Infection with the AIDS virus has led to an expanding global health crisis, with over 45 million persons currently living with HIV infection. Ineffective immune control and resultant disease progression is a hallmark of this infection, despite the induction of vigorous virus-specific CD8 T cell responses in most infected persons. Although the features of protective immunity in HIV infection remain to be defined, growing evidence points to a critical role for virus-specific CD4 cells. This is consistent with observations in murine models that maintenance of effective antiviral CTL responses in chronic viral infections is critically dependent on virus-specific T helper cells (1–8).
A potentially simple explanation for the lack of effective immune control in chronic HIV infection is that HIV selectively infects activated CD4 cells and thereby destroys the very cells being generated to coordinate the adaptive immune response (9). However, simple deletion of antigen-specific CD4 cells cannot be the reason for immune failure, since it has been shown that large numbers of virus-specific CD4 cells that secrete interferon gamma (IFN-γ) persist in most persons with uncontrolled viremia (10–12). In contrast, the ability of CD4 cells to proliferate in response to viral antigenic challenge is impaired in persons with progressive infection and high viral loads (13–15). This apparent paradox implies the presence of a functional CD4 defect, the definition of which is of critical importance in understanding the immunologic control of viral replication.
A paper published by Younes et al. in this issue (16), and a series of recent publications (17–19), provide new insights into CD4 cells that are associated with lack of HIV immune control and suggest that the presence of antigen-specific CD4 cells able to produce IL-2 is a key component of effective immunity. Younes et al. examined virus-specific CD4 T cell responses in two groups of newly HIV-infected persons (16). One group consisted of persons treated in the earliest stages of acute infection in whom viremia was consistently suppressed by effective antiviral therapy (aviremic subjects), whereas a second group was likewise treated in the earliest stages of acute infection but experienced persistent antigenic stimulation due to treatment interruptions or treatment failure (viremic subjects). Focusing on persons treated in acute infection makes sense, given that early treatment is associated with generation of HIV-specific CD4 cell responses (15, 20–23), and comparison of the two groups thus allowed assessment of the role of persistent antigen exposure on immune responses. Although both groups had readily detectable HIV-specific CD4 cell responses by intracellular IFN-γ staining, there were striking differences in the ability of these cells to produce IL-2. In aviremic subjects, the antigen-specific cells not only produced IL-2 in addition to INF-γ but also proliferated in vitro, as assessed in a flow cytometric analysis in which decreases in CFSE labeling are indicative of cell division. In viremic subjects, longitudinal analysis of these responses in persons failing antiretroviral therapy demonstrated that persistent exposure to virus was associated with a marked decline in the proliferative capacity of HIV-specific CD4 cells to undetectable levels (16). The defective proliferative capacity of HIV-specific CD4 cells could be corrected in vitro by the addition of exogenous IL-2 (16, 19), suggesting that HIV-specific CD4 cells in individuals with persistent HIV infection are not simply deleted by the virus but are deficient in their ability to produce IL-2 and proliferate.
These results, which demonstrate distinct subsets of HIV-specific CD4 cells defined by proliferative capacity and ability to produce IL-2 and/or IFN-γ in response to cognate antigen exposure, are consistent with findings in murine models of chronic viral infections and with data from several recent human studies (17–19, 24, 25). In untreated HIV-infected persons with progressive and nonprogressive disease, Harari et al. recently reported three functionally distinct populations of CD4 cells: a subset secreting IL-2, a subset secreting IFN-γ, and a subset able to secrete both IL-2 and IFN-γ in response to cognate antigen (18). In untreated persons with progressive disease, the antigen-specific CD4 cells were skewed toward those cells secreting IFN-γ alone, and IL-2–secreting cells were almost absent, findings similar to those of Younes et al. in their studies of treated viremic and aviremic subjects (16). Moreover, Harari et al. found a negative correlation between the frequencies of IL-2 and IL-2/IFN-γ–secreting CD4 cells and viral load (18). This same relationship between viral load and CD4 cells secreting both IFN-γ and IL-2 was recently reported by Boaz et al. (17), suggesting a critical role of this specific population of CD4 cells in viral containment. Other recent studies provide evidence that viremia directly impairs virus-specific proliferative responses by showing that interruption in antiretroviral therapy and subsequent increased viremia in persons with strong virus-specific CD4 proliferative responses led to a loss of proliferative responses but not the ability to secrete IFN-γ in an antigen-specific manner, and the proliferative responses recovered rapidly with resuppression of virmeia (14, 19). Coupled with the demonstration that virus-specific proliferative responses can be corrected in vitro by the addition of exogenous IL-2, these studies provide firm evidence that these cells are present but dysfunctional (16, 19). However, the inability of IL-2 treatment of HIV-infected persons to restore functional immunity suggests that the relationship is not simple (26, 27).
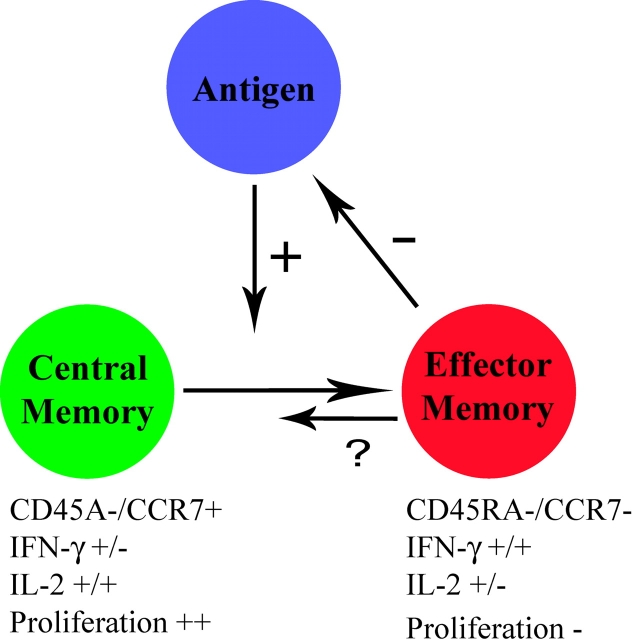
The observed lack of IL-2–producing cells during persistent HIV exposure is of particular interest in light of recent studies regarding development of T cell memory. Memory CD4 and CD8 cells can be divided into different subsets based on their effector functions and migratory capacity (28–30). T cells expressing the LN homing markers L-selectin (CD62L) and CCR7 have been termed central memory cells, whereas cells that are CD62L−/CCR7− have been termed effector memory cells and are thought to have the capacity to migrate to sites of viral replication in the tissues (Fig. 1) . Central memory and effector memory T cells have also been described to differ in their cytokine production capacity, with central memory cells producing predominately IL-2 and effector memory cells producing both IFN-γ and IL-2 (28). The publications by Younes et al. (16) and Harari et al. (18) indicate that HIV-specific CD4 cells that produce IFN-γ alone or IFN-γ and IL-2 are CD45RA−/CCR7− and are thought to have poor proliferative capacity and thereby belong to the effector memory subset. In contrast, HIV-specific CD4+/CD45RA−/ CCR7+ central memory cells produce primarily IL-2 after stimulation with cognate antigen (16, 18) and are the subset thought to be capable of rapid proliferation. The presence of persistent antigen in HIV-infected persons with ongoing viremia is associated with a paucity of HIV-specific central memory CD4 cells compared with aviremic individuals with suppressed viral loads. Similar findings were reported recently in hepatitis C virus (HCV)–infected humans using magnetic bead enrichment of CD4+/tetramer+ cells, showing the phenotype of HCV-specific CD4 cells to display a distinct CD45RA−/CCR7+ central memory phenotype ex vivo in individuals with resolved HCV infection (31).
Figure 1.
Potential model of the relationship between central and effector memory cell differentiation and antigen load in chronic viral infections. The presence of viral antigen drives the generation of effector memory cells capable of producing IFN-γ but lacking significant proliferative capacity, which are short lived in vivo. Effector memory cells in turn drive down antigen levels. Persistent exposure to high levels of antigen is thought to drive differentiation to short lived effector cells and impair development of central memory cells capable of proliferating and producing IL-2.
These studies indicate that chronic antigen exposure leads to impaired virus-specific CD4 T cell function. Previous studies in murine models of lymphocytic choriomeningitis virus (LCMV) infection have indicated that LCMV-specific CD4 cells lose the ability to produce IL-2 over time in LCMV-infected perforin knockout mice that establish chronic infection compared with mice with acute LCMV infection that is subsequently resolved (32). These data are consistent with previous studies in murine models of chronic viral infections, indicating loss of CD4 and CD8 T cell responsiveness, including decreased proliferation and cytokine production, during the establishment of viral persistence (33–35). Furthermore, chronically LCMV-infected perforin knockout mice are able to maintain IFN-γ–producing LCMV-specific CD4 cells, consistent with the observations in the recently reported human studies of HIV-infected individuals that maintain IFN-γ–producing but not IL-2–producing HIV-specific CD4 cells during periods of viremia (16, 18, 19). It is interesting to note that the impairment of proliferative responses in HIV-infected individuals appears to be restricted to HIV-specific CD4 cells, as several studies have indicated no significant difference between proliferation to cytomegalovirus (CMV) or other positive control antigens in persons with suppressed or uncontrolled HIV viremia (14, 16, 19, 36). This may possibly be due to the relative differences in CMV and HIV viral loads, since CMV viral loads are often undetectable by current assays. Similar to the suppressed proliferative responses in individuals with high HIV viral loads, persons with chronic HCV infection, which results in similar levels of uncontrolled viremia, display weak or absent HCV-specific CD4 proliferative responses compared with individuals with spontaneously resolved HCV infection (37–39). Recent evidence in LCMV-infected mice indicates the presence of antigen may drive the generation of effector memory cells that convert to central memory cells after antigen clearance, implying a complex and dynamic relationship exists between the virus and host immune response (40, 41).
The current findings are also of particular interest given recent advances in understanding the role of CD4 cells in the development of effective CD8 T cell responses. Although the need for CD4 help in maintenance of effective antiviral CD8 T cell responses has been known for some time, recent studies in mice show that the development of functional CD8 T cell memory is dependent on the presence of functional CD4 help during priming (2, 3, 5). CD4 cells are clearly dispensable for primary expansion of CD8 cells during initial antigen encounter, but memory CD8 cells capable of subsequent rapid expansion in response to secondary challenge are not generated. This notion is further supported by recent reports in chimpanzee models of HCV infection where the depletion of CD4 memory cells in immune animals before reinfection resulted in persistent HCV infection rather than clearance, and the emergence of viral escape mutations in MHC class I–restricted epitopes (36). These findings may have important implications in HIV infection, since the HIV reverse transcriptase lacks proofreading function and viral variants continue to arise in vivo. Impaired CD4 responses during “priming” to these new variants may contribute to the lack of effective long-term immune control in this infection.
These studies describing a functional defect in virus-specific CD4 T cells may also help to explain the lack of effective antiviral CD8 T cell function in HIV infection. A recent study in HIV-infected persons demonstrated a defect in the ability of CD8 cells from persons with high viral loads to expand upon encounter with antigen, suggesting a potential link between dysfunctional CD8 cells and the CD4 cell defects now being reported (42). Recent evidence further indicates an important role of the expression of the costimulatory molecule CD28 on antigen-specific CD8 cells: restoration of CD28 expression on HIV and CMV-specific CD28−/CD8+ cells reconstituted the ability of these cells to produce IL-2 (43). However, the possible link between expression of CD28 and IL-2 production by antigen-specific CD4 cells remains to be defined. Such studies need now to examine the relationship between the proliferative capacity of CD8 cells and the presence of antigen-specific IL-2–producing CD4 cells, which will require assays that measure function of these cells rather than just the ability to produce IFN-γ. It is important to note that multiple studies have failed to define differences in the frequency of HIV-specific IFN-γ–producing CD8 cells in subjects with progressive and nonprogressive HIV infection, or to identify a correlation between frequencies of HIV-specific CD8+/IFN-γ+ cells and viral load (10, 42, 44), similar to the lack of correlation between HIV-specific CD4+/IFN-γ+ T cells and viral load (10, 18). Defining the relationship between IL-2–producing HIV-specific CD4 cells and various parameters of frequency and function of HIV-specific CD8 T cell responses within the same individuals may provide greater insight into the interactions of CD4 and CD8 cells in chronic viral infections and the contribution of subsets of virus-specific T cells with different functional properties to the overall control of viremia.
Despite the important demonstration that chronic antigen exposure such as persistent HIV viremia may suppress the development of central memory cells capable of proliferating and producing IL-2, important questions remain. Among these is the mechanism whereby high viral loads suppress proliferation and IL-2 production by virus-specific CD4 and CD8 cells. Recent evidence generated in mouse studies has indicated effector memory cells producing IFN-γ only, similar to that described in individuals with high HIV viral loads (16, 18, 19), are short lived in vivo and do not efficiently develop into long-term memory cells (40, 45). Since even prolonged antiviral therapy is associated with only a partial recovery of IL-2–secreting virus-specific CD4 cells (18, 19), it remains to be determined whether additional approaches such as therapeutic immunization might lead to an augmentation of these responder cells and whether this would confer an antiviral benefit. The growing body of evidence supporting the imperative role of CD4 cells in antiviral immunity underscores the importance of boosting virus-specific CD4 memory cells in vaccine design for the generation of effective immune control in chronic viral infections and for the ultimate conquest of HIV.
Acknowledgments
We thank Paul Klenerman for helpful comments and discussion.
References
- 1.Zajac, A.J., J.N. Blattman, K. Murali-Krishna, D.J. Sourdive, M. Suresh, J.D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shedlock, D.J., and H. Shen. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 300:337–339. [DOI] [PubMed] [Google Scholar]
- 3.Sun, J.C., and M.J. Bevan. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 300:339–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matloubian, M., R.J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056–8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janssen, E.M., E.E. Lemmens, T. Wolfe, U. Christen, M.G. von Herrath, and S.P. Schoenberger. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 421:852–856. [DOI] [PubMed] [Google Scholar]
- 6.Battegay, M., D. Moskophidis, A. Rahemtulla, H. Hengartner, T.W. Mak, and R.M. Zinkernagel. 1994. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J. Virol. 68:4700–4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Planz, O., S. Ehl, E. Furrer, E. Horvath, M.A. Brundler, H. Hengartner, and R.M. Zinkernagel. 1997. A critical role for neutralizing-antibody-producing B cells, CD4(+) T cells, and interferons in persistent and acute infections of mice with lymphocytic choriomeningitis virus: implications for adoptive immunotherapy of virus carriers. Proc. Natl. Acad. Sci. USA. 94:6874–6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomsen, A.R., J. Johansen, O. Marker, and J.P. Christensen. 1996. Exhaustion of CTL memory and recrudescence of viremia in lymphocytic choriomeningitis virus-infected MHC class II-deficient mice and B cell-deficient mice. J. Immunol. 157:3074–3080. [PubMed] [Google Scholar]
- 9.Douek, D.C., J.M. Brenchley, M.R. Betts, D.R. Ambrozak, B.J. Hill, Y. Okamoto, J.P. Casazza, J. Kuruppu, K. Kunstman, S. Wolinsky, et al. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 417:95–98. [DOI] [PubMed] [Google Scholar]
- 10.Betts, M.R., D.R. Ambrozak, D.C. Douek, S. Bonhoeffer, J.M. Brenchley, J.P. Casazza, R.A. Koup, and L.J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75:11983–11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harari, A., G.P. Rizzardi, K. Ellefsen, D. Ciuffreda, P. Champagne, P.A. Bart, D. Kaufmann, A. Telenti, R. Sahli, G. Tambussi, et al. 2002. Analysis of HIV-1- and CMV-specific memory CD4 T-cell responses during primary and chronic infection. Blood. 100:1381–1387. [DOI] [PubMed] [Google Scholar]
- 12.Pitcher, C.J., C. Quittner, D.M. Peterson, M. Connors, R.A. Koup, V.C. Maino, and L.J. Picker. 1999. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat. Med. 5:518–525. [DOI] [PubMed] [Google Scholar]
- 13.Palmer, B.E., E. Boritz, N. Blyveis, and C.C. Wilson. 2002. Discordance between frequency of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon-producing CD4(+) T cells and HIV-1-specific lymphoproliferation in HIV-1-infected subjects with active viral replication. J. Virol. 76:5925–5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNeil, A.C., W.L. Shupert, C.A. Iyasere, C.W. Hallahan, J.A. Mican, R.T. Davey, Jr., and M. Connors. 2001. High-level HIV-1 viremia suppresses viral antigen-specific CD4(+) T cell proliferation. Proc. Natl. Acad. Sci. USA. 98:13878–13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg, E.S., J.M. Billingsley, A.M. Caliendo, S.L. Boswell, P.E. Sax, S.A. Kalams, and B.D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 278:1447–1450. [DOI] [PubMed] [Google Scholar]
- 16.Younes, S.-A., Y.-D. Bader, A.R. Dumont, R. Boulassel, Z. Grossman, J.-P. Routy, and R.-P. Sekaly. 2003. HIV-1 viremia prevents the establishment of IL-2–producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J. Exp. Med. 198:1909–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boaz, M.J., A. Waters, S. Murad, P.J. Easterbrook, and A. Vyakarnam. 2002. Presence of HIV-1 Gag-specific IFN-gamma+IL-2+ and CD28+IL-2+ CD4 T cell responses is associated with nonprogression in HIV-1 infection. J. Immunol. 169:6376–6385. [DOI] [PubMed] [Google Scholar]
- 18.Harari, A., S. Petitpierre, F. Vallelian, and G. Pantaleo. 2003. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood. 10.1182/blood-2003-04-1203. [DOI] [PubMed]
- 19.Iyasere, C., J.C. Tilton, A.J. Johnson, S. Younes, B. Yassine-Diab, R.P. Sekaly, W.W. Kwok, S.A. Migueles, A.C. Laborico, W.L. Shupert, et al. 2003. Diminished proliferation of human immunodeficiency virus-specific CD4+ T cells is associated with diminished interleukin-2 (IL-2) production and is recovered by exogenous IL-2. J. Virol. 77:10900–10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg, E.S., M. Altfeld, S.H. Poon, M.N. Phillips, B.M. Wilkes, R.L. Eldridge, G.K. Robbins, R.T. D'Aquila, P.J. Goulder, and B.D. Walker. 2000. Immune control of HIV-1 after early treatment of acute infection. Nature. 407:523–526. [DOI] [PubMed] [Google Scholar]
- 21.Malhotra, U., S. Holte, T. Zhu, E. Delpit, C. Huntsberry, A. Sette, R. Shankarappa, J. Maenza, L. Corey, and M.J. McElrath. 2003. Early induction and maintenance of Env-specific T-helper cells following human immunodeficiency virus type 1 infection. J. Virol. 77:2663–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oxenius, A., S. Fidler, M. Brady, S.J. Dawson, K. Ruth, P.J. Easterbrook, J.N. Weber, R.E. Phillips, and D.A. Price. 2001. Variable fate of virus-specific CD4(+) T cells during primary HIV-1 infection. Eur. J. Immunol. 31:3782–3788. [DOI] [PubMed] [Google Scholar]
- 23.Malhotra, U., M.M. Berrey, Y. Huang, J. Markee, D.J. Brown, S. Ap, L. Musey, T. Schacker, L. Corey, and M.J. McElrath. 2000. Effect of combination antiretroviral therapy on T-cell immunity in acute human immunodeficiency virus type 1 infection. J. Infect. Dis. 181:121–131. [DOI] [PubMed] [Google Scholar]
- 24.Whitmire, J.K., M.S. Asano, K. Murali-Krishna, M. Suresh, and R. Ahmed. 1998. Long-term CD4 Th1 and Th2 memory following acute lymphocytic choriomeningitis virus infection. J. Virol. 72:8281–8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varga, S.M., and R.M. Welsh. 2000. High frequency of virus-specific interleukin-2-producing CD4(+) T cells and Th1 dominance during lymphocytic choriomeningitis virus infection. J. Virol. 74:4429–4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dybul, M., B. Hidalgo, T.W. Chun, M. Belson, S.A. Migueles, J.S. Justement, B. Herpin, C. Perry, C.W. Hallahan, R.T. Davey, et al. 2002. Pilot study of the effects of intermittent interleukin-2 on human immunodeficiency virus (HIV)-specific immune responses in patients treated during recently acquired HIV infection. J. Infect. Dis. 185:61–68. [DOI] [PubMed] [Google Scholar]
- 27.Davey, R.T., Jr., N. Bhat, C. Yoder, T.W. Chun, J.A. Metcalf, R. Dewar, V. Natarajan, R.A. Lempicki, J.W. Adelsberger, K.D. Miller, et al. 1999. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc. Natl. Acad. Sci. USA. 96:15109–15114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 401:708–712. [DOI] [PubMed] [Google Scholar]
- 29.Reinhardt, R.L., A. Khoruts, R. Merica, T. Zell, and M.K. Jenkins. 2001. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 410:101–105. [DOI] [PubMed] [Google Scholar]
- 30.Masopust, D., V. Vezys, A.L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 291:2413–2417. [DOI] [PubMed] [Google Scholar]
- 31.Day, C.L., N.P. Seth, M. Lucas, H. Appel, L. Gauthier, G.M. Lauer, G.K. Robbins, Z.M. Szczepiorkowski, D.R. Casson, R.T. Chung, et al. 2003. Ex vivo analysis of human memory CD4 T cells specific for hepatitis C virus using MHC class II tetramers. J. Clin. Invest. 112:831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuller, M.J., and A.J. Zajac. 2003. Ablation of CD8 and CD4 T cell responses by high viral loads. J. Immunol. 170:477–486. [DOI] [PubMed] [Google Scholar]
- 33.Oxenius, A., R.M. Zinkernagel, and H. Hengartner. 1998. Comparison of activation versus induction of unresponsiveness of virus-specific CD4+ and CD8+ T cells upon acute versus persistent viral infection. Immunity. 9:449–457. [DOI] [PubMed] [Google Scholar]
- 34.Ciurea, A., L. Hunziker, P. Klenerman, H. Hengartner, and R.M. Zinkernagel. 2001. Impairment of CD4(+) T cell responses during chronic virus infection prevents neutralizing antibody responses against virus escape mutants. J. Exp. Med. 193:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wherry, E.J., J.N. Blattman, K. Murali-Krishna, R. van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grakoui, A., N.H. Shoukry, D.J. Woollard, J.-H. Han, H.L. Hanson, J. Ghrayeb, K.K. Murthy, C.M. Rice, and C.M. Walker. 2003. HCV persistence and immune evasion in the absence of memory T cell help. Science. 302:659–662. [DOI] [PubMed] [Google Scholar]
- 37.Gerlach, J.T., H.M. Diepolder, M.C. Jung, N.H. Gruener, W.W. Schraut, R. Zachoval, R. Hoffmann, C.A. Schirren, T. Santantonio, and G.R. Pape. 1999. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology. 117:933–941. [DOI] [PubMed] [Google Scholar]
- 38.Missale, G., R. Bertoni, V. Lamonaca, A. Valli, M. Massari, C. More, M.G. Rumi, M. Houghton, F. Fiaccadori, and C. Ferrari. 1996. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J. Clin. Invest. 98:706–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diepolder, H.M., R. Zachoval, R.M. Hoffmann, E.A. Wierenga, T. Santantonio, M.C. Jung, D. Eichenlaub, and G.R. Pape. 1995. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet. 346:1006–1007. [DOI] [PubMed] [Google Scholar]
- 40.Wherry, E.J., V. Teichgraber, T.C. Becker, D. Masopust, S.M. Kaech, R. Antia, U.H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225–234. [DOI] [PubMed] [Google Scholar]
- 41.Seder, R.A., and R. Ahmed. 2003. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat. Immunol. 4:835–842. [DOI] [PubMed] [Google Scholar]
- 42.Migueles, S.A., A.C. Laborico, W.L. Shupert, M.S. Sabbaghian, R. Rabin, C.W. Hallahan, D. Van Baarle, S. Kostense, F. Miedema, M. McLaughlin, et al. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3:1061–1068. [DOI] [PubMed] [Google Scholar]
- 43.Topp, M.S., S.R. Riddell, Y. Akatsuka, M.C. Jensen, J.N. Blattman, and P.D. Greenberg. 2003. Restoration of CD28 expression in CD28− CD8+ memory effector T cells reconstitutes antigen-induced IL-2 production. J. Exp. Med. 198:947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Addo, M.M., X.G. Yu, A. Rathod, D. Cohen, R.L. Eldridge, D. Strick, M.N. Johnston, C. Corcoran, A.G. Wurcel, C.A. Fitzpatrick, et al. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 77:2081–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu, C.Y., J.R. Kirman, M.J. Rotte, D.F. Davey, S.P. Perfetto, E.G. Rhee, B.L. Freidag, B.J. Hill, D.C. Douek, and R.A. Seder. 2002. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat. Immunol. 3:852–858. [DOI] [PubMed] [Google Scholar]