Abstract
To dissect the influence of CD21/CD35 and FcγRIIB in antigen retention and humoral memory, we used an adoptive transfer model in which antigen-primed B and T lymphocytes were given to sublethally irradiated wild-type mice or mice deficient in CD21/CD35 (Cr2−/−) or FcγRIIB receptors (FcγRIIB−/−). Cr2−/− chimeras showed impaired memory as characterized by a decrease in antibody titer, reduced frequency of antibody secreting cells, an absence of affinity maturation, and significantly reduced recall response. The impaired memory in Cr2−/− chimeras corresponded with the reduced frequency of antigen-specific memory B cells. Interestingly, FcγRIIB−/− chimeras showed a differential phenotype with impaired splenic but normal bone marrow responses. These data suggest that CD21/CD35 on stroma, including follicular dendritic cells, is critical to the maintenance of long-term B lymphocyte memory.
Keywords: B lymphocytes, memory, complement receptors, FcγRIIB, follicular dendritic cells
Introduction
Long-term humoral memory is defined as the ability to generate a more effective antibody response after encounter with the original stimulating T-dependent antigen. In humans, humoral memory can persist for decades after initial antigen exposure as evidenced by the continued presence of antigen-specific immunoglobulin (1). Because serum immunoglobulins have half-lives for 1–3 wk (–4), the B lymphocyte compartment must continually produce specific immunoglobulin for long-term protection.
Effective recall responses to recurrent antigen exposure are postulated to require both preexisting, circulating antigen-specific antibody and memory B lymphocytes (5). Antibody-secreting cells (ASCs)*generate antigen-specific serum immunoglobulin that neutralizes harmful antigens and promotes efficient antigen trapping important for recall responses. Memory B lymphocytes quickly respond to cognate antigen, giving rise to new ASCs producing higher affinity immunoglobulins. Traditional models of humoral memory explain antibody persistence by continued differentiation of memory B lymphocytes into terminally differentiated ASCs (6). These models are called into question by the recent observation that some ASCs are long-lived, residing predominantly in the bone marrow (BM) and spleen (7, 8). Therefore, antigen-specific serum immunoglobulin titers could be maintained by these long-lived ASCs in the absence of continued differentiation of memory B lymphocytes. However, several classic studies suggest that continued differentiation of memory B lymphocytes is critical for optimal memory. Not only do serum immunoglobulin levels decrease more dramatically in the absence of antigen (9, 10), but the average affinity of serum immunoglobulin increases markedly with time after initial antigen encounter (i.e., affinity maturation; 11, 12), suggesting that the selection of high affinity B lymphocytes is an ongoing process. Therefore, despite the long-lived nature of some ASCs, continued differentiation of memory B lymphocytes likely plays a major role in humoral memory. Also implicit in these classic observations is that antigen is required for affinity maturation and for maintaining antibody titers. Therefore, understanding how antigen is retained long-term is paramount to understanding how affinity maturation and antibody titers continue after antigen exposure.
Memory B lymphocytes and long-lived ASCs are generated as end products of germinal center (GC) reactions (1, 13). Antigen sequestered on follicular dendritic cells (FDCs) within GC light zones provides the substrate for selection of high affinity B lymphocytes into the memory compartment. FDCs harbor antigen over a long term, suggesting that these cells have a critical role in humoral memory. Consistent with this idea is the recent observation that LTβRnull mice, whose principally defined mutation is a lack of detectable FDCs and GCs, are incapable of maintaining high titer serum immunoglobulin levels (14). Despite the implied role for FDCs in sustaining humoral memory by making antigen available long-term, the mechanism of antigen retention is unknown.
Two classes of receptor molecules capable of trapping antigen on FDCs are complement receptors, i.e., CD21/CD35 and FcγRIIB receptors. CD21/CD35 have a major role in generating the memory B lymphocyte compartment. The depletion of complement component C3 before immunization led to diminished immune complexes on FDC surfaces, suggesting that C3-tagged antigen and/or immune complexes are trapped via CD21/CD35 (15). In addition, mice deficient in complement components C3 and C4 or receptors CD21/CD35 have reduced abilities to trap a wide array of both T-independent and T-dependent antigens, leading to markedly impaired short-term humoral responses (16–19). Short-term humoral responses appear less dependent on FDC-derived CD21/CD35 because chimeric mice (i.e., WT BM into Cr2−/− recipient mice) have normal to slightly reduced antibody responses (20). The role of CD21/CD35 in antigen deposition for long-term, i.e., memory responses, is less defined, although chimeric mice lacking CD21/CD35 on FDCs, but not on B lymphocytes, generate reduced antibody titers after secondary challenge (20, 21). Unlike CD21/CD35, FcγRIIB is not constitutive on the surface of FDCs and instead is inducibly up-regulated after immunization (22). FcγRIIB-deficient mice are hyperresponsive (23), although this likely results from diminished regulatory signaling on B lymphocytes rather than any perturbation in antigen deposition (24). Because FcγRIIB is up-regulated on FDCs after immunization and because persisting antibody titers are considered important for immune complex deposition, it was suggested that FcγRIIB might be critical for trapping IgG–antigen immune complexes required for maintaining humoral memory as well as for recall responses.
In this study, we investigate the influence of antigen retention on antibody titers and affinity maturation. We find that complement receptors CD21/CD35 on recipient stroma are critical in ensuring normal ASC and memory B lymphocyte frequency, high antibody titers, and continued affinity maturation. Moreover, FDC expression of CD21/CD35 is critical for effective recall responses. In contrast, antibody persistence and the recall response are less dependent on stromal cell expression of FcγRIIB receptors, although frequencies of splenic ASCs and memory B lymphocytes are reduced relative to WT chimeras.
Materials and Methods
Mice.
Mice were housed at Harvard Medical School in a specific pathogen-free facility. C57BL/6 mice (Jackson ImmunoResearch Laboratories) and C57BL/6-CD45.1 congenic mice (National Cancer Institute) were used as sources of memory T and B lymphocytes. Recipient mice included the following genotypes: C57BL/6, Cr2−/− backcrossed 5 and 10 generations to C57BL/6, and FcγRIIB−/− mice backcrossed 3 generations on a C57BL/6 background (Taconic). Notably, no experimental differences were found in recipient mice with different degrees of C57BL/6 backcrossing.
Adoptive Transfer Protocol and Immunization Protocol.
4-hydroxy- 3-nitrophenyl (NP)-specific memory B lymphocytes were generated using C57BL/6-CD45.1 congenic mice. First immunized with 50 μg 10% alum-precipitated (Sigma-Aldrich) NP5-BSA (Biosearch Technologies) intraperitoneally, these mice were then boosted with the same antigen dose after 3 wk. Splenic mononuclear cells were isolated by ficoll-hypaque density centrifugation. To enrich for B lymphocytes, splenic mononuclear cells from NP-BSA–primed CD45.1 congenic mice were incubated with an antibody cocktail consisting of rat IgG anti–mouse CD4, CD8, CD11b/CD18, and IgD (Caltag and Amersham Biosciences) at concentrations of 107 cells/ml and 1 μg/ml/antibody. Anti–mouse IgD was added to the cocktail to restrict the recovery of predominating naive B lymphocytes. Cells were then panned twice on Petri dishes coated with goat anti–rat IgG (mouse adsorbed; Southern Biotechnologies) for 1 h at 4°C. B lymphocyte purity ranged from 70–80% with <10% T lymphocyte and <10% macrophage contamination (unpublished data). ∼10% of adoptively transferred B lymphocytes were NP specific as determined by FACS®. The NP-specific memory response was IgG1+λ+ as determined by ELISA and BIAcore.
Keyhole limpet hemocyanin (KLH)-specific T lymphocytes were generated from C57BL/6 mice immunized intraperitoneally with 50 μg alum-precipitated KLH as described above. Similarly, splenic mononuclear cells were enriched for T lymphocytes by incubation with an antibody cocktail consisting of rat IgG anti–mouse B220, CD19, CD11b/CD18, and IgD. Splenocytes were then panned as described above. T lymphocyte purity was 50–70% in the transferred population with 10–30% B lymphocyte contamination and <10% macrophage contamination.
Single cell suspensions of 107 B and 5 × 106 T lymphocytes were adoptively transferred with 50 μg soluble NP5-KLH intravenously into sublethally irradiated (650 RAD, Cs source) MHC-identical recipient mice (CD45.2). Irradiated control mice were given antigen but no memory B or T lymphocytes after irradiation. 3 or 16 wk after adoptive transfer, recipient and control mice were challenged intravenously with 50 μg soluble NP5-KLH and 1 wk after mice were killed, tissues were isolated to measure recall responses. Recipient and control mice were bled periodically throughout the 16-wk rest period. Serum was analyzed by ELISA for specific antibody production to NP (see below).
For secondary adoptive transfers, single cell suspensions of 107 B lymphocytes from the primary chimeras were given intravenously with 5 × 106 T lymphocytes from KLH-primed mice and 50 μg NP5-KLH were given to sublethally irradiated WT or Cr2−/− recipients. These mice were analyzed 3 wk later.
Anti-NP Response and Affinity Measurements by ELISA.
Serum was collected from individual mice and NP-specific antibody titers were determined by sandwich ELISA. 96-well plates (Immulon 1B) were coated with 5 μg/well NP5-BSA or NP15-BSA. Plates were blocked with the addition of 5% dry milk (Carnation®) in PBS (Blotto). NP-specific IgG serum antibody in serially diluted samples was detected by alkaline phosphatase–conjugated goat anti–mouse IgG (Sigma-Aldrich). Between incubations, plates were washed with PBS containing 0.1% Tween. Color was developed using p-nitrophenyl phosphate (Sigma-Aldrich) as substrate and absorbance was measured at 405 nm using Softmax software package. Antibody titer was determined as the reciprocal of the greatest dilution whose absorbance remained above background. For relative affinity measurements, the ratio of titers to NP5-BSA and NP15-BSA was calculated for individual mice using OD405 in the linear ranges of the assays as previously described (25).
Relative Serum Antibody Affinity to NP Determined by BIAcore.
A BIAcore 2000™ was used to study the kinetics of anti-NP serum IgG binding to NP. Standard amine coupling was used to immobilize goat anti–mouse IgG Fcγ (Jackson ImmunoResearch Laboratories) diluted to 30 μg/ml in 10 mM acetic acid buffer, pH 4.0, to a CM5 sensor chip (Biosensor AB; Amersham Biosciences). A second flow cell on the chip was prepared using the same procedure, without goat anti–mouse IgG Fcγ, to subtract out significant nonspecific responses. Experiments were performed at 25°C with a 10-μl/minute flow rate. Serum samples diluted 1:1,000 in HBS (BIAcore) containing 1% BSA were injected over the chip. Captured serum IgG was tested for antigen-binding kinetics by injection with NP5-BSA. Apparent kinetic rate constants (kd and ka) for NP5 binding to serum IgG antibodies were estimated using nonlinear curve fitting with BIAevaluation 3.1 software (BIAcore). Equilibrium dissociation constants (KD) were calculated as kd/ka. All values were determined by first normalizing to the amount of IgG bound to the chip.
Enzyme-linked Immunospot Assay for NP-specific ASCs.
Frequencies of NP-specific ASCs were quantitated as previously described (26). In brief, 24-well polystyrene plates (Costar) were coated with NP5-BSA. After extensive washing, plates were blocked with 1% BSA in PBS for 2 h. Serially diluted splenic mononuclear cells or BM cells (106 to 103 cells/well) were added in DMEM media with 2% fetal bovine serum and incubated overnight at 37°C. Each dilution of cells was assayed in duplicate. Plates were then washed with PBS containing 0.1% Tween and incubated with alkaline phosphatase–conjugated goat anti–mouse IgG antibody (Sigma-Aldrich). Plates were developed using 5-bromo-4-chloro-3-indolyl phosphate at 1 mg/ml in 0.6% agarose to produce blue-colored spots identifying NP-specific ASCs. Spots were counted to determine ASC frequency. As controls, each sample was also plated in wells coated with BSA or KLH. Few BSA-specific ASCs were detected in the BM or spleen and <100 KLH-specific ASCs were observed per recipient spleen (unpublished data).
Immunohistology and Antigen Deposition.
Chimeric mice were given 50 μg soluble NP5-BSA biotin intravenously 16 wk after adoptive transfer. Spleens were isolated 16–18 h after injection and prepared for immunohistology as previously described (27). 5-μm thick sections were examined for antigen retention within splenic compartments using streptavidin-coupled TRITC (Sigma- Aldrich). Peanut agglutinin (PNA; E-Y Laboratories) coupled to FITC was used to identify GCs and B lymphocyte zones were delineated with Cy5-coupled anti-IgM antibody (BD Biosciences). Staining was visualized by confocal microscopy (Radiance 2000; Bio-Rad Laboratories).
FACS® Analysis and Identification of NP-specific Memory B Cells.
Single cell suspensions of splenocytes and BM cells were incubated on ice with rat anti–mouse CD16/CD32 (2.4G2; BD Biosciences). Cells were then treated with a cocktail of NP coupled to allophycocyanin (Molecular Probes), FITC-conjugated goat anti–mouse Igλ (Southern Biotechnology Associates, Inc.), PE-conjugated rat anti–mouse CD19 (1D3; BD Biosciences), and biotinylated rat anti–mouse CD45.1 (A20; BD Biosciences). After washing, cells were incubated on ice with streptavidin-PerCP (BD Biosciences). Cells were analyzed with a FACSCalibur® (Becton Dickinson). CD19+CD45.1+λ+NP+ cells within the lymphocyte gate were considered memory B lymphocytes. These cells were negative for cell surface expression of CD138, IgM, CD3, and CD11b.
Statistical Analysis.
All statistical comparisons reported used the standard two-tailed t test assuming unequal variance.
Results
The mechanism of long-term antigen retention participating in humoral memory was examined using a hapten carrier adoptive transfer model. A similar adoptive transfer system was used previously in rats to demonstrate that persisting antibody titers and recall responses depended on the presence of antigen (9). This approach was used to create chimeric mice with differential expression of complement and FcγRIIB receptors on FDC stroma and B lymphocytes. To test the importance of CD21/CD35 and FcγRIIB in harboring antigen for long-term memory, NP-specific memory B lymphocytes, KLH-primed T lymphocytes, and antigen (NP-KLH) were transferred into sublethally irradiated recipient mice deficient in CD21/CD35 (Cr2−/−) or FcγRIIB (FcγRIIB−/−), as well as WT controls. Thus, chimeric mice have normal complement and FcγRIIB-sufficient B lymphocytes but their stromal cells and radioresistant myeloid cells are receptor deficient. To control for endogenous responses, parallel sets of recipient mice were treated identically except that they did not receive memory lymphocytes. Finally, to identify transferred memory B lymphocytes C57BL/6 mice congenic for the CD45.1 allotypic marker were used as donors, whereas recipient mice expressed CD45.2 exclusively.
Short-Term Responses.
Short-term antibody responses were examined in the presence or absence of CD21/CD35 or FcγRIIB. All groups of chimeric mice had comparable anti-NP titers 3 wk after lymphocyte transfer with mean titers ranging from 16.7 × 103 to 22.7 × 103 (Table I). NP titers in WT chimeras deprived of antigen were substantially reduced. When WT and Cr2−/− chimeric mice were challenged with NP5-KLH 3 wk after the initial transfer of antigen and memory lymphocytes, specific IgG titers were again comparable between WT and Cr2−/− recipient mice (unpublished data). These data suggest that the adoptively transferred memory B lymphocytes were generating equivalent short-term antibody responses irrespective of stromal expression of CD21/CD35.
Table I.
Persistence of NP Titers after Adoptive Transfer of Memory B Lymphocytes and During the Recall Response
| Chimera | Time after transfer of NP-specific memory B lymphocytes (NP-specific IgG titers, ×103) | ||||
|---|---|---|---|---|---|
| 2–3 wk | 6–8 wk | 10–12 wk | 16 wk | 17 wk | |
| WT (n = 34) | 22.7 ± 3.8 | 19.7 ± 3.8 | 10.4 ± 1.9 | 6.4 ± 1.2 | 67.2 ± 19.1 |
| Cr2−/− (n = 31) | 16.7 ± 2.7 | 9.5 ± 1.7* | 5.8 ± 1.1* | 2.9 ± 0.7* | 6.4 ± 1.8* |
| FcγRIIB−/− (n = 32) | 22.3 ± 5.2 | 15.4 ± 4.4 | 7.3 ± 1.6 | 4.6 ± 1.5 | 38.4 ± 14.9 |
| WT, no antigen (n = 8) | 8.9 ± 3.4* | 7.7 ± 3.0* | 5.1 ± 2.3* | 2.1 ± 0.7* | 1.6 ± 2.1* |
| Irradiated controls (n = 41) | <1.6* | <1.6* | <1.6* | <1.6* | <1.6* |
Numbers represent mean anti-NP IgG titers (×103) ± SEM at the indicated time points after adoptive transfer of NP-specific memory B lymphocytes.
, statistically significant differences upon comparison to WT. Results are pooled from six independent experiments.
Long-Term Antibody Persistence.
To measure long-term antibody responses, changes in serum anti-NP titers were monitored over 16 wk for each chimeric mouse (Table I). WT mice receiving adoptively transferred cells in the absence of antigen generated two to three times less antibody compared with WT chimeras receiving antigen, demonstrating that optimal responses are antigen dependent. 6–8 wk after adoptive transfer, antibody titers in WT and FcγRIIB−/− chimeras dropped by ∼25% of the initial titer, whereas anti-NP titers fell ∼50% in chimeric mice lacking CD21/CD35+ stroma. WT and FcγRIIB−/− chimeric mice maintained significantly higher titers compared with Cr2−/− chimeras until the end of the experimental protocol (P < 0.015; Table I). Importantly, anti-NP titers were negligible in irradiated control mice, suggesting that donor B lymphocytes were the principle source of responding B lymphocytes in experimental mice (Table I).
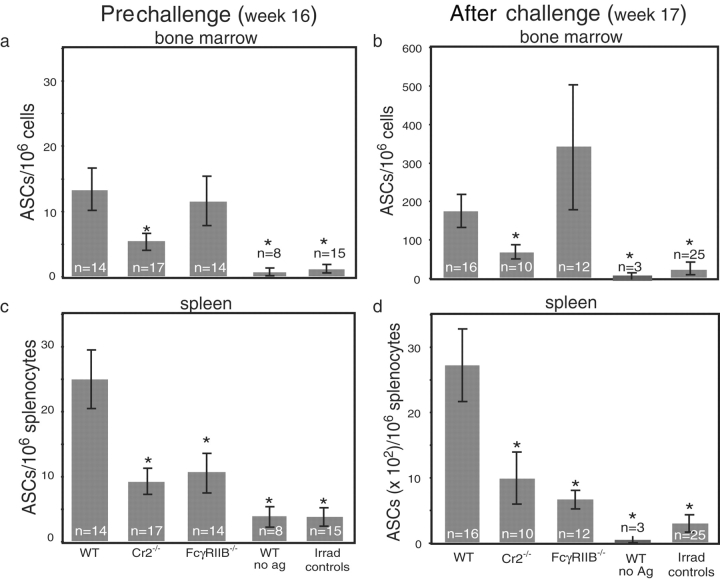
The significant decrease in antibody titers in Cr2−/− chimeric mice suggested that the frequency and/or number of plasma cells was impaired in the absence of CD21/CD35. To examine this possibility, BM and spleens from recipient mice 16 wk after transfer were analyzed for NP-specific ASCs by ELISPOT. The BM of WT and FcγRIIB−/− chimeric mice had similar frequencies of NP-specific ASCs (13.4 ± 3.2 and 11.6 ± 3.7 ASCs/106 BM cells, respectively; Fig. 1 a ). In contrast, the BM of Cr2−/− chimeras had two- to threefold fewer NP-specific ASCs (5.6± 1.1 ASCs/106 BM cells, P < 0.035). Similar reductions were found in splenic NP-specific ASCs of mice lacking CD21/CD35 (25.1± 4.4 vs. 9.4 ± 1.6 ASCs/106 splenocytes in WT and Cr2−/− chimeras, P < 0.004; Fig. 1 b). Unlike the frequency of NP-specific ASCs observed in BM, FcγRIIB−/− chimeric mice had reduced frequencies of ASCs in the spleen when compared with WT chimeras (10.9 ± 3.1/106 splenocytes, P < 0.016). In both the BM and spleen, the ASCs were likely donor cell derived because irradiated control mice from each genotype tested failed to produce NP-specific ASCs (all recipient genotype controls are pooled in the bar shown in Fig. 1). As expected, negligible numbers of ASCs were observed in the BM and spleen of WT chimeric mice deprived of antigen. Therefore, the presence of CD21/CD35 on recipient stroma is required for optimal production and/or sustenance of plasma cells.
Figure 1.
Frequency of NP-specific ASCs in chimeric mice 16 wk after receiving memory B lymphocytes. Recipient BM (a and c) and spleen (b and d) before (a and b) or 1 wk after antigen challenge intravenously with 50 μg NP5-KLH (c and d) were analyzed by ELISPOT for IgG-secreting plasma cells specific for NP. Data are represented as means ± SEM (a and b shows three independent experiments and c and d shows four independent experiments). n = number of mice in each group. *, statistically significant differences upon comparisons to WT chimeras (P < 0.035 BM and P < 0.02 spleen).
One explanation for reduced ASCs in Cr2−/− chimeras is that ASCs are short-lived and require replacement by antigen-dependent precursors (28). To determine if there was a reduction in memory B lymphocytes in the Cr2−/− chimeras, frequencies of CD45.1+ (donor-derived) NP-binding λ+ (CD138−) B lymphocytes were assessed 16 wk after transfer. CD138− staining was used to distinguish memory B lymphocytes from ASCs. Based on FACS® analysis, a significant reduction in memory B lymphocytes was observed in the spleens of receptor-deficient chimeras, i.e., 310 ± 55 vs. 125 ± 25, 129 ± 29, and 63 ± 18 per 106 splenocytes for WT, Cr2−/−, FcγRIIB−/−, and WT no antigen, respectively (Fig. 2, a and c ). Similar reductions in donor-derived NP-specific memory B lymphocytes were found in BM, although only chimeras lacking CD21/CD35+ stroma and WT no antigen chimeras had significant reductions relative to WT chimeras (Fig. 2 b). Therefore, reduced ASC frequency and serum antibody titers in Cr2−/− chimeras might be caused by reduced frequencies of memory B lymphocytes.
Figure 2.
Frequency of donor-derived, NP-specific memory B lymphocytes in chimeric mice 16 wk after adoptive transfer. (a) Representative scheme to identify antigen-specific, donor-derived memory B lymphocytes by FACS®. Frequency of NP-binding CD45.1+Igλ+ B lymphocytes (CD19+) was determined from mouse BM (b and c) and spleen (d and e) before (b and d) or 1 wk after antigen challenge intravenously with 50 μg NP-KLH (c and e). Cells were also confirmed as CD3−, IgM−, CD138−, and CD11b−. Data are represented as means ± SEM of three independent experiments. n = number of mice in each group. *, statistically significant differences upon comparisons to WT chimeras (P < 0.01 BM and spleen prechallenge and P < 0.04 after challenge).
Another potential contributing factor for reduced frequency of NP-specific ASCs and memory B lymphocytes in the absence of CD21/CD35 on recipient stroma is a general reduction in chimerism relative to WT recipients. The degree of chimerism was determined by comparing the ratio of donor B lymphocytes (CD45.1+CD19+) versus total B lymphocytes (total CD19+). On average, the frequency of donor B lymphocytes was slightly greater in WT mice compared with Cr2−/− mice, although the difference was not significant (35.8 ± 4.75%, 32.6 ± 4.1%, and 23.8 ± 3.3% in the spleens of WT, Cr2−/−, and FcγRIIB−/− chimeras, respectively). Therefore, the degree of chimerism does not explain the differences in frequencies of NP-specific ASCs and memory B lymphocytes between recipient groups.
Affinity Maturation.
Given the importance of antigen in affinity maturation and the implication that CD21/CD35-bearing stroma is important for long-term antigen retention, it was proposed that affinity maturation might also be impaired in the absence of CD21/CD35. To estimate relative serum affinity, two approaches were taken. The first approach used ELISA to determine the ratio of antibody titers binding low (NP5) to highly (NP15) haptenated BSA. No significant differences in the ratio of NP5/NP15 titers were detected between the recipient groups 3 wk after the transfer of memory B lymphocytes (Fig. 3) . In WT chimeras, the relative serum affinity increased modestly with time (from 52 ± 5% at 3 wk after transfer to 70 ± 7% at 16 wk, P < 0.03). Relative serum affinities did not change significantly with time in Cr2−/− chimeras (53 ± 4% to 48 ± 6%, from 3 to 16 wk, respectively). The most significant changes in relative serum affinities were observed in FcγRIIB−/− chimeras (60 ± 6% to 84 ± 6%, P < 0.006).
Figure 3.
Changes in relative serum affinity with time after antigen exposure. Sera collected from chimeric mice were titrated in ELISA for the amount of high affinity (NP5-BSA) and the amount of total (NP15-BSA) IgG. The ratio of NP5:NP15 titers is expressed as mean ratios ± SEM and are compiled from four independent experiments. ▪, WT chimeras (n = 34); ♦, Cr2−/− chimeras (n = 31); •, FcγRIIB−/− chimeras (n = 32); *, statistically significant differences. Statistical comparisons were made to the 2–3-wk time point for each group (P < 0.01).
To confirm that relative serum affinities in FcγRIIB−/− chimeras increase markedly with time after the transfer of memory B lymphocytes, surface plasmon resonance was used to examine the real-time kinetics of anti-NP serum IgG binding to NP-BSA. Real-time measurements obtained by this technique allow an estimate of affinity based on relative on/off rates (KD = kd/ka). WT chimeras demonstrated modest yet incremental increases in affinity whereas Cr2−/− chimeras failed to show statistically significant changes (for WT, ΔKD of 1.5 × 10−8 over 16 wk after cell transfer, P < 0.03; Table II) . In contrast to Cr2−/−, FcγRIIB−/− chimeras demonstrated significant increases in affinity constants with time after cell transfer (ΔKD of 4.8 × 10−8 over 16 wk after transfer, P < 0.003). Therefore, the absence of FcγRIIB-bearing stroma potentiates affinity maturation.
Table II.
Changes in Relative Serum Affinity after Adoptive Transfer of Memory B Lymphocytes
| Mean ΔKD (BIAcore)
|
||
|---|---|---|
| Chimera | 1–12 wk | 14–16 wk |
| WT | 1.6 × 10−8 * | 1.5 × 10−8 * |
| Cr2−/− | 0.8 × 10−8 | 1.4 × 10−8 |
| FcγRIIB−/− | 0.2 × 10−8 | 4.8 × 10−8 * |
Mean changes in relative affinity (ΔKD; left side of table) were determined by calculating equilibrium dissociation constants (KD) for each time point serum sample, followed by subtraction of starting KD values 2–3 wk after cell transfer (n = 20 for WT, 11 each for Cr2−/− and FcγRIIB−/− chimeras). Results are summarized from two independent experiments. *, statistically significant differences upon comparison to mean 2–3-wk time point values (P < 0.03 for WT chimeras and P < 0.003 for FcγRIIB−/− chimeras).
Recall Responses.
By definition, memory responses are faster and more robust than primary responses. The contribution of CD21/CD35 and FcγRIIB receptors on recall responses was tested in the current model by challenging chimeric mice intravenously with soluble NP5-KLH 4 mo after adoptive transfer. 1 wk later, mice were analyzed for antibody titers and frequency of ASCs and memory B lymphocytes. Anti-NP titers increased approximately 10-fold in WT and FcγRIIB−/− chimeric mice after challenge (mean prechallenge to postchallenge titers = 6.4 × 103 to 67.2 × 103 for WT and 4.6 × 103 to 38.4 × 103 for FcγRIIB−/− chimeras; Table I). By contrast, the mean anti-NP titer increased approximately twofold in Cr2−/− chimeras after antigen challenge and was approximately 10-fold less than that observed in WT chimeras (from 2.9 × 103 to 6.4 × 103; Table I). Therefore, the recall response of Cr2−/− chimeric mice was substantially reduced compared with WT and FcγRIIB−/− chimeras.
To determine whether antibody titers correlated with the frequency of effector cells, NP-specific ASCs from the BM and spleen from all groups of mice were measured by ELISPOT after antigen challenge. The frequency of NP-specific ASCs increased ∼10-fold in the BM and 100-fold in the spleen in all groups (compare Fig. 1, a to c and b to d). WT and FcγRIIB−/− chimeras produced similar frequencies of NP-specific BM ASCs, consistent with similar antibody titers generated as part of the memory response (Fig. 1 c). In contrast, the relative frequency of NP-specific ASCs was reduced two- to threefold in the spleen and BM of Cr2−/− chimeras compared with WT chimeras. This suggested that reductions in memory B lymphocyte frequency and/or reduced antigen localization via CD21/CD35 contributed to reduced recall responsiveness. Despite a near 10-fold increase in antibody titer, FcγRIIB−/− chimeras had significantly reduced frequency of splenic ASCs compared with WT chimeras.
To assess whether the renewed production of NP-specific memory B lymphocytes was affected in the absence of CD21/CD35- or FcγRIIB-containing stroma, splenic B lymphocytes isolated from chimeric mice were analyzed by FACS®. Again, as predicted for memory responses, an increase in the frequency of NP-specific memory B lymphocytes was observed both in the BM and spleen after antigen challenge in all chimeras (compare Fig. 2, b to d and c to e). For example, the frequency of NP-specific memory B lymphocytes in WT chimeras increased from a mean of 82 ± 12 to 420 ± 92 per 106 cells in the BM and from 310 ± 55 to 3,280 ± 601 per 106 splenocytes. The relative frequency of memory B lymphocytes was significantly reduced in the BM (160 ± 40/106 cells) and spleen (1,720 ± 526/106 cells) of CD21/CD35-deficient chimeric mice. Consistent with the reduced frequency of splenic ASCs, the relative frequency of memory B lymphocytes was reduced in spleens (1,560 ± 526/106 cells) of FcγRIIB-deficient chimeric mice.
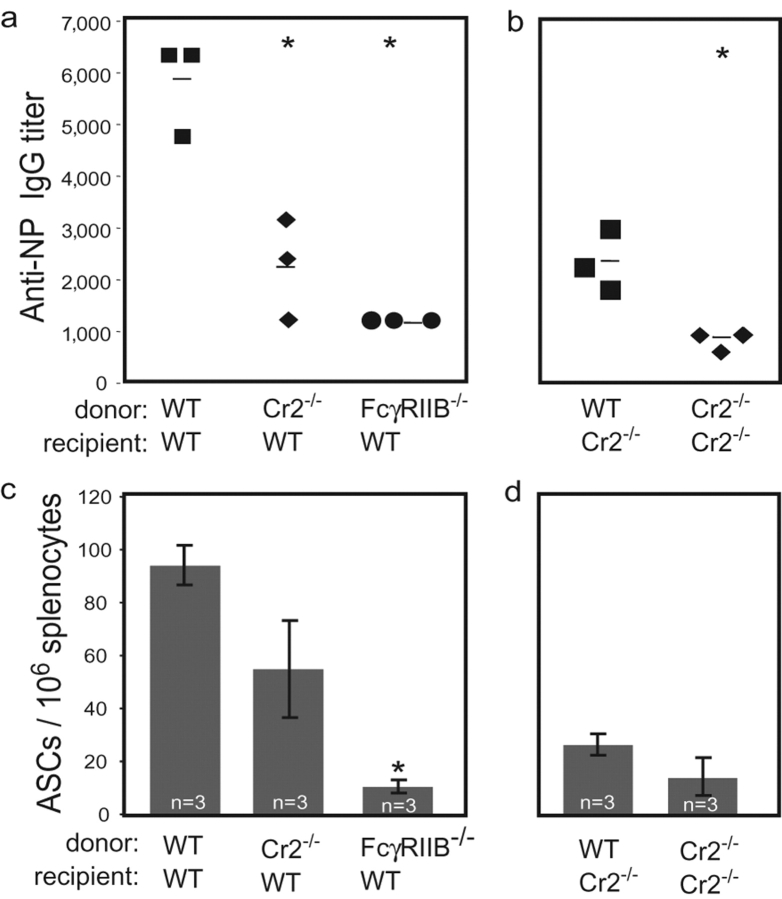
The finding of 10-fold differences in antibody production between WT and Cr2−/− chimeras, but only two- to threefold differences in memory B lymphocytes and ASC frequencies, suggested that donor-derived memory B lymphocytes in Cr2−/− mice might be functionally unresponsive. To address this question, serial adoptive transfers were performed. Splenic B lymphocytes from primary recipient mice were adoptively transferred along with antigen and T cells into sublethally irradiated WT mice. 3 wk after transfer, anti-NP titers and splenic ASC frequencies were determined. Consistent with earlier results, the mean anti-NP titer was approximately two- to threefold less in secondary chimeric mice receiving primed B lymphocytes from Cr2−/− primary chimeras compared with WT primary chimeras (2,267 ± 581 vs. 5,867 ± 533, P < 0.01; Fig. 4 a ). Similarly, analysis of the frequency of splenic ASCs revealed an approximate twofold reduction in mice receiving primed B lymphocytes from Cr2−/− versus WT primary chimeras (55 ± 18.2 vs. 94 ± 7.4, P < 0.07; Fig. 4 c). Thus, the frequency of functional memory B lymphocytes was reduced in Cr2−/− chimeras as evidenced by their failure to respond optimally in WT recipients. The NP titer of recipients of primed B lymphocytes from primary FcγRIIB−/− donors was reduced by over fourfold relative to primary WT donors (1,200 ± 0 vs. 5,867 ± 533). Moreover, even greater reduction was observed in the frequency of NP-specific ASCs in secondary chimeras receiving B lymphocytes from FcγRIIB−/− primary chimeras (10.2 ± 1.8, P < 0.008; Fig. 4 c). Therefore, as observed in the spleens of primary FcγRIIB−/− chimeras, there was an impaired response after secondary transfer into WT recipients.
Figure 4.
Memory responses after secondary transfer. Splenic B lymphocytes from original chimeric mice were adoptively transferred intravenously with KLH-primed T cells and NP-KLH into sublethally irradiated WT (a and c) and Cr2−/− (b and d) recipients. 3 wk after secondary transfer into WT (a) and Cr2−/− (b) mice, NP-binding serum IgG was measured by ELISA. Each symbol represents a single mouse. Horizontal bars represent mean antibody titers. (c and d) ELISPOTs were performed on recipient splenocytes to determine ASC frequency. Data are represented as means ± SEM from a single experiment. *, statistically significant differences. Statistical comparisons to WT chimeras were made (P < 0.01).
An additional factor in the diminished recall response of Cr2−/− chimeras is impaired antigen localization on challenge. To test this possibility, serial transfers were also performed using Cr2−/− mice as secondary recipients. An approximate twofold reduction in antibody titer was observed in Cr2−/− versus WT recipients (1,067 ± 133 vs. 2,533 ± 353, P < 0.03; Fig. 4 b). The difference in antibody titers between Cr2−/− secondary chimeras receiving either splenocytes from WT or Cr2−/− primary chimeras was again reflected at the level of ASC frequency (17 ± 4.9 for Cr2−/− vs. 25.8 ± 1.7 for WT; Fig. 4 d). Therefore, the 10-fold reduction in recall response of Cr2−/− chimeras was due in large part to both a reduction in memory B lymphocytes and a lack of complement receptors on recipient stroma.
Antigen Deposition in the Absence of CD21/CD35.
To examine whether the ability to trap antigen efficiently contributes to differences in recall responsiveness between chimeric mice, the groups of recipient mice were injected with biotinylated NP-BSA 16 h before harvesting. Immunohistochemical analyses of splenic sections from WT and FcγRIIB−/− chimeras identified antigen within PNA+ GCs (Fig. 5, a and b ). By contrast, antigen deposition was negligible within the GC of Cr2−/− chimeras (Fig. 5, c and d). The lack of localized biotinylated antigen in Cr2−/− mice was observed at multiple time points, ranging from 12–20 h after administration, suggesting that kinetic differences in antigen trapping are not responsible for the absence of detectable antigen in Cr2−/− chimeras (unpublished data). Importantly, antigen detected in WT and FcγRIIB−/− chimeras colocalized with FDCs within the light zones of GCs (unpublished data).
Figure 5.
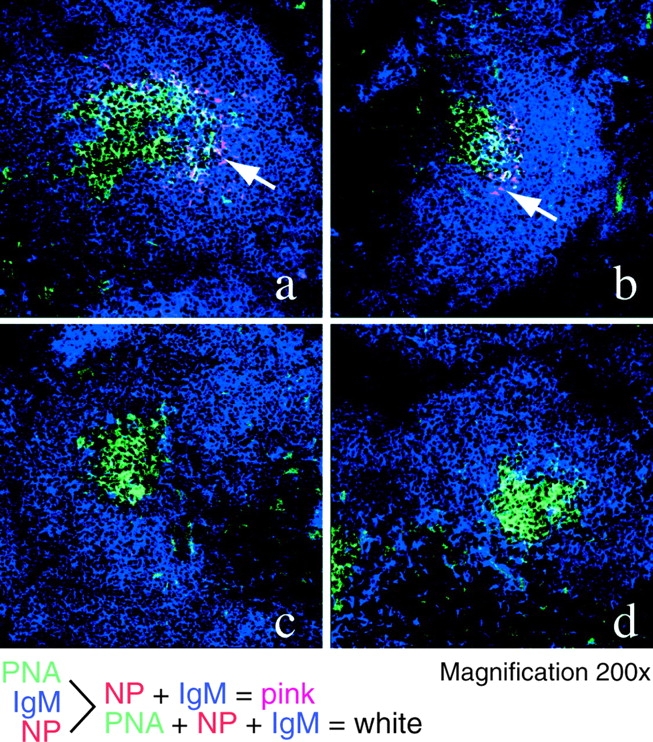
Dependence on CD21/CD35+ stroma for localizing antigen in GCs. 16 wk after adoptive transfer of memory B lymphocytes, WT (a), FcgRIIB−/− (b), and Cr2−/− (c) chimeric mice were injected with biotinylated NP-BSA. (d) WT chimeras not receiving biotinylated antigen. 5-μM splenic cryosections were stained with anti-IgM (blue) and PNA (green) to delineate B lymphocyte zones and GCs, respectively. Arrows point to detectable antigen, as visualized using SA-TRITC (red). Colocalized IgM, PNA, and antigen are detected in white. Staining patterns shown are representative of multiple sections (>5) from five mice per chimeric group.
Discussion
The role of antigen in the maintenance of long-term B lymphocyte memory is subject to debate. In this study, the mechanism of antigen retention leading to long-term antibody production, persistence of memory B lymphocytes, and affinity maturation was addressed using an adoptive transfer model similar to that pioneered by Gray and Skarvall (9). Because memory responses using this model depend on introduced antigen, it is amenable to examining the contributions of receptors believed critical for capturing and retaining antigen by adoptively transferring memory B lymphocytes into mice deficient in either CD21/CD35 or FcγRIIB. We demonstrated that CD21/CD35 expression on radioresistant stomal cells (including FDCs) is important for maintaining serum titers (Table I), high frequencies of ASCs (Fig. 1) and memory B lymphocytes (Fig. 2), as well as for optimal affinity maturation (Fig. 3, Table II). Moreover, recall responses were severely compromised in the absence of CD21/CD35-bearing stroma (Figs. 1, c and d, 2, d and e, and 4; Table I). In contrast, although splenic responses were impaired in FcγRIIB−/− chimeras, the absence of FcγRIIB on recipient cells did not affect overall antibody persistence or frequency of BM ASCs and surprisingly led to significantly increased serum affinity. These observations suggest that although both CD21/CD35 and FcγRIIB participate in trapping antigen, overall memory B lymphocyte responses are more dependent on stromal cell–associated complement receptors.
Antigen-dependent maintenance of NP titers.
The experimental model used depends on antigen because WT mice receiving memory B and T lymphocytes without antigen at the time of cell transfer fail to sustain significant anti-NP titers. In addition, they are unable to mount significant recall responses 16 wk after the transfer of memory B lymphocytes (Table I). These data suggest that memory B lymphocytes are not maintained (in the absence of antigen) to the extent that they efficiently differentiate into antibody-producing plasma cells after antigen challenge. Interestingly, antibody titers in Cr2− / −chimeras were similar to WT chimeras not receiving antigen after 16 wk, suggesting that antigen retention via CD21/CD35 is critical for antibody persistence. However, chimeras lacking CD21/CD35+ stroma contained limited NP-specific ASCs. CD21/CD35 involvement in antibody persistence was examined previously, where it was reported that Cr2− / −mice immunized with T-dependent antigens generated reduced antibody responses (29, 30). In one report, it was noted that the decay of serum antibody after antigen challenge was faster in Cr2− / −mice compared with WT mice (29), consistent with the thesis that the ASC pool is not efficiently replenished in the absence of CD21/CD35. However, because these studies used Cr2− / −mice whose B lymphocytes and follicular stroma lacked CD21/CD35, they were unable to distinguish CD21/CD35-dependent antigen trapping from its role as a B lymphocyte coreceptor. In this study, the responding memory B lymphocyte pool had intact B lymphocyte receptor signaling complexes and therefore the contribution of stroma-derived CD21/CD35 for antigen sequestration was isolated from coreceptor-associated B cell receptor signaling. The implication of reduced antibody titers in Cr2− / −chimeras in this study is that CD21/CD35 on FDCs is critical in long-term antigen retention, although other radioresistant CD21/CD35-expressing myeloid cells may also participate.
Differences in serum antibody levels in the various chimeric groups directly correlated with the frequency of NP-specific ASCs in BM. ASC frequency is reduced in Cr2−/− chimeras relative to WT chimeras, supporting the idea that active ASC replacement contributes to effective long-term B lymphocyte memory. Several recent reports addressed the possibility of long-lived ASCs. One report using bromodeoxyuridine labeling concluded that 60% of BM ASCs can survive in excess of 90 d without turnover (8). Another study demonstrated that a fraction of adoptively transferred ASCs continue to produce antibody for longer than 1 yr (7). The interpretations of the latter study were questioned, however, by another study using the same lymphocytic choriomeningitis virus model antigen system that failed to observe “antigen-independent long-lived persisting antibody” (28). Although experiments in this study did not address ASC lifespan directly, the observation that WT chimeras not receiving antigen still produced measurable, albeit significantly reduced, anti-NP titers is consistent with the presence of low numbers of long-lived ASCs (negligible ASCs were detected in the BM).
Interestingly, differences in antibody titers between WT chimeras and Cr2−/− chimeras was significant after 6–8 wk, which suggests that either fewer long-lived ASCs are generated initially after transfer and/or that continued production of ASCs is less efficient in mice without CD21/CD35+ stroma. Long-lived ASCs, as well as memory B lymphocytes, are products of GC responses. Based on results using a Bcl-2 transgenic model, Smith et al. (31) proposed that predominantly high affinity ASCs produced in GCs are recruited to the BM. In the current model, the observation of similar antibody titers after 2–3 wk suggests that the initial GC responses between the chimeric groups are comparable. The subsequent differences in antibody titers observed between WT and Cr2−/− chimeras after 6 wk (i.e., after GC wane) could then be explained by less efficient replenishment of the BM ASC pool.
Affinity Maturation.
To delineate whether post-GC selection contributed to altered antibody titers in WT and Cr2−/− chimeras, relative serum affinity changes were monitored. Early evaluation of affinity maturation demonstrated that affinity increases with time after initial antigen contact and that the degree of increase depends on the amount of antigen encountered (11, 32, 33). Because affinity increases with time, one presumes that either high affinity ASCs are selectively surviving or that the production and/or selection of new ASCs is ongoing (33, 34). Long-lived ASCs possess little to no surface Ig and are not responsive to antigen. Therefore, they are unlikely subject to selection (8, 24, 35, 36). In light of the data presented here, it appears that post-GC selection establishes new ASCs important for increases in relative serum affinity. Relative serum affinity was measured in this study using two independent assays. Previous work using the ELISA-based assay demonstrated that affinity measurements correlated with mutations within VDJ genes conferring high affinity binding (37, 38). Both techniques showed that over the course of 16 wk relative serum affinity increased modestly in WT chimeras whereas no statistically significant increase was observed in Cr2−/− chimeras (Fig. 3, Table II). Therefore, our results imply that antigen deposition via CD21/CD35 is important for post-GC selection, a thesis supported by the observation that Cr2−/− chimeras were less able to trap injected antigen (Fig. 5 c). Previous work using LTα-deficient mice concluded that FDCs are not required for affinity maturation (39). However, differences between these results versus those of this study are likely explained by the immunization protocols. Matsumoto et al. (39) immunized with much larger and more frequent doses of antigen in addition to using adjuvant, likely diminishing the importance of antigen deposition on FDCs for affinity maturation.
Memory B Lymphocyte Persistence.
The longevity of memory B lymphocytes is proposed as antigen independent based on an experimental system in which the antigen specificity was switched (40). Additional support for antigen-independent persistence of memory B lymphocytes comes from reports that memory B lymphocytes are generally resting cells without measurable cell proliferation (41). Moreover, they persist in the absence of T cell help (42, 43) or chemokines and chemokine receptors required for FDC development (10, 39). In this study, the frequency of memory B lymphocytes is reduced significantly in WT chimeras not receiving antigen, although low frequencies were detectable predominantly in the spleen (Fig. 2 c). These memory B lymphocytes were donor derived because they were identified using an allo-specific marker and in addition, because irradiated control mice contained a negligible number of memory B lymphocytes. These data are consistent with reports suggesting that at least a fraction of the memory B lymphocyte pool is maintained in the absence of antigen. However, significantly more memory B lymphocytes were detected in WT chimeras receiving antigen, suggesting that a substantial fraction of the memory B lymphocyte pool is replenished with time. These frequencies of memory B lymphocytes detected by FACS® are similar to frequencies reported by Takahashi et al. (44). Similar to differences found with ASCs, optimal memory B lymphocyte frequency depended on stroma-derived CD21/CD35, a finding that was supported by three independent observations (Figs. 2 and 4). The consistent reductions in both ASC and memory B lymphocyte frequency observed between WT and Cr2−/− chimeras with and without antigen, in concert with changes in relative serum affinities, suggest that clonal selection continues after GC wane. Thus, it is suggested that both antigen-dependent and antigen-independent mechanisms drive B lymphocyte memory.
Role of Stroma-derived FcγRIIB.
The importance of Fcγ-RIIB expression on stroma was investigated by comparing memory responses in FcγRIIB−/− chimeras to WT chimeras. It was previously demonstrated that secondary responses in FcγRIIB−/− mice, as well as in Fcγc −/− mice (45), were normal (23). However, because these mice lack FcγRIIB on B lymphocytes and FDCs, these studies were unable to distinguish their relative contributions to the immune response. FcγRIIB is inducible on FDCs (22), making it a candidate for long-term retention of antigen via IgG immune complexes. Myeloid cells (including macrophages, monocytes, neutrophils, and mast cells) also express FcγRIIB along with FcγRI and RIII, and it is therefore possible that other radioresistant cells contribute to long-term antigen retention in the current system (46). Antibody persistence appears independent of FcγRIIB on FDCs because WT and FcγRIIB−/− chimeras had similar antibody titers and BM ASCs. However, both splenic ASC and memory B lymphocyte frequency were reduced in FcγRIIB−/− chimeras, suggesting either that antigen deposition via FcγRIIB is required for post-GC production of both splenic populations or that selection is more stringent in the absence of FcγRIIB (Figs. 1 and 2). Measurements of relative serum affinity seem to favor the latter hypothe-sis because affinity increases dramatically with time in FcγRIIB−/− chimeras (Fig. 3, Table II). One explanation is that selective pressure might be greater in FcγRIIB−/− chimeras due to increased negative signaling on B lymphocytes by unoccupied Fcγ portions of immune complexes not bound by FcγRIIB on FDCs. This possibility is based on the assumption that FcγRIIB on FDCs compete with FcγRIIB on B lymphocytes for binding IgG. Interestingly, FcγRIIB−/− chimeras are also similar to WT chimeras in their ability to trap injected antigen (Fig. 5 b). This might be due to increased trapping of antigen via CD21/CD35, although we did not directly address this possibility. An alternative hypothesis is that FcγRIIB has a signaling role on splenic FDCs that enhances the release of chemokines for retention of memory B lymphocytes and ASCs. FDCs are a source of at least one such chemokine, B lymphocyte chemokine-1, which promotes the localization of B lymphocytes within the splenic follicles (47, 48).
Recall Responses.
B lymphocyte memory is best assessed functionally by measuring the recall response. Similar to findings of Gray and Skarvall (9), efficient recall responses in this study require antigen at the time of adoptive transfer. Despite detectable donor-derived memory B lymphocytes in WT chimeras deprived of antigen, these mice did not generate a significant recall antibody response (Table I). Significantly, Cr2−/− chimeras demonstrated only a twofold increase in antibody titer after challenge whereas titers in WT and FcγRIIB−/− chimeras increased 8–10-fold. Therefore, the recall response was impaired in the absence of CD21/CD35-bearing stroma. This result was additionally substantiated by secondary transfer of splenocytes from WT and Cr2−/− chimeras into WT secondary chimeras (Fig. 4, a and c). It is likely that several factors contributed to diminished recall responses in Cr2−/− chimeras. They had reduced serum antibody titers that potentially contributed to less efficient antigen trapping, as well as lower frequencies of memory B lymphocytes, necessary to mount a recall response. Interestingly, only two- to threefold reductions in ASCs and memory B lymphocytes were observed in Cr2−/− chimeras compared with WT chimeras, yet 10-fold differences in antibody titers were observed. This suggested that memory B lymphocytes in Cr2−/− chimeras were less responsive compared with those in WT chimeras due to less efficient trapping of antigen. This possibility seems likely because memory B lymphocytes from WT mice respond less efficiently after transfer into Cr2−/− recipients. Thus, the combined effects of fewer memory B lymphocytes and less efficient response in the absence of CD21/CD35 would explain the overall 10-fold reduction in antibody titers in Cr2−/− chimeras. Also intriguing is that despite a near normal antibody response in the FcγRIIB−/− chimeras, the frequency of splenic ASCs was significantly reduced both in the primary (Fig. 1 b) and secondary chimeras (Fig. 4 b). Thus, as discussed above, Fcγ-RIIB seems to be required for the maintenance of memory B lymphocytes in the spleen independent of the amount of antigen trapping (Fig. 5 b).
In conclusion, the data presented here support a model whereby humoral memory is maintained by continuous antigen-dependent selection and/or differentiation of memory B lymphocytes and ASCs. FcγRIIB on splenic FDCs (or on radioresistant myeloid cells) also appears important for the maintenance of splenic memory B lymphocytes but is not required for overall memory recall responses. The implication from Cr2−/− chimeras is that ongoing clonal selection depends on antigen retained long-term on CD21/CD35-bearing FDCs. Lastly, the availability of CD21/CD35-bearing stroma provides the appropriate microenvironment for generating substantial recall responses.
Acknowledgments
We thank M. McHeyzer-Williams and D. Driver for helpful advice on NP conjugations and the detection of NP-specific B lymphocytes by FACS®. For critical review of the manuscript, we thank K. Rajewsky, K.L. Knight, and E. Paul.
This work was supported by grants from the National Arthritis Foundation to R.A. Barrington and from the National Institutes of Health to M.C. Carroll (grant no. A139246).
Footnotes
Abbreviations used in this paper: ASC, antibody-secreting cell; BM, bone marrow; FDC, follicular dendritic cell; GC, germinal center; KLH, keyhole limpet hemocyanin; NP, 4-hydroxy-3-nitrophenyl; PNA, peanut agglutinin.
References
- 1.Ahmed, R., and D. Gray. 1996. Immunological memory and protective immunity: understanding their relation. Science. 272:54–60. [DOI] [PubMed] [Google Scholar]
- 2.Talbot, P.J., and M.J. Buchmeier. 1987. Catabolism of homologous murine monoclonal hybridoma IgG antibodies in mice. Immunology. 60:485–489. [PMC free article] [PubMed] [Google Scholar]
- 3.Vieira, P., and K. Rajewsky. 1988. The half-lives of serum immunoglobulins in adult mice. Eur. J. Immunol. 18:313–316. [DOI] [PubMed] [Google Scholar]
- 4.Vieira, P., and K. Rajewsky. 1986. The bulk of endogenously produced IgG2a is eliminated from the serum of adult C57BL/6 mice with a half-life of 6–8 days. Eur. J. Immunol. 16:871–874. [DOI] [PubMed] [Google Scholar]
- 5.Gray, D., and T. Leanderson. 1990. Expansion, selection and maintenance of memory B-cell clones. Curr. Top. Microbiol. Immunol. 159:1–17. [DOI] [PubMed] [Google Scholar]
- 6.Zinkernagel, R.M., M.F. Bachmann, T.M. Kundig, S. Oehen, H. Pirchet, and H. Hengartner. 1996. On immunological memory. Annu. Rev. Immunol. 14:333–367. [DOI] [PubMed] [Google Scholar]
- 7.Slifka, M.K., R. Antia, J.K. Whitmire, and R. Ahmed. 1998. Humoral immunity due to long-lived plasma cells. Immunity. 8:363–372. [DOI] [PubMed] [Google Scholar]
- 8.Manz, R.A., A. Thiel, and A. Radbruch. 1997. Lifetime of plasma cells in the bone marrow. Nature. 388:133–134. [DOI] [PubMed] [Google Scholar]
- 9.Gray, D., and H. Skarvall. 1988. B-cell memory is short-lived in the absence of antigen. Nature. 336:70–73. [DOI] [PubMed] [Google Scholar]
- 10.Karrer, U., C. Lopez-Macias, A. Oxenius, B. Odermatt, M.F. Bachmann, U. Kalinke, H. Bluethmann, H. Hengartner, and R.M. Zinkernagel. 2000. Antiviral B cell memory in the absence of mature follicular dendritic cell networks and classical germinal centers in TNFR1−/− mice. J. Immunol. 164:768–778. [DOI] [PubMed] [Google Scholar]
- 11.Steiner, L.A., and H.N. Eisen. 1967. Sequential changes in the relative affinity of antibodies synthesized during the immune response. J. Exp. Med. 126:1161–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siskind, G.W., and H.N. Eisen. 1965. Effect of variation in antibody-hapten association constant upon the biologic activity of the antibody. J. Immunol. 95:436–441. [PubMed] [Google Scholar]
- 13.MacLennan, I.C. 1994. Germinal centers. Annu. Rev. Immunol. 12:117–139. [DOI] [PubMed] [Google Scholar]
- 14.Futterer, A., K. Mink, A. Luz, M.H. Kosco-Vilbois, and K. Pfeffer. 1998. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 9:59–70. [DOI] [PubMed] [Google Scholar]
- 15.Papamichail, M., C. Gutierrez, P. Embling, P. Johnson, E.J. Holborow, and M.B. Pepys. 1975. Complement dependence of localisation of aggregated IgG in germinal centres. Scand. J. Immunol. 4:343–347. [DOI] [PubMed] [Google Scholar]
- 16.Fischer, M.B., M. Ma, S. Goerg, X. Zhou, J. Xia, O. Finco, S. Han, G. Kelsoe, R.G. Howard, T.L. Rothstein, et al. 1996. Regulation of the B cell response to T-dependent antigens by classical pathway complement. J. Immunol. 157:549–556. [PubMed] [Google Scholar]
- 17.Fischer, M.B., M. Ma, N.C. Hsu, and M.C. Carroll. 1998. Local synthesis of C3 within the splenic lymphoid compartment can reconstitute the impaired immune response in C3-deficient mice. J. Immunol. 160:2619–2625. [PubMed] [Google Scholar]
- 18.Molina, H., V.M. Holers, B. Li, Y. Fung, S. Mariathasan, J. Goellner, J. Strauss-Schoenberger, R.W. Karr, and D.D. Chaplin. 1996. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc. Natl. Acad. Sci. USA. 93:3357–3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guinamard, R., M. Okigaki, J. Schlessinger, and J.V. Ravetch. 2000. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat. Immunol. 1:31–36. [DOI] [PubMed] [Google Scholar]
- 20.Ahearn, J.M., M.B. Fischer, D. Croix, S. Goerg, M. Ma, J. Xia, X. Zhou, R.G. Howard, T.L. Rothstein, and M.C. Carroll. 1996. Disruption of the Cr2 locus results in a re-duction in B-1a cells and in an impaired B cell response to T-dependent antigen. Immunity. 4:251–262. [DOI] [PubMed] [Google Scholar]
- 21.Fang, Y., C. Xu, Y.X. Fu, V.M. Holers, and H. Molina. 1998. Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a strong antigen-specific IgG response. J. Immunol. 160:5273–5279. [PubMed] [Google Scholar]
- 22.Qin, D., J. Wu, K.A. Vora, J.V. Ravetch, A.K. Szakal, T. Manser, and J.G. Tew. 2000. Fc gamma receptor IIB on follicular dendritic cells regulates the B cell recall response. J. Immunol. 164:6268–6275. [DOI] [PubMed] [Google Scholar]
- 23.Takai, T., M. Ono, M. Hikida, H. Ohmori, and J.V. Ravetch. 1996. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 379:346–349. [DOI] [PubMed] [Google Scholar]
- 24.Bolland, S., and J.V. Ravetch. 2000. Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity. 13:277–285. [DOI] [PubMed] [Google Scholar]
- 25.Han, S., B. Zheng, J. Dal Porto, and G. Kelsoe. 1995. In situ studies of the primary immune response to (4-hydroxy-3- nitrophenyl)acetyl. IV. Affinity-dependent, antigen-driven B cell apoptosis in germinal centers as a mechanism for maintaining self-tolerance. J. Exp. Med. 182:1635–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lalor, P.A., G.J. Nossal, R.D. Sanderson, and M.G. McHeyzer-Williams. 1992. Functional and molecular characterization of single, (4-hydroxy-3-nitrophenyl)acetyl (NP)-specific, IgG1+ B cells from antibody-secreting and memory B cell pathways in the C57BL/6 immune response to NP. Eur. J. Immunol. 22:3001–3011. [DOI] [PubMed] [Google Scholar]
- 27.Fischer, M.B., S. Goerg, L. Shen, A.P. Prodeus, C.C. Goodnow, G. Kelsoe, and M.C. Carroll. 1998. Dependence of germinal center B cells on expression of CD21/CD35 for survival. Science. 280:582–585. [DOI] [PubMed] [Google Scholar]
- 28.Ochsenbein, A.F., D.D. Pinschewer, S. Sierro, E. Horvath, H. Hengartner, and R.M. Zinkernagel. 2000. Protective long-term antibody memory by antigen-driven and T help-dependent differentiation of long-lived memory B cells to short-lived plasma cells independent of secondary lymphoid organs. Proc. Natl. Acad. Sci. USA. 97:13263–13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen, Z., S.B. Koralov, M. Gendelman, M.C. Carroll, and G. Kelsoe. 2000. Humoral immune responses in Cr2−/− mice: enhanced affinity maturation but impaired antibody persistence. J. Immunol. 164:4522–4532. [DOI] [PubMed] [Google Scholar]
- 30.Wu, X., N. Jiang, Y.F. Fang, C. Xu, D. Mao, J. Singh, Y.X. Fu, and H. Molina. 2000. Impaired affinity maturation in Cr2−/− mice is rescued by adjuvants without improvement in germinal center development. J. Immunol. 165:3119–3127. [DOI] [PubMed] [Google Scholar]
- 31.Smith, K.G., A. Light, L.A. O'Reilly, S.M. Ang, A. Strasser, and D. Tarlinton. 2000. bcl-2 transgene expression inhibits apoptosis in the germinal center and reveals differences in the selection of memory B cells and bone marrow antibody-forming cells. J. Exp. Med. 191:475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siskind, G.W., P. Dunn, and J.G. Walker. 1968. Studies on the control of antibody synthesis. II. Effect of antigen dose and of suppression by passive antibody on the affinity of antibody synthesized. J. Exp. Med. 127:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eisen, H.N., and G.W. Siskind. 1964. Variations in affinities of antibodies during the immune response. Biochemistry. 3:996–1008. [DOI] [PubMed] [Google Scholar]
- 34.Steiner, L.A., and H.N. Eisen. 1967. The relative affinity of antibodies synthesized in the secondary response. J. Exp. Med. 126:1185–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abney, E.R., M.D. Cooper, J.F. Kearney, A.R. Lawton, and R.M. Parkhouse. 1978. Sequential expression of immunoglobulin on developing mouse B lymphocytes: a systematic survey that suggests a model for the generation of immunoglobulin isotype diversity. J. Immunol. 120:2041–2049. [PubMed] [Google Scholar]
- 36.Halper, J., S.M. Fu, C.Y. Wang, R. Winchester, and H.G. Kunkel. 1978. Patterns of expression of human “Ia-like” antigens during the terminal stages of B cell development. J. Immunol. 120:1480–1484. [PubMed] [Google Scholar]
- 37.Herzenberg, L.A., S.J. Black, and T. Tokuhisa. 1980. Memory B cells at successive stages of differentiation. Affinity maturation and the role of IgD receptors. J. Exp. Med. 151:1071–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Radmacher, M.D., G. Kelsoe, and T.B. Kepler. 1998. Predicted and inferred waiting times for key mutations in the germinal centre reaction: evidence for stochasticity in selection. Immunol. Cell Biol. 76:373–381. [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto, M., S.F. Lo, C.J. Carruthers, J. Min, S. Mariathasan, G. Huang, D.R. Plas, S.M. Martin, R.S. Geha, M.H. Nahm, et al. 1996. Affinity maturation without germinal centres in lymphotoxin-alpha-deficient mice. Nature. 382:462–466. [DOI] [PubMed] [Google Scholar]
- 40.Maruyama, M., K.P. Lam, and K. Rajewsky. 2000. Memory B-cell persistence is independent of persisting immunizing antigen. Nature. 407:636–642. [DOI] [PubMed] [Google Scholar]
- 41.Schittek, B., and K. Rajewsky. 1990. Maintenance of B-cell memory by long-lived cells generated from proliferating precursors. Nature. 346:749–751. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi, Y., P.R. Dutta, D.M. Cerasoli, and G. Kelsoe. 1998. In situ studies of the primary immune response to (4-hydroxy-3- nitrophenyl)acetyl. V. Affinity maturation develops in two stages of clonal selection. J. Exp. Med. 187:885–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vieira, P., and K. Rajewsky. 1990. Persistence of memory B cells in mice deprived of T cell help. Int. Immunol. 2:487–494. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi, Y., H. Ohta, and T. Takemori. 2001. Fas is required for clonal selection in germinal centers and the subsequent establishment of the memory B cell repertoire. Immunity. 14:181–192. [DOI] [PubMed] [Google Scholar]
- 45.Vora, K.A., J.V. Ravetch, and T. Manser. 1997. Amplified follicular immune complex deposition in mice lacking the Fc receptor gamma-chain does not alter maturation of the B cell response. J. Immunol. 159:2116–2124. [PubMed] [Google Scholar]
- 46.Ravetch, J.V., and J.P. Kinet. 1991. Fc receptors. Annu. Rev. Immunol. 9:457–492. [DOI] [PubMed] [Google Scholar]
- 47.Cyster, J.G., K.M. Ansel, K. Reif, E.H. Ekland, P.L. Hyman, H.L. Tang, S.A. Luther, and V.N. Ngo. 2000. Follicular stromal cells and lymphocyte homing to follicles. Immunol. Rev. 176:181–193. [DOI] [PubMed] [Google Scholar]
- 48.Husson, H., S.M. Lugli, P. Ghia, A. Cardoso, A. Roth, K. Brohmi, E.G. Carideo, Y.S. Choi, J. Browning, and A.S. Freedman. 2000. Functional effects of TNF and lymphotoxin alpha1beta2 on FDC-like cells. Cell. Immunol. 203:134–143. [DOI] [PubMed] [Google Scholar]