C1q, we observed that ∼1/3 of C3b/bi deposition occurred independently of classical pathway in cells exposed to medium or to sPLA2 (unpublished data).
Figure 5.
IgM antibodies bind to components of lysophospholipids on the cell surface membrane. (A) Apoptotic cells were incubated in medium alone or medium containing 1 U of PLs (PLA2 or PLD) for 30 min at 37°C or on ice (0°C). The cells were washed and then incubated with purified IgM or the TEPC15 anti-PC mAb for 20 min at 37°C. IgM binding to live cells is shown for comparison. Antibody binding was detected by flow cytometry and is expressed as the mean ± SD of 4 experiments. (B) Thymocytes were rendered apoptotic as in Fig. 1 and then exposed to 1U of type I or type III sPLA2 or 1U PLD for 30 min at 37°C. They were then washed and incubated with wild-type (WT) or IgM-deficient (sIgM−/−) serum followed by flow cytometry analysis for C3 binding. The results are expressed as the percentage of cells staining for C3 and are representative of two experiments. Cells incubated with heat-inactivated serum (HIS) or anti-Thy1 were used as negative and positive controls, respectively. (C) NHS was incubated with solid phase adsorbed lysophospholipids and then tested for binding to apoptotic cells. The results are expressed as inhibition of IgM binding, calculated as in Fig. 4 and are the mean ± SD of 3 experiments. (D) NHS was preincubated with varying concentration of PC, PS, or PE as shown for 30 min at 37°C. Inhibition of IgM binding to apoptotic cells was calculated as above. The results are expressed as the mean ± SD of 3 experiments. (E) Purified IgM or NHS was incubated with PC (PC-Cl) and the percentage inhibition of IgM binding to apoptotic cells determined by flow cytometry as in C. TEPC15, and an anti-β2 microglobulin mAb, were used as positive and negative controls, respectively. The results are expressed as the mean ± SD of 4 experiments.
The findings above suggested the possibility that IgM antibodies bound to lysophospholipids but the results could also be explained by an effect of PLA2 increasing membrane exposure to an unrelated antigen. To determine whether IgM was directly targeting lysophospholipids, apoptotic cells were incubated with PLD, an enzyme that cleaves the head groups from phospholipids. As shown in Fig. 5, A and B, PLD strikingly reduced IgM (Fig. 5 A) and C3 binding to apoptotic cells suggesting that the antibodies had specificity for the polar head group of phospholipids. To further address IgM specificity, IgM was preabsorbed with lysophosphatidylcholine (lysoPtC), lysophosphatidylserine (lysoPtS), or lysophosphatidylethanolamine (lysoPtE) containing micelles. However, these results were inconclusive due to the high detergent activity of residual lysophospholipids on apoptotic cells. As an alternative approach, IgM was preabsorbed on plates coated with lysophospholipids and the supernates tested for binding to apoptotic cells. As shown in Fig. 5 C, whereas preadsorption with lysoPtE and lysoPtS had little effect, preadsorption with lysoPtC resulted in ∼50% inhibition of IgM binding to apoptotic cells. These results support the observation that IgM bound to lysophospholipids on apoptotic cells and indicate that a significant proportion of the antibodies bound to lysoPtC.
Loss of antibody binding to PLD-treated apoptotic cells suggested that IgM antibodies recognized the polar head group of lysophospholipids. To examine this directly, IgM was pre incubated with PC, PE or PS and tested for binding to apoptotic cells. As shown in (Fig. 5 D), PC, but not PS or PE, reduced IgM binding to apoptotic cells in a dose-dependent response. Inhibition was relatively specific in that preincubation of anti-β2 microglobulin with PC had no effect on binding (Fig. 5 E). Taken together with the results above, these findings indicate that a substantial fraction of the polyclonal IgM in human serum that binds to apoptotic cells recognizes lysoPtC.
Inhibition of iPLA2 Attenuates IgM Binding to Apoptotic Cells.
PLA2 has been implicated in membrane remodeling during apoptosis (24, 25). To determine whether endogenous PLs were responsible for inducing the cell membrane changes that led to the binding of IgM, inhibitors of PLA2 were used to attenuate PLA2 hydrolysis. BEL (an inhibitor of the Ca2+-independent type VI PLA2, iPLA2), but not Shionogi-1 (an inhibitor of Ca2+-dependent type IV PLA2, cPLA2) attenuated the binding of both IgM (Fig. 6 A) and annexin V (Fig. 6 B) to the cell surface membrane. This effect on the cell membrane can be attributed to attenuation of iPLA2 rather than phosphatidate phosphohydrolase, because propranolol failed to effect binding of either IgM or annexin V (26). To determine whether attenuation of IgM binding by BEL could be due to a general inhibition of apoptosis, rather than a selective surface membrane effect, we asked whether BEL impeded other downstream effects of the apoptotic cascade. Since BEL did not inhibit cleavage of the caspase-3 substrates, Phi-phi Lux or PARP nor did it attenuate nuclear condensation (Fig. 6, C–E), we conclude that the BEL effect on cell surface membranes of apoptotic cells was relatively selective. In sum, these results strongly suggest that the endogenous calcium independent PLA2, iPLA2, is involved in the hydrolysis of plasma membrane phospholipids and exposure of the epitope(s) recognized by IgM.
Figure 6.
BEL, an inhibitor of endogenous iPLA2 attenuates IgM binding to apoptotic cells. (A) PBMT were incubated with staurosporine together with medium alone or medium containing the PLA2 inhibitors, 10 μM BEL or 5 μM Shionogi-1 for 6 h (A and C) or the phosphatidate phosphohydrolase inhibitor, propranolol (12.5 μM) (B). The cells were then stained with Annexin V-FITC (A and B) and/or purified human IgM followed by FITC-anti–human IgM (B). To determine the effects of BEL on intracellular apoptotic events, cells were examined for caspase-3 activity by flow cytometry (C), PARP cleavage by Western blot analysis (D), or nuclear condensation by Hoechst staining and immunofluorescence (E) in the presence or absence of 10 μM BEL as described in Materials and Methods. For Western blot analysis (D), protein loading was compared by probing the same membrane with antiribosomal P antiserum (38 kD). A–C are mean ± SD of 3 experiments; D and E are representative of two experiments with identical results.
Discussion
Previous studies have shown that complement opsonization is required for efficient phagocytosis of dying cells in vitro (6, 7) and that uptake of these opsonized cells is associated with expression of the antiinflammatory cytokine, TGF-β (6). Three observations, namely that C1q binds to apoptotic cells in vitro (10), that apoptotic cells accumulate in the kidneys of mice deficient in C1q (4), and that complement activation on apoptotic cells promotes phagocytosis of these cells by macrophages in vitro, suggest a pivotal role for complement in noninflammatory clearance of dying cells. Here, we report that IgM antibodies in normal individuals are quantitatively most important for C1q binding and C3b/bi activation on the apoptotic cell surface and that these natural antibodies bind to lysophosphorylcholine that is exposed on cells undergoing apoptosis.
Since IgM bound to apoptotic cells by the Fab, rather than the Fc, portion of the Ig, binding could be attributed to antibody recognition of an antigen(s) exposed on dying cells. A potential candidate antigen was PS, a membrane phospholipid that translocates from the inner to the outer side of the cell membrane during apoptosis and is recognized by at least some anticardiolipin autoantibodies that occur in the serum of patients with diseases such as systemic lupus erythematosus (27, 28). Failure of PS-containing liposomes to absorb IgM reactivity, and the lack of a correlation between anticardiolipin autoantibody titers and IgM binding to apoptotic cells, indicated that the apoptotic cell-reactive IgM antibodies and anticardiolipin autoantibodies had different specificities.
Failure to identify cell surface protein autoantigens by immunoprecipitation or by Western blot analysis suggested that IgM antibodies were binding to nonprotein antigens exposed on apoptotic cell membranes. Clues to the specificity of the IgM antibodies were provided by enzymatic hydrolysis of membrane components and antibody inhibition experiments. Apoptotic cell membranes are known to be highly susceptible to type II sPLA2, resulting in the hydrolysis of membrane phospholipids and the release of arachidonic acid (29). Incubation of apoptotic cells with either type II or type III sPLA2 strikingly increased IgM and C3 binding to the cell surface. This finding suggested that the IgM antibodies recognized lysophospholipid(s) generated by PLA2 itself or recognized other structures made more accessible by membrane disruption.
To distinguish between these two possibilities, we exposed apoptotic cells to PLD, a lipase that removes the head group from phospholipids. The striking loss of reactivity of both IgM and C3 binding to PLD treated apoptotic cells coupled with the inhibition of IgM binding by PC-Cl, PC- KLH, and lyso-PC (but not by PE nor PS) strongly suggested that IgM antibodies in normal sera directly and, relatively selectively, target the PC moiety on lysophosphatidylcholine. This specificity is strikingly similar to that described for CRP (30), an acute phase protein that is also implicated in the clearance of self-antigens during inflammation and tissue injury (31). Based on observations in HGS or IgM-deficient sera, it should be noted that 25–50% of C3 binding to apoptotic cells occurred independently of IgM, presumably indicating that CRP or other complement activators were responsible. Quantitative estimates of the contribution of classical versus other pathways of C3 binding to apoptotic cells revealed that alternative (7) and/or MBL (32) pathways contributed ∼1/3 of C3b/bi binding.
Previous studies have suggested that some natural antibodies have specificity for phospholipids. All strains of mice produce idiotypically restricted anti-PtC, predominantly by B1 B cells (33, 34). Witztum and colleagues demonstrated that ∼30% of IgM mAbs that develop spontaneously in Apo-E deficient mice, bind to oxidized LDL (35). Furthermore, a subset of these monoclonal antibodies (EO6) bound to apoptotic porcine aortic endothelial cells (23). Since the EO6 IgM anti-oxLDL antibodies were also inhibited by PC (21), these antibodies bear striking similarities to the IgM autoantibodies detected in the current study. However, the IgM antibodies reported here were minimally absorbed by intentionally oxidized apoptotic cells or oxLDL suggesting subtle differences in binding specificity.
Recent studies have shown that, amongst the 10 groups of PLA2, the calcium-independent type VIA cytosolic PLA2, iPLA2, plays an important role in lipid remodeling (36). Although both cPLA2 and iPLA2 are cleaved by caspase-3 during apoptosis, the cleaved form of cPLA2 acts as a dominant negative inhibitor of function whereas cleaved iPLA2 activity is increased resulting in increased phospholipid turnover (37), although the precise roles of cPLA2 and iPLA2 may be cell type and context specific (for a review, see reference 20). BEL is a PLA2 inhibitor that has 1,000-fold greater selectivity for iPLA2 compared with either cPLA2 or sPLA2 (38, 39). Here, we demonstrate that BEL significantly attenuated cell surface membrane changes such as PtS exposure on apoptotic cells consistent with a previous studies (37). Associated with this membrane alteration, IgM binding to apoptotic cells was reduced by ∼50%. This finding indicates that activation of endogenous iPLA2 during apoptosis contributes to exposure of the phospholipid antigen, lyso-PC, on the cell surface. Furthermore, it supports the identity of lyso-PC as an antigen recognized by IgM antibodies since antisense studies revealed that iPLA2 selectively inhibited steady-state levels of cellular lyso-PtC (40).
Numerous studies in the past have suggested that at least some IgM antibodies in normal individuals have autoreactivity. Examples include low titer anti-ssDNA (41), rheumatoid factor, and antibodies reactive with bromelain-treated red blood cells (42, 34). In mice, reactivity to self appears to be more prominent with IgM antibodies produced by CD5+ B1 lymphocytes that are most abundant in the peritoneum (43–45). Of particular relevance to this study, anti-PC antibodies are detected in normal mouse serum (46) as well as NHS (46). The relationship between anti-PC and autoantibodies has long been of interest. A single base change in the S37 T15 idiotype positive hybridoma, altered the specificity of the antibody from PC to DNA. In addition, some anti-DNA antibodies cross react with PC (47). Although it has been suggested that anti-PC antibodies generated in response to pneumococcal infections may mutate to anti-DNA leading to disease (48), vaccination with pneumococcal polysaccharide failed to induce anti-DNA antibodies in humans (47).
The findings in the current manuscript offer a possible different explanation for the origin and function of anti-PC antibodies in health and disease (Fig. 7) . Under normal circumstances, anti-PC antibodies would perform a housekeeping role by promoting the clearance of dying cells through activation of classical complement pathway. If, however, classical complement components or opsonins such as the acute phase protein, CRP, are limiting, IgM would bind to dying cells but the cells would be inefficiently cleared leading to postapoptotic necrosis and/or coating by IgG antibodies (IgG antibodies are produced, albeit with delayed kinetics, in serum IgM-deficient mice (49). Phagocytosis of necrotic cells (50) or engagement of macrophage Fc receptors by IgG (51) leads to production of proinflammatory cytokines, including TNF-α. CRP (6) and IgM bind to necrotic cells, but do not prevent proinflammatory cytokine production since production seems to be initiated by other pathways (52). These cytokines activate and lead to the differentiation of antigen presenting cells (53) promoting the engagement of low affinity self-reactive T lymphocytes. This scenario could readily promote a T cell driven anti-PC response with the potential for isotype switching to IgG and for somatic mutation. Whether anti-PC antibodies described here are germ line encoded and positively selected, whether they bind to bacterial PC and whether they can evolve to become anti-DNA autoantibodies are important questions that remain to be determined.
Figure 7.
Proposed role of IgM in the binding and clearance of apoptotic cells. Apoptotic cells activate iPLA2, which results in exposure of lysophospholipids, including lyso-PtC, on the cell membrane. Under normal circumstances, lysoPtC is recognized by IgM which activates the classical complement pathway (A). As shown, not all complement activation is IgM dependent. Macrophages or dendritic cells phagocytose complement-coated cells, and produce immunosuppressive cytokines such as TGF-β (see references 50, 6, and 54; A). In contrast, when little IgM is available (B), either the cells undergo post-apoptotic necrosis and/or are seen by IgG antibodies. In either case, phagocytosis of this cargo leads to proinflammatory cytokine production (see references 50, and 6) and, possibly, maturation of dendritic cells (reference 55).
Acknowledgments
We thank Drs. Michael Gelb and John Atkinson for helpful discussion, and colleagues for providing reagents.
This work was supported, in part, by grants from the National Institutes of Health, AR46582 and AR45482.
Footnotes
Abbreviations used in this paper: BEL, bromoeonol lactone; FPLC, Fast Performance Liquid Chromatography; HGS, hypogammaglobulinemia serum; NHS, normal human serum; MAC, membrane attack complex; PBT, peripheral blood–derived T cell; PC, phosphorylcholine; PE, phosphorylethanolamine; PL, phospholipase; PS, phosphorylserine.
References
- 1.Wakeland, E.K., K. Liu, R.R. Graham, and T.W. Behrens. 2001. Delineating the genetic basis of systemic lupus erythematosus. Immunity. 15:397–408. [DOI] [PubMed] [Google Scholar]
- 2.Scott, R.S., E.J. McMahon, S.M. Pop, E.A. Reap, R. Caricchio, P.L. Cohen, H.S. Earp, and G.K. Matsushima. 2001. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 411:207–211. [DOI] [PubMed] [Google Scholar]
- 3.Napirei, M., H. Karsunky, B. Zevnik, H. Stephan, H.G. Mannherz, and T. Moroy. 2000. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat. Genet. 25:177–181. [DOI] [PubMed] [Google Scholar]
- 4.Botto, M., C. Dell'Agnola, A.E. Bygrave, E.M. Thompson, T. Cook, F. Petry, M. Loos, P.P. Pandolfi, and M.J. Walport. 1998. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Gen. 19:56–59. [DOI] [PubMed] [Google Scholar]
- 5.Bickerstaff, M.C.M., M. Botto, W.L. Hutchinson, J. Herbert, G.A. Tennent, A. Bybee, D.A. Mitchell, H.T. Cook, P.J.G. Butler, M.J. Walport, et al. 1999. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nat. Med. 5:694–697. [DOI] [PubMed] [Google Scholar]
- 6.Gershov, D., S. Kim, N. Brot, and K.B. Elkon. 2000. C-reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J. Exp. Med. 192:1353–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mevorach, D., J. Mascarenhas, D.A. Gershov, and K.B. Elkon. 1998. Complement-dependent clearance of apoptotic cells by human macrophages. J. Exp. Med. 188:2313–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boes, M., T. Schmidt, K. Linkemann, B.C. Beaudette, A. Marshak-Rothstein, and J. Chen. 2000. Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc. Natl. Acad. Sci. USA. 97:1184–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrenstein, M.R., H.T. Cook, and M.S. Neuberger. 2000. Deficiency in serum immunoglobulin (Ig)M predisposes to development of IgG autoantibodies. J. Exp. Med. 191:1253–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korb, L.C., and J.M. Ahearn. 1997. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes. J. Immunol. 158:4525–4528. [PubMed] [Google Scholar]
- 11.Gharavi, A.E., E.N. Harris, M.D. Lockshin, G.R.V. Hughes, and K.B. Elkon. 1988. IgG subclass and light chain distribution of anticardiolipin and anti-DNA antibodies in SLE. Ann. Rheum. Dis. 47:286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaishnaw, A.K., J.R. Orlinick, J.L. Chu, P.H. Krammer, M.V. Chao, and K.B. Elkon. 1999. Molecular basis for the apoptotic defects in patients with CD95 (Fas/Apo-1) mutations. J. Clin. Invest. 103:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casiano, C.A., R.L. Ochs, and E.M. Tan. 1998. Distinct cleavage products of nuclear proteins in apoptosis and necrosis revealed by autoantibody probes. Cell Death Differ. 5:183–190. [DOI] [PubMed] [Google Scholar]
- 14.Elkon, K.B., A.P. Parnassa, and C.L. Foster. 1985. Lupus autoantibodies target the ribosomal P proteins. J. Exp. Med. 162:459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghomashchi, F., A. Stewart, Y. Hefner, S. Ramanadham, J. Turk, C.C. Leslie, and M.H. Gelb. 2001. A pyrrolidine-based specific inhibitor of cytosolic phospholipase A(2)α blocks arachidonic acid release in a variety of mammalian cells. Biochim. Biophys. Acta. 1513:160–166. [DOI] [PubMed] [Google Scholar]
- 16.Celli, C.M., A.E. Gharavi, and H. Chaimovich. 1999. Opposite β2-glycoprotein I requirement for the binding of infectious and autoimmune antiphospholipid antibodies to cardiolipin liposomes is associated with antibody avidity. Biochim. Biophys. Acta. 1416:225–238. [DOI] [PubMed] [Google Scholar]
- 17.Boes, M. 2000. Role of natural and immune IgM antibodies in immune responses. Mol. Immunol. 37:1141–1149. [DOI] [PubMed] [Google Scholar]
- 18.Buttke, T.M., and P.A. Sandstrom. 1994. Oxidative stress as a mediator of apoptosis. Immunol. Today. 15:7–10. [DOI] [PubMed] [Google Scholar]
- 19.Hildeman, D.A., T. Mitchell, T.K. Teague, P. Henson, B.J. Day, J. Kappler, and P.C. Marrack. 1999. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 10:735–744. [DOI] [PubMed] [Google Scholar]
- 20.Cummings, B.S., J. McHowat, and R.G. Schnellmann. 2000. Phospholipase A(2)s in cell injury and death. J. Pharmacol. Exp. Ther. 294:793–799. [PubMed] [Google Scholar]
- 21.Shaw, P.X., S. Horkko, M.K. Chang, L.K. Curtiss, W. Palinski, G.J. Silverman, and J.L. Witztum. 2000. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J. Clin. Invest. 105:1731–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desaymard, C., A.M. Giusti, and M.D. Scharff. 1984. Rat anti-T15 monoclonal antibodies with specificity for VH- and VH-VL epitopes. Mol. Immunol. 21:961–967. [DOI] [PubMed] [Google Scholar]
- 23.Chang, M.K., C. Bergmark, A. Laurila, S. Horkko, K.H. Han, P. Friedman, E.A. Dennis, and J.L. Witztum. 1999. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc. Natl. Acad. Sci. USA. 96:6353–6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atsumi, G., M. Tajima, A. Hadano, Y. Nakatani, M. Murakami, and I. Kudo. 1998. Fas-induced arachidonic acid release is mediated by Ca2+-independent phospholipase A2 but not cytosolic phospholipase A2, which undergoes proteolytic inactivation. J. Biol. Chem. 273:13870–13877. [DOI] [PubMed] [Google Scholar]
- 25.Wissing, D., H. Mouritzen, M. Egeblad, G.G. Poirier, and M. Jaattela. 1997. Involvement of caspase-dependent activation of cytosolic phospholipase A2 in tumor necrosis factor-induced apoptosis. Proc. Natl. Acad. Sci. USA. 94:5073–5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson, C.A., M.A. Balboa, J. Balsinde, and E.A. Dennis. 1999. Regulation of cyclooxygenase-2 expression by phosphatidate phosphohydrolase in human amnionic WISH cells. J. Biol. Chem. 274:27689–27693. [DOI] [PubMed] [Google Scholar]
- 27.Horkko, S., E. Miller, E. Dudl, P. Reaven, L.K. Curtiss, N.J. Zvaifler, R. Terkeltaub, S.S. Pierangeli, D.W. Branch, W. Palinski, et al. 1996. Antiphospholipid antibodies are directed against epitopes of oxidized phospholipids. Recognition of cardiolipin by monoclonal antibodies to epitopes of oxidized low density lipoprotein. J. Clin. Invest. 98:815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price, B.E., J. Rauch, M.A. Shia, M.T. Walsh, W. Lieberthal, and H.M. Gilligan. 1996. Antiphospholipid autoantibodies bind to apoptotic, but not viable, thymocytes in a β2-glycoprotein I-dependent manner. J. Immunol. 157:2201–2208. [PubMed] [Google Scholar]
- 29.Atsumi, G., M. Murakami, M. Tajima, S. Shimbara, N. Hara, and I. Kudo. 1997. The perturbed membrane of cells undergoing apoptosis is susceptible to type II secretory phospholipase A2 to liberate arachidonic acid. Biochim. Biophys. Acta. 1349:43–54. [DOI] [PubMed] [Google Scholar]
- 30.Volanakis, J.E., and K.W.A. Wirtz. 1979. Interaction of C-reactive protein with artificial phosphatidylcholine bilayers. Nature. 281:155–157. [DOI] [PubMed] [Google Scholar]
- 31.Kushner, I., and M.H. Kaplan. 1961. Studies of acute phase protein. J. Exp. Med. 114:961–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogden, C.A., A. deCathelineau, P.R. Hoffmann, D. Bratton, B. Ghebrehiwet, V.A. Fadok, and P.M. Henson. 2001. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J. Exp. Med. 194:781–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayakawa, K., R.R. Hardy, M. Honda, L.A. Herzenberg, and A.D. Steinberg. 1984. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc. Natl. Acad. Sci. USA. 81:2494–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mercolino, T.J., L.W. Arnold, L.A. Hawkins, and G. Haughton. 1988. Normal mouse peritoneum contains a large population of Ly-1+ (CD5) B cells that recognize phosphatidyl choline. Relationship to cells that secrete hemolytic antibody specific for autologous erythrocytes. J. Exp. Med. 168:687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palinski, W., S. Horkko, E. Miller, U.P. Steinbrecher, H.C. Powell, L.K. Curtiss, and J.L. Witztum. 1996. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J. Clin. Invest. 98:800–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balsinde, J., I.D. Bianco, E.J. Ackermann, K. Conde-Frieboes, and E.A. Dennis. 1995. Inhibition of calcium-independent phospholipase A2 prevents arachidonic acid incorporation and phospholipid remodeling in P388D1 macrophages. Proc. Natl. Acad. Sci. USA. 92:8527–8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atsumi, G., M. Murakami, K. Kojima, A. Hadano, M. Tajima, and I. Kudo. 2000. Distinct roles of two intracellular phospholipase A2s in fatty acid release in the cell death pathway. Proteolytic fragment of type IVA cytosolic phospholipase A2alpha inhibits stimulus-induced arachidonate release, whereas that of type VI Ca2+-independent phospholipase A2 augments spontaneous fatty acid release. J. Biol. Chem. 275:18248–18258. [DOI] [PubMed] [Google Scholar]
- 38.Balsinde, J., and E.A. Dennis. 1996. Distinct roles in signal transduction for each of the phospholipase A2 enzymes present in P388D1 macrophages. J. Biol. Chem. 271:6758–6765. [DOI] [PubMed] [Google Scholar]
- 39.Hazen, S.L., L.A. Zupan, R.H. Weiss, D.P. Getman, and R.W. Gross. 1991. Suicide inhibition of canine myocardial cytosolic calcium-independent phospholipase A2. Mechanism-based discrimination between calcium-dependent and -independent phospholipases A2. J. Biol. Chem. 266:7227–7232. [PubMed] [Google Scholar]
- 40.Balsinde, J., M.A. Balboa, and E.A. Dennis. 1997. Antisense inhibition of group VI Ca2+- independent phospholipase A2 blocks phospholipid fatty acid remodeling in murine P388D1 macrophages. J. Biol. Chem. 272:29317–29321. [DOI] [PubMed] [Google Scholar]
- 41.Pisetsky, D.S. 2000. Anti-DNA and autoantibodies. Curr. Opin. Rheumatol. 12:364–368. [DOI] [PubMed] [Google Scholar]
- 42.Cox, K.O., and S.J. Hardy. 1985. Autoantibodies against mouse bromelain-modified RBC are specifically inhibited by a common membrane phospholipid, phosphatidylcholine. Immunology. 55:263–269. [PMC free article] [PubMed] [Google Scholar]
- 43.Casali, P., and E.W. Schettino. 1996. Structure and function of natural antibodies. Curr. Top. Microbiol. Immunol. 210:167–179. [DOI] [PubMed] [Google Scholar]
- 44.Foerster, J. 1993. Autoimmune hemolytic anemias. 9th edition, Wintrobe's Clinical Hematology. Lea & Febiger, Philadelphia. pp. 1170–1196.
- 45.Hayakawa, K., and R.R. Hardy. 1988. Normal, autoimmune, and malignant CD5+ B cells: the LY-1 B lineage? Rev. Immunol. 6:197–218. [DOI] [PubMed] [Google Scholar]
- 46.Briles, D.E., M. Nahm, K. Schroer, J. Davie, P. Baker, J. Kearney, and R. Barletta. 1981. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 streptococcus pneumoniae. J. Exp. Med. 153:694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma, A., D.A. Isenberg, and B. Diamond. 2001. Crossreactivity of human anti-dsdna antibodies to phosphorylcholine: clues to their origin. J. Autoimmun. 16:479–484. [DOI] [PubMed] [Google Scholar]
- 48.Diamond, B., J.B. Katz, E. Paul, C. Aranow, and D. Lustgarten. 1992. The role of somatic mutations in the pathogenic anti-DNA response. Annu. Rev. Immunol. 10:731–742. [DOI] [PubMed] [Google Scholar]
- 49.Ehrenstein, M.R., T.L. O'Keefe, S.L. Davies, and M.S. Neuberger. 1998. Targeted gene disruption reveals a role for natural secretory IgM in the maturation of the primary immune response. Proc. Natl. Acad. Sci. USA. 95:10089–10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fadok, V.A., D.L. Bratton, A. Konowal, P.W. Freed, J.Y. Westcott, and P.M. Henson. 1998. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Invest. 101:890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ravetch, J.V., and S. Bolland. 2001. IgG Fc receptors. Annu. Rev. Immunol. 19:275–290. [DOI] [PubMed] [Google Scholar]
- 52.Li, M., D.F. Carpio, Y. Zheng, P. Bruzzo, V. Singh, F. Ouaaz, R.M. Medzhitov, and A.A. Beg. 2001. An essential role of the NF-κ B/Toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J. Immunol. 166:7128–7135. [DOI] [PubMed] [Google Scholar]
- 53.Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. [DOI] [PubMed] [Google Scholar]
- 54.Steinman, R.M., S. Turley, I. Mellman, and K. Inaba. 2000. The induction of tolerance by dendritic cells that have captured apoptotic cells. J. Exp. Med. 191:411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gallucci, S., M. Lolkema, and P. Matzinger. 1999. Natural adjuvants: endogenous activators of dendritic cells. Nat. Med. 5:1249–1255. [DOI] [PubMed] [Google Scholar]