Abstract
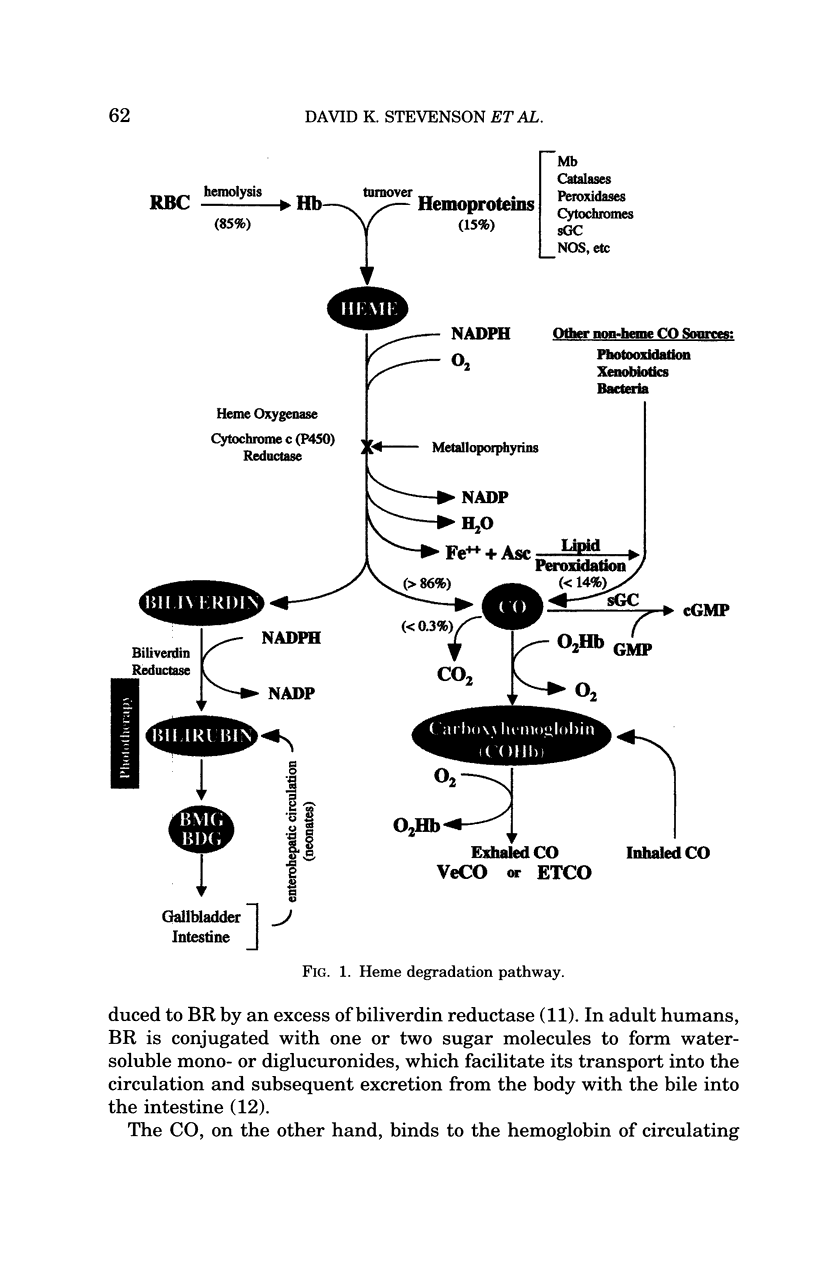
Even though the heme degradation pathway consists of only two reactions, it and its major enzyme (i.e. HO), nonetheless, impact other processes not only through the removal of excess heme, but also through the production of several metabolically active compounds. Thus CO and biliverdin along with reactive iron, Fe2, are the primordial products of this ancient, highly conserved reaction. That every component of the heme catabolic pathway is directly or indirectly related to other reactions involving oxygen or light is, perhaps, no accident of nature. That a fundamentally destructive event can be linked with a multiplicity of synthetic events and various biological effects, depending on the timing and location of the HO activity, is testament to the economy and the ultimate beauty of nature. Furthermore, the interaction of the heme catabolic pathway with that of the NOS system may lead to even more exciting avenues of research. It may be shown that the integrity of the heme catabolic pathway, which is ever present and plays a role in every tissue, is central to the existence of most complex organisms.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acevedo C. H., Ahmed A. Hemeoxygenase-1 inhibits human myometrial contractility via carbon monoxide and is upregulated by progesterone during pregnancy. J Clin Invest. 1998 Mar 1;101(5):949–955. doi: 10.1172/JCI927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam J., Cai J., Smith A. Isolation and characterization of the mouse heme oxygenase-1 gene. Distal 5' sequences are required for induction by heme or heavy metals. J Biol Chem. 1994 Jan 14;269(2):1001–1009. [PubMed] [Google Scholar]
- Appleton S. D., Chretien M. L., McLaughlin B. E., Vreman H. J., Stevenson D. K., Brien J. F., Nakatsu K., Maurice D. H., Marks G. S. Selective inhibition of heme oxygenase, without inhibition of nitric oxide synthase or soluble guanylyl cyclase, by metalloporphyrins at low concentrations. Drug Metab Dispos. 1999 Oct;27(10):1214–1219. [PubMed] [Google Scholar]
- Bartoletti A. L., Stevenson D. K., Ostrander C. R., Johnson J. D. Pulmonary excretion of carbon monoxide in the human infant as an index of bilirubin production. I. Effects of gestational and postnatal age and some common neonatal abnormalities. J Pediatr. 1979 Jun;94(6):952–955. doi: 10.1016/s0022-3476(79)80231-0. [DOI] [PubMed] [Google Scholar]
- Berk P. D., Blaschke T. F., Scharschmidt B. F., Waggoner J. G., Berlin N. I. A new approach to quantitation of the various sources of bilrubin in man. J Lab Clin Med. 1976 May;87(5):767–780. [PubMed] [Google Scholar]
- Berk P. D., Rodkey F. L., Blaschke T. F., Collison H. A., Waggoner J. G. Comparison of plasma bilirubin turnover and carbon monoxide production in man. J Lab Clin Med. 1974 Jan;83(1):29–37. [PubMed] [Google Scholar]
- Braughler J. M., Duncan L. A., Chase R. L. The involvement of iron in lipid peroxidation. Importance of ferric to ferrous ratios in initiation. J Biol Chem. 1986 Aug 5;261(22):10282–10289. [PubMed] [Google Scholar]
- COBURN R. F., DANIELSON G. K., BLAKEMORE W. S., FORSTER R. E., 2nd CARBON MONOXIDE IN BLOOD: ANALYTICAL METHOD AND SOURCES OF ERROR. J Appl Physiol. 1964 May;19:510–515. doi: 10.1152/jappl.1964.19.3.510. [DOI] [PubMed] [Google Scholar]
- Chan G. C., Lau Y. L., Yeung C. Y. End tidal carbon monoxide concentration in childhood haemolytic disorders. J Paediatr Child Health. 1998 Oct;34(5):447–450. doi: 10.1046/j.1440-1754.1998.00270.x. [DOI] [PubMed] [Google Scholar]
- Chowdhury J. R., Jansen P. L., Fischberg E. B., Daniller A., Arias I. M. Hepatic conversion of bilirubin monoglucuronide to diglucuronide in uridine diphosphate-glucuronyl transferase-deficient man and rat by bilirubin glucuronoside glucuronosyltransferase. J Clin Invest. 1978 Jul;62(1):191–196. doi: 10.1172/JCI109105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn R. F. Endogenous carbon monoxide production and body CO stores. Acta Med Scand Suppl. 1967;472:269–282. doi: 10.1111/j.0954-6820.1967.tb12633.x. [DOI] [PubMed] [Google Scholar]
- Coburn R. F., Williams W. J., White P., Kahn S. B. The production of carbon monoxide from hemoglobin in vivo. J Clin Invest. 1967 Mar;46(3):346–356. doi: 10.1172/JCI105536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R. S., Hopper A. O., Ostrander C. R., Stevenson D. K. Total bilirubin production in infants of Chinese, Japanese, and Korean ancestry. Taiwan Yi Xue Hui Za Zhi. 1982 Dec;81(12):1524–1529. [PubMed] [Google Scholar]
- Cornejo J., Beale S. I. Algal heme oxygenase from Cyanidium caldarium. Partial purification and fractionation into three required protein components. J Biol Chem. 1988 Aug 25;263(24):11915–11921. [PubMed] [Google Scholar]
- Dawson T. M., Snyder S. H. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J Neurosci. 1994 Sep;14(9):5147–5159. doi: 10.1523/JNEUROSCI.14-09-05147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doré S., Takahashi M., Ferris C. D., Zakhary R., Hester L. D., Guastella D., Snyder S. H. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc Natl Acad Sci U S A. 1999 Mar 2;96(5):2445–2450. doi: 10.1073/pnas.96.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fällström S. P. Endogenous formation of carbon monoxide in newborn infants. IV. On the relation between the blood carboxyhaemoglobin concentration and the pulmonary elimination of carbon monoxide. Acta Paediatr Scand. 1968 Jul;57(4):321–329. doi: 10.1111/j.1651-2227.1968.tb07300.x. [DOI] [PubMed] [Google Scholar]
- Hintz S. R., Vreman H. J., Stevenson D. K. Mortality of metalloporphyrin-treated neonatal rats after light exposure. Dev Pharmacol Ther. 1990;14(3):187–192. [PubMed] [Google Scholar]
- Holden H. M., Rypniewski W. R., Law J. H., Rayment I. The molecular structure of insecticyanin from the tobacco hornworm Manduca sexta L. at 2.6 A resolution. EMBO J. 1987 Jun;6(6):1565–1570. doi: 10.1002/j.1460-2075.1987.tb02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hronis T. S., Traugh J. A. Structural requirements for porphyrin inhibition of the hemin-controlled protein kinase and maintenance of protein synthesis in reticulocytes. J Biol Chem. 1986 May 15;261(14):6234–6238. [PubMed] [Google Scholar]
- Ignarro L. J., Ballot B., Wood K. S. Regulation of soluble guanylate cyclase activity by porphyrins and metalloporphyrins. J Biol Chem. 1984 May 25;259(10):6201–6207. [PubMed] [Google Scholar]
- Johnson R. A., Kozma F., Colombari E. Carbon monoxide: from toxin to endogenous modulator of cardiovascular functions. Braz J Med Biol Res. 1999 Jan;32(1):1–14. doi: 10.1590/s0100-879x1999000100001. [DOI] [PubMed] [Google Scholar]
- Kaplan M., Beutler E., Vreman H. J., Hammerman C., Levy-Lahad E., Renbaum P., Stevenson D. K. Neonatal hyperbilirubinemia in glucose-6-phosphate dehydrogenase-deficient heterozygotes. Pediatrics. 1999 Jul;104(1 Pt 1):68–74. doi: 10.1542/peds.104.1.68. [DOI] [PubMed] [Google Scholar]
- Kaplan M., Vreman H. J., Hammerman C., Leiter C., Rudensky B., MacDonald M. G., Stevenson D. K. Combination of ABO blood group incompatibility and glucose-6-phosphate dehydrogenase deficiency: effect on hemolysis and neonatal hyperbilirubinemia. Acta Paediatr. 1998 Apr;87(4):455–457. doi: 10.1080/08035259850157093. [DOI] [PubMed] [Google Scholar]
- Kappas A., Drummond G. S., Henschke C., Valaes T. Direct comparison of Sn-mesoporphyrin, an inhibitor of bilirubin production, and phototherapy in controlling hyperbilirubinemia in term and near-term newborns. Pediatrics. 1995 Apr;95(4):468–474. [PubMed] [Google Scholar]
- Kappas A., Drummond G. S., Manola T., Petmezaki S., Valaes T. Sn-protoporphyrin use in the management of hyperbilirubinemia in term newborns with direct Coombs-positive ABO incompatibility. Pediatrics. 1988 Apr;81(4):485–497. [PubMed] [Google Scholar]
- Kim C. B., Hintz S. R., Vreman H. J., Stevenson D. K. In vitro carbon monoxide production by the small intestine of suckling and adult Wistar rats: effect of parenteral tin-protoporphyrin. Dev Pharmacol Ther. 1988;11(3):166–172. doi: 10.1159/000457684. [DOI] [PubMed] [Google Scholar]
- Longo L. D. The biological effects of carbon monoxide on the pregnant woman, fetus, and newborn infant. Am J Obstet Gynecol. 1977 Sep 1;129(1):69–103. doi: 10.1016/0002-9378(77)90824-9. [DOI] [PubMed] [Google Scholar]
- Maines M. D. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988 Jul;2(10):2557–2568. [PubMed] [Google Scholar]
- Maines M. D., Ibrahim N. G., Kappas A. Solubilization and partial purification of heme oxygenase from rat liver. J Biol Chem. 1977 Aug 25;252(16):5900–5903. [PubMed] [Google Scholar]
- Maines M. D. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- Maines M. Carbon monoxide and nitric oxide homology: differential modulation of heme oxygenases in brain and detection of protein and activity. Methods Enzymol. 1996;268:473–488. doi: 10.1016/s0076-6879(96)68049-5. [DOI] [PubMed] [Google Scholar]
- Marks G. S., Brien J. F., Nakatsu K., McLaughlin B. E. Does carbon monoxide have a physiological function? Trends Pharmacol Sci. 1991 May;12(5):185–188. doi: 10.1016/0165-6147(91)90544-3. [DOI] [PubMed] [Google Scholar]
- McCoubrey W. K., Jr, Huang T. J., Maines M. D. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur J Biochem. 1997 Jul 15;247(2):725–732. doi: 10.1111/j.1432-1033.1997.00725.x. [DOI] [PubMed] [Google Scholar]
- McDonagh A. F., Palma L. A. Heme catabolism in fish. Bile pigments in gallbladder bile of the electric torpedo, Torpedo californicus. Comp Biochem Physiol B. 1982;73(3):501–507. doi: 10.1016/0305-0491(82)90066-9. [DOI] [PubMed] [Google Scholar]
- Meffert M. K., Haley J. E., Schuman E. M., Schulman H., Madison D. V. Inhibition of hippocampal heme oxygenase, nitric oxide synthase, and long-term potentiation by metalloporphyrins. Neuron. 1994 Nov;13(5):1225–1233. doi: 10.1016/0896-6273(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Meneghini R. Iron homeostasis, oxidative stress, and DNA damage. Free Radic Biol Med. 1997;23(5):783–792. doi: 10.1016/s0891-5849(97)00016-6. [DOI] [PubMed] [Google Scholar]
- Morita T., Perrella M. A., Lee M. E., Kourembanas S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1475–1479. doi: 10.1073/pnas.92.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami T., Toyomura K., Kinoshita T., Morisawa S. A beneficial role of bile pigments as an endogenous tissue protector: anti-complement effects of biliverdin and conjugated bilirubin. Biochim Biophys Acta. 1993 Oct 3;1158(2):189–193. doi: 10.1016/0304-4165(93)90013-x. [DOI] [PubMed] [Google Scholar]
- Prabhakar N. R., Dinerman J. L., Agani F. H., Snyder S. H. Carbon monoxide: a role in carotid body chemoreception. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):1994–1997. doi: 10.1073/pnas.92.6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers P. A., Seidman D. S., Wei P. L., Dennery P. A., Stevenson D. K. Duration of action and tissue distribution of zinc protoporphyrin in neonatal rats. Pediatr Res. 1996 Jun;39(6):1041–1049. doi: 10.1203/00006450-199606000-00018. [DOI] [PubMed] [Google Scholar]
- Rodgers P. A., Stevenson D. K. Developmental biology of heme oxygenase. Clin Perinatol. 1990 Jun;17(2):275–291. [PubMed] [Google Scholar]
- Rodgers P. A., Vreman H. J., Dennery P. A., Stevenson D. K. Sources of carbon monoxide (CO) in biological systems and applications of CO detection technologies. Semin Perinatol. 1994 Feb;18(1):2–10. [PubMed] [Google Scholar]
- Rodgers P. A., Vreman H. J., Stevenson D. K. Heme catabolism in rhesus neonates inhibited by zinc protoporphyrin. Dev Pharmacol Ther. 1990;14(4):216–222. [PubMed] [Google Scholar]
- Ruggiero-Lopez D., Servetto C., Lopez E., Lenoir D., Alallon W., Biol M. C., Louisot P., Martin A. Comparative effects of dietary corn, fish and Krill oils on intestinal glycosylation. Biochem Mol Biol Int. 1994 Aug;33(5):1001–1010. [PubMed] [Google Scholar]
- SJOSTRAND T. The formation of carbon monoxide by the decomposition of haemoglobin in vivo. Acta Physiol Scand. 1952;26(4):338–344. doi: 10.1111/j.1748-1716.1952.tb00915.x. [DOI] [PubMed] [Google Scholar]
- Seidman D. S., Shiloh M., Stevenson D. K., Vreman H. J., Gale R. Role of hemolysis in neonatal jaundice associated with glucose-6 phosphate dehydrogenase deficiency. J Pediatr. 1995 Nov;127(5):804–806. doi: 10.1016/s0022-3476(95)70177-x. [DOI] [PubMed] [Google Scholar]
- Slusher T. M., Vreman H. J., McLaren D. W., Lewison L. J., Brown A. K., Stevenson D. K. Glucose-6-phosphate dehydrogenase deficiency and carboxyhemoglobin concentrations associated with bilirubin-related morbidity and death in Nigerian infants. J Pediatr. 1995 Jan;126(1):102–108. doi: 10.1016/s0022-3476(95)70510-4. [DOI] [PubMed] [Google Scholar]
- Stevenson D. K., Bartoletti A. L., Ostrander C. R., Johnson J. D. Pulmonary excretion of carbon monoxide in the human infant as an index of bilirubin production. II. Infants of diabetic mothers. J Pediatr. 1979 Jun;94(6):956–958. doi: 10.1016/s0022-3476(79)80232-2. [DOI] [PubMed] [Google Scholar]
- Stevenson D. K., Rodgers P. A., Vreman H. J. The use of metalloporphyrins for the chemoprevention of neonatal jaundice. Am J Dis Child. 1989 Mar;143(3):353–356. doi: 10.1001/archpedi.1989.02150150111027. [DOI] [PubMed] [Google Scholar]
- Stocker R., Yamamoto Y., McDonagh A. F., Glazer A. N., Ames B. N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987 Feb 27;235(4792):1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Suttner D. M., Dennery P. A. Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J. 1999 Oct;13(13):1800–1809. doi: 10.1096/fasebj.13.13.1800. [DOI] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969 Dec 10;244(23):6388–6394. [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler R. F. Synthesis of bile pigments in plants. Formation of carbon monoxide and phycocyanobilin in wild-type and mutant strains of the alga, Cyanidium caldarium. Biochemistry. 1972 Nov 7;11(23):4235–4242. doi: 10.1021/bi00773a007. [DOI] [PubMed] [Google Scholar]
- Uetani Y., Nakamura H., Okamoto O., Yamazaki T., Vreman H. J., Stevenson D. K. Carboxyhemoglobin measurements in the diagnosis of ABO hemolytic disease. Acta Paediatr Jpn. 1989 Apr;31(2):171–176. doi: 10.1111/j.1442-200x.1989.tb01285.x. [DOI] [PubMed] [Google Scholar]
- Vallier H. A., Rodgers P. A., Castillo R. O., Stevenson D. K. Absorption of zinc deuteroporphyrin IX 2,4-bis-glycol by the neonatal rat small intestine in vivo. Dev Pharmacol Ther. 1991;17(1-2):109–115. doi: 10.1159/000457506. [DOI] [PubMed] [Google Scholar]
- Vreman H. J., Cipkala D. A., Stevenson D. K. Characterization of porphyrin heme oxygenase inhibitors. Can J Physiol Pharmacol. 1996 Mar;74(3):278–285. [PubMed] [Google Scholar]
- Vreman H. J., Ekstrand B. C., Stevenson D. K. Selection of metalloporphyrin heme oxygenase inhibitors based on potency and photoreactivity. Pediatr Res. 1993 Feb;33(2):195–200. doi: 10.1203/00006450-199302000-00021. [DOI] [PubMed] [Google Scholar]
- Vreman H. J., Gillman M. J., Downum K. R., Stevenson D. K. In vitro generation of carbon monoxide from organic molecules and synthetic metalloporphyrins mediated by light. Dev Pharmacol Ther. 1990;15(2):112–124. doi: 10.1159/000457630. [DOI] [PubMed] [Google Scholar]
- Vreman H. J., Hintz S. R., Kim C. B., Castillo R. O., Stevenson D. K. Effects of oral administration of tin and zinc protoporphyrin on neonatal and adult rat tissue heme oxygenase activity. J Pediatr Gastroenterol Nutr. 1988 Nov-Dec;7(6):902–906. doi: 10.1097/00005176-198811000-00019. [DOI] [PubMed] [Google Scholar]
- Vreman H. J., Mahoney J. J., Stevenson D. K. Carbon monoxide and carboxyhemoglobin. Adv Pediatr. 1995;42:303–334. [PubMed] [Google Scholar]
- Vreman H. J., Rodgers P. A., Gale R., Stevenson D. K. Carbon monoxide excretion as an index of bilirubin production in rhesus monkeys. J Med Primatol. 1989;18(6):449–460. [PubMed] [Google Scholar]
- Vreman H. J., Rodgers P. A., Stevenson D. K. Zinc protoporphyrin administration for suppression of increased bilirubin production by iatrogenic hemolysis in rhesus neonates. J Pediatr. 1990 Aug;117(2 Pt 1):292–297. doi: 10.1016/s0022-3476(05)80550-5. [DOI] [PubMed] [Google Scholar]
- Vreman H. J., Stevenson D. K., Henton D., Rosenthal P. Correlation of carbon monoxide and bilirubin production by tissue homogenates. J Chromatogr. 1988 Jun 3;427(2):315–319. doi: 10.1016/0378-4347(88)80134-8. [DOI] [PubMed] [Google Scholar]
- Vreman H. J., Wong R. J., Sanesi C. A., Dennery P. A., Stevenson D. K. Simultaneous production of carbon monoxide and thiobarbituric acid reactive substances in rat tissue preparations by an iron-ascorbate system. Can J Physiol Pharmacol. 1998 Dec;76(12):1057–1065. doi: 10.1139/cjpp-76-12-1057. [DOI] [PubMed] [Google Scholar]
- Vreman H. J., Wong R. J., Stevenson D. K., Route R. K., Reader S. D., Fejer M. M., Gale R., Seidman D. S. Light-emitting diodes: a novel light source for phototherapy. Pediatr Res. 1998 Nov;44(5):804–809. doi: 10.1203/00006450-199811000-00027. [DOI] [PubMed] [Google Scholar]
- Wang R. Resurgence of carbon monoxide: an endogenous gaseous vasorelaxing factor. Can J Physiol Pharmacol. 1998 Jan;76(1):1–15. doi: 10.1139/cjpp-76-1-1. [DOI] [PubMed] [Google Scholar]
- Wolff D. G. The formation of carbon monoxide during peroxidation of microsomal lipids. Biochem Biophys Res Commun. 1976 Dec 20;73(4):850–857. doi: 10.1016/0006-291x(76)90199-6. [DOI] [PubMed] [Google Scholar]
- Wranne L. Studies on erythro-kinetics in infancy. VII. Quantitative estimation of the haemoglobin catabolism by carbon monoxide technique in young infants. Acta Paediatr Scand. 1967 Jul;56(4):381–390. doi: 10.1111/j.1651-2227.1967.tb15396.x. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Noguchi M., Kikuchi G. The step of carbon monoxide liberation in the sequence of heme degradation catalyzed by the reconstituted microsomal heme oxygenase system. J Biol Chem. 1982 Aug 25;257(16):9345–9348. [PubMed] [Google Scholar]