Abstract
In type 1 diabetes, autoimmune T cells cause destruction of pancreatic β cells by largely unknown mechanism. Previous analyses have shown that β cell destruction is delayed but can occur in perforin-deficient nonobese diabetic (NOD) mice and that Fas-deficient NOD mice do not develop diabetes. However, because of possible pleiotropic functions of Fas, it was not clear whether the Fas receptor was an essential mediator of β cell death in type 1 diabetes. To directly test this hypothesis, we have generated a β cell–specific knockout of the Fas gene in a transgenic model of type 1 autoimmune diabetes in which CD4+ T cells with a transgenic TCR specific for influenza hemagglutinin (HA) are causing diabetes in mice that express HA under control of the rat insulin promoter. Here we show that the Fas-deficient mice develop autoimmune diabetes with slightly accelerated kinetics indicating that Fas-dependent apoptosis of β cells is a dispensable mode of cell death in this disease.
Keywords: Fas receptor, diabetes, β cell death, autoimmunity, conditional knockout
Introduction
Nonobese diabetic (NOD) mice are thought to represent a suitable animal model of human type 1 diabetes (1, 2). In this model, the disease depends on the activation of autoimmune T cells by peptides presented by the particular NOD class II (g7) MHC allele that controls disease. In addition, several T cell receptor transgenic models of type 1 diabetes have been developed in which particularly high frequencies of CD4 or CD8 T cells specific for antigens expressed in β cells cause pancreatic islet cell infiltration with subsequent destruction of β cells (3, 4). In the NOD and in some transgenic models (3, 5, 6), the disease can be precipitated when CD4 T cells from diabetic mice are transferred into immunodeficient recipients where diabetes develops within 1–2 wk. Nevertheless, the mechanism of β cell destruction has remained obscure. It became clear that perforin-deficient NOD mice exhibited a delayed onset of disease (7, 8), but it was not obvious whether the late phase of β cell destruction was affected. On the other hand, it was shown that Fas-deficient lpr/lpr mice on the NOD background did not develop diabetes (9). Additional analyses had suggested that Fas was present on β cells (9) and that expression increased during development of the disease. Other studies, however, also revealed that in Fas-deficient NOD mice there was no infiltration of pancreatic islets by mononuclear cells (10), thus leaving open the question of whether Fas was directly involved in β cell death. More recent studies using a dominant negative Fas mutation (11) or in vivo anti-Fas L antibody treatment (12) were interpreted to indicate that Fas may have a role in promoting predominantly early stages of the disease. To address a potential role of Fas in β cell destruction conclusively, we have produced a conditional Fas allele (fasfl) in which the death domain-encoding exon 9 is flanked by loxP sites, allowing for cell type–specific Fas inactivation (unpublished data). To analyze whether Fas inactivation in pancreatic β cells resulted in resistance to the development of diabetes, the fasfl allele and a rat insulin promoter (RIP)–controlled Cre transgene (13) were introduced into a transgenic model of type 1 diabetes in which a transgenic TCR recognizes peptide 111–119 of influenza hemagglutinin (HA) expressed under control of the RIP and presented by class II Ed MHC molecules (TCR-HA, Ins-HA) (5). The onset and development of diabetes in I-Ed homozygous mice carrying the five transgenes (TCR-HA+/−, Ins-HA+/−, Fasfl/fl, RIP-Cre+/− mice) was compared with that in mice with only four transgenes lacking the RIP-cre transgene (TCR-HA+/−, Ins-HA+/−, Fasfl/fl, RIP-Cre−/− mice) and thereby expressing Fas in β cells.
Materials and Methods
Mice and Genotyping.
TCR-HA mice and Ins-HA mice were bred as heterozygous transgenic mice and are on the BALB/c background. Fasfl mice derived from C57Bl/6 were created by homologous recombination using a conditional Fas allele in which the exon 9 has been flanked by loxP sites. RIP-Cre mice were on a mixed 129sv, C57Bl/6, and DBA-2 background (13). All animals were maintained in a pathogen-free facility in accordance with the guidelines of the Committee on Animals of Harvard Medical School. Genotyping of transgenes was determined by PCR on tail DNA. Primer sequences were 5′-GGCTACCATGCGAACAATTCACCCG-3′ and 5′-CTCCGTCAGCCATAGCAAATTTCTG-3′ for the HA transgene, 5′-ACAAGGTGGCAGTAACAGGA-3′ and 5′-ACAGTCAGTCTGGTTCCTGA-3′ for the TCR-HA transgene, 5′-CGATGCAACGAGTGATGAGG-3′ and 5′-CATCGCTCGACCAGTTTAGT-3′ for the Cre transgene, and 5′-TGCAGTTGCTGAGATGAACCATTTTCTCTGTCT-3′ (P1) and 5′-GGCTTTGGAAAGGAATTTCCTCCTAAGAGG-3′ (P2) for WT and floxed Fas alleles. A 430-bp amplified product indicated the presence of a WT Fas allele, whereas a 470-bp amplified product indicated the presence of a floxed Fas allele, since the sense primer was located in exon 9 and the antisense one 3′ to the loxP site downstream of exon 9 (Fig. 1 A). Homozygosity for I-Ed was determined by staining peripheral blood leukocytes.
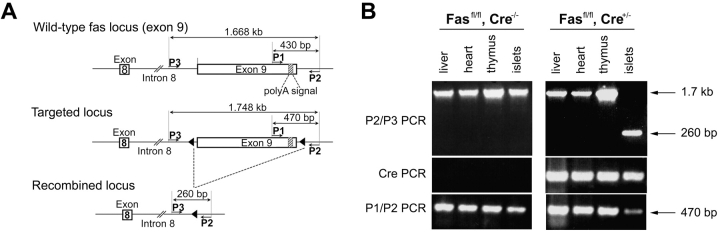
Figure 1.
Successful elimination of the floxed Fas–exon 9 by homologous recombination in pancreatic β cells. (A) Representation of WT Fas, floxed Fas, and recombined floxed Fas alleles, and location of the primers used in PCR analyses. Cre-mediated recombination of the floxed Fas allele results in the deletion of exon 9. (B) PCR analysis on DNA from liver, heart, thymus, and purified islets of one Fasfl homozygous and one Fasfl homozygous, RIP-Cre heterozygous mouse.
RIP-Cre–mediated Fas Recombination in Islet DNA.
DNA was prepared from the thymus, liver, heart, and islets isolated at the Harvard Medical School Islet Core Rodent Isolation. RIP-Cre–mediated Fas recombination was assessed using a sense primer with the sequence 5′-GTCCTCTATTATCCTCATCATGAG-3′ (P3) located upstream of the 5′ loxP site and the antisense primer located downstream of the 3′ loxP site (P2). A 1.7-kb amplified product indicated the presence of intact exon 9, whereas a 260-bp amplified product indicated the presence of a deleted exon 9.
Diabetes Monitoring and Cyclophosphamide Administration.
Development of spontaneous diabetes was assessed by measuring blood glucose twice a week with an automatic glucometer (Accu-chek Advantage; Roche). Cyclophosphamide (Sigma-Aldrich) in PBS was injected intraperitoneally at a dose of 200 mg/kg. Blood glucose levels of cyclophosphamide-treated mice were monitored daily.
Adoptive Transfer of Diabetes.
CD4+ TCR-HA–expressing cells were purified by sorting lymphocytes from spleen and LN of RAG-2−/−, TCR-HA+/− mice stained with the 6.5 (anti–TCR-HA) and anti-CD4 (GK1.5; BD Biosciences) mAbs. 105 sorted 6.5+CD4+ cells were i.v. injected into either RAG-2−/−, Fasfl/fl, Rip-cre+/−, Ins-HA+/− or RAG-2−/−, Fasfl/fl, Rip-cre−/−, Ins-HA+/− mice. Blood glucose levels of the recipients were tested daily.
Results and Discussion
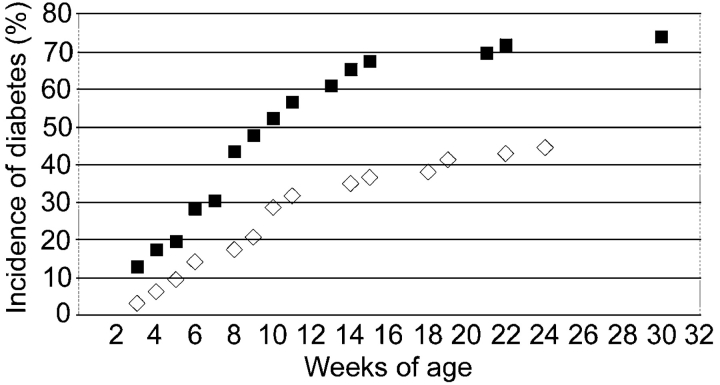
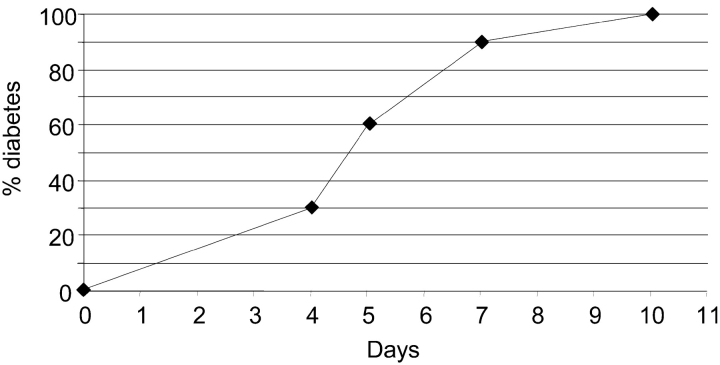
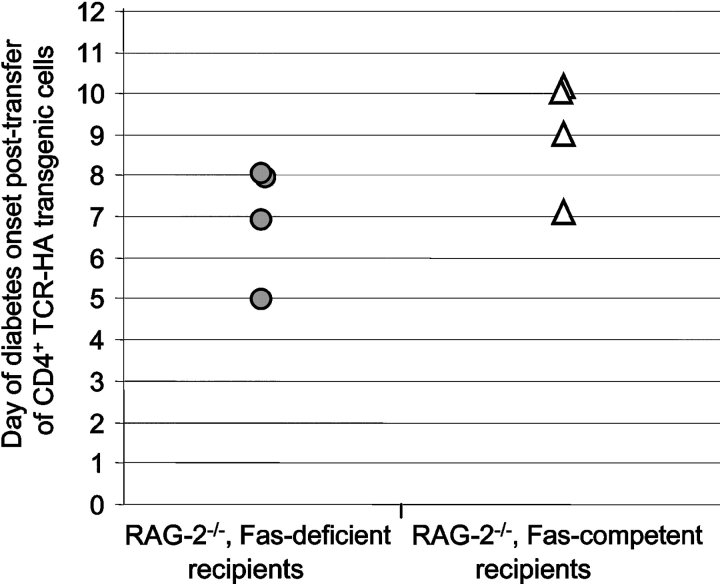
The efficacy and tissue specificity of Cre-mediated deletion of Fas–exon 9 was assessed by PCR on DNA prepared from purified islets and various tissues from Cre-expressing and -nonexpressing Fasfl homozygous mice. Fig. 1 A depicts a schematic representation of mutated and WT Fas alleles and the position of the primers used to assess littermate genotype and β cell-specific loss of functional Fas allele. Fig. 1 B shows that, regardless of Cre expression, DNA of nonpancreatic origin yielded a single 1.7-kb P2/P3 PCR product indicative of the sole presence of Fas–exon 9 (Fig. 1 A). Even traces of recombined Fas–exon 9 would have been detected by the presence of a 260-bp fragment, which because of its short size would be favored over the 1.7-kb fragment. Evidence for islet-specific Cre-mediated deletion of exon 9 came from the single 260-bp P2/P3 PCR product that was obtained from islets of Cre-expressing mice, whereas a single 1.7-kb band was detected from the islets of Cre-nonexpressing mice. PCR analysis of the same samples using the P1/P2 primers that hybridize to WT nonrecombined but not to recombined Fas alleles (Fig. 1 A) yielded a single 470-bp band in nonpancreatic tissues of mice irrespective of RIP-Cre expression and in islets of mice lacking RIP-Cre (Fig. 1 B). A much fainter 470-bp band was seen in islets of Cre-expressing mice (Fig. 1 B). This faint band is likely to reflect the fact that whole islets such as those used in the present study contain cells other than β cells (∼30%), e.g., non–β endocrine cells, macrophages, endothelial cells, etc., lacking Cre expression. Thus, the absence of amplification of a 260-bp P2/P3 PCR product (which will be favored over the 1.7-kb P2/P3 PCR product) in nonpancreatic tissues and its detection only in Cre-expressing islets and the diminution of the 470-bp fragment intensity in Cre-expressing islets strongly suggest that the RIP-Cre–mediated recombination resulted in tissue-specific deletion of functional Fas in the vast majority of β cells. Since the development of diabetes with blood sugar levels exceeding 200 mg/dl requires destruction of well over 50% of all β cells, the conditional knockout was suitable to reveal a possible essential role of Fas in the development of diabetes, since any Fas-dependent destruction of β cells could not exceed 50% in these particular mice, and thus be insufficient to result in diabetes. Previous work using a RIP-Cre transgenic mouse line identical to that of the present study could not find evidence for a toxic effect of the transgene to the β cells, their function, or the glucose tolerance of RIP-Cre transgenic mice (13, 14). Furthermore, the Fasfl heterozygous, TCR-HA, Ins-HA, RIP-Cre transgenic breeders used in this study to generate the Fas-competent and -deficient TCR-HA, Ins-HA mice behaved identically to TCR-HA, Ins-HA double transgenic mice with respect to diabetes development, fertility, and frequency of the expected genotypes among their litter. Thus, we could exclude a role for the RIP-Cre transgene in potential phenotypical differences between RIP-Cre–expressing and –nonexpressing mice. To assess the impact of Fas expression by β cells in their T cell–mediated destruction, we compared the development of spontaneous autoimmune diabetes in the TCR-HA, Ins-HA transgenic model in which the floxed Fas alleles are recombined by the Cre recombinase (TCR-HA+/−, Ins-HA+/−, Fasfl/fl, RIP-Cre+/− mice) to that of transgenic littermates lacking Cre expression (TCR-HA+/−, Ins-HA+/−, Fasfl/fl, RIP-Cre−/− mice) (Fig. 2) . The incidence and onset of diabetes in the latter quadruple transgenic littermates was not different from that in TCR-HA, Ins-HA double transgenic mice, indicating that neither the introduced floxed Fas alleles nor the mixed genetic background brought by the Fas floxed and RIP-Cre transgenic mice did perturb autoimmunity. The elimination of Fas by Cre recombinase did not produce a delay in the onset but some acceleration of the onset of diabetes (Fig. 2). A similar conclusion, namely that Fas deficiency did not delay the onset of diabetes, was reached when diabetes was analyzed in TCR-HA, Ins-HA mice transgenic mice with a β cell–specific deletion of Fas that were treated with 200 mg/kg cyclosphosphamide. This treatment is known to precipitate diabetes in NOD and TCR-HA, Ins-HA mice with similar kinetics, perhaps by interfering with regulatory (suppressive) T cells that control the development of disease. The RIP-Cre–expressing mice came down with diabetes 4–5 d after treatment with the drug, indicating that also under these conditions Fas did not play a discernible role in β cell death caused by autoimmune T cells (Fig. 3) . Finally, it was tested whether purified CD4+ TCR-HA–expressing T cells from TCR-HA transgenic RAG-2−/− mice could cause β cell destruction in the absence of CD8+ T cells by transferring them into Ins-HA transgenic mice on the RAG-2−/− background lacking or expressing fas on β cells. As shown in Fig. 4 , diabetes developed in both types of recipients within a short time period after injection of 105 CD4+ T cells.
Figure 2.
Effective destruction of β-islet cells from Fas-deficient and Fas-competent TCR-HA, Ins-HA transgenic mice. Blood glucose levels of Fasfl/fl, Rip-Cre+/−, TCR-HA+/−, Ins-HA+/− (▪, n = 46) and Fasfl/fl, Rip-Cre−/−, TCR-HA+/−, Ins-HA+/− (⋄, n = 63) mice were assessed over a period of 32 wk. Mice were considered diabetic after two successive blood glucose measurements ≥300 mg/dl.
Figure 3.
Cyclophosphamide-induced diabetes in TCR-HA+/−, Ins-HA+/−, Fasfl/fl, RIP-Cre+/− mice. Mice (n = 10) were injected with 200 mg/kg of cyclophosphamide on day 0 and monitored for their blood glucose levels every day.
Figure 4.
Development of diabetes in Fas-deficient and Fas-competent RAG-2−/−, Ins-HA+/− recipients injected with CD4+ TCR-HA transgenic cells. 6.5+CD4+ cells from RAG-2−/−, TCR-HA+/− mice were sorted, and 105 cells were transferred into RAG-2−/−, Fasfl/fl, Rip-cre+/−, Ins-HA+/− (•, n = 4) and RAG-2−/−, Fasfl/fl, Rip-cre−/−, Ins-HA+/− (▵, n = 4) on day zero. Blood glucose levels were monitored daily.
In both the NOD model (2) and in several TCR transgenic models (3, 5, 6), the autoimmune disease can be transferred with purified CD4 T cells into immunodeficient mice that themselves do not have any T cells. Since only a few activated CD4+ T cells express perforin but most express Fas ligand, it was conceivable that such T cells destroyed β cells by a Fas L–Fas interaction that required direct cell contact. Our findings refute this hypothesis and are consistent with the notion that β cells from Fas-deficient mice that were transplanted into Fas-competent mice are effectively destroyed by diabetogenic T cells (14–16) even though it is not clear in the latter scenario whether destruction of the transplanted β cells occurs by the same mechanism as that of β cells in situ. Our results are also consistent with the finding that a dominant negative Fas transgene in NOD mice had little effect on the development of diabetes when splenocytes from diabetic animals were transferred into x-irradiated transgenic nondiabetic recipients (11).
By eliminating the Fas L–Fas apoptotic system as the essential mediator of β cell death in type 1 diabetes, our results should shift the focus to other mediators of apoptotic cell death that do not require direct contact between T cells and β cells. With regard to this notion, we may point out that there is apparently no significant expression of class II MHC on β islet cells (1) and perhaps even more conclusively that antigen-specific contact between T cells and β cells is not required for the T cell–mediated destruction of β cells (17). Thus, the search for mediators of β cell destruction needs to focus on molecules that play an essential role in β cell destruction in type 1 autoimmune diabetes and differ from Fas. The identification of such essential mediators of β cell death would perhaps permit interference with the disease once the process of destruction has begun and first symptoms are detectable.
Acknowledgments
We thank R.N. Kulkarni and C.R. Kahn (Joslin Diabetes Center, Boston, MA) for providing the RIP-Cre mice.
H. von Boehmer and K. Rajewsky were supported by the Koerber Foundation, Hamburg, Germany, in the form of the Koerber Prize for European Science in 1997 and by the Juvenile Diabetes Research Foundation.
Abbreviations used in this paper: HA, hemagglutinin; NOD, nonobese diabetic; RIP, rat insulin promoter.
References
- 1.Bach, J.F., and D. Mathis. 1997. The NOD mouse. Res. Immunol. 148:285–286. [DOI] [PubMed] [Google Scholar]
- 2.Tisch, R., and H. McDevitt. 1996. Insulin-dependent diabetes mellitus. Cell. 85:291–297. [DOI] [PubMed] [Google Scholar]
- 3.Degermann, S., C. Reilly, B. Scott, L. Ogata, H. von Boehmer, and D. Lo. 1994. On the various manifestations of spontaneous autoimmune diabetes in rodent models. Eur. J. Immunol. 24:3155–3160. [DOI] [PubMed] [Google Scholar]
- 4.Scott, B., R. Liblau, S. Degermann, L.A. Marconi, L. Ogata, A.J. Caton, H.O. McDevitt, and D. Lo. 1994. A role for non-MHC genetic polymorphism in susceptibility to spontaneous autoimmunity. Immunity. 1:73–83. [DOI] [PubMed] [Google Scholar]
- 5.Sarukhan, A., A. Lanoue, A. Franzke, N. Brousse, J. Buer, and H. von Boehmer. 1998. Changes in function of antigen-specific lymphocytes correlating with progression towards diabetes in a transgenic model. EMBO J. 17:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz, J.D., C. Benoist, and D. Mathis. 1995. T helper cell subsets in insulin-dependent diabetes. Science. 268:1185–1188. [DOI] [PubMed] [Google Scholar]
- 7.Kagi, D., B. Odermatt, P. Seiler, R.M. Zinkernagel, T.W. Mak, and H. Hengartner. 1997. Reduced incidence and delayed onset of diabetes in perforin-deficient nonobese diabetic mice. J. Exp. Med. 186:989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amrani, A., J. Verdaguer, B. Anderson, T. Utsugi, S. Bou, and P. Santamaria. 1999. Perforin-independent beta-cell destruction by diabetogenic CD8(+) T lymphocytes in transgenic nonobese diabetic mice. J. Clin. Invest. 103:1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chervonsky, A.V., Y. Wang, F.S. Wong, I. Visintin, R.A. Flavell, C.A. Janeway, Jr., and L.A. Matis. 1997. The role of Fas in autoimmune diabetes. Cell. 89:17–24. [DOI] [PubMed] [Google Scholar]
- 10.Itoh, N., A. Imagawa, T. Hanafusa, M. Waguri, K. Yamamoto, H. Iwahashi, M. Moriwaki, H. Nakajima, J. Miyagawa, M. Namba, et al. 1997. Requirement of Fas for the development of autoimmune diabetes in nonobese diabetic mice. J. Exp. Med. 186:613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savinov, A.Y., A. Tcherepanov, E.A. Green, R.A. Flavell, and A.V. Chervonsky. 2003. Contribution of Fas to diabetes development. Proc. Natl. Acad. Sci. USA. 100:628–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakayama, M., M. Nagata, H. Yasuda, K. Arisawa, R. Kotani, K. Yamada, S.A. Chowdhury, S. Chakrabarty, Z.Z. Jin, H. Yagita, et al. 2002. Fas/Fas ligand interactions play an essential role in the initiation of murine autoimmune diabetes. Diabetes. 51:1391–1397. [DOI] [PubMed] [Google Scholar]
- 13.Kulkarni, R.N., J.C. Bruning, J.N. Winnay, C. Postic, M.A. Magnuson, and C.R. Kahn. 1999. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 96:329–339. [DOI] [PubMed] [Google Scholar]
- 14.Allison, J., and A. Strasser. 1998. Mechanisms of beta cell death in diabetes: a minor role for CD95. Proc. Natl. Acad. Sci. USA. 95:13818–13822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim, Y.H., S. Kim, K.A. Kim, H. Yagita, N. Kayagaki, K.W. Kim, and M.S. Lee. 1999. Apoptosis of pancreatic beta-cells detected in accelerated diabetes of NOD mice: no role of Fas-Fas ligand interaction in autoimmune diabetes. Eur. J. Immunol. 29:455–465. [DOI] [PubMed] [Google Scholar]
- 16.Pakala, S.V., M. Chivetta, C.B. Kelly, and J.D. Katz. 1999. In autoimmune diabetes the transition from benign to pernicious insulitis requires an islet cell response to tumor necrosis factor alpha. J. Exp. Med. 189:1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarukhan, A., O. Lechner, and H. von Boehmer. 1999. Autoimmune insulitis and diabetes in the absence of antigen-specific contact between T cells and islet beta-cells. Eur. J. Immunol. 29:3410–3416. [DOI] [PubMed] [Google Scholar]