Abstract
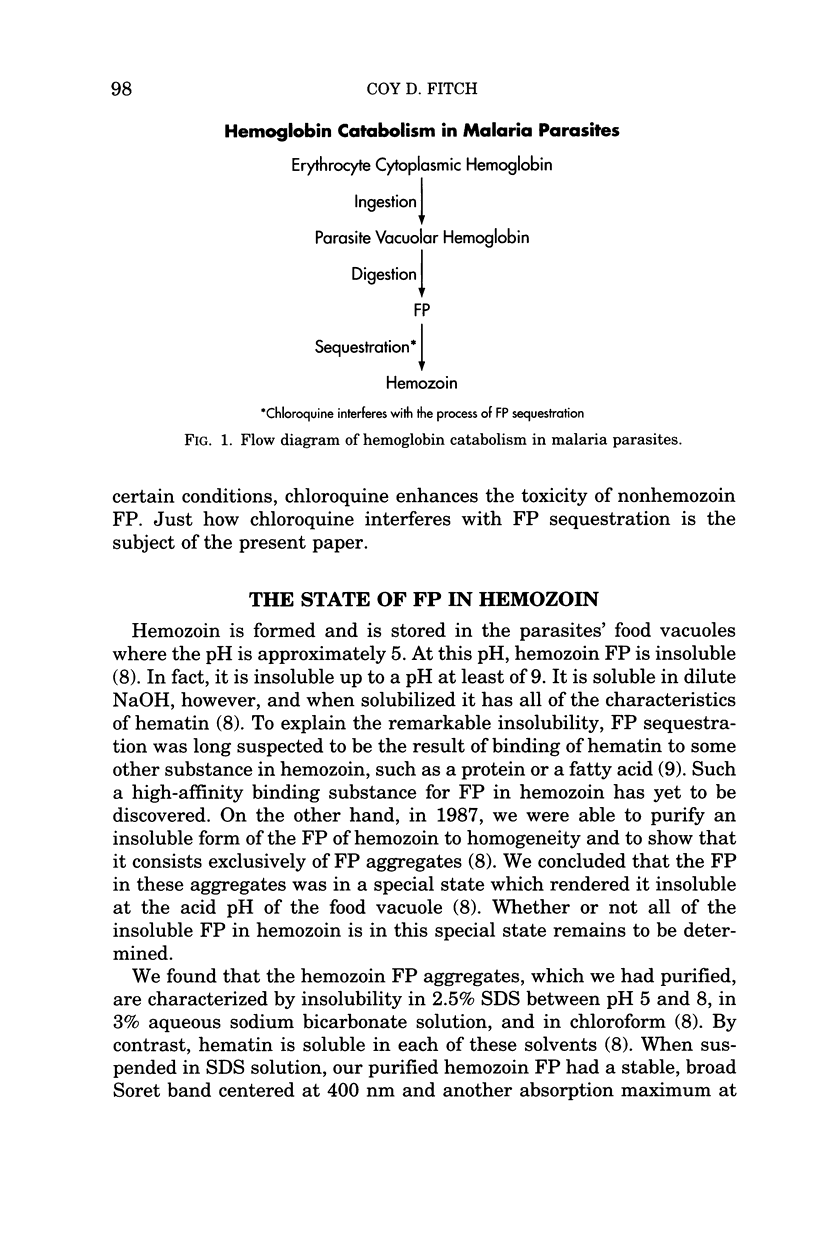
When malaria parasites digest hemoglobin, they release FP intracellularly. FP is an oxidized form of heme which is toxic for biological membranes. The parasites are not poisoned when they digest hemoglobin, however, because they sequester FP in hemozoin. In fact, the refractile, dark brown substance in hemozoin is sequestered FP. Chloroquine binds tightly to nonhemozoin FP and, under certain circumstances, enhances its toxicity. In addition, chloroquine interferes with FP sequestration and causes toxic nonhemozoin FP to accumulate to lethal levels in erythrocytes parasitized with malaria parasites. Evidently, this is how chloroquine kills malaria parasites. It is desirable, therefore, to know more about FP sequestration and how it is affected by chloroquine. Malaria parasites possess a catalyst for FP sequestration which is modulated by treatment with quinoline antimalarial drugs such as chloroquine and quinine. Chloroquine treatment causes the activity of the catalyst to decrease by 80 to 90 percent. Quinine treatment has no obvious direct effect on the catalyst for FP sequestration. Nevertheless, quinine treatment antagonizes and reverses the chloroquine-induced loss of ability to sequester FP. The effect of chloroquine treatment also is antagonized by various metabolic inhibitors, including inhibitors of protein biosynthesis such as cycloheximide. These findings indicate that chloroquine, like quinine, does not interact directly with the catalyst for FP sequestration. Instead, they are evidence that chloroquine acts by increasing the amount, accessibility, or reactivity of a regulator of the catalyst for FP sequestration. I propose that chloroquine increases the amount of the regulator, which inactivates the catalyst for FP sequestration, which leads to accumulation of nonhemozoin FP, which binds with high-affinity to chloroquine and which ultimately kills the malaria parasite.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
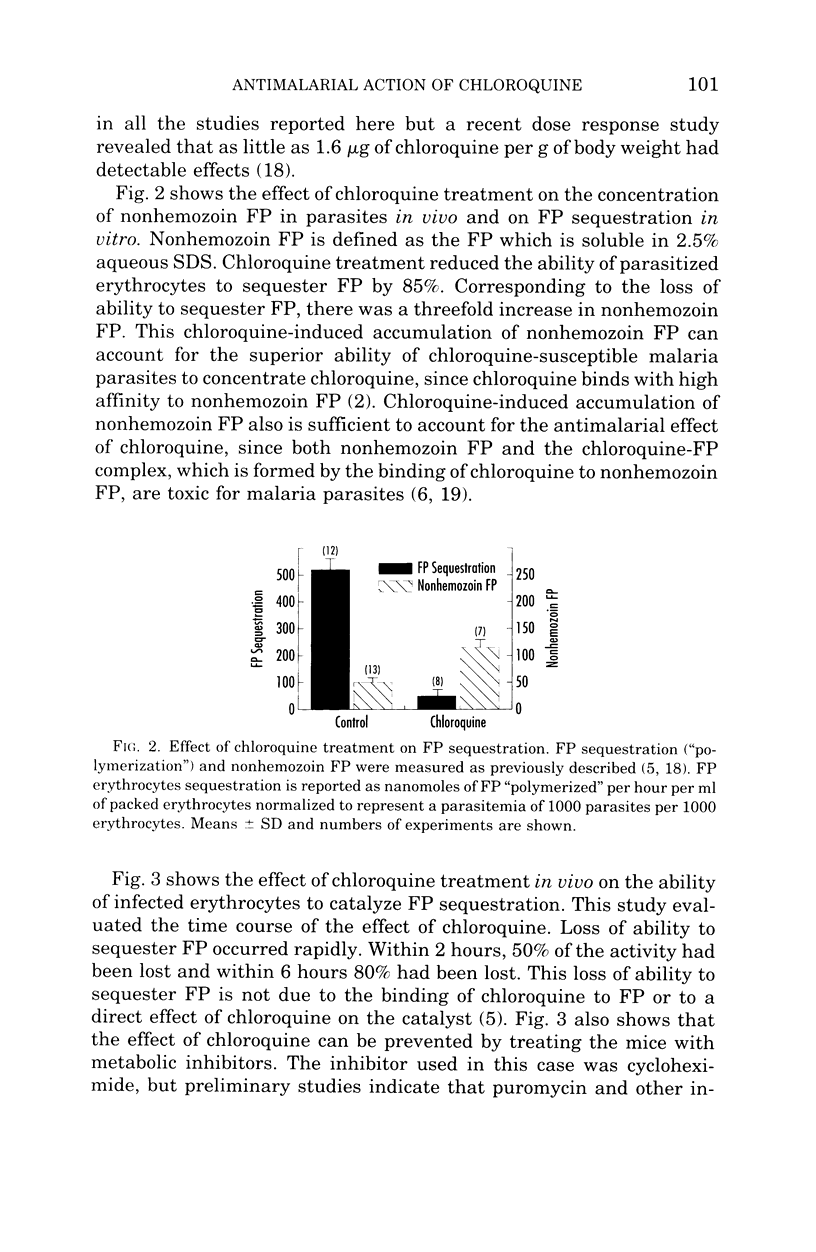
- Bendrat K., Berger B. J., Cerami A. Haem polymerization in malaria. Nature. 1995 Nov 9;378(6553):138–139. doi: 10.1038/378138a0. [DOI] [PubMed] [Google Scholar]
- Bohle D. S., Dinnebier R. E., Madsen S. K., Stephens P. W. Characterization of the products of the heme detoxification pathway in malarial late trophozoites by X-ray diffraction. J Biol Chem. 1997 Jan 10;272(2):713–716. doi: 10.1074/jbc.272.2.713. [DOI] [PubMed] [Google Scholar]
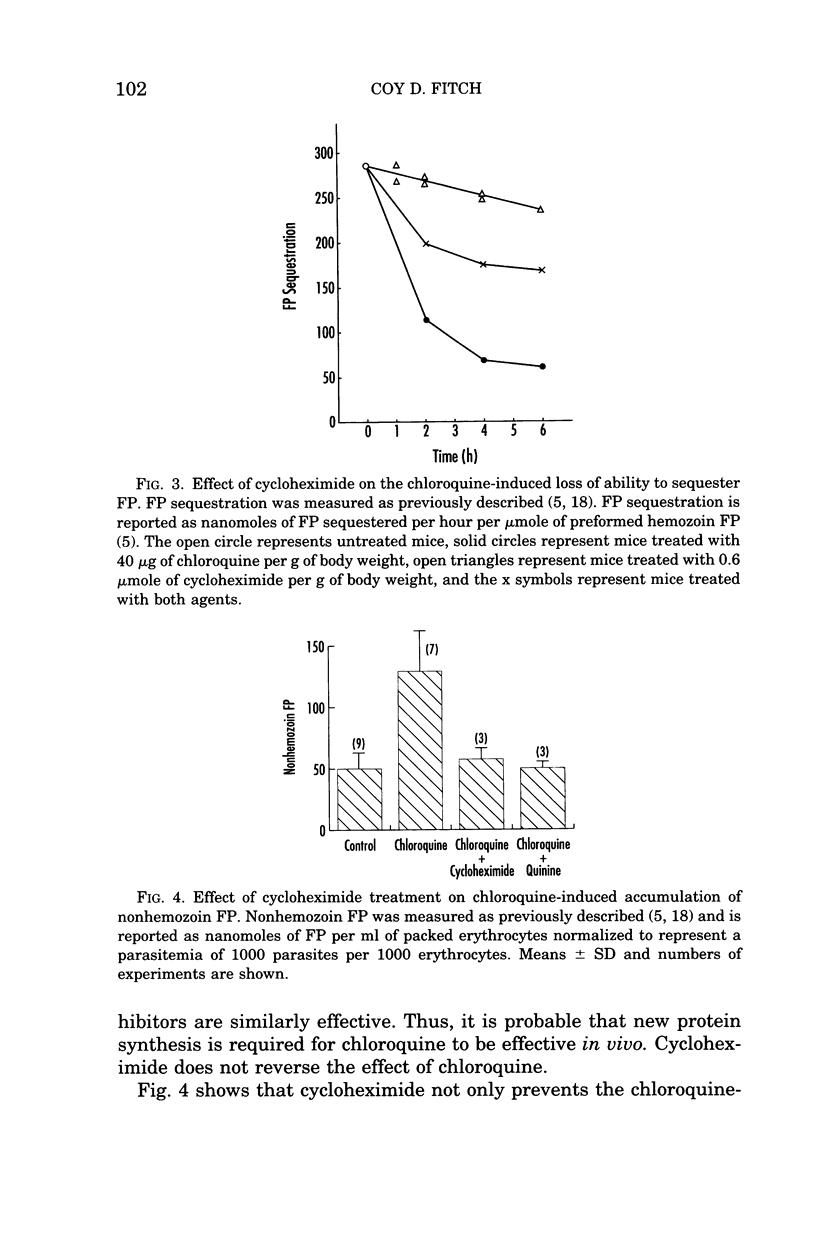
- Chou A. C., Chevli R., Fitch C. D. Ferriprotoporphyrin IX fulfills the criteria for identification as the chloroquine receptor of malaria parasites. Biochemistry. 1980 Apr 15;19(8):1543–1549. doi: 10.1021/bi00549a600. [DOI] [PubMed] [Google Scholar]
- Chou A. C., Fitch C. D. Control of heme polymerase by chloroquine and other quinoline derivatives. Biochem Biophys Res Commun. 1993 Aug 31;195(1):422–427. doi: 10.1006/bbrc.1993.2060. [DOI] [PubMed] [Google Scholar]
- Chou A. C., Fitch C. D. Heme polymerase: modulation by chloroquine treatment of a rodent malaria. Life Sci. 1992;51(26):2073–2078. doi: 10.1016/0024-3205(92)90158-l. [DOI] [PubMed] [Google Scholar]
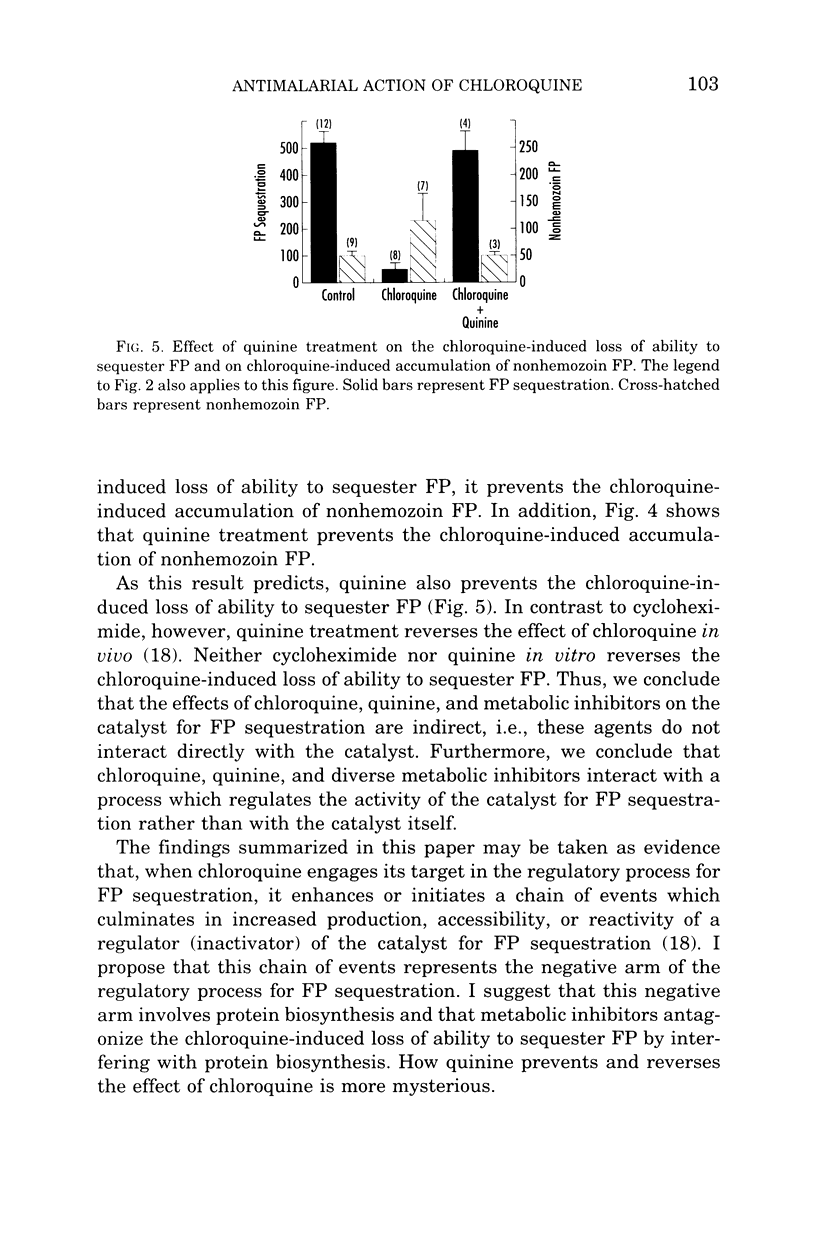
- Dorn A., Stoffel R., Matile H., Bubendorf A., Ridley R. G. Malarial haemozoin/beta-haematin supports haem polymerization in the absence of protein. Nature. 1995 Mar 16;374(6519):269–271. doi: 10.1038/374269a0. [DOI] [PubMed] [Google Scholar]
- Fitch C. D., Chevli R., Banyal H. S., Phillips G., Pfaller M. A., Krogstad D. J. Lysis of Plasmodium falciparum by ferriprotoporphyrin IX and a chloroquine-ferriprotoporphyrin IX complex. Antimicrob Agents Chemother. 1982 May;21(5):819–822. doi: 10.1128/aac.21.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch C. D., Chou A. C. Heat-labile and heat-stimulable heme polymerase activities in Plasmodium berghei. Mol Biochem Parasitol. 1996 Nov 25;82(2):261–264. doi: 10.1016/0166-6851(96)02744-2. [DOI] [PubMed] [Google Scholar]
- Fitch C. D., Chou A. C. Regulation of heme polymerizing activity and the antimalarial action of chloroquine. Antimicrob Agents Chemother. 1997 Nov;41(11):2461–2465. doi: 10.1128/aac.41.11.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch C. D., Kanjananggulpan P. The state of ferriprotoporphyrin IX in malaria pigment. J Biol Chem. 1987 Nov 15;262(32):15552–15555. [PubMed] [Google Scholar]
- Fitch C. D. Plasmodium falciparum in owl monkeys: drug resistance and chloroquine binding capacity. Science. 1970 Jul 17;169(3942):289–290. doi: 10.1126/science.169.3942.289. [DOI] [PubMed] [Google Scholar]
- Macomber P. B., O'Brien R. L., Hahn F. E. Chloroquine: physiological basis of drug resistance in Plasmodium berghei. Science. 1966 Jun 3;152(3727):1374–1375. doi: 10.1126/science.152.3727.1374. [DOI] [PubMed] [Google Scholar]
- Olliaro P. L., Goldberg D. E. The plasmodium digestive vacuole: metabolic headquarters and choice drug target. Parasitol Today. 1995 Aug;11(8):294–297. doi: 10.1016/0169-4758(95)80042-5. [DOI] [PubMed] [Google Scholar]
- Orjih A. U., Banyal H. S., Chevli R., Fitch C. D. Hemin lyses malaria parasites. Science. 1981 Nov 6;214(4521):667–669. doi: 10.1126/science.7027441. [DOI] [PubMed] [Google Scholar]
- Slater A. F., Cerami A. Inhibition by chloroquine of a novel haem polymerase enzyme activity in malaria trophozoites. Nature. 1992 Jan 9;355(6356):167–169. doi: 10.1038/355167a0. [DOI] [PubMed] [Google Scholar]
- Slater A. F., Swiggard W. J., Orton B. R., Flitter W. D., Goldberg D. E., Cerami A., Henderson G. B. An iron-carboxylate bond links the heme units of malaria pigment. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):325–329. doi: 10.1073/pnas.88.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan D. J., Jr, Gluzman I. Y., Goldberg D. E. Plasmodium hemozoin formation mediated by histidine-rich proteins. Science. 1996 Jan 12;271(5246):219–222. doi: 10.1126/science.271.5246.219. [DOI] [PubMed] [Google Scholar]


