Abstract
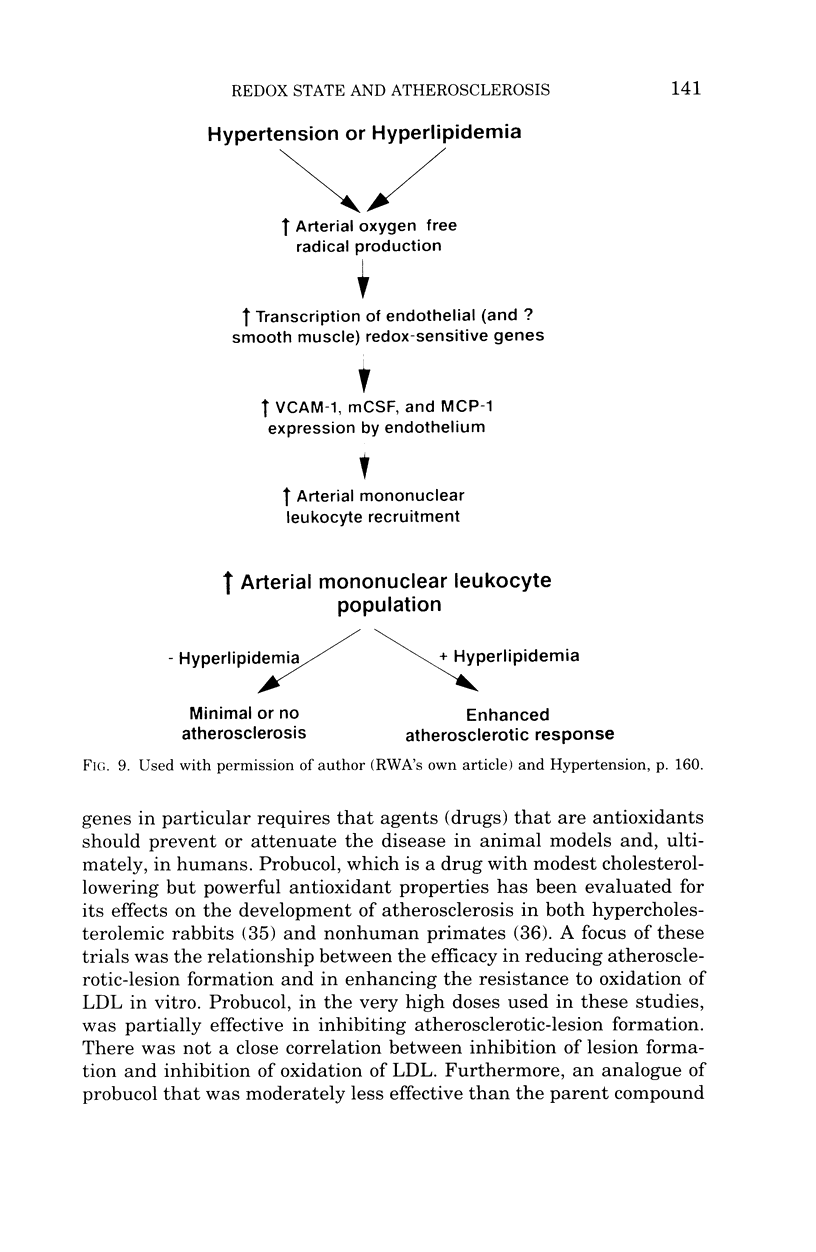
Accumulating evidence provides a compelling case that one of the major pathophysiologic mechanisms involved in the pathogenesis of atherosclerosis is enhanced oxidative stress and that the most important manifestation of this altered redox state is the modulation of a set(s) of proinflammatory genes that are regulated directly or indirectly by reactive oxygen species. Viewed in this perspective the oxidation of LDL is but one important consequence of a generalized metabolic abnormality of the arterial wall in atherosclerosis rather than being the core pathophysiological feature. The fact that hypercholesterolemia, hypertension, and AGEs related to diabetes mellitus all activate similar redox-sensitive proinflammatory genes associated with the pathogenesis of atherosclerosis provides the potential for the development of unifying concepts concerning the etiology of the disease. These concepts also provide additional evidence that antioxidants may be potentially attractive therapeutic agents.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander R. W. Theodore Cooper Memorial Lecture. Hypertension and the pathogenesis of atherosclerosis. Oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension. 1995 Feb;25(2):155–161. doi: 10.1161/01.hyp.25.2.155. [DOI] [PubMed] [Google Scholar]
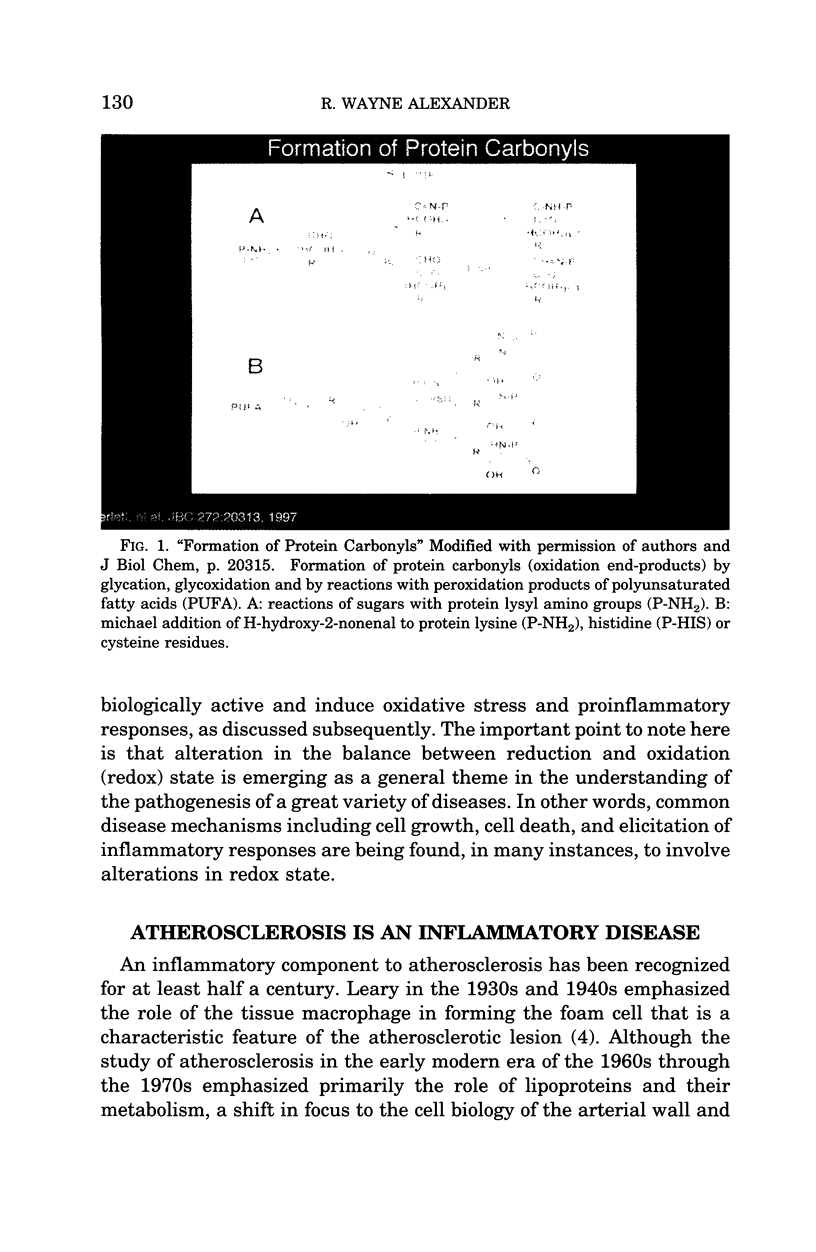
- Berlett B. S., Stadtman E. R. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997 Aug 15;272(33):20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
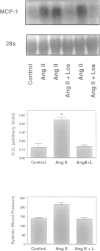
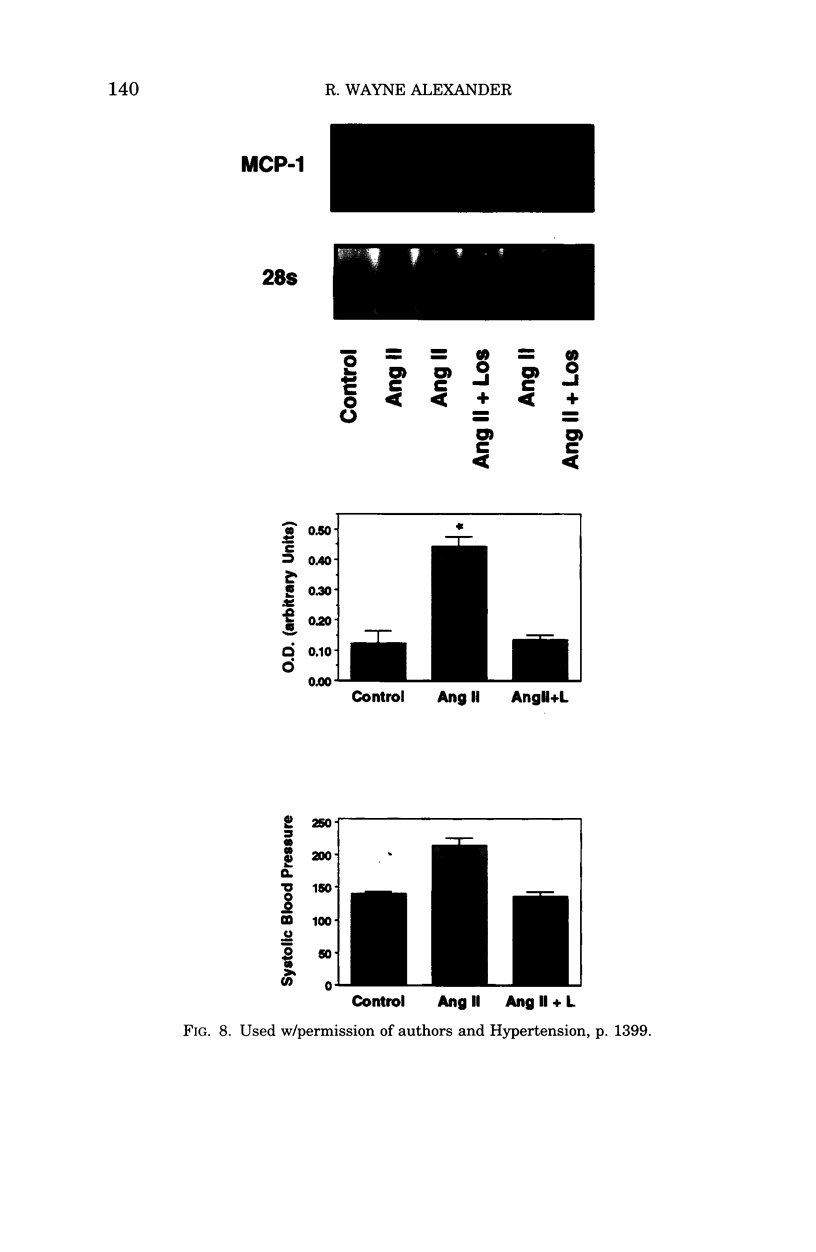
- Capers Q., 4th, Alexander R. W., Lou P., De Leon H., Wilcox J. N., Ishizaka N., Howard A. B., Taylor W. R. Monocyte chemoattractant protein-1 expression in aortic tissues of hypertensive rats. Hypertension. 1997 Dec;30(6):1397–1402. doi: 10.1161/01.hyp.30.6.1397. [DOI] [PubMed] [Google Scholar]
- Cybulsky M. I., Gimbrone M. A., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991 Feb 15;251(4995):788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- Faggiotto A., Ross R., Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984 Jul-Aug;4(4):323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- Fruebis J., Steinberg D., Dresel H. A., Carew T. E. A comparison of the antiatherogenic effects of probucol and of a structural analogue of probucol in low density lipoprotein receptor-deficient rabbits. J Clin Invest. 1994 Jul;94(1):392–398. doi: 10.1172/JCI117334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Galis Z. S., Sukhova G. K., Lark M. W., Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994 Dec;94(6):2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. Am J Pathol. 1981 May;103(2):191–200. [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Bevilacqua M. P., Cybulsky M. I. Endothelial-dependent mechanisms of leukocyte adhesion in inflammation and atherosclerosis. Ann N Y Acad Sci. 1990;598:77–85. doi: 10.1111/j.1749-6632.1990.tb42279.x. [DOI] [PubMed] [Google Scholar]
- Harrison D. G., Freiman P. C., Armstrong M. L., Marcus M. L., Heistad D. D. Alterations of vascular reactivity in atherosclerosis. Circ Res. 1987 Nov;61(5 Pt 2):II74–II80. [PubMed] [Google Scholar]
- Iademarco M. F., McQuillan J. J., Rosen G. D., Dean D. C. Characterization of the promoter for vascular cell adhesion molecule-1 (VCAM-1). J Biol Chem. 1992 Aug 15;267(23):16323–16329. [PubMed] [Google Scholar]
- Libby P. Atheroma: more than mush. Lancet. 1996 Nov;348 (Suppl 1):s4–s7. doi: 10.1016/s0140-6736(96)98002-2. [DOI] [PubMed] [Google Scholar]
- Ludmer P. L., Selwyn A. P., Shook T. L., Wayne R. R., Mudge G. H., Alexander R. W., Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986 Oct 23;315(17):1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
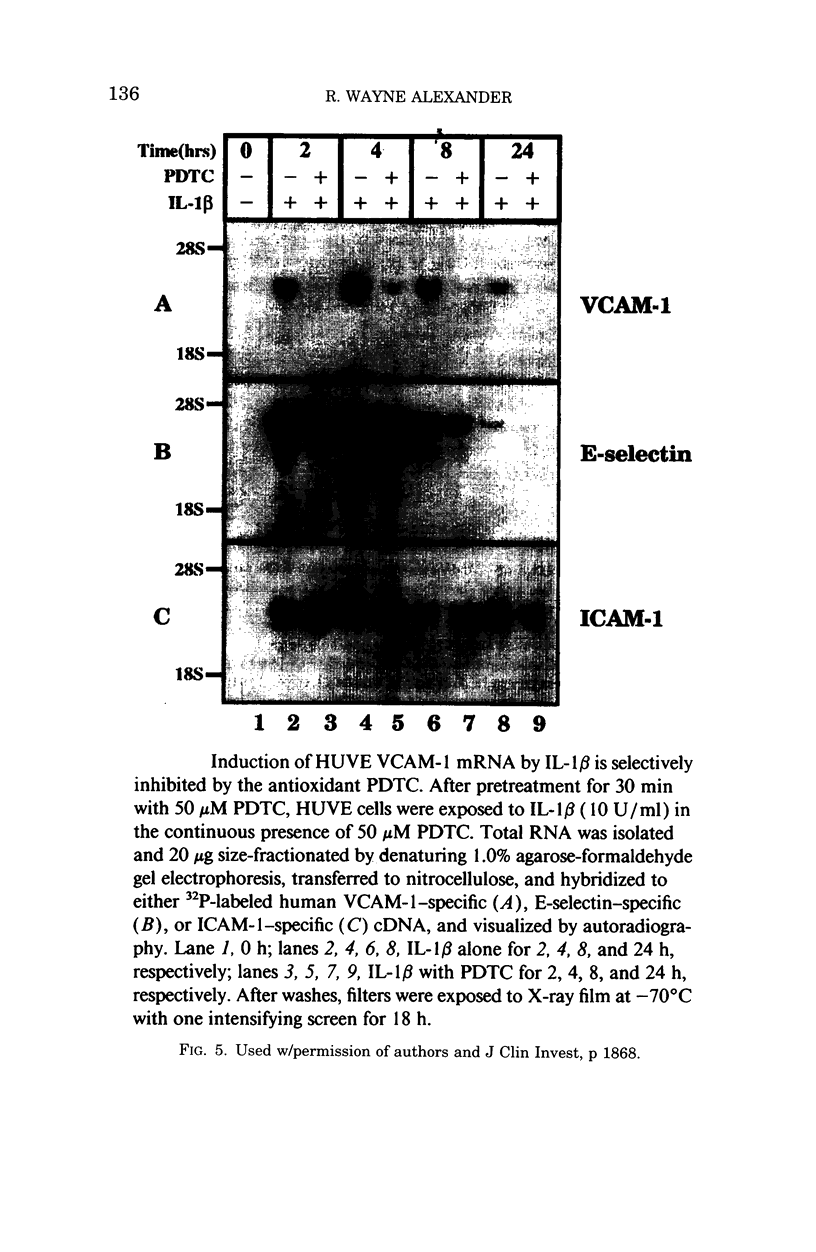
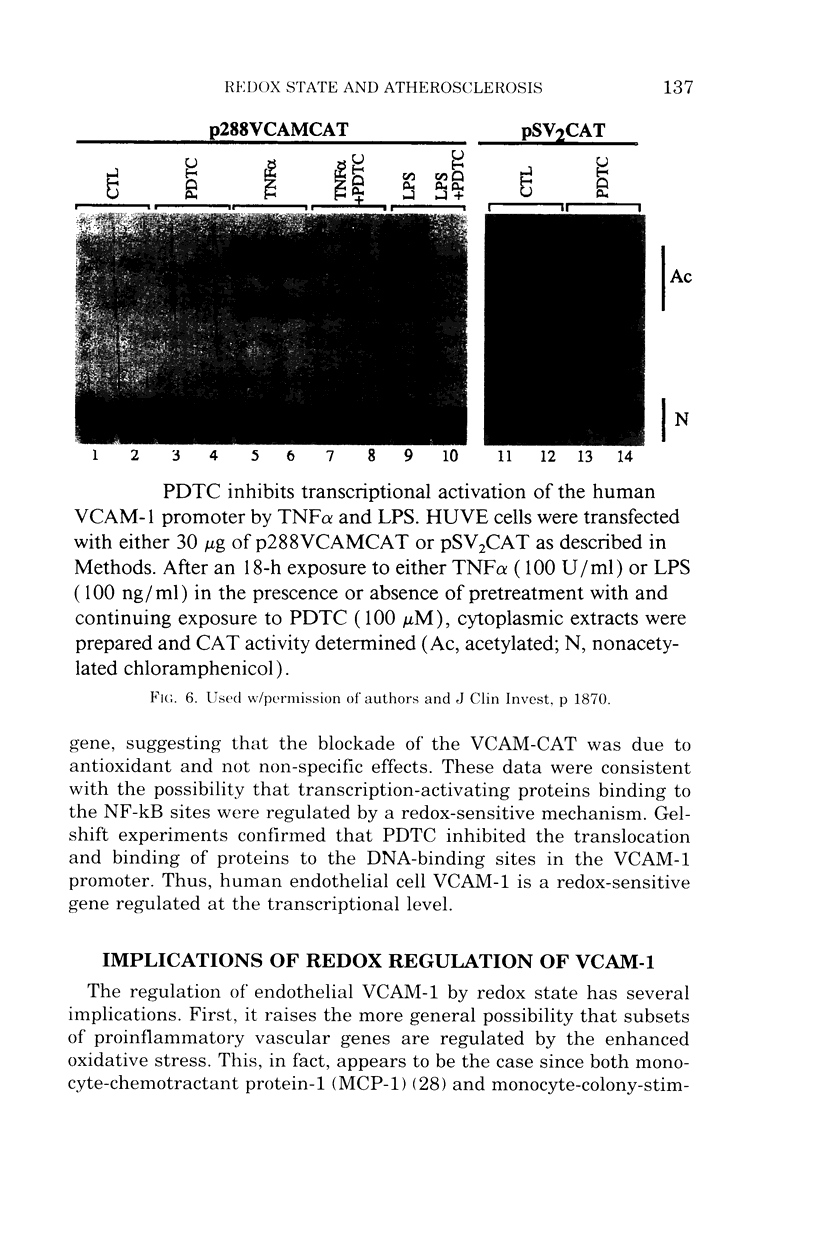
- Marui N., Offermann M. K., Swerlick R., Kunsch C., Rosen C. A., Ahmad M., Alexander R. W., Medford R. M. Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J Clin Invest. 1993 Oct;92(4):1866–1874. doi: 10.1172/JCI116778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro J. M., Cotran R. S. The pathogenesis of atherosclerosis: atherogenesis and inflammation. Lab Invest. 1988 Mar;58(3):249–261. [PubMed] [Google Scholar]
- Mügge A., Elwell J. H., Peterson T. E., Hofmeyer T. G., Heistad D. D., Harrison D. G. Chronic treatment with polyethylene-glycolated superoxide dismutase partially restores endothelium-dependent vascular relaxations in cholesterol-fed rabbits. Circ Res. 1991 Nov;69(5):1293–1300. doi: 10.1161/01.res.69.5.1293. [DOI] [PubMed] [Google Scholar]
- Neish A. S., Williams A. J., Palmer H. J., Whitley M. Z., Collins T. Functional analysis of the human vascular cell adhesion molecule 1 promoter. J Exp Med. 1992 Dec 1;176(6):1583–1593. doi: 10.1084/jem.176.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara Y., Peterson T. E., Harrison D. G. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993 Jun;91(6):2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara Y., Peterson T. E., Sayegh H. S., Subramanian R. R., Wilcox J. N., Harrison D. G. Dietary correction of hypercholesterolemia in the rabbit normalizes endothelial superoxide anion production. Circulation. 1995 Aug 15;92(4):898–903. doi: 10.1161/01.cir.92.4.898. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S., Kurz S., Münzel T., Tarpey M., Freeman B. A., Griendling K. K., Harrison D. G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996 Apr 15;97(8):1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Williamson J. R., Brownlee M. Glucose and diabetic vascular disease. FASEB J. 1992 Aug;6(11):2905–2914. doi: 10.1096/fasebj.6.11.1644256. [DOI] [PubMed] [Google Scholar]
- Sasahara M., Raines E. W., Chait A., Carew T. E., Steinberg D., Wahl P. W., Ross R. Inhibition of hypercholesterolemia-induced atherosclerosis in the nonhuman primate by probucol. I. Is the extent of atherosclerosis related to resistance of LDL to oxidation? J Clin Invest. 1994 Jul;94(1):155–164. doi: 10.1172/JCI117301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A. M., Hori O., Chen J. X., Li J. F., Crandall J., Zhang J., Cao R., Yan S. D., Brett J., Stern D. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995 Sep;96(3):1395–1403. doi: 10.1172/JCI118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A. M., Vianna M., Gerlach M., Brett J., Ryan J., Kao J., Esposito C., Hegarty H., Hurley W., Clauss M. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992 Jul 25;267(21):14987–14997. [PubMed] [Google Scholar]
- Schreck R., Meier B., Männel D. N., Dröge W., Baeuerle P. A. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992 May 1;175(5):1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Stemme S., Holm J., Hansson G. K. T lymphocytes in human atherosclerotic plaques are memory cells expressing CD45RO and the integrin VLA-1. Arterioscler Thromb. 1992 Feb;12(2):206–211. doi: 10.1161/01.atv.12.2.206. [DOI] [PubMed] [Google Scholar]
- Wilson S. K. Role of oxygen-derived free radicals in acute angiotensin II--induced hypertensive vascular disease in the rat. Circ Res. 1990 Mar;66(3):722–734. doi: 10.1161/01.res.66.3.722. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Palinski W., Butler S. W., Picard S., Steinberg D., Witztum J. L. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb. 1994 Jan;14(1):32–40. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Palinski W., Rosenfeld M. E., Parthasarathy S., Carew T. E., Butler S., Witztum J. L., Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989 Oct;84(4):1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wal A. C., Becker A. E., van der Loos C. M., Das P. K. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. 1994 Jan;89(1):36–44. doi: 10.1161/01.cir.89.1.36. [DOI] [PubMed] [Google Scholar]