Abstract
In this study, we investigated the requirement of the Listeria monocytogenes broad-range phospholipase C (PC-PLC) during infection of human epithelial cells. L. monocytogenes is a facultative intracellular bacterial pathogen of humans and a variety of animal species. After entering a host cell, L. monocytogenes is initially surrounded by a membrane-bound vacuole. Bacteria promote their escape from this vacuole, grow within the host cell cytosol, and spread from cell to cell via actin-based motility. Most infection studies with L. monocytogenes have been performed with mouse cells or an in vivo mouse model of infection. In all mouse-derived cells tested, the pore-forming cytolysin listeriolysin O (LLO) is absolutely required for lysis of primary vacuoles formed during host cell entry. However, L. monocytogenes can escape from primary vacuoles in the absence of LLO during infection of human epithelial cell lines Henle 407, HEp-2, and HeLa. Previous studies have shown that the broad-range phospholipase C, PC-PLC, promotes lysis of Henle 407 cell primary vacuoles in the absence of LLO. Here, we have shown that PC-PLC is also required for lysis of HEp-2 and HeLa cell primary vacuoles in the absence of LLO expression. Furthermore, our results indicated that the amount of PC-PLC activity is critical for the efficiency of vacuolar lysis. In an LLO-negative derivative of L. monocytogenes strain 10403S, expression of PC-PLC has to increase before or upon entry into human epithelial cells, compared to expression in broth culture, to allow bacterial escape from primary vacuoles. Using a system for inducible PC-PLC expression in L. monocytogenes, we provide evidence that phospholipase activity can be increased by elevated expression of PC-PLC or Mpl, the enzyme required for proteolytic activation of PC-PLC. Lastly, by using the inducible PC-PLC expression system, we demonstrate that, in the absence of LLO, PC-PLC activity is not only required for lysis of primary vacuoles in human epithelial cells but is also necessary for efficient cell-to-cell spread. We speculate that the additional requirement for PC-PLC activity is for lysis of secondary double-membrane vacuoles formed during cell-to-cell spread.
Listeria monocytogenes is a gram-positive, facultative intracellular bacterial pathogen of humans and a variety of animals. L. monocytogenes can infect a variety of cell types, including macrophages, epithelial cells, fibroblasts, and hepatocytes (57). After entering a host cell, L. monocytogenes promotes its escape from primary single-membrane vacuoles formed during entry, allowing bacteria access to the host cell cytosol. Bacteria replicate within the cytosol and utilize actin-based motility to spread into neighboring cells. This cell-to-cell spreading event results in the formation of secondary double-membrane vacuoles, from which bacteria rapidly escape to gain access to the cytosol of the secondary infected cell, where continued replication occurs (39, 54).
The virulence of L. monocytogenes is directly related to its ability to escape from vacuoles and spread from cell to cell without leaving the intracellular milieu. Many factors required for intracellular growth and spread of L. monocytogenes have been identified, and their roles as virulence determinants have been studied primarily in mouse models of infection (5, 43, 53, 57). In all mouse-derived cells tested, which include both professional and nonprofessional phagocytic cells, the pore-forming cytolysin listeriolysin O (LLO), encoded by hly, is absolutely required for vacuolar lysis (11, 44, 57). In addition to LLO, L. monocytogenes secretes two phospholipases C (PLCs), PI-PLC and PC-PLC, encoded by plcA and plcB, respectively (7, 29, 34, 55). Using L. monocytogenes mutants to infect mouse-derived cell lines, it has been shown that PI-PLC and PC-PLC act synergistically to assist LLO in lysing primary and secondary vacuoles, respectively (17, 53). Interestingly, the absolute requirement of LLO for vacuolar lysis depends on the cell type and species of origin. Previous studies have shown that L. monocytogenes can access the host cell cytosol in the absence of LLO during infection of the human-derived fibroblast cell line WS1, the human-derived epithelial cell line Henle 407, and human-derived dendritic cells (42, 44). Recently, the epithelial cell lines HEp-2 and HeLa have also been identified as human-derived host cells in which LLO is not required for lysis of L. monocytogenes-containing primary vacuoles (24, 40). Prior studies have shown that PC-PLC mediates LLO-independent escape from primary vacuoles in Henle 407 cells (31). PC-PLC is a broad-range PLC that is secreted as an inactive 33-kDa proenzyme and cleaved to an enzymatically active 29-kDa form by the L. monocytogenes secreted metalloprotease Mpl (12, 18, 36, 45, 46). Alternatively, within host cells PC-PLC can be activated by a host-derived vacuolar cysteine protease (32).
Most of the genes required for the intracellular lifestyle of L. monocytogenes, including hly, plcA, and plcB, are clustered within an ∼10-kb region on the bacterial chromosome (43, 56). The plcB gene is cotranscribed with the actA gene, which encodes a bacterial surface protein required for actin-based motility within the host cell cytosol (12, 27, 55). The expression of all virulence genes described above is coordinately regulated by the transcriptional activator PrfA (9, 30, 35). In general, expression of PrfA-regulated genes is low when bacteria are grown in broth culture (48). However, L. monocytogenes strains that synthesize a mutant form of PrfA (PrfA*) maintain constitutive overexpression of PrfA-regulated genes in broth culture (47, 48, 58). Nonetheless, an increase in PrfA-regulated gene expression is seen when bacteria are grown in medium treated with activated charcoal or in tissue culture medium or upon infection of host cells (15, 38, 48, 50). However, the extent and timing of the PrfA-mediated increase in gene expression varies for individual virulence genes (3, 6, 38, 49). For example, during intracellular infection of the mouse macrophage-like cell line J774, transcription from the PrfA-regulated hly gene promoter is induced ∼20-fold, whereas the actA-plcB promoter is induced ∼200-fold in the cytosol compared to growth in broth culture (38). However, most virulence gene expression studies have been performed with mouse-derived cell lines, and thus differences in PrfA-regulated virulence gene expression may occur during infection of host cells derived from other species (6).
We sought to determine the requirement of PC-PLC throughout intracellular infection of human-derived cells in the absence of LLO. Since the majority of studies leading to our current understanding of the roles of LLO and PC-PLC during intracellular infection have been based on mouse models of infection, the requirement of PC-PLC for optimal intracellular growth and cell-to-cell spread during infection of human cells may have been underestimated. To address these questions, we have removed transcriptional control of plcB from its native PrfA-dependent promoter and placed transcription of plcB under an inducible control mechanism. This system allowed for regulated expression of PC-PLC when bacteria were grown in broth culture or during infection of host cells. Using L. monocytogenes strains that expressed various amounts of PC-PLC, we found that in the absence of LLO the amount of PC-PLC activity is critical for the efficiency of lysis of primary vacuoles in human-derived epithelial cells. Our results indicated that, in an LLO-negative derivative of L. monocytogenes strain 10403S, expression of PC-PLC from its native promoter has to increase compared to expression in broth culture to allow bacterial escape from primary vacuoles. Furthermore, by shutting off PC-PLC expression after LLO-negative bacteria have entered the host cell cytosol, we show that after escape from primary vacuoles, PC-PLC activity is required for facilitating cell-to-cell spread during infection of human epithelial cells.
MATERIALS AND METHODS
Bacterial and eukaryotic cell growth conditions.
The bacterial strains used in the present study are listed in Table 1. L. monocytogenes strains were grown in brain heart infusion (BHI) medium (Difco, Detroit, Mich.). Escherichia coli strains were grown in Luria-Bertani (LB) medium at 37°C with shaking. All bacterial strains were stored at −80°C in BHI or LB medium with 40% glycerol. Antibiotics were used at the following concentrations: ampicillin at 100 μg/ml; chloramphenicol at 20 μg/ml for selection of pAM401 derivatives and pPL2 derivatives in E. coli, at 10 μg/ml for selection of pAM401 derivatives in L. monocytogenes, and at 7.5 μg/ml for selection of integrated pPL2 derivatives in L. monocytogenes; kanamycin at 30 μg/ml; streptomycin at 200 μg/ml; and nalidixic acid at 40 μg/ml. Host cells were infected in the absence of antibiotic selection. The human-derived epithelial cell lines Henle 407 (American Type Culture Collection [ATCC] CCL-6), HeLa (ATCC CCL-2), and HEp-2 (ATCC CCL-23) were propagated in RPMI 1640 l-glutamine medium (Mediatech, Herndon, Va.) supplemented with 10% FBS (HyClone, Logan, Utah), 55 μM 2-mercaptoethanol, 1 mM sodium pyruvate, and 2 mM glutamine. Tissue culture cells were maintained at 37°C in a 5% CO2-air atmosphere.
TABLE 1.
Strains and plasmids
| Strain | Genotype and relevant featuresa | Source or reference |
|---|---|---|
| L. monocytogenes | ||
| 10403S | Wild-type strain (PrfA) | 2 |
| NF-L476 | 10403S actA:gus:plcB | 50 |
| DP-L2161 | 10403S Δhly | 25 |
| DP-L2318 | 10403S Δhly ΔplcB | 31 |
| DP-L3078 | 10403S ΔactA | 52 |
| DH-L616 | DP-L2161 i-hly | 11 |
| DH-L718 | DP-L2318 i-plcB | This study |
| DH-L726 | DP-L2318 pPL2 | This study |
| DH-L727 | DP-L2161 pPL2 | This study |
| DH-L728 | DP-L2161 pAMspacOid | This study |
| DH-L729 | DP-L2318 pAMspacOid | This study |
| DH-L824 | DP-L2318 pAMiplcB | This study |
| SLCC-5764 | Wild-type strain (PrfA*) | 8 |
| DH-L377 | SLCC-5764 Δhly | This study |
| DH-L419 | SLCC-5764 Δhly ΔplcB | This study |
| DH-L683 | DH-L377 pAMspacOid | This study |
| DH-L687 | DH-L419 pAMspacOid | This study |
| DH-L693 | DH-L419 pAMspac-plcB | This study |
| DH-L699 | DH-L419 i-plcB | This study |
| DH-L735 | DH-L419 pAMiplcB | This study |
| DH-L858 | DH-L377 i-hly | This study |
| E. coli | ||
| DH-E123 | pCON1 in JM109 | 14 |
| DH-E182 | XL1-Blue [F′ proAB lacIq Δ(lacZ)M15 Tn10] recA1 endA1 gyrA96 thi-1 hsdR17 supE relA1 lac | Stratagene |
| DH-E375 | CLG190 (F′ lac pro lacIq) Δ(malF)3 Δ(phoA) PvuII phoR Δ(lac)X74 Δ(ara leu)7697 araD139 galE galK pcnB zad::Tn10 recA; Strr | D. Boyd |
| DH-E384 | pLIV1 in E. coli K-12 dam− recA::Cam | 11 |
| DH-E474 | SM10 {F−thi-1 thr-1 leuB6 recA tonA21 lacY1 supE44 Mu+C λ− [RP4-2 (Tc::Mu)] Kmrtra+} | 51 |
| DH-E487 | pCON1Δhly in XL1-Blue | This study |
| DH-E585 | pPL2 in SM10 | 28 |
| DH-E618 | pPL2-i-hly in SM10 | 11 |
| DH-E659 | pAMspacOid in XL1-Blue | This study |
| DH-E668 | pTrc99A in XL1-Blue | Pharmacia |
| DH-E716 | pPL2-i-plcB in SM10 | This study |
| DH-E723 | pPL2-PactA:plcB in SM10 | This study |
| DH-E733 | pPL2-SPO-1-lacI in SM10 | This study |
| DH-E739 | pLIV1-plcB in CLG190 | This study |
| DH-E784 | pPL2-SPO-1 in SM10 | This study |
| DP-E1888 | pDP1888 (pKSV7ΔplcB) in DH5α | 53 |
| DP-E2316 | pAM401 in E. coli K-12 | 60 |
Kmr, kanamycin resistance; strr, streptomycin resistance.
Plasmid and strain construction.
PFU polymerase (Stratagene, La Jolla, Calif.) was used for PCRs when DNA fragments were subsequently used for plasmid construction. All other enzymes were purchased from New England Biolabs (Beverly, Mass.) and used according to the manufacturer's instructions. Plasmids with plcB-containing inserts were initially cloned in E. coli CLG190, with the exception of plasmids pAMspac-plcB and pAMiplcB, which were cloned in E. coli CLG190 containing plasmid pTrc99A as an additional source of the Lac repressor protein. E. coli strain XL1-Blue was used as a cloning strain for all other plasmid ligations.
Construction of SLCC-5764-derived strains containing in-frame deletions in hly or hly plus plcB.
The primer pair For_1kb_hly (XbaI) CCTCTAGACGGGGAAGTCCATGATTAGTATGCC and Rev_1kb_hly (EcoRI) TGGAATTCGCAATCGGTTGGCTCCTTTACCAAGCG and chromosomal DNA of strain DP-L2161 were used to amplify by PCR the hly gene containing an in-frame deletion. Relevant restriction sites in primer sequences are underlined in the text. The resulting PCR product was cut with the restriction enzymes XbaI and EcoRI and ligated with the allelic exchange vector pCON1 (14), which had been cut with the same enzymes. The resulting plasmid was named pCON1Δhly and used to create strain DH-L377 (SLCC-5764 Δhly). Allelic exchange was performed essentially as previously described (8); however, a chloramphenicol concentration of 5 μg/ml was used to select for plasmid integration. The allelic exchange vector pDP1888 (53), containing a large in-frame deletion in plcB, was used for allelic exchange in strain DH-L377 to create strain DH-L419 (SLCC-5764 Δhly ΔplcB), containing in-frame deletions in hly and plcB.
Construction of L. monocytogenes strains for single-copy inducible expression of LLO and PC-PLC.
Chromosomal DNA of strain DP-L3078 containing a large in-frame deletion in actA was used as a template to amplify by PCR the plcB gene, lacking the actA-plcB promoter, with the primer pair 5′actA (XbaI) GCTCTAGAAACGGAATAATTAGTG and 3′plcB (XbaI) CGTCTAGAGCTAACGAGTGGATAAGAATGTATTCCT. The resulting PCR product was cut with XbaI and ligated with the inducible expression vector pLIV1 (11), which was linearized with XbaI. The correct orientation of the insert, in which transcription of plcB was placed under inducible SPAC/lacOid promoter/operator control, was determined by PCR. The resulting vector for inducible expression of PC-PLC was named pLIV1-plcB. Next, pLIV1-plcB was cut with KpnI, and the KpnI fragment harboring the inducible expression cassette was cloned into the unique KpnI site of the site-specific integration vector pPL2 (28). The plasmid, in which inducible plcB (i-plcB) was transcribed in the same direction as the gram-negative and gram-positive cat genes of pPL2, was named pPL2-i-plcB and was used for further analysis after verification of the correct promoter and plcB gene sequence by automated fluorescence sequencing. Site-specific integration was performed as described previously (28) with plasmid pPL2-i-plcB and strain DP-L2318 (10403S Δhly ΔplcB). The resulting strain yielding single-copy inducible expression of PC-PLC was named DH-L718 (10403S Δhly ΔplcB i-plcB). Strain DH-L699 (SLCC-5764 Δhly ΔplcB i-plcB) was constructed by integrating pPL2-i-plcB into the chromosome of strain DH-L419 (SLCC-5764 Δhly ΔplcB), followed by selection on BHI plates containing 40 μg of nalidixic acid and 7.5 μg of chloramphenicol/ml. Strain DH-L858 (SLCC-5764 Δhly i-hly) was constructed by integrating the previously described pPL2-derived vector pDH618 (11) containing the inducible LLO expression cassette into the tRNAArg gene of strain DH-L377 (SLCC-5764 Δhly).
Construction of plasmid pAMiplcB for multicopy inducible expression of PC-PLC in L. monocytogenes.
Initially, the SPAC/lacOid promoter/operator region of plasmid pLIV1 (11) was cloned into the multicopy E. coli-L. monocytogenes shuttle vector pAM401 (60). The primer pair 5′-EcoRV-spac AAGATATCCTAACAGCACAAGAGCGGAAAG and 3′-XbaI dam− pLIV1 ACTTTAGGTCGACTCTAGAACACCTCCTTAAGC was used to amplify the SPAC/lacOid promoter region from plasmid pLIV1. The resulting PCR product was cut with EcoRV and XbaI and cloned into pAM401, which had been cut with the same enzymes. The resulting plasmid was named pAMspacOid. Next, chromosomal DNA of strain DP-L3078 containing a large in-frame deletion in actA and the primer pair 5′ actA (XbaI) GCTCTAGAAACGGAATAATTAGTG and 3′ plcB (XbaI) CGTCTAGAGCTAACGAGTGGATAAGAATGTATTCCT were used to amplify the plcB gene lacking the actA-plcB promoter. The resulting PCR product was cut with XbaI and ligated with the XbaI-linearized plasmid pAMspacOid. Orientation of inserts was determined by PCR analysis and a plasmid in which plcB was orientated to place transcription under SPAC/lacOid promoter control was named pAMspac-plcB. For construction of the inducible pAMiplcB plasmid vector, the E. coli lacI gene was initially cloned under SPO-1 promoter control into plasmid pPL2-SPO-1. The 5′ phosphorylated primer pair 5′SacI spac (−40-+1) EagI P-CAATTTTGCAAAAAGTTGTTGACTTTATCTACAAGGTGTGGCATAATGTGTGGC and 3′SacI spac (−40-+1) EagI P-GGCCGCCACACATTATGCCACACCTTGTAGATAAAGTCAACAACTTTTTGCAAAATTGAGCT containing the SPO-1 promoter sequence was hybridized and ligated with vector pPL2, which had been cut with SacI and EagI. The resulting plasmid was named pPL2-SPO-1. Next, the lacI gene was amplified from plasmid pLIV1 with the primer pair 5′PstI-lacI AACTGCAGATTCAAACGGAGGGAGACGATTTTGATG and 3′ SalI-lacI ACGCGTCGACCGCTCACTGCCCGCTTTCCAGTCGGG. The PCR product was cut with PstI and SalI and ligated with the plasmid pPL2-SPO-1, which had been cut with the same enzymes. The resulting plasmid was named pPL2-SPO-1-lacI. Plasmid pPL2-SPO-1-lacI was used as a template to amplify the SPO-1-lacI fragment with the primer pair 5′SphI-spac ACATGCATGCTGGAGCTCAATTTTGCAAAAAGTTGTTGAC and 3′NruI-lacI ACGCTCGCGACGCTCACTGCCCGCTTTCCAGTCGGG. The PCR product was digested with NruI and SphI and ligated with the plasmid pAMspac-plcB, which had been cut with the same enzymes. Correct promoter and plcB sequence was confirmed by automated fluorescence sequencing, and the resulting plasmid was named pAMiplcB. L. monocytogenes strains DP-L2318 (10403S Δhly ΔplcB) and DH-L419 (SLCC-5764 Δhly ΔplcB) were transformed by electroporation (41) with plasmid pAMiplcB, resulting in strains DH-L824 and DH-L735, respectively.
Hemolytic activity assay.
L. monocytogenes overnight cultures were diluted 1:10 into fresh BHI medium, which was supplemented with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for strains containing the inducible LLO expression cassette, and then grown 5 h at 37°C with shaking. Hemolytic activities were determined as previously described (11, 44). Hemolytic units were defined as the reciprocal of the culture supernatant dilution that yielded 50% lysis of sheep red blood cells.
PC-PLC activity assay.
L. monocytogenes strains were grown overnight in 2 to 3 ml of BHI medium, diluted 1:10 into fresh BHI medium with or without IPTG at the indicated concentration, and grown for 5 h at 37°C with shaking. The optical densities at 600 nm were determined to confirm that cultures had reached similar densities. Proteins from culture supernatants were precipitated on ice in the presence of 10% trichloroacetic acid (TCA), resuspended in 1% of the original volume in 1× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer containing 0.2 N NaOH, and separated on 10% SDS-PAGE gels. PC-PLC activities were detected as previously described by using an egg yolk overlay of SDS-PAGE gels, and activities were seen as zones of opacity (26, 32). Figures are presented in negative contrast for clarity.
GUS activity assays.
An overnight culture of L. monocytogenes strain NF-L476 (10403S actA:gus:plcB) was diluted 1:10 into fresh BHI medium and grown for 3 h at 37°C with shaking. A 1-ml aliquot was removed and centrifuged for 5 min at 16,000 × g to collect bacteria. The pellet was resuspended in 100 μl of ABT assay buffer (0.1 M potassium phosphate [pH 7.0], 0.1 M NaCl, 0.1% Triton X-100) and then quick-frozen in an ethanol dry ice bath and stored at −80°C to allow determination of β-Glucuronidase (GUS) activity values at a later time. Dilutions of the bacterial culture were plated on BHI plates to determine the CFU per milliliter of culture. In addition, 2 ml of culture was collected by centrifugation, washed once with phosphate-buffered saline (PBS) buffer, resuspended in 10 ml of RPMI 10% FBS tissue culture medium, and incubated for 2 h at 37°C in a 5% CO2-air atmosphere. After this incubation, samples were removed to determine the CFU per milliliter and then prepared and frozen for the determination of GUS activity values as described above. GUS activities were determined as previously described (61) with some modifications. Bacterial pellets were thawed and adjusted to 108 bacteria per 50 μl of ABT buffer for BHI grown bacteria and to 107 bacteria per 50 μl of ABT buffer for RPMI-grown bacteria and then mixed with 10 μl of 4-methylumbilliferyl-β-d-glucuronide at a concentration of 0.4 mg/ml. Samples were incubated at room temperature for 80 min. After this incubation, 4 μl were removed and diluted into 196 μl of ABT assay buffer, and fluorescence values were determined by using a SpectraMAX GeminiXS instrument (Molecular Devices) at excitation and emission wavelengths of 366 and 445 nm, respectively. Known concentrations of the fluorescent 4-methylumbelliferone product ranging from 15.6 to 4000 nM were used to obtain a standard curve. GUS activities are given in picomoles of product formed per minute per 106 bacteria. Means and standard deviations of four independently grown cultures were determined.
Intracellular growth assay in human epithelial cells.
A total of 1.5 × 106 to 2.0 × 106 host cells were seeded 1 day prior to infection in 60-mm-diameter culture dishes containing 12-mm-diameter round glass coverslips. Before seeding HeLa and HEp-2 cells, coverslips were treated for 1 h at room temperature with 6 ml of 0.02 N acetic acid containing 10 μg of rat tail collagen (BD Biosciences, Bedford, Mass.)/ml. L. monocytogenes strains were grown overnight in 2 to 3 ml of BHI medium at 30°C without shaking. L. monocytogenes overnight cultures grown under these conditions contained ∼2 × 109 bacteria/ml. Bacterial cultures were washed once with PBS (pH 7.1) and used to infect monolayers of host cells at a multiplicity of infection (MOI) of 50:1 (bacterium/host cell ratio) or of 67:1 in RPMI-10% FBS medium. Alternatively, bacterial overnight cultures were diluted 1:10 into fresh BHI medium containing IPTG at the indicated concentration and grown for 2 h at 37°C with shaking. Dilutions of these mid-log-phase cultures were plated on BHI plates, and it was determined that an optical density at 600 nm of 0.4 corresponds to ∼5 × 108 bacteria/ml. Aliquots of these mid-log-phase cultures corresponding to ∼5 × 108 bacteria were centrifuged for 5 min at 16,000 × g, and the bacterial pellets were resuspended in 100 μl of PBS. These bacterial suspensions were used to infect host cell monolayers at the indicated MOI. At 1 h after infection, monolayers were washed three times with PBS buffer, and RPMI-10% FBS medium containing 50 μg of gentamicin/ml was added. The numbers of CFU per coverslip were determined at the time points indicated in each figure by placing coverslips, in triplicate, into 15-ml conical tubes containing 5 ml of sterile water and then vortexing and plating appropriate dilutions onto LB agar plates.
Plaquing assay in Henle 407 cells.
At 1 day prior to infection, 1.2 × 106 to 1.5 × 106 Henle 407 cells were seeded in each well of six-well dishes. L. monocytogenes overnight cultures were diluted 1:10 into fresh BHI medium with or without 1 mM IPTG and then grown for 2 h at 37°C with shaking. Approximately 5 × 108 bacteria were centrifuged for 5 min at 16,000 × g, and bacterial pellets were resuspended in 100 μl of PBS. Then, 2 μl of a 1:50 dilution was used to infect monolayers of Henle 407 cells in 3 ml of RPMI-10% FBS (heat-inactivated and dialyzed) medium with or without 0.01, 0.1, or 1 mM IPTG. At 1 h after infection, monolayers were washed twice with cold PBS and then overlaid with an agarose-medium mixture containing 0.7% agarose, 1× Dulbecco modified Eagle medium, 5% FBS (heat inactivated and dialyzed), 10 μg of gentamicin/ml, and IPTG at the concentrations described above. At 4 days after infection, a second agarose-medium overlay was applied that contained 187 μg of neutral red/ml, 10 μg of gentamicin/ml, and IPTG at the concentrations described above in 1× Dulbecco modified Eagle medium. The following day, plates were scanned to digital images, and the diameters of 15 plaques per well were determined by using Adobe Photoshop 6.0 software.
Vacuolar lysis assay.
One day prior to infection, 5 × 105 Henle 407 cells were seeded onto 18-mm-square glass coverslips placed in the wells of a six-well dish. L. monocytogenes strains were grown for 2 h in BHI in the absence or presence of 1 or 10 mM IPTG and then prepared for infections as described for the plaquing assays. Henle 407 cells were infected at an MOI of 100:1 in the absence or presence of 1 or 10 mM IPTG. At 1 h after infection, host cells were washed three times with PBS, and RPMI-10% FBS medium containing 50 μg of gentamicin/ml was added. At 2 h after infection, monolayers were washed three times with PBS and then fixed in PBS containing 3.2% paraformaldehyde. The percentage of bacteria that had escaped from primary vacuoles and were surrounded with actin filaments was determined by immunofluorescence staining as described previously (25). Next, 50 to 150 bacteria were analyzed for each sample, and the percent vacuolar lysis was calculated by dividing the number of cytosolic bacteria by the total number of bacteria analyzed per sample and multiplying that value by 100.
Homologous Henle 407 to Henle 407 cell-to-cell spreading assay.
A total of 1.5 × 106 Henle 407 cells were seeded in 60-mm-diameter dishes as primary cells or seeded in wells of six-well plates with or without 18-mm-square coverslips as secondary cells for immunofluorescence and plaquing analysis, respectively. Strain DH-L735 (SLCC-5764 Δhly ΔplcB, pAMiplcB) was grown for 2 h at 37°C with or without 1 mM IPTG and prepared for infections as described for plaquing assays. Primary Henle 407 cells were infected at an MOI of 200:1 with or without 1 mM IPTG in RPMI-10% FBS (heat-inactivated and dialyzed) medium. At 1 h after infection, monolayers were washed three times with PBS, and serum-free RPMI medium containing 50 μg of gentamicin and 2 μg of Cell Tracker Blue (Molecular Probes, Eugene, Oreg.)/ml was added to differentially label primary cells. At 1.5 h after infection, monolayers were washed three times with PBS to remove excess CellTracker, and serum-containing medium supplemented with 50 μg of gentamicin/ml was added. At 2 h postinfection, host cells were removed from dishes and counted, and 1,000 Henle 407 cells (primary CellTracker Blue-labeled cells) were placed in duplicate on monolayers of uninfected Henle 407 cells (secondary unlabeled cells) in the presence of 1 mM IPTG. Alternatively, 5,000 primary Henle 407 cells were placed in duplicate on monolayers of secondary Henle 407 cells in the absence of IPTG. To determine the number of primary Henle 407 cells that initially contained bacteria in the cytosol, secondary monolayers, which had been seeded on coverslips, were fixed 8 h after the primary infected Henle 407 cells were places onto the secondary monolayer. Fixed samples were prepared for immunofluorescence microscopy as described for vacuolar lysis assays, and the numbers of primary infected host cells (CellTracker Blue labeled) containing bacteria surrounded with actin filaments, and therefore in the cytosol, were determined by visually scanning the 18-mm-square coverslip. For plaquing assays, secondary monolayers were overlaid 2 h after primary infected cells were placed onto secondary cell monolayers with an agarose-medium mixture (see plaquing assay) containing 10 μg of gentamicin/ml with or without 1 mM IPTG. At 4 days after infection, a second agarose-medium overlay containing 187 μg of neutral red/ml and 10 μg of gentamicin/ml with or without 1 mM IPTG was added. Images of plaques were obtained after overnight incubation.
Henle 407 cell infection for 24 h.
A total of 106 Henle 407 cells were seeded into each well of a six-well dish containing an 18-mm-square coverslip. Strain DH-L735 (SLCC-5764 Δhly ΔplcB, pAMiplcB) was grown for 2 h in BHI medium containing 1 mM IPTG and then prepared for infections as described for the plaquing assays. Henle 407 cells were infected at MOIs of 1:1 or 100:1 in the presence or absence of 1 mM IPTG, respectively. After 1 h of infection, monolayers were washed three times with PBS, and RPMI-10% FBS medium with or without 1 mM IPTG containing 30 μg of gentamicin/ml was added. At 24 h after infection, coverslips were removed, stained with Diff-Quik (DADE-Behring), and analyzed by light microscopy.
Nucleotide sequence accession numbers.
The DNA sequence of the prfA* allele of strain SLCC-5764 was determined by automated fluorescence sequencing at the Dana-Farber-Harvard Cancer Center High-Throughput DNA Sequencing Facility and deposited in the EMBL/GenBank/DDBJ databases under accession number AY318750.
RESULTS
In the absence of LLO, PC-PLC is required for vacuolar lysis in HEp-2 and HeLa cells.
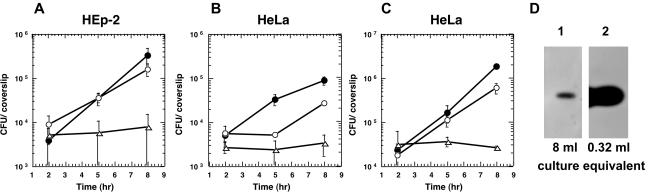
During infection of the human-derived epithelial cell line Henle 407, PC-PLC promotes lysis of primary vacuoles in the absence of LLO (31). Previous studies have shown that LLO-negative L. monocytogenes strains can also escape from primary vacuoles in the human-derived epithelial cell lines HEp-2 and HeLa (24, 40). However, an L. monocytogenes strain with deletions of LLO and both phospholipases, PI-PLC and PC-PLC, fails to escape from the primary vacuole in these cells (24, 40). Here, we set out to determine whether PC-PLC is specifically required for lysis of HeLa and Hep-2 cell primary vacuoles in the absence of LLO. We performed intracellular growth assays (gentamicin protection assays) in HEp-2 and HeLa cells with the wild-type L. monocytogenes strain 10403S and the isogenic LLO-negative (DP-L2161) or LLO-, PC-PLC-negative (DP-L2318) strains. Only bacteria that are able to lyse primary vacuoles and access the cytosol can grow within the host cell, leading to an increase in the number of intracellular gentamicin-protected bacteria. As expected, the LLO-negative strain was able to escape from primary vacuoles and grow within the host cell cytosol of both HEp-2 and HeLa cell lines (Fig. 1A and B). However, an LLO-, PC-PLC-negative L. monocytogenes strain was unable to grow within HEp-2 or HeLa cells. This indicated that in the absence of LLO, PC-PLC is required for lysis of primary vacuoles of HEp-2 and HeLa cells (Fig. 1A and B), and it therefore may be a general phenomenon that PC-PLC can promote vacuolar lysis in human epithelial cells. Interestingly, we observed a delay in the initiation of intracellular growth when HeLa cells were infected with the LLO-negative DP-L2161 strain. An increase in the number of intracellular bacteria was not detected until after 5 h postinfection. This lag in the initiation of intracellular growth was not seen with an LLO-negative derivative of L. monocytogenes strain SLCC-5764 (Fig. 1C), which contains the prfA* allele, resulting in increased expression of PrfA-regulated virulence genes in broth culture (47, 48, 58). As shown in Fig. 1D, a drastic increase in PC-PLC activity was detected in culture supernatants of strain DH-L377 (PrfA*) compared to strain DH-L2161 (PrfA). This increase in PC-PLC activity correlated with an increase in PC-PLC protein level as detected by Western blotting (data not shown). This result suggested that the efficiency of primary vacuolar lysis is dependent on PC-PLC activity levels.
FIG. 1.
Intracellular growth in human epithelial cells and PC-PLC activity. (A) Monolayers of HEp-2 cells were infected at an MOI of 50:1 with strains 10403S (•), DP-L2161 (10403S Δhly) (○), and DP-L2318 (10403S Δhly ΔplcB) (▵). Intracellular growth was determined as described in Materials and Methods. (B) Monolayers of HeLa cells were infected at an MOI of 67:1 with strains 10403S (•), DP-L2161 (10403S Δhly) (○), and DP-L2318 (10403S Δhly ΔplcB) (▵). (C) Monolayers of HeLa cells were infected at an MOI of 67:1 with PrfA* strains SLCC-5764 (•), DH-L377 (SLCC-5764 Δhly) (○), and DH-L419 (SLCC-5764 Δhly ΔplcB) (▵). The data points in growth curves represent the means ± the standard deviations of three coverslips from one of two experiments. (D) Overnight cultures of strains DP-L2161 (10403S Δhly) and DH-L377 (SLCC-5764 Δhly) were diluted 1:10 in BHI medium and grown for 5 h at 37°C. Proteins from culture supernatants were TCA precipitated and separated by SDS-PAGE. PC-PLC activities were determined by using an egg yolk overlay assay as described in Materials and Methods. Lane 1, the equivalent of 8 ml of DP-L2161 culture supernatant was loaded; lane 2, the equivalent of 0.32 ml of DH-L377 culture supernatant was loaded (1/25 the amount of lane 1).
SPAC/lacOid-regulated gene expression is neither PrfA nor background strain dependent.
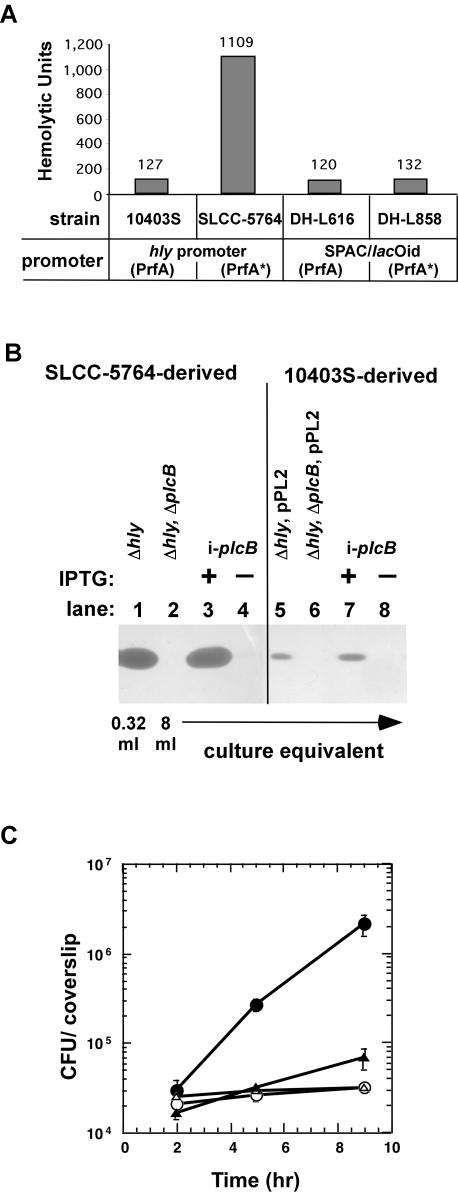
In addition to lysis of the primary vacuole in the absence of LLO, we sought to determine the requirement of PC-PLC for the intracellular growth and spread of L. monocytogenes during infection of human epithelial cells. We have recently developed an inducible expression system for determining the temporal requirement of virulence factors during intracellular infection by L. monocytogenes (11). The inducible expression system allows transcription of a virulence gene to be removed from the normal bacterial control mechanism and placed under the control of an IPTG-inducible promoter, yielding IPTG dose-dependent expression of virulence genes during intracellular infection. Using this system, the inducible virulence gene is placed in an ectopic location on the chromosome within a L. monocytogenes strain containing an in-frame deletion of the native virulence gene. In a previous study, transcription of hly was removed from the native PrfA-dependent control and placed under control of the inducible SPAC/lacOid promoter/operator within the tRNAArg locus (11). To confirm that expression of L. monocytogenes virulence genes controlled by SPAC/lacOid within the tRNAArg locus is indeed PrfA and background strain independent, we compared LLO expression resulting from native and SPAC/lacOid promoter control in L. monocytogenes strains 10403S (PrfA) and SLCC-5764 (PrfA*). As previously mentioned, SLCC-5764 contains a mutation within prfA, leading to increased expression of PrfA-regulated virulence genes in broth culture. LLO expression can be easily detected by measuring the hemolytic activity of culture supernatants by means of a sheep red blood cell lysis assay (44). Furthermore, hemolytic activity has been shown to strictly correlate with LLO protein levels as determined by Western blot analysis (11). As previously reported, similar hemolytic activities were observed in culture supernatants of 10403S strains in which LLO was expressed from the native hly promoter or the inducible SPAC/lacOid promoter in the presence of 1 mM IPTG (Fig. 2A, compare 10403S and DH-L616). An ∼10-fold-higher hemolytic activity was observed when LLO was expressed from the native PrfA*-activated hly promoter in SLCC-5764 compared to the native PrfA-activated hly promoter in 10403S or to the inducible SPAC/lacOid promoter in the 10403S background (Fig. 2A, compare SLCC-5764 to 10403S and DH-L616). However, similar hemolytic activities were obtained when LLO was expressed from the inducible SPAC/lacOid promoter in the 10403S (PrfA) or SLCC-5764 (PrfA*) strains (Fig. 2A, compare DH-L616 and DH-L858), indicating that LLO expression from the inducible promoter is indeed PrfA and background strain independent.
FIG. 2.
Inducible expression of LLO and PC-PLC in L. monocytogenes. (A) Hemolytic activity assay. Hemolytic activities were determined from culture supernatants of L. monocytogenes as described in Materials and Methods. LLO was expressed from the inducible SPAC/lacOid promoter in the presence of 1 mM IPTG or from the native PrfA- or PrfA*-regulated hly promoter in strains 10403S and SLCC-5764, respectively. (B) PC-PLC activity assay. PC-PLC was expressed under the control of the inducible SPAC/lacOid promoter (i-plcB) or the native PrfA-regulated actA-plcB promoter in the L. monocytogenes10403S (PrfA) and SLCC-5764 (PrfA*) backgrounds. Overnight cultures were diluted 1:10 in BHI medium and grown for 5 h at 37°C in the presence or absence of 1 mM IPTG. Culture supernatants were TCA precipitated, and an equivalent of 8 or 0.32 ml of culture supernatant was separated by SDS-PAGE. PC-PLC activities were detected as described in Materials and Methods. Lane 1, DH-L377 (SLCC-5764 Δhly); lane 2, DH-L419 (SLCC-5764 Δhly ΔplcB); lane 3, DH-L699 (SLCC-5764 Δhly ΔplcB i-plcB) with 1 mM IPTG; lane 4, DH-L699 (SLCC-5764 Δhly ΔplcB i-plcB) without IPTG; lane 5, DH-L727 (10403S Δhly, pPL2); lane 6, DP-L726 (10403S Δhly ΔplcB, pPL2); lane 7, DH-L718 (10403S Δhly ΔplcB i-plcB) with 1 mM IPTG; lane 8, DH-L718 (10403S Δhly ΔplcB i-plcB) without IPTG. (C) Intracellular growth in Henle 407 cells. Overnight cultures of L. monocytogenes strains were diluted 1:10 in BHI medium and grown for 2 h at 37°C in the presence or absence of 10 mM IPTG. Monolayers of Henle 407 cells were infected at an MOI of 50:1, and intracellular growth was measured in the presence or absence of 10 mM IPTG as described in Materials and Methods. Symbols: •, DH-L377 (SLCC-5764 Δhly); ○, DH-L419 (SLCC-5764 Δhly ΔplcB); ▵, DH-L699 (SLCC-5764 Δhly ΔplcB i-plcB) without IPTG; ▴, DH-L699 (SLCC-5764 Δhly ΔplcB i-plcB) with 10 mM IPTG. The data points in growth curves represent the means ± standard deviations of three coverslips from one of two experiments.
Inducible PC-PLC expression in strains 10403S (PrfA) and SLCC-5764 (PrfA*).
To determine the requirement of PC-PLC for the intracellular growth and spread of L. monocytogenes during infection of human epithelial cells, we placed the transcription of plcB under control of the SPAC/lacOid promoter on the chromosome of LLO-, PC-PLC-negative L. monocytogenes strains. In both 10403S (PrfA) and SLCC-5764 (PrfA*) background strains, IPTG-dependent PC-PLC activities were detected by using the inducible expression system (Fig. 2B). The inducible PC-PLC activity obtained from the SPAC/lacOid promoter in the 10403S background was similar to that observed when plcB was transcribed from the native PrfA-dependent promoter in 10403S (Fig. 2B, compare lanes 5 and 7). Significantly higher PC-PLC activity (∼25-fold) was detected when plcB was transcribed from the native PrfA* activated promoter than from the inducible SPAC/lacOid promoter in the SLCC-5764 background strain (Fig. 2B, compare lanes 1 and 3; note that 25-fold-less protein was loaded in lane 1). However, despite detecting similar levels of plcB specific transcripts (data not shown), significantly higher PC-PLC activity was detected when plcB was transcribed from the inducible SPAC/lacOid promoter in the SLCC-5764 (PrfA*) background than in the 10403S (PrfA) background (Fig. 2B, compare lanes 3 and 7). We reasoned that this increase in PC-PLC activity was due to PrfA-dependent posttranscriptional regulation, most likely at the level of proteolytic activation of proPC-PLC by Mpl. Indeed, we detected significantly higher amounts of Mpl protein in the supernatants of SLCC-5764-derived strains compared to those of 10403S-derived strains (data not shown). Taken together, our results suggested that the amount or activity of Mpl (or other PrfA-regulated gene products) limits PC-PLC activity in strain 10403S when grown in broth culture.
Single-gene-copy, inducible PC-PLC expression does not allow complementation of PC-PLC activity within host cells.
We initially confirmed that PC-PLC activity could be complemented during infection of human epithelial cells when PC-PLC is expressed from its native promoter from the tRNAArg locus (data not shown). Next, we determined whether inducible PC-PLC expression would allow complementation of PC-PLC activity during infection of host cells. Monolayers of Henle 407 cells were infected with the DH-L699 (SLCC-5764 Δhly ΔplcB i-plcB) strain, and intracellular complementation of PC-PLC activity was measured based on the ability of bacteria to escape the primary vacuole and replicate within host cells in the presence of IPTG. As shown in Fig. 2C, only minimal intracellular growth of DH-L699 bacteria was seen during infection in the presence of 10 mM IPTG. To determine whether the failure to grow within Henle 407 cells resulted from an inability to escape from primary vacuoles, we determined the number of bacteria that had escaped from primary vacuoles by using immunofluorescence microscopy (for experimental details, see Materials and Methods; see also reference 25). By 2 h postinfection, 72% (36 of 50 bacteria analyzed) of DH-L377 (SLCC-5764 Δhly) had escaped the primary vacuole. However, no DH-L699 (SLCC-5764 Δhly ΔplcB i-plcB) bacteria had escaped the primary vacuole in the presence or absence of 10 mM IPTG (0 of 50 bacteria analyzed) by 2 h postinfection. Nonetheless, the slight increase in the number of intracellular DH-L699 bacteria observed in the intracellular growth curve (Fig. 2C) suggested that, in the presence of IPTG, a few bacteria had escaped from the primary vacuole and reached the host cell cytosol over the 9-h infection period.
Intracellular infections with strain DH-L718 (10403S Δhly ΔplcB i-plcB) yielded similar results as described for strain DH-L699 (SLCC-5764 Δhly ΔplcB i-plcB). In the absence or presence of IPTG, DH-L718 bacteria were unable to escape the primary vacuole or replicate within Henle 407 cells (data not shown). As shown in Fig. 2B, we detected nearly identical PC-PLC activities during growth in broth culture when plcB was transcribed from the native PrfA-regulated promoter or the inducible promoter in the 10403S background (Fig. 2B, compare lanes 5 and 7). Therefore, the inability of DH-L718 bacteria to escape from vacuoles and grow within Henle 407 cells suggested that in strain 10403S the expression of PC-PLC must increase compared to the expression in broth culture to allow bacterial escape from Henle 407 cell primary vacuoles in the absence of LLO. Indeed, using the 10403S-derived L. monocytogenes strain NF-L476, which contains the gus reporter gene under transcriptional control of the actA-plcB promoter, we detected an approximately 20-fold increase in glucuronidase (GUS) activity when bacteria were shifted from BHI medium to the RPMI tissue culture medium used for host cell infections. Strain NF-L476 produced 0.11 ± 0.03 pmol of product per min per 106 bacteria in BHI medium compared to 2.42 ± 0.85 pmol of product per min per 106 bacteria when shifted to RPMI medium for 2 h (for experimental details, see Materials and Methods). This result was consistent with previously described measurements of transcript levels and enzyme activity values using reporter gene fusions (4, 6, 50) and indicated that the native PrfA-regulated actA-plcB promoter responds to environmental changes prior to host cell entry or entry into the cytosol.
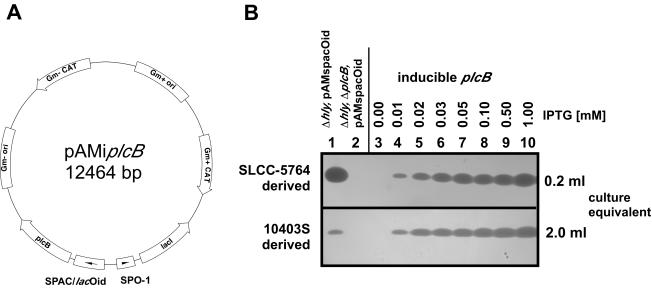
Inducible PC-PLC expression from a multicopy plasmid vector.
To allow bacterial escape from primary vacuoles in human epithelial cells in the absence of LLO, we found that relatively high amounts of active PC-PLC are required. To achieve high-level inducible PC-PLC expression, we placed transcription of plcB under the control of the inducible SPAC/lacOid promoter on the multicopy plasmid pAM401, resulting in plasmid pAMiplcB (Fig. 3A). Initially, we transformed LLO-, PC-PLC-negative derivatives of strains 10403S and SLCC-5764 with plasmid pAMiplcB and analyzed inducible PC-PLC expression when bacteria were grown in broth culture. We found that expression of PC-PLC was tightly controlled by the presence or absence of IPTG and was IPTG dose dependent as determined by PC-PLC activity assays (Fig. 3B). Using the multicopy inducible PC-PLC strain DH-L735 (SLCC-5764 Δhly ΔplcB, pAMiplcB), we obtained PC-PLC activity at an IPTG concentration of 0.01 mM that was similar to the PC-PLC activity observed from the induced (1 mM IPTG) single-copy inducible PC-PLC strain DH-L699 (SLCC-5764 Δhly ΔplcB i-plcB) (data not shown). When induced with 1 mM IPTG, DH-L735 (SLCC-5764 Δhly ΔplcB, pAMiplcB) yielded PC-PLC activity that was slightly lower but comparable to the PC-PLC activity detected from strain DH-L683 (SLCC-5764 Δhly, pAMspacOid), in which plcB was transcribed from the native PrfA*-regulated promoter (Fig. 3B, compare upper-panel lanes 1 and 10). In strain DH-L824 (10403S Δhly ΔplcB, pAMiplcB) we obtained PC-PLC activity at an IPTG concentration of 0.01 mM that was similar to the PC-PLC activity observed from DH-L728 (10403S Δhly, pAMspacOid), in which plcB was transcribed from the native PrfA-activated promoter (Fig. 3B, compare lower-panel lanes 1 and 4). However, the PC-PLC activity from DH-L824 increased to levels significantly higher than those obtained from DH-L728 as IPTG concentrations were increased over a range of 0.02 to 1.0 mM IPTG (Fig. 3B, compare lower-panel lane 1 to lanes 5 to 10).
FIG. 3.
Multicopy inducible PC-PLC expression system for L. monocytogenes. (A) Schematic representation of the inducible PC-PLC expression vector pAMiplcB. plcB was cloned into plasmid pAM401 under SPAC/lacOid promoter/operator control, together with lacI under constitutive SPO-1 promoter control. (B) PC-PLC activity assays of SLCC-5764 (PrfA*) and 10403S (PrfA) derived strains. Overnight cultures of L. monocytogenes strains were diluted 1:10 in BHI medium with or without IPTG at the indicated concentrations and grown for 5 h at 37°C. Culture supernatants were TCA precipitated, an equivalent of 0.2 or 2 ml of culture supernatant was separated by SDS-PAGE, and PC-PLC activities were detected by egg yolk overlay assays. In the upper panel are SLCC-5764 (PrfA*) strain derivatives. Lane 1, DH-L683 (SLCC-5764 Δhly, pAMspacOid); lane 2, DH-L687 (SLCC-5764 Δhly ΔplcB, pAMspacOid); lanes 3 to 10, DH-L735 (SLCC-5764 Δhly ΔplcB, pAMiplcB; inducible plcB) grown in the presence of increasing concentrations of IPTG as indicated above the figure. In the lower panel are 10403S (PrfA) strain derivatives. Lane 1, DH-L728 (10403S Δhly, pAMspacOid); lane 2, DH-L729 (10403S Δhly ΔplcB, pAMspacOid); lanes 3 to 10, DH-L824 (10403S Δhly ΔplcB, pAMiplcB; inducible plcB) grown in the presence of increasing concentrations of IPTG as indicated above the figure.
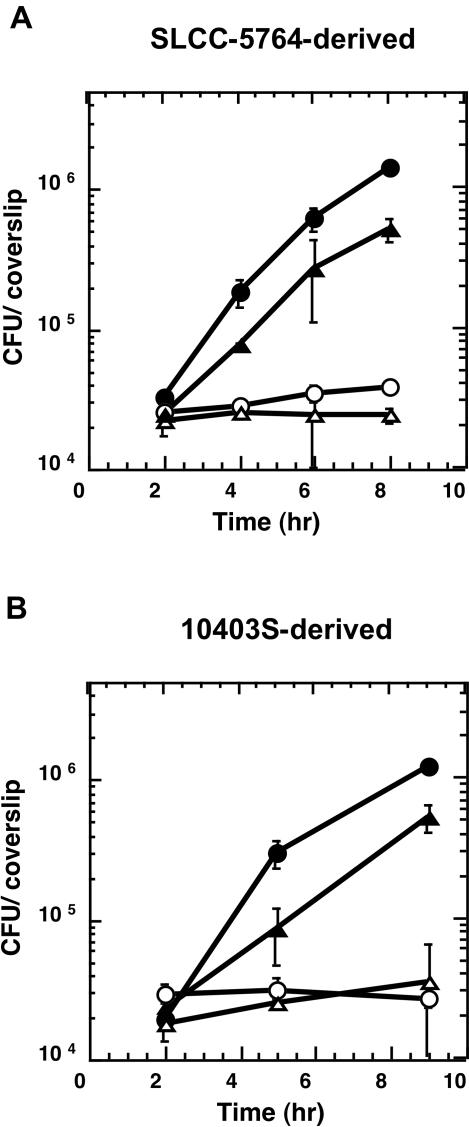
Furthermore, strains DH-L735 (SLCC-5764 Δhly ΔplcB, pAMiplcB) and DH-L824 (10403S Δhly ΔplcB, pAMiplcB) containing the multicopy inducible PC-PLC expression vector were both able to grow in an IPTG-dependent manner in Henle 407 cells. The observed growth rates were similar to L. monocytogenes strains in which plcB was transcribed from its native PrfA* or PrfA-regulated promoter (Fig. 4). Using vacuolar lysis assays, we found that 2 h postinfection of Henle 407 cells 58% of DH-L728 (10403S Δhly, pAMspacOid) bacteria had escaped the primary vacuole. Moreover, 41% of DH-L824 (10403S Δhly ΔplcB, pAMiplcB) bacteria grown in the presence of 1 mM IPTG had reached the host cell cytosol at 2 h postinfection. Therefore, upon induction of PC-PLC expression from the multicopy plasmid at 1 mM IPTG, similar but less efficient escape from primary vacuoles of Henle 407 cells was observed with strain DH-L824 in comparison to 10403S bacteria that expressed PC-PLC under native PrfA-regulated control. This observation suggested that strain 10403S produced an increase in PC-PLC activity derived from the native PrfA-regulated promoter that resulted in PC-PLC activity at least as high as that produced from the fully induced SPAC/lacOid promoter on the multicopy plasmid (Fig. 3B, lower panel, compare lanes 1 and 10). However, it should be kept in mind that the plasmid copy number might vary per bacterium, and therefore PC-PLC activity detected in BHI broth on a population level might not completely reflect PC-PLC activities per bacterium during infection, a parameter that is important for vacuolar lysis.
FIG. 4.
Intracellular growth of multicopy inducible PC-PLC L. monocytogenes strains in Henle 407 cells. Overnight cultures of L. monocytogenes strains were diluted 1:10 in BHI medium and grown for 2 h at 37°C in the presence or absence of 10 mM IPTG. Monolayers of Henle 407 cells were infected at an MOI of 50:1, and intracellular growth was measured in the presence or absence of 10 mM IPTG as described in Materials and Methods. (A) SLCC-5764 (PrfA*) strain derivatives. Symbols: •, DH-L683 (SLCC-5764 Δhly, pAMspacOid); ○, DH-L687 (SLCC-5764 Δhly ΔplcB, pAMspacOid); ▵, DH-L735 (SLCC-5764 Δhly ΔplcB, pAMiplcB) without IPTG; ▴, DH-L735 (SLCC-5764 Δhly ΔplcB, pAMiplcB) with 10 mM IPTG. (B) 10403S (PrfA) strain derivatives. Symbols: •, DH-L728 (10403S Δhly, pAMspacOid); ○, DH-L729 (10403S Δhly ΔplcB, pAMspacOid); ▵, DH-L824 (10403S Δhly ΔplcB, pAMiplcB) without IPTG; ▴, DH-L824 (10403S Δhly ΔplcB, pAMiplcB) with 10 mM IPTG. The data points in the growth curves in panels A and B represent the means ± standard deviations of three coverslips from one of three experiments and from one experiment, respectively.
PC-PLC activity in the absence of LLO is required for cell-to-cell spread during infection of Henle 407 cells.
Using the tightly regulated PC-PLC expression system from the multicopy plasmid, we were able to determine whether, in the absence of LLO, PC-PLC activity was required for cell-to-cell spread during infection of human epithelial cells. We first assessed the ability of LLO-negative, inducible PC-PLC bacteria to spread from cell to cell by using plaquing assays in the presence of different concentrations of IPTG. Strain DH-L735 (SLCC-5764 Δhly ΔplcB, pAMiplcB) was grown in BHI broth culture for 2 h in the absence or presence of 1 mM IPTG to preinduce PC-PLC expression. These cultures were subsequently used for plaque formation assays in Henle 407 cells at various concentrations of IPTG. Strain DH-L735 showed a plaque size of 89% compared to DH-L683 (SLCC-5764 Δhly, pAMspacOid) in the presence of 1 mM IPTG. No further increase in plaque size was seen by increasing the inducer concentration to 10 mM IPTG. However, plaque sizes decreased to 70 and 31% when the concentration of IPTG was decreased to 0.1 and 0.01 mM IPTG, respectively. No visible plaques were formed in the complete absence of IPTG. This result demonstrated that in the absence of LLO, continuous high-level expression of PC-PLC is required for maximal cell-to-cell spread in Henle 407 cells.
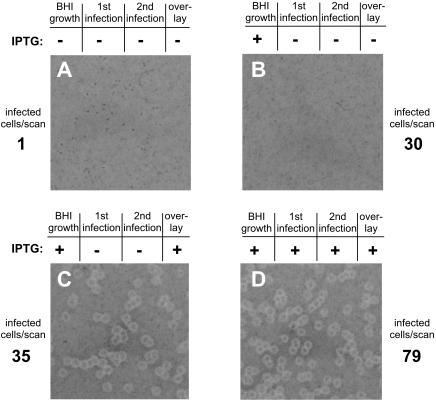
Next, we used strain DH-L735 (SLCC-5764 Δhly ΔplcB, pAMiplcB) for a homologous Henle 407 to Henle 407 cell-to-cell spreading assay to specifically investigate the effect of halting PC-PLC expression after lysis of primary vacuoles (Fig. 5). Strain DH-L735 was grown in BHI broth for 2 h in the presence of 1 mM IPTG to preinduce PC-PLC production prior to infection of Henle 407 cells. The preinduced DH-L735 bacteria were then used to infect Henle 407 cells in the absence or presence of 1 mM IPTG. Using immunofluorescence microscopy, we confirmed that under these infection conditions preinduced DH-L735 bacteria were able to escape from primary vacuoles of Henle 407 cells even in the absence of added inducer during the infection (see Materials and Methods for experimental details). At 2 h postinfection, primary infected Henle 407 cells were collected and added to a secondary monolayer of Henle 407 cells in the presence or absence of IPTG. The ability of DH-L735 to spread from primary infected cells to cells in the secondary monolayer and then continue to spread from cell to cell in the secondary monolayer was determined by the formation of plaques in the secondary cell monolayer. Preinduced DH-L735 bacteria were only able to spread from cell to cell and form visible plaques in a monolayer of secondary Henle 407 cells when maintained in the presence of IPTG (Fig. 5C and D). Secondary Henle 407 cell monolayers that were infected via primary Henle 407 cells containing preinduced DH-L735 bacteria did not result in plaque formation in the absence of IPTG (Fig. 5B). Nonetheless, immunofluorescence microscopy indicated that equivalent numbers of infected primary Henle 407 cells were added to the secondary cell monolayer that received no IPTG during host cell infection (Fig. 5B and C). This result demonstrated that after lysis of primary vacuoles, LLO-negative L. monocytogenes bacteria can only spread from cell to cell to form visible plaques if PC-PLC is expressed.
FIG. 5.
Henle 407 to Henle 407 cell-to-cell spread. Strain DH-L735 (SLCC-5764 Δhly ΔplcB, pAMiplcB) was grown for 2 h at 37°C in BHI medium in the presence or absence of 1 mM IPTG. Monolayers of Henle 407 cells were infected at an MOI of 200:1 in the presence or absence of 1 mM IPTG (first infection). At 1 h after infection, the monolayers were washed, and serum-free medium containing 50 μg of gentamicin/ml and 2 μg of CellTracker Blue/ml was added to differentially label primary infected cells. At 1.5 h after infection, the monolayers were washed to remove excess CellTracker, and serum-containing medium supplemented with 50 μg of gentamicin/ml was added. At 2 h postinfection, host cells were removed from dishes and counted, and 1,000 Henle 407 cells (primary CellTracker Blue labeled cells) were placed in duplicate on monolayers of uninfected, unlabeled Henle 407 cells in the presence of 1 mM IPTG (second infection). Alternatively, 5,000 primary Henle 407 cells were placed in duplicate on monolayers of secondary Henle 407 cells in the absence of IPTG. The number of primary Henle 407 cells that initially contained bacteria in the cytosol was determined microscopically as described in Materials and Methods and is noted next to each panel (infected cells/scan). For plaquing assays, monolayers were overlaid 2 h after primary infected cells were placed onto secondary cell monolayers with an agarose-medium mixture containing 10 μg of gentamicin/ml with or without 1 mM IPTG. At 4 days after infection, a second overlay containing neutral red and 10 μg of gentamicin/ml with or without 1 mM IPTG was added. Images of plaques were obtained after overnight incubation. The presence or absence of 1 mM IPTG during growth in BHI medium, primary cell infection, secondary cell infection, or the overlay is indicated above each panel.
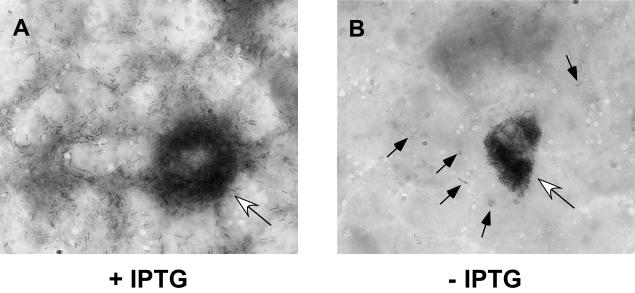
Immunofluorescence microscopy analysis indicated that preinduced DH-L735 bacteria are capable of initiating an infection in Henle 407 cells. Therefore, three likely explanations for the observed defect in cell-to-cell spread in the absence of continuous PC-PLC induction are: (i) bacteria are unable to grow within the host cell cytosol in the absence of PC-PLC activity; (ii) in the absence of PC-PLC activity bacteria can replicate within the host cell cytosol, but cannot spread to secondary host cells; or (iii) PC-PLC activity is required for lysis of double-membrane vacuoles formed during cell-to-cell spread. We infected Henle 407 cells on coverslips with preinduced DH-L735 (SLCC-5764 Δhly ΔplcB, pAMiplcB) bacteria in the presence or absence of IPTG. At 24 h postinfection, coverslips were stained and analyzed by light microscopy (Fig. 6). In the presence of inducer, we observed extended foci of infected host cells (Fig. 6A). In the absence of inducer, only the primary infected host cell contained numerous bacteria (Fig. 6B). Some bacteria were observed in secondary neighboring cells, but no extended growth in these cells was seen (Fig. 6B). Therefore, we speculate that in the absence of LLO, PC-PLC is required for lysis of double-membrane vacuoles formed during cell-to-cell spread in Henle 407 cells but is not required for bacterial replication or the actual spreading event into secondary Henle 407 cells.
FIG. 6.
PC-PLC is required for cell-to-cell spread in Henle 407 cells in the absence of LLO. Strain DH-L735 (SLCC-5764 Δhly ΔplcB, pAMiplcB) was diluted 1:10 in BHI medium containing 1 mM IPTG and grown for 2 h at 37°C to induce PC-PLC expression. Monolayers of Henle 407 cells seeded onto glass coverslips were infected at an MOI of 1:1 in the presence of 1 mM IPTG (A) or at an MOI of 100:1 in the absence of IPTG (B). At 1 h after infection, monolayers were washed, and medium containing 30 μg of gentamicin/ml was added. At 24 h after infection, coverslips were stained with Diff-Quik (DADE-Behring) and analyzed by light microscopy. Open arrows indicate heavily infected primary host cells. The solid arrows in panel B indicate bacteria within neighboring cells. Bacteria are present throughout neighboring cells in panel A and were therefore not indicated by arrows.
DISCUSSION
After entry into host cells, L. monocytogenes must escape the phagocytic vacuole in order to replicate within the host cell cytosol. During intracellular infection, L. monocytogenes promotes its escape from two different vacuolar compartments: a single-membrane vacuole formed upon initial host cell entry and secondary double-membrane vacuoles formed during cell-to-cell spread (39, 54). L. monocytogenes secretes three known factors that interact with membranes: the pore-forming cytolysin LLO and the phospholipases PI-PLC and PC-PLC (8, 10, 16, 29, 53, 55). Differences in the requirement of these determinants for vacuolar lysis have been described depending upon the host cell type infected (31, 44, 53). In the present study, we further examined the requirement of the L. monocytogenes broad-range PLC, PC-PLC, during infection of human epithelial cells. We found that PC-PLC can promote lysis of primary vacuoles in several human-derived epithelial cell lines in the absence of LLO. However, relatively high levels of PC-PLC activity were necessary for lysis of primary vacuoles.
In the present study, we removed expression of PC-PLC from its native transcriptional control mechanism and placed the expression of PC-PLC under IPTG-inducible control. We observed stringent and IPTG dose-dependent production of PC-PLC when bacteria were grown in broth culture. Using inducible PC-PLC expression, we found that in the absence of LLO, continuous high-level expression of PC-PLC is required for optimal cell-to-cell spread within human epithelial cells. Our results indicated that, after escape from primary vacuoles, PC-PLC is not required for intracellular bacterial replication or to mediate spread into neighboring cells during infection of human epithelial cells but is necessary for lysis of secondary spreading vacuoles in the absence of LLO.
Previous studies have shown that in addition to all mouse-derived cells examined, LLO is required for primary vacuole lysis in several human-derived cells, including human umbilical vein endothelial cells and human brain microvascular endothelial cells (13, 23). However, L. monocytogenes can escape from primary vacuoles in the absence of LLO during infection of the human-derived epithelial cell lines Henle 407, HEp-2, and HeLa; the human-derived fibroblast cell line WS1; and human-derived dendritic cells (24, 40, 42, 44). Prior studies have shown that PC-PLC mediates the LLO-independent escape from primary vacuoles in Henle 407 cells (31). Here, we have shown that PC-PLC is also required for lysis of primary vacuoles in HEp-2 and HeLa cells in the absence of LLO (Fig. 1). Hence, we suggest that it may be a general occurrence that PC-PLC can promote vacuolar lysis in human epithelial cells. This observation raises several interesting questions and suggests that vacuoles of human epithelial cells differ from all murine cells evaluated. This difference may be due to differences in phospholipid composition, intravacuolar pH, or protein factors in the membrane or due to altered expression and/or activation of PC-PLC in some human cell vacuoles versus mouse cell vacuoles.
The exact mechanism of action of both L. monocytogenes PLCs, PI-PLC and PC-PLC, is not well understood. The phospholipases may serve to degrade host cell membranes directly, or initial phospholipid degradation may initiate a chain of events to activate host cell activities that serve to degrade vacuolar membranes (20, 59). However, it has been shown that both phospholipases must retain their enzymatic activity for their biological function in mouse-derived cells (1, 62). Since PC-PLC acts on a broad range of substrates, including phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, and sphingomyelin, it seems plausible that PC-PLC can actively disrupt vacuolar membranes and that this membrane-damaging activity is sufficient to allow bacterial escape from Henle 407, HEp-2, and HeLa cell primary vacuoles (18, 21). Previous studies have shown that amino acid substitutions altering the substrate specificity of PC-PLC without decreasing enzymatic activity had a negative effect on vacuolar lysis efficiency in Henle 407 cells (62). Therefore, it was speculated that substrate specificity might be more important than the actual activity level of PC-PLC to achieve membrane lysis. However, our results strongly indicate that without changing substrate specificity, the amount of PC-PLC activity is important for bacterial escape from human epithelial cell primary vacuoles. We removed the expression of PC-PLC from the native PrfA-regulated actA-plcB promoter and placed transcription of plcB under control of the IPTG-inducible SPAC/lacOid promoter/operator on the chromosome of an LLO-, PC-PLC-negative 10403S-derived strain. Although we obtained similar PC-PLC activities from the inducible and the native promoter during growth in BHI medium (Fig. 2B), only the strain that expressed PC-PLC from the native actA-plcB promoter was capable of escaping from Henle 407 cell primary vacuoles. These results suggest that in 10403S-derived strains, expression of PC-PLC from the native PrfA-regulated actA-plcB promoter is increased before or upon bacterial entry into host cells and that the resulting increased amount of PC-PLC activity is required for vacuolar lysis in Henle 407 cells in the absence of LLO. Indeed, we were able to complement PC-PLC activity for lysis of Henle 407 cell primary vacuoles after high-level expression of PC-PLC from the inducible SPAC/lacOid promoter on a high-copy-number plasmid (Fig. 3 and 4). We favor a model in which PC-PLC is actively degrading primary vacuoles of human epithelial cells, yet high levels of PC-PLC are required for membrane disruption.
The studies presented here and previous reports have indicated that transcription from the actA-plcB promoter is increased when bacteria are shifted from BHI medium to tissue culture medium (4, 6, 50). Our results suggest that this increase (∼20-fold) yields expression levels of PC-PLC that are essential to allow bacterial escape from Henle 407 cell primary vacuoles in the absence of LLO. It would be interesting to determine directly the activation level of the actA-plcB promoter within primary vacuoles of human epithelial cells in comparison to mouse-derived cells or other cell lines in which PC-PLC activity is not sufficient to promote vacuolar lysis. We have attempted to address this question by using a previously described gus reporter gene fusion to the actA-plcB promoter (50). However, low infection efficiencies in human epithelial cells have hampered our ability to determine GUS activity values for bacteria specifically within primary vacuoles. However, preliminary results suggest that overexpression of PC-PLC cannot relieve the LLO requirement for vacuolar lysis in mouse-derived cells. Thus, the observed difference in the ability of PC-PLC to mediate vacuolar lysis in human epithelial cells may not be due solely to insufficient expression of PC-PLC in mouse cell primary vacuoles (A. Gründling and D. E. Higgins, unpublished results).
In the present study, we have provided evidence that an increase in PC-PLC activity is required to allow LLO-negative, 10403S-derived bacteria to escape from Henle 407 cell primary vacuoles. By placing expression of PC-PLC under dose-dependent IPTG control, we showed that PC-PLC activity can be increased by increasing expression of PC-PLC (Fig. 3B). Furthermore, significantly higher PC-PLC activity was detected when plcB was transcribed from the inducible promoter in the SLCC-5764 (PrfA*) background strain than in the 10403S (PrfA) background strain (Fig. 2B, compare lanes 3 and 7). We hypothesize that this increase in PC-PLC activity is due to PrfA-dependent posttranscriptional regulation, most likely at the level of proteolytic activation of proPC-PLC by Mpl. It has already been shown that transcription from the actA-plcB promoter increases upon bacterial entry into the cytosol of host cells (6, 15, 38, 50). It is not clear at the moment whether the expression of Mpl is increased within host cells. Transcription of the mpl gene is not increased when bacteria are shifted from BHI to tissue culture medium (4). However, whole-genome transcriptome analysis has shown that transcription of mpl is regulated in a manner similar to that of many other PrfA-regulated genes during growth in broth (37). Furthermore, it has been shown that activation of PC-PLC is sensitive to bafilomycin A1, an inhibitor of the vacuolar proton pump ATPase required for acidification of vacuolar compartments (33). In that study it was also observed that the increase in active PC-PLC at low pH is dependent on Mpl. Therefore, the increase in Mpl-dependent activation of PC-PLC coupled with an increase in PC-PLC expression within vacuoles leads to an amplification of PC-PLC activity in the compartment where phospholipase activity is needed most.
Moreover, we were able to show that continuous high-level expression of PC-PLC is necessary for optimal cell-to-cell spread during infection of Henle 407 cells in the absence of LLO. The inducible PC-PLC expression system allowed us to shut off PC-PLC expression after initiating intracellular infection. Preinduced PC-PLC expressing bacteria were used to infect Henle 407 cells in the absence of inducer. Using microscopic analysis, we confirmed that preinduced bacteria were able to escape from primary vacuoles of Henle 407 cells (Fig. 5 and 6). Substantial bacterial growth was seen in the primary infected host cell even without further induction of PC-PLC expression (Fig. 6). However, phenotypically LLO- and PC-PLC-negative bacteria were not able to form extended foci of infection in monolayers of Henle 407 cells in the absence of continued PC-PLC induction (Fig. 6B). These results are most consistent with the idea that PC-PLC is not required for growth within the host cell cytosol but is necessary for continued cell-to-cell spread. It has been reported that L. monocytogenes strains deleted for PI-PLC and PC-PLC show a decrease in intracellular growth rate after bacteria are microinjected directly into the host cell cytosol of Caco-2 cells (19). Our results indicated that LLO-, PC-PLC-negative bacteria can grow efficiently within the cytosol of Henle 407 cells. However, we have not ruled out the possibility that PC-PLC activity is required for optimal intracellular bacterial replication. We plan to use green fluorescent protein-expressing L. monocytogenes strains, together with our inducible expression system and time-lapse video microscopy, to determine the contribution of the three membrane-active determinants for optimal intracellular bacterial replication. Our results are consistent with a model that, in the absence of LLO, PC-PLC is required for lysis of secondary double-membrane vacuoles in Henle 407 cells. Therefore, PI-PLC, the phosphatidylinositol-specific PLC, or other L. monocytogenes proteins are not sufficient to lyse Henle 407 cell spreading vacuoles in the absence of LLO or PC-PLC. We are now attempting to confirm by electron microscopy whether, after PC-PLC expression is shut off, LLO-, PC-PLC-negative bacteria are indeed trapped within spreading vacuoles after cell-to-cell spread.
In conclusion, slight differences in membrane composition may account for the observed differences in the requirement of LLO or PC-PLC for vacuolar lysis. These differences may also contribute to the susceptibility of different host species to infection by L. monocytogenes. Listeria ivanovii, a second pathogenic Listeria species, secretes, in addition to a pore-forming cytolysin and two PLCs, a sphingomyelinase C (SmcL) that has also been implicated in vacuolar lysis (22). L. monocytogenes and L. ivanovii share many virulence properties but differ in their pathogenicities. Whereas L. monocytogenes causes infections in a wide range of animals, L. ivanovii predominantly infects ruminants, especially sheep. It is intriguing to speculate that the occurrence of a sphingomyelinase might be an adaptation to the primary host of L. ivanovii since there is an increased sphingomyelin/phosphatidylcholine ratio in membranes of ruminants compared to humans and rodents (57).
Acknowledgments
We gratefully acknowledge Aimee Shen for construction of plasmid pPL2-SPO-1 and Hélène Marquis for technical advice, Mpl antibody, and critical review of the manuscript. We also acknowledge Aimee Shen and Christiaan van Ooij for helpful review of the manuscript. We thank Howard Goldfine for providing PC-PLC antibody and Dana Boyd for providing the essential cloning strain CLG190.
This work was supported by U.S. Public Health Service grant AI-53669 from the National Institutes of Health (D.E.H.) and by the Austrian Science Foundation FWF Erwin Schrödinger postdoctoral fellowships J2032 and J2183 (A.G.).
REFERENCES
- 1.Bannam, T., and H. Goldfine. 1999. Mutagenesis of active-site histidines of Listeria monocytogenes phosphatidylinositol-specific phospholipase C: effects on enzyme activity and biological function. Infect. Immun. 67:182-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bishop, D. K., and D. J. Hinrichs. 1987. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 139:2005-2009. [PubMed] [Google Scholar]
- 3.Bohne, J., H. Kestler, C. Uebele, Z. Sokolovic, and W. Goebel. 1996. Differential regulation of the virulence genes of Listeria monocytogenes by the transcriptional activator PrfA. Mol. Microbiol. 20:1189-1198. [DOI] [PubMed] [Google Scholar]
- 4.Bohne, J., Z. Sokolovic, and W. Goebel. 1994. Transcriptional regulation of prfA and PrfA-regulated virulence genes in Listeria monocytogenes. Mol. Microbiol. 11:1141-1150. [DOI] [PubMed] [Google Scholar]
- 5.Brundage, R. A., G. A. Smith, A. Camilli, J. A. Theriot, and D. A. Portnoy. 1993. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Proc. Natl. Acad. Sci. USA 90:11890-11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bubert, A., Z. Sokolovic, S. K. Chun, L. Papatheodorou, A. Simm, and W. Goebel. 1999. Differential expression of Listeria monocytogenes virulence genes in mammalian host cells. Mol. Gen. Genet. 261:323-336. [DOI] [PubMed] [Google Scholar]
- 7.Camilli, A., H. Goldfine, and D. A. Portnoy. 1991. Listeria monocytogenes mutants lacking phosphatidylinositol-specific phospholipase C are avirulent. J. Exp. Med. 173:751-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camilli, A., L. G. Tilney, and D. A. Portnoy. 1993. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol. Microbiol. 8:143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakraborty, T., M. Leimeister-Wachter, E. Domann, M. Hartl, W. Goebel, T. Nichterlein, and S. Notermans. 1992. Coordinate regulation of virulence genes in Listeria monocytogenes requires the product of the prfA gene. J. Bacteriol. 174:568-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cossart, P., M. F. Vicente, J. Mengaud, F. Baquero, J. C. Perez-Diaz, and P. Berche. 1989. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect. Immun. 57:3629-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dancz, C. E., A. Haraga, D. A. Portnoy, and D. E. Higgins. 2002. Inducible control of virulence gene expression in Listeria monocytogenes: temporal requirement of listeriolysin O during intracellular infection. J. Bacteriol. 184:5935-5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domann, E., M. Leimeister-Wachter, W. Goebel, and T. Chakraborty. 1991. Molecular cloning, sequencing, and identification of a metalloprotease gene from Listeria monocytogenes that is species specific and physically linked to the listeriolysin gene. Infect. Immun. 59:65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drevets, D. A. 1998. Listeria monocytogenes virulence factors that stimulate endothelial cells. Infect. Immun. 66:232-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freitag, N. E. 2000. Genetic tools for use with Listeria monocytogenes, p. 488-498. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 15.Freitag, N. E., and K. E. Jacobs. 1999. Examination of Listeria monocytogenes intracellular gene expression by using the green fluorescent protein of Aequorea victoria. Infect. Immun. 67:1844-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaillard, J. L., P. Berche, J. Mounier, S. Richard, and P. Sansonetti. 1987. In vitro model of penetration and intracellular growth of L. monocytogenes in human enterocyte-like cell line Caco-2. Infect. Immun. 55:2822-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gedde, M. M., D. E. Higgins, L. G. Tilney, and D. A. Portnoy. 2000. Role of listeriolysin O in cell-to-cell spread of Listeria monocytogenes. Infect. Immun. 68:999-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geoffroy, C., J. Raveneau, J. Beretti, A. Lecroisey, J. Vazquez-Boland, J. E. Alouf, and P. Berche. 1991. Purification and characterization of an extracellular 29-kilodalton phospholipase C from Listeria monocytogenes. Infect. Immun. 59:2382-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goetz, M., A. Bubert, G. F. Wang, I. Chico-Calero, J. A. Vazquez-Boland, M. Beck, J. Slaghuis, A. A. Szalay, and W. Goebel. 2001. Microinjection and growth of bacteria in the cytosol of mammalian host cells. Proc. Natl. Acad. Sci. USA 98:12221-12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldfine, H., T. Bannam, N. C. Johnston, and W. R. Zuckert. 1998. Bacterial phospholipases and intracellular growth: the two distinct phospholipases C of Listeria monocytogenes. Symp. Ser. Soc. Appl. Microbiol. 27:7S-14S. [DOI] [PubMed] [Google Scholar]
- 21.Goldfine, H., N. C. Johnston, and C. Knob. 1993. Nonspecific phospholipase C of Listeria monocytogenes: activity on phospholipids in Triton X-100-mixed micelles and in biological membranes. J. Bacteriol. 175:4298-4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Zorn, B., G. Dominguez-Bernal, M. Suarez, M. T. Ripio, Y. Vega, S. Novella, and J. A. Vazquez-Boland. 1999. The smcL gene of Listeria ivanovii encodes a sphingomyelinase C that mediates bacterial escape from the phagocytic vacuole. Mol. Microbiol. 33:510-523. [DOI] [PubMed] [Google Scholar]
- 23.Greiffenberg, L., W. Goebel, K. S. Kim, I. Weiglein, A. Bubert, F. Engelbrecht, M. Stins, and M. Kuhn. 1998. Interaction of Listeria monocytogenes with human brain microvascular endothelial cells: InlB-dependent invasion, long-term intracellular growth, and spread from macrophages to endothelial cells. Infect. Immun. 66:5260-5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hense, M., E. Domann, S. Krusch, P. Wachholz, K. E. Dittmar, M. Rohde, J. Wehland, T. Chakraborty, and S. Weiss. 2001. Eukaryotic expression plasmid transfer from the intracellular bacterium Listeria monocytogenes to host cells. Cell. Microbiol. 3:599-609. [DOI] [PubMed] [Google Scholar]
- 25.Jones, S., and D. A. Portnoy. 1994. Characterization of Listeria monocytogenes pathogenesis in a strain expressing perfringolysin O in place of listeriolysin O. Infect. Immun. 62:5608-5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kathariou, S., L. Pine, V. George, G. M. Carlone, and B. P. Holloway. 1990. Nonhemolytic Listeria monocytogenes mutants that are also noninvasive for mammalian cells in culture: evidence for coordinate regulation of virulence. Infect. Immun. 58:3988-3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kocks, C., E. Gouin, M. Tabouret, P. Berche, H. Ohayon, and P. Cossart. 1992. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell 68:521-531. [DOI] [PubMed] [Google Scholar]
- 28.Lauer, P., M. Y. N. Chow, M. J. Loessner, D. A. Portnoy, and R. Calendar. 2002. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J. Bacteriol. 184:4177-4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leimeister-Wachter, M., E. Domann, and T. Chakraborty. 1991. Detection of a gene encoding a phosphatidylinositol-specific phospholipase C that is co-ordinately expressed with listeriolysin in Listeria monocytogenes. Mol. Microbiol. 5:361-366. [DOI] [PubMed] [Google Scholar]
- 30.Leimeister-Wachter, M., C. Haffner, E. Domann, W. Goebel, and T. Chakraborty. 1990. Identification of a gene that positively regulates expression of listeriolysin, the major virulence factor of Listeria monocytogenes. Proc. Natl. Acad. Sci. USA 87:8336-8340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marquis, H., V. Doshi, and D. A. Portnoy. 1995. The broad-range phospholipase C and a metalloprotease mediate listeriolysin O-independent escape of Listeria monocytogenes from a primary vacuole in human epithelial cells. Infect. Immun. 63:4531-4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marquis, H., H. Goldfine, and D. A. Portnoy. 1997. Proteolytic pathways of activation and degradation of a bacterial phospholipase C during intracellular infection by Listeria monocytogenes. J. Cell Biol. 137:1381-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marquis, H., and E. J. Hager. 2000. pH-regulated activation and release of a bacteria-associated phospholipase C during intracellular infection by Listeria monocytogenes. Mol. Microbiol. 35:289-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mengaud, J., C. Braun-Breton, and P. Cossart. 1991. Identification of phosphatidylinositol-specific phospholipase C activity in Listeria monocytogenes: a novel type of virulence factor? Mol. Microbiol. 5:367-372. [DOI] [PubMed] [Google Scholar]
- 35.Mengaud, J., S. Dramsi, E. Gouin, J. A. Vazquez-Boland, G. Milon, and P. Cossart. 1991. Pleiotropic control of Listeria monocytogenes virulence factors by a gene that is autoregulated. Mol. Microbiol. 5:2273-2283. [DOI] [PubMed] [Google Scholar]
- 36.Mengaud, J., C. Geoffroy, and P. Cossart. 1991. Identification of a new operon involved in Listeria monocytogenes virulence: its first gene encodes a protein homologous to bacterial metalloproteases. Infect. Immun. 59:1043-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milohanic, E., P. Glaser, J. Y. Coppee, L. Frangeul, Y. Vega, J. A. Vazquez-Boland, F. Kunst, P. Cossart, and C. Buchrieser. 2003. Transcriptome analysis of Listeria monocytogenes identifies three groups of genes differently regulated by PrfA. Mol. Microbiol. 47:1613-1625. [DOI] [PubMed] [Google Scholar]
- 38.Moors, M. A., B. Levitt, P. Youngman, and D. A. Portnoy. 1999. Expression of listeriolysin O and ActA by intracellular and extracellular Listeria monocytogenes. Infect. Immun. 67:131-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mounier, J., A. Ryter, M. Coquis-Rondon, and P. J. Sansonetti. 1990. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect. Immun. 58:1048-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Riordan, M., C. H. Yi, R. Gonzales, K. D. Lee, and D. A. Portnoy. 2002. Innate recognition of bacteria by a macrophage cytosolic surveillance pathway. Proc. Natl. Acad. Sci. USA 99:13861-13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park, S. F., and G. S. Stewart. 1990. High-efficiency transformation of Listeria monocytogenes by electroporation of penicillin-treated cells. Gene 94:129-132. [DOI] [PubMed] [Google Scholar]
- 42.Paschen, A., K. E. Dittmar, R. Grenningloh, M. Rohde, D. Schadendorf, E. Domann, T. Chakraborty, and S. Weiss. 2000. Human dendritic cells infected by Listeria monocytogenes: induction of maturation, requirements for phagolysosomal escape and antigen presentation capacity. Eur. J. Immunol. 30:3447-3456. [DOI] [PubMed] [Google Scholar]
- 43.Portnoy, D. A., T. Chakraborty, W. Goebel, and P. Cossart. 1992. Molecular determinants of Listeria monocytogenes pathogenesis. Infect. Immun. 60:1263-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Portnoy, D. A., P. S. Jacks, and D. J. Hinrichs. 1988. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 167:1459-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poyart, C., E. Abachin, I. Razafimanantsoa, and P. Berche. 1993. The zinc metalloprotease of Listeria monocytogenes is required for maturation of phosphatidylcholine phospholipase C: direct evidence obtained by gene complementation. Infect. Immun. 61:1576-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raveneau, J., C. Geoffroy, J. L. Beretti, J. L. Gaillard, J. E. Alouf, and P. Berche. 1992. Reduced virulence of a Listeria monocytogenes phospholipase-deficient mutant obtained by transposon insertion into the zinc metalloprotease gene. Infect. Immun. 60:916-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ripio, M. T., G. Dominguez-Bernal, M. Lara, M. Suarez, and J. A. Vazquez-Boland. 1997. A Gly145Ser substitution in the transcriptional activator PrfA causes constitutive overexpression of virulence factors in Listeria monocytogenes. J. Bacteriol. 179:1533-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ripio, M. T., G. Dominguez-Bernal, M. Suarez, K. Brehm, P. Berche, and J. A. Vazquez-Boland. 1996. Transcriptional activation of virulence genes in wild-type strains of Listeria monocytogenes in response to a change in the extracellular medium composition. Res. Microbiol. 147:371-384. [DOI] [PubMed] [Google Scholar]
- 49.Sheehan, B., A. Klarsfeld, T. Msadek, and P. Cossart. 1995. Differential activation of virulence gene expression by PrfA, the Listeria monocytogenes virulence regulator. J. Bacteriol. 177:6469-6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shetron-Rama, L. M., H. Marquis, H. G. Bouwer, and N. E. Freitag. 2002. Intracellular induction of Listeria monocytogenes actA expression. Infect. Immun. 70:1087-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vitro genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 52.Skoble, J., D. A. Portnoy, and M. D. Welch. 2000. Three regions within ActA promote Arp2/3 complex-mediated actin nucleation and Listeria monocytogenes motility. J. Cell Biol. 150:527-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith, G. A., H. Marquis, S. Jones, N. C. Johnston, D. A. Portnoy, and H. Goldfine. 1995. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect. Immun. 63:4231-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tilney, L. G., and D. A. Portnoy. 1989. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J. Cell Biol. 109:1597-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vazquez-Boland, J., C. Kocks, S. Dramsi, H. Ohayon, C. Geoffroy, J. Mengaud, and P. Cossart. 1992. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect. Immun. 60:219-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vazquez-Boland, J. A., G. Dominguez-Bernal, B. Gonzalez-Zorn, J. Kreft, and W. Goebel. 2001. Pathogenicity islands and virulence evolution in Listeria. Microbes Infect. 3:571-584. [DOI] [PubMed] [Google Scholar]
- 57.Vazquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. Gonzalez-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vega, Y., C. Dickneite, M. T. Ripio, R. Bockmann, B. Gonzalez-Zorn, S. Novella, G. Dominguez-Bernal, W. Goebel, and J. A. Vazquez-Boland. 1998. Functional similarities between the Listeria monocytogenes virulence regulator PrfA and cyclic AMP receptor protein: the PrfA* (Gly145Ser) mutation increases binding affinity for target DNA. J. Bacteriol. 180:6655-6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wadsworth, S. J., and H. Goldfine. 2002. Mobilization of protein kinase C in macrophages induced by Listeria monocytogenes affects its internalization and escape from the phagosome. Infect. Immun. 70:4650-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wirth, R., F. Y. An, and D. B. Clewell. 1986. Highly efficient protoplast transformation system for Streptococcus faecalis and a new Escherichia coli-S. faecalis shuttle vector. J. Bacteriol. 165:831-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Youngman, P. 1987. Plasmid vectors for recovering and exploiting Tn917 transpositions in Bacillus and other gram-positive bacteria, p. 79-103. In K. Hardy (ed.), Plasmids: a practical approach. IRL Press, Oxford, England.
- 62.Zuckert, W. R., H. Marquis, and H. Goldfine. 1998. Modulation of enzymatic activity and biological function of Listeria monocytogenes broad-range phospholipase C by amino acid substitutions and by replacement with the Bacillus cereus ortholog. Infect. Immun. 66:4823-4831. [DOI] [PMC free article] [PubMed] [Google Scholar]